Effect of Soil Organic Matters in Dredged Soils to Utilization of their Mixtures Made with a Steel Slag
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Dredged Soils
2.1.2. Steel Slag
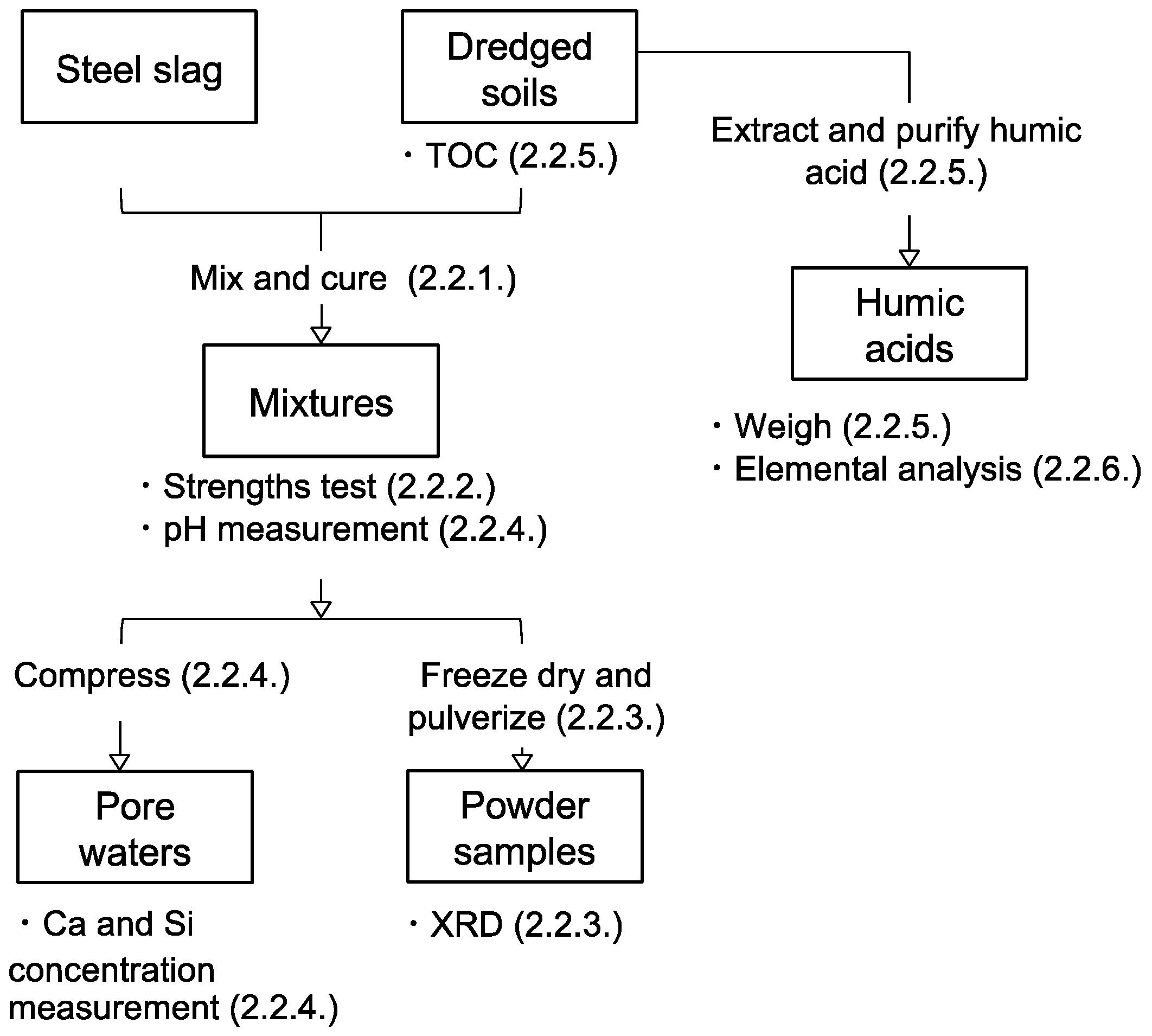
2.2. Methods
2.2.1. Preparation of Steel Slag-Dredged Soil Mixtures
2.2.2. Unconfined Compressive Strength Tests
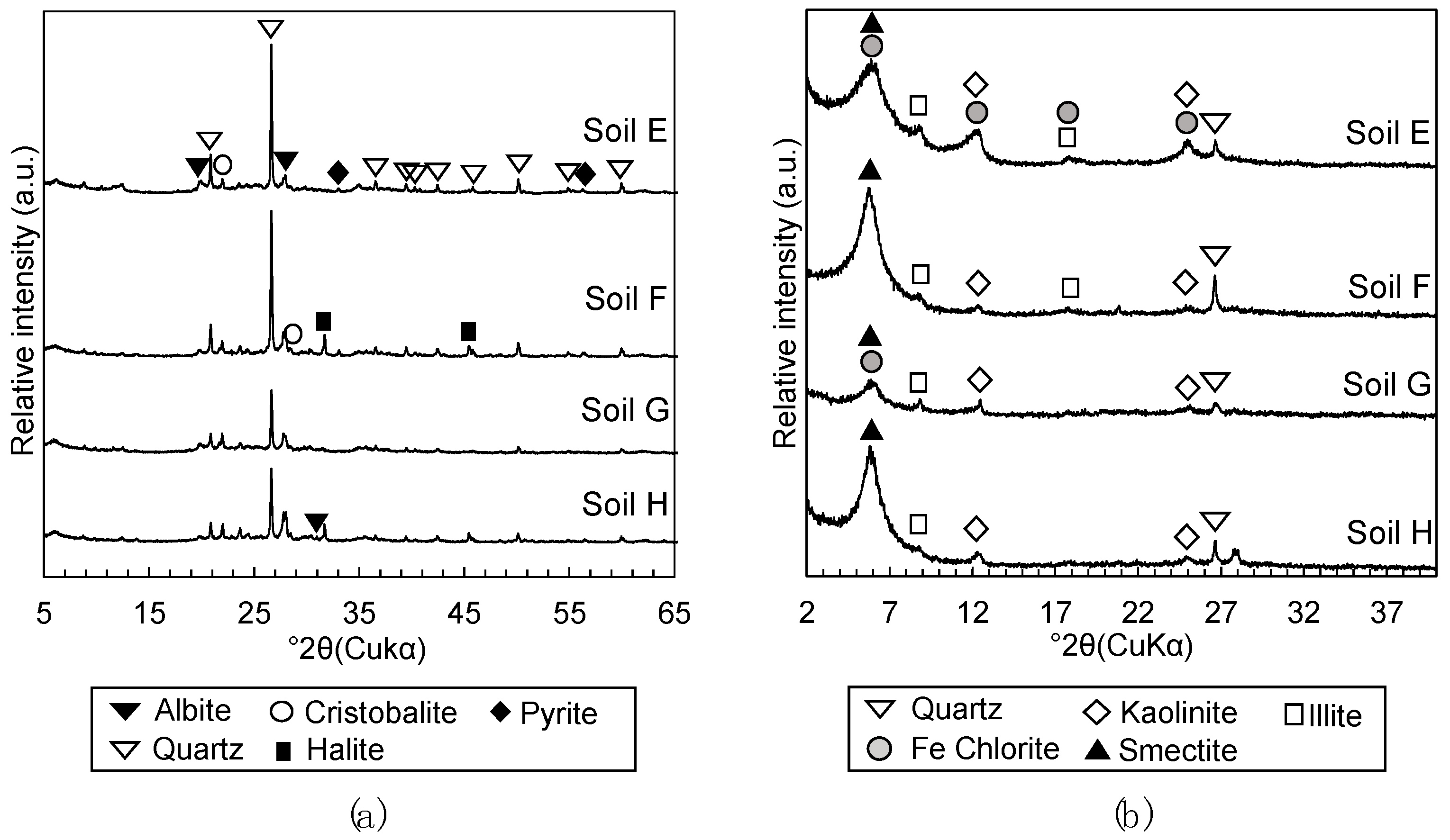
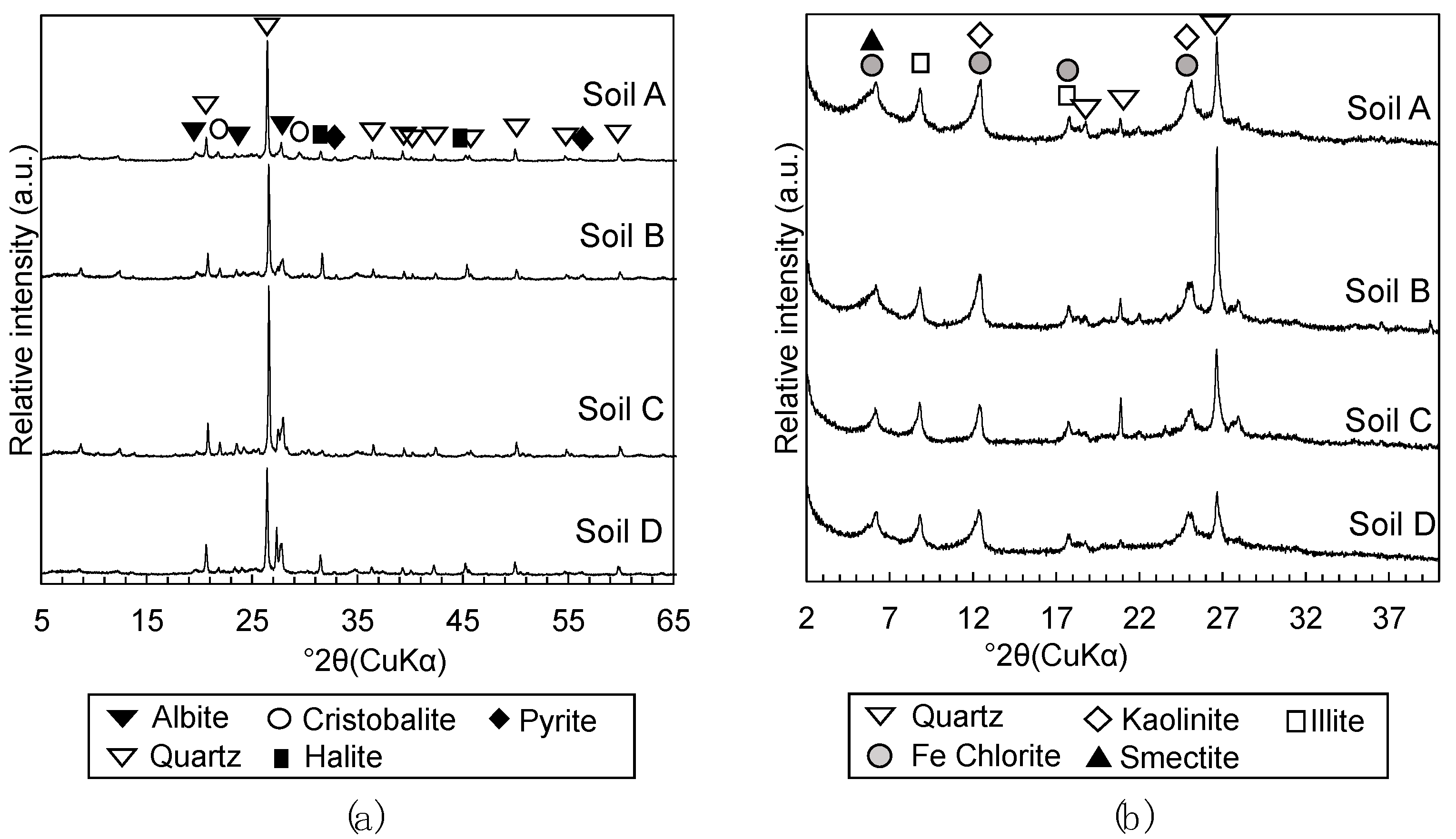
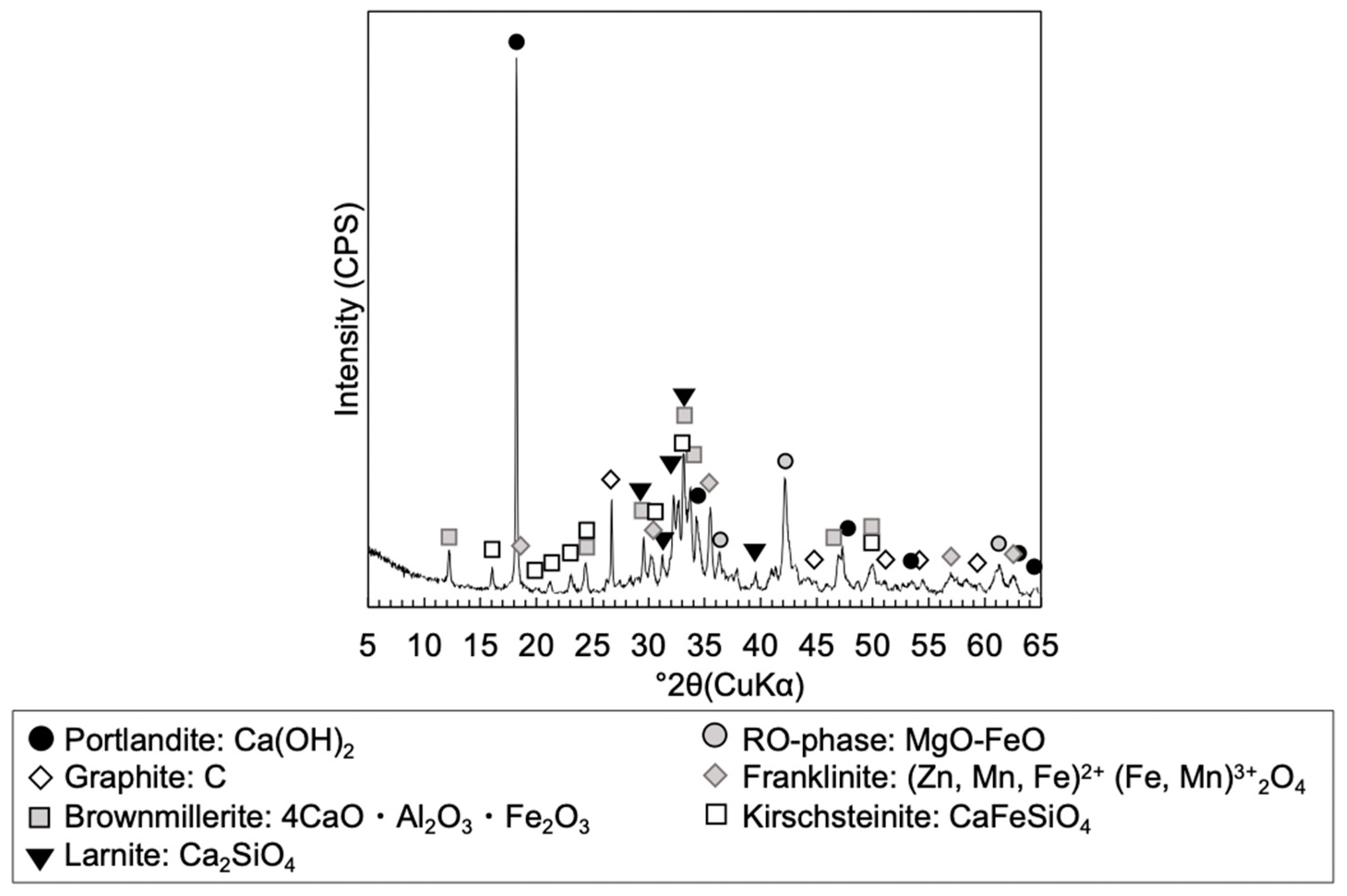
2.2.3. Mineralogical Phases Assemblage
2.2.4. Chemistry of the Pore Water Solutions
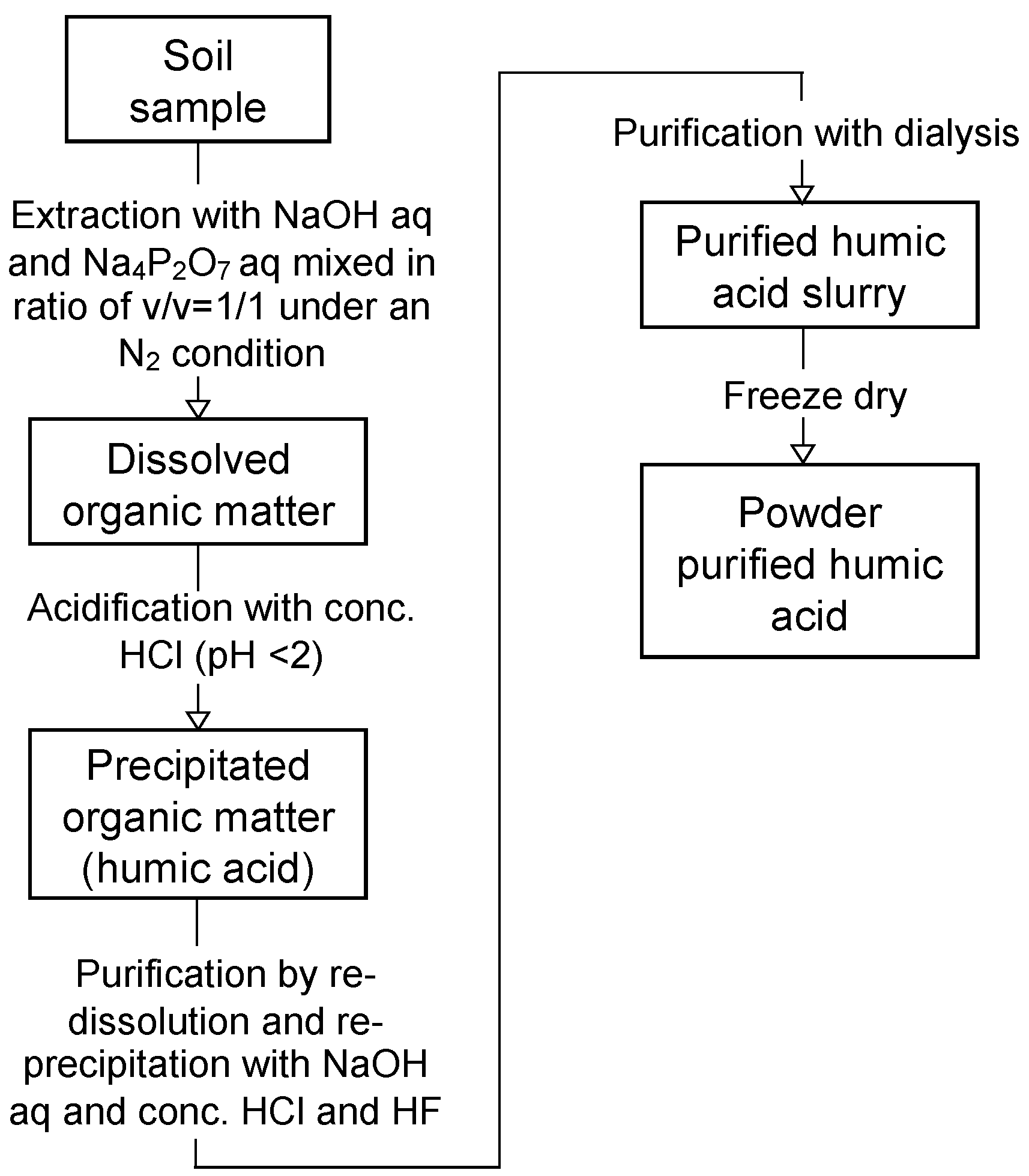
2.2.5. Quantification of Soil Organic Matter and Humic Acids in the Dredged Soils
2.2.6. Elemental Compositions of Humic Acids
3. Results
3.1. Characterization of the Mixtures
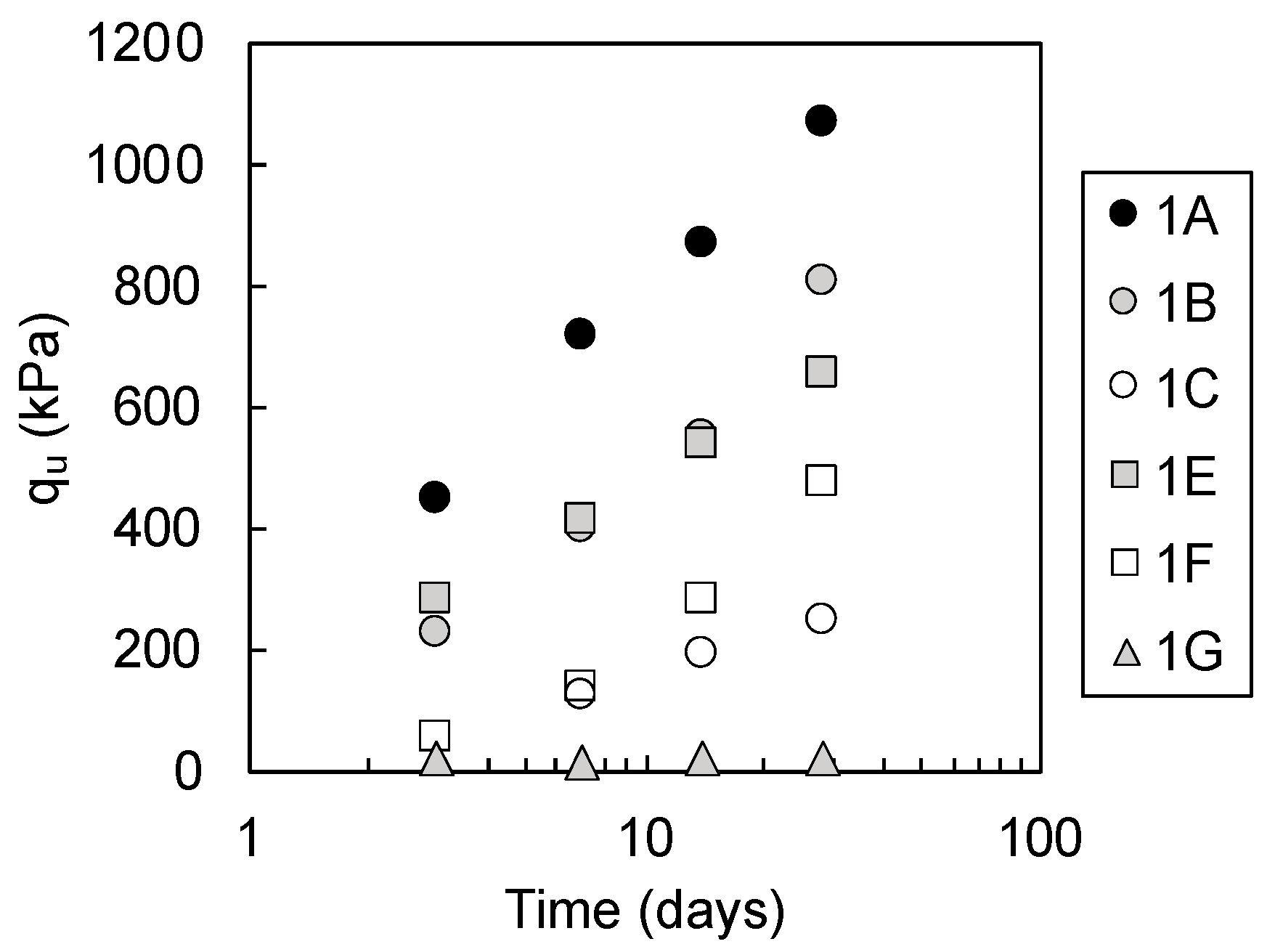
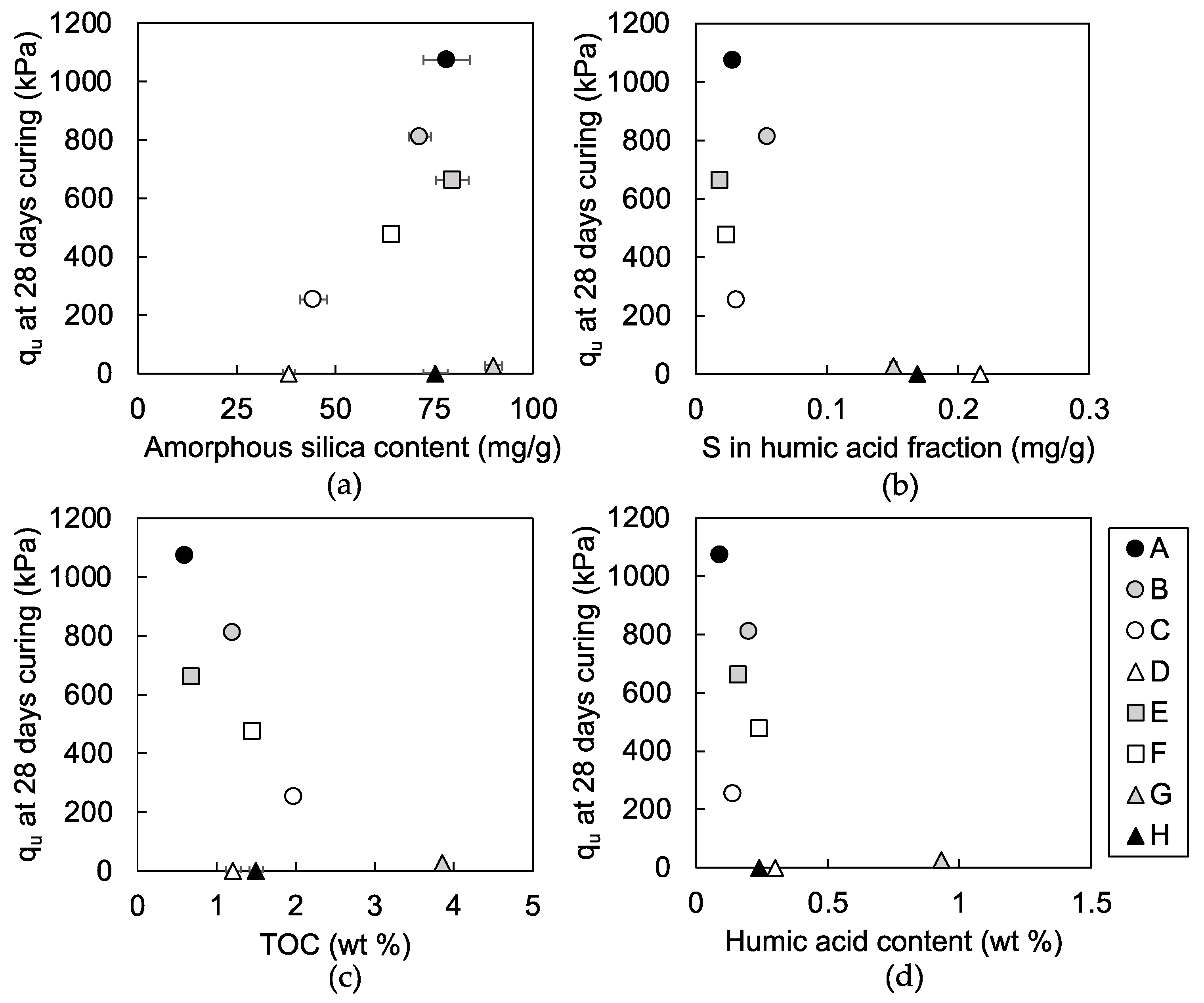
3.1.1. Unconfined Compressive Strength
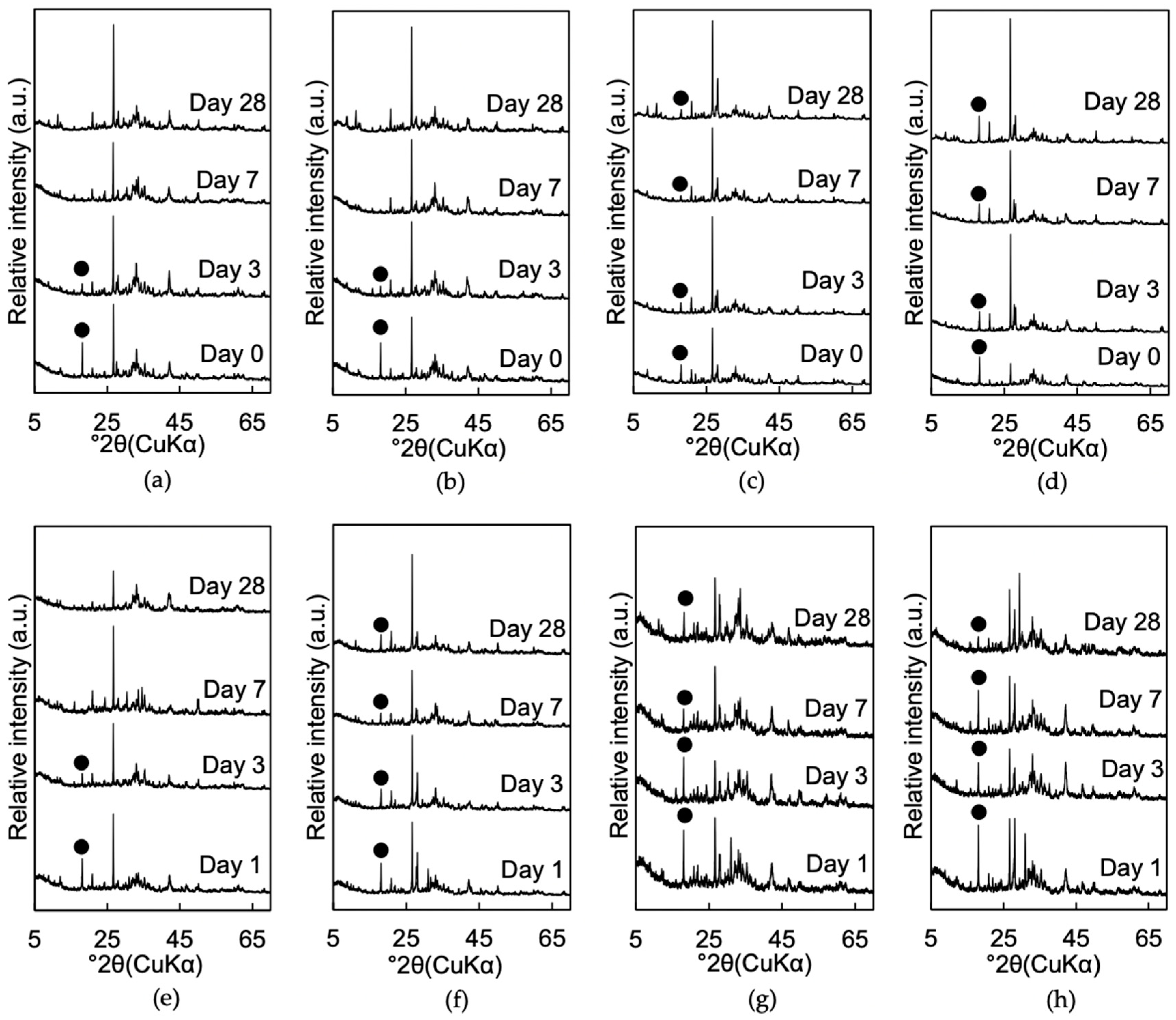
3.1.2. Mineralogical Phase Assemblages
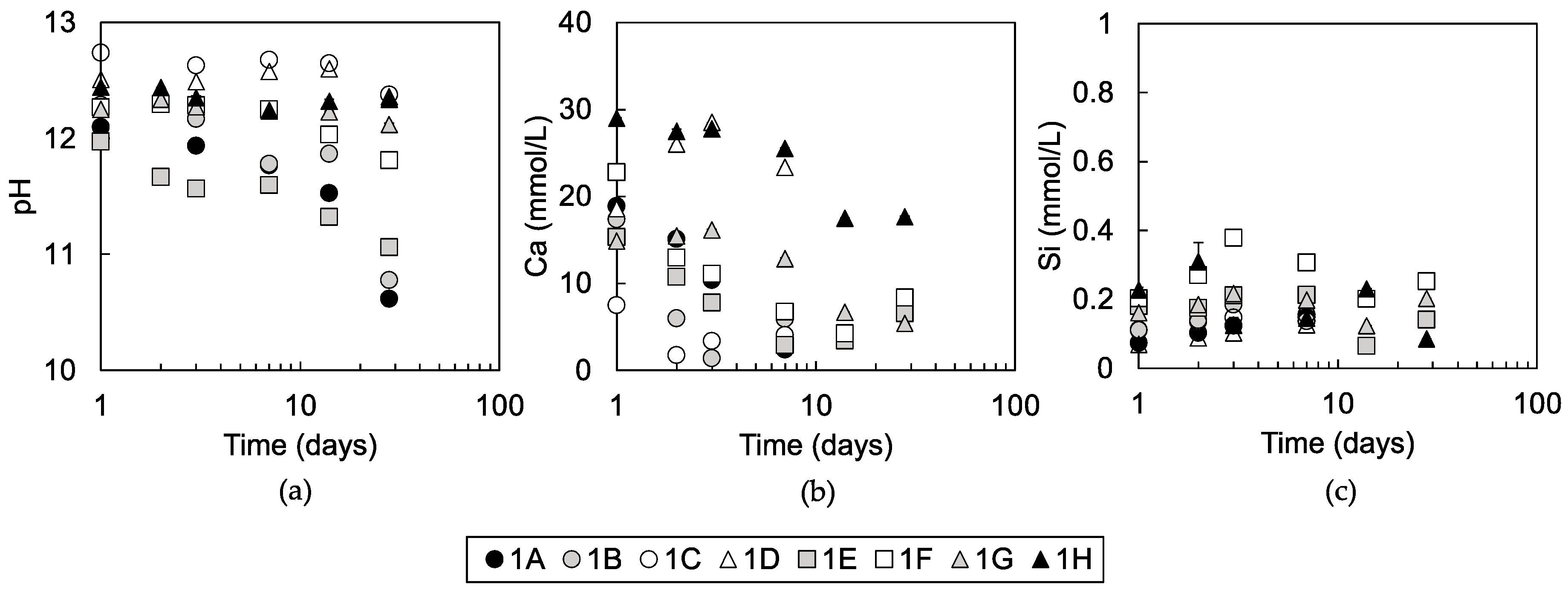
3.1.3. Solution Chemistry of Pore Water
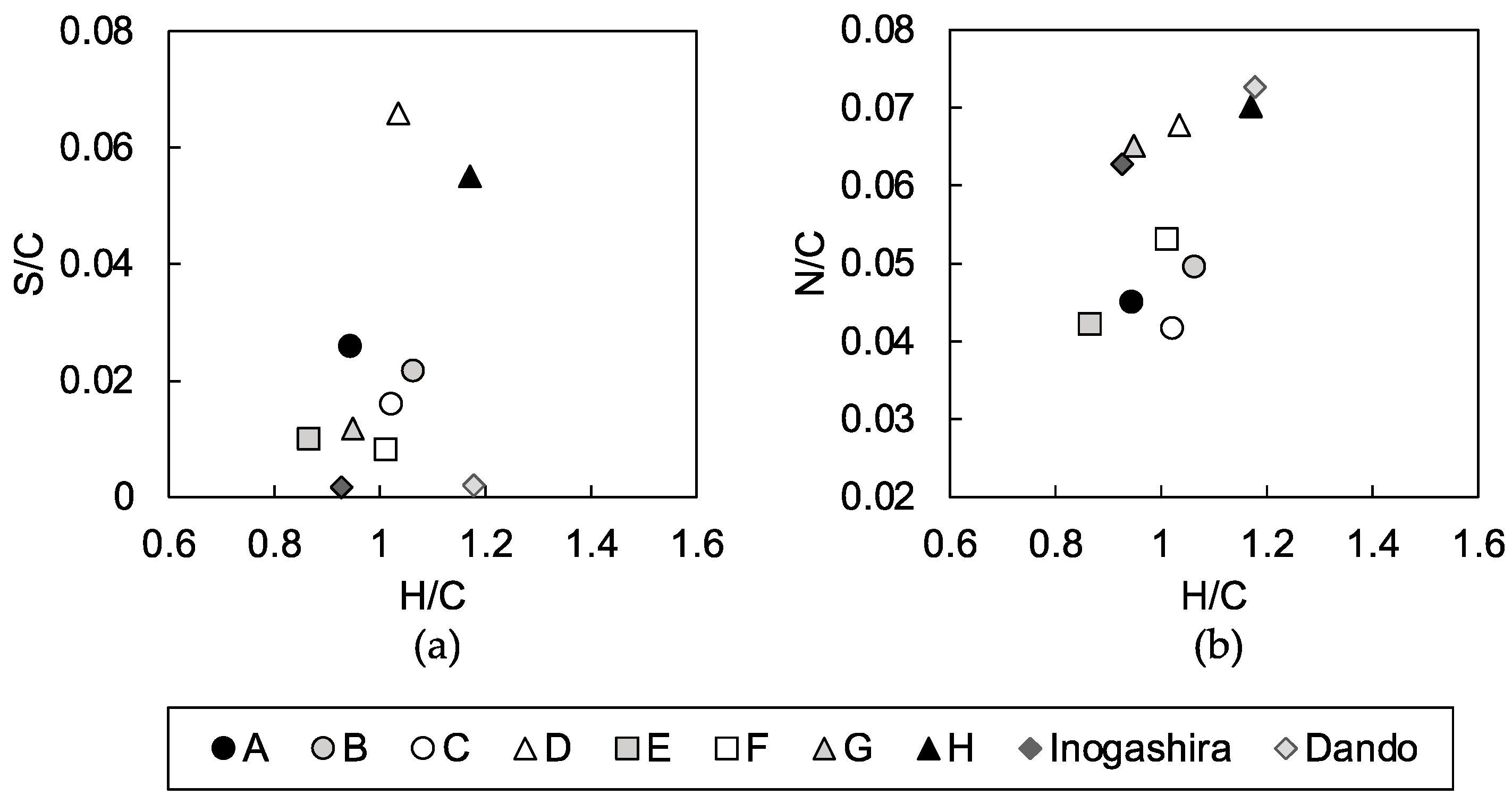
3.2. Characterization of Soil Organic Matter in Dredged Soils
4. Discussion
4.1. Indicators for Strength Development of the Steel Slag-Dredged Soil Mixtures
4.2. Characteristics of Humic Acids that Inhibit Strength Development of the Mixtures
4.3. Effects of Soil Organic Matters on Pozzolanic Reaction
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Onorati, F.; Mugnai, C.; Pulcini, M.; Gabellini, M. A framework for the integrated assessment and management of dredged materials in Italy: A case study based on the application of Local Sediment Quality Guidelines. J. Soils Sediments 2013, 13, 474–487. [Google Scholar] [CrossRef]
- Bates, M.E.; Fox-Lent, C.; Seymour, L.; Wender, B.A.; Linkov, I. Life cycle assessment for dredged sediment placement strategies. Sci. Total Environ. 2015, 511, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Land, Infrastructures, Transport and Tourism, Japan. On the Development of Technical Guideline on Ocean Disposal and Effective use of the Dredged Soils. 2006. Available online: https://www.mlit.go.jp/kisha/kisha06/11/110619/01.pdf (accessed on 15 October 2020). (In Japanese)
- Ministry of land, Infrastructures, Transport and Tourism, Ports, and Harbors Bureau. Recycle Guidelines for Port/Airport Maintenance (Revised). 2018. Available online: https://www.mlit.go.jp/common/001231697.pdf (accessed on 13 October 2020). (In Japanese)
- Mymrin, V.; Stella, J.C.; Scremim, C.B.; Pan, R.C.Y.; Sanches, F.G.; Alekseev, K.; Pedroso, D.E.; Molinetti, A.; Fortini, O.M. Utilization of sediments dredged from marine ports as a principal component of composite material. J. Clean. Prod. 2017, 142, 4041–4049. [Google Scholar] [CrossRef]
- Lirer, S.; Liguori, B.; Capasso, I.; Flora, A.; Caputo, D. Mechanical and chemical properties of composite materials made of dredged sediments in a fly-ash based geopolymer. J. Environ. Manag. 2017, 191, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mymrin, V.; Pan, R.C.Y.; Alekseev, K.; Avanci, M.A.; Stella, J.C.; Scremim, C.B.; Schiavini, D.N.; Pinto, L.S.; Berton, R.; Weber, S.L. Overburden soil and marine dredging sludge utilization for production of new composites as highly efficient environmental management. J. Environ. Manag. 2019, 236, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Ahn, J.; Han, W.J.; Gabr, M.A. Experimental evaluation of strength characteristics of stabilized dredged soil. J. Mater. Civ. Eng. 2010, 22, 539–544. [Google Scholar] [CrossRef]
- Tsuchida, T.; Porbaha, A.; Yamane, N. Development of a geomaterial from dredged bay mud. J. Mater. Civ. Eng. 2001, 13, 152–160. [Google Scholar] [CrossRef]
- Kiso, E.; Tsujii, M.; Ito, K.; Nakagawa, M.; Gomyo, M.; Nagatome, T. Method of dredged soil improvement by mixing with converter steel-making slag. Proc. Civ. Eng. Ocean. 2008, 24, 327–332. [Google Scholar] [CrossRef]
- Miraoui, M.; Zentar, R.; Abriak, N.E. Road material basis in dredged sediment and basic oxygen furnace steel slag. Constr. Build. Mater. 2012, 30, 309–319. [Google Scholar] [CrossRef]
- Weerakoon, N.R.; Nishimura, S.; Sato, H.; Toda, K.; Sato, T.; Arai, Y. Stiffness and strength mobilisation in steel-slag-mixed dredged clays in early curing. Proc. Inst. Civ. Eng. Gr. Improv. 2018, 1–17. [Google Scholar] [CrossRef]
- Toda, K.; Sato, H.; Weerakoon, N.; Otake, T.; Nishimura, S.; Sato, T. Key factors affecting strength development of steel slag-dredged soil mixtures. Minerals 2018, 8, 174. [Google Scholar] [CrossRef]
- Lothenbach, B.; Nonat, A. Calcium silicate hydrates: Solid and liquid phase composition. Cem. Concr. Res. 2015, 78, 57–70. [Google Scholar] [CrossRef]
- Tremblay, H.; Duchesne, J.; Locat, J.; Leroueil, S. Influence of the nature of organic compounds on fine soil stabilization with cement. Can. Geotech. J. 2002, 39, 535–546. [Google Scholar] [CrossRef]
- Kang, G.-O.; Tsuchida, T.; Kim, Y.-S.; Baek, W.-J. Influence of humic acid on the strength behavior of cement-treated clay during various curing stages. J. Mater. Civ. Eng. 2017, 29, 1–18. [Google Scholar] [CrossRef]
- Schulten, H.R.; Schnitzer, M. Three-dimensional models for humic acids and soil organic matter. Naturwissenschaften 1995, 82, 487–498. [Google Scholar] [CrossRef]
- Sutton, R.; Sposito, G. Molecular structure in soil humic substances: The new view. Environ. Sci. Technol. 2005, 39, 9009–9015. [Google Scholar] [CrossRef]
- Rice, J.A.; MacCarthy, P. Statistical evaluation of the elemental composition of humic substances. Org. Geochem. 1991, 17, 635–648. [Google Scholar] [CrossRef]
- Avena, M.J.; Koopal, L.K.; Van Riemsdijk, W.H. Proton binding to humic acids: Electrostatic and intrinsic interactions. J. Colloid Interface Sci. 1999, 217, 37–48. [Google Scholar] [CrossRef]
- Blake, G.R. Particle density. In Encyclopedia of Soil Science; Chesworth, W., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 504–505. ISBN 978-1-4020-3995-9. [Google Scholar] [CrossRef]
- Mortlock, R.A.; Froelich, P.N. A simple method for the rapid determination of biogenic opal in pelagic marine sediments. Deep Sea Res. Part. A Oceanogr. Res. Pap. 1989, 36, 1415–1426. [Google Scholar] [CrossRef]
- Kodama, H. Identification and quantification of non-crystalline inorganic minerals in soils by selective chemical dissolution method. Chisitsu News 1995, 496, 26–35. [Google Scholar]
- Fukushima, M.; Yamamoto, M.; Komai, T.; Yamamoto, K. Studies of structural alterations of humic acids from conifer bark residue during composting by pyrolysis-gas chromatography/mass spectrometry using tetramethylammonium hydroxide (TMAH-py-GC/MS). J. Anal. Appl. Pyrolysis 2009, 86, 200–206. [Google Scholar] [CrossRef]
- Fukushima, M.; Yamamoto, K.; Ootsuka, K.; Komai, T.; Aramaki, T.; Ueda, S.; Horiya, S. Effects of the maturity of wood waste compost on the structural features of humic acids. Bioresour. Technol. 2009, 100, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Obara, H.; Takata, Y.; Kohyama, K.; Ohkura, T.; Maejima, Y.; Watabayashi, S.; Kanda, T. A new soil map of Japan based on comprehensive soil classification system of Japan First Approximation. Bull. Natl. Inst. Agro-Environ. Sci. 2016, 37, 133–148. [Google Scholar]
- Kuwatsuka, S.; Watanabe, A.; Itoh, K.; Arai, S. Comparision of Two Methods of Preparation of Humic and Fulvic Acids, IHSS Method and NAGOYA Method. Soil Sci. Plant. Nutr. 1992, 38, 23–30. [Google Scholar] [CrossRef]
- Watanabe, A.; Itoh, K.; Arai, S.; Kuwatsuka, S. Comparison of the composition of humic and fulvic acids prepared by the IHSS method and NAGOYA method. Soil Sci. Plant. Nutr. 1994, 40, 601–608. [Google Scholar] [CrossRef]
- Coastal Development Institute of Technology. Technical Manual for Utilizing Calcia Modified Soil at Ports, Airports and Coasts; Coastal Development Institute of Technology: Tokyo, Japan, 2017; pp. 8:2–8:4. (In Japanese) [Google Scholar]
- Grangeon, S.; Claret, F.; Roosz, C.; Sato, T.; Gaboreau, S.; Linard, Y. Structure of nanocrystalline calcium silicate hydrates: Insights from X-ray diffraction, synchrotron X-ray absorption and nuclear magnetic resonance research papers. J. Appl. Crystallogr. 2016, 49, 1–14. [Google Scholar] [CrossRef]
- Stuermer, D.H.; Peters, K.E.; Kaplan, I.R. Source indicators of humic substances and proto-kerogen. Stable isotope ratios, elemental compositions and electron spin resonance spectra. Geochim. Cosmochim. Acta 1978, 42, 989–997. [Google Scholar] [CrossRef]
- Giovanela, M.; Parlanti, E.; Soriano-Sierra, E.J.; Soldi, M.S.; Sierra, M.M.D. Elemental compositions, FT-IR spectral and thermal behavior of sedimentary fulvic and humic acids from aquatic and terrestrial environments. Geochem. J. 2004, 38, 255–264. [Google Scholar] [CrossRef]
- Vairavamurthy, A.; Mopper, K. Geochemical formation of organosulphur compounds (thiols) by addition of H2S to sedimentary organic matter. Nature 1987, 329, 623–625. [Google Scholar] [CrossRef]
- Ferdelman, T.G.; Church, T.M.; Luther, G.W. Sulfur enrichment of humic substances in a Delaware salt marsh sediment core. Geochim. Cosmochim. Acta 1991, 55, 979–988. [Google Scholar] [CrossRef]
- Vairavamurthy, M.A.; Maletic, D.; Wang, S.; Manowitz, B.; Eglinton, T.; Lyons, T. Characterization of sulfur-containing functional groups in sedimentary humic substances by X-ray absorption near-edge structure spectroscopy. Energy Fuels 1997, 11, 546–553. [Google Scholar] [CrossRef]
- Milne, C.J.; Kinniburgh, D.G.; De Wit, J.C.; Van Riemsdijk, W.H.; Koopal, L.K. Analysis of metal-ion binding by a peat humic acid using a simple electrostatic model. J. Colloid Interface Sci. 1995, 175, 448–460. [Google Scholar] [CrossRef]
- Plank, J.; Gretz, M. Study on the interaction between anionic and cationic latex particles and Portland cement. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 330, 227–233. [Google Scholar] [CrossRef]
- Thomas, N.L.; Birchall, J.D. The retarding action of sugars on cement hydration. Cem. Concr. Res. 1983, 13, 830–842. [Google Scholar] [CrossRef]
- Plank, J.; Hirsch, C. Impact of zeta potential of early cement hydration phases on superplasticizer adsorption. Cem. Concr. Res. 2007, 37, 537–542. [Google Scholar] [CrossRef]
| Samples | Soil Particle Density (g/cm3) | Liquid Limit | Content of Fine Particle (<0.075 mm) (%) |
|---|---|---|---|
| Soil A (1) | 2.777 | 73.4 | 83.2 |
| Soil B (1) | 2.737 | 89.8 | 99.3 |
| Soil C (1) | 2.709 | 44.1 | 91.1 |
| Soil D (1) | 2.707 | 66.2 | 58.6 |
| Soil E | 2.721 | 80.0 | 75.5 |
| Soil F | 2.655 | 54.5 | 86.3 |
| Soil G | 2.544 | 96.1 | 99.3 |
| Soil H | 2.629 | 66.1 | 63.5 |
| Samples | TOC | Humic Acid Content | Humic Acid Elemental Composition (wt.%) (1) | ||||
|---|---|---|---|---|---|---|---|
| (wt. %) | (wt. %) | C | H | N | S | O (3) | |
| Soil A | 0.60 ± 0.01 | 0.09 (2) | 45.55 | 3.61 | 2.39 | 3.14 | 45.31 |
| Soil B | 1.20 ± 0.01 | 0.20 (2) | 47.26 | 4.22 | 2.73 | 2.72 | 43.07 |
| Soil C | 1.97 ± 0.01 | 0.14 (2) | 52.03 | 4.46 | 2.53 | 2.20 | 38.78 |
| Soil D | 1.21 ± 0.10 | 0.30 (2) | 41.12 | 3.57 | 3.25 | 7.23 | 44.83 |
| Soil E | 0.68 ± 0.01 | 0.16 | 43.33 | 3.15 | 2.13 | 1.14 | 50.25 |
| Soil F | 1.46 ± 0.04 | 0.24 | 46.17 | 3.92 | 2.86 | 0.99 | 46.06 |
| Soil G | 3.86 ± 0.01 | 0.93 | 51.76 | 4.12 | 3.93 | 1.62 | 38.57 |
| Soil H | 1.50 ± 0.08 | 0.24 | 47.88 | 4.70 | 3.92 | 7.04 | 36.46 |
| Inogashira (4) | 16.7 | 5.10 | 54.83 | 4.27 | 4.01 | 0.26 | 36.63 |
| Dando (4) | 6.19 | 0.69 | 53.04 | 5.25 | 4.49 | 0.29 | 36.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toda, K.; Kikuchi, R.; Otake, T.; Nishimura, S.; Akashi, Y.; Aimoto, M.; Kokado, T.; Sato, T. Effect of Soil Organic Matters in Dredged Soils to Utilization of their Mixtures Made with a Steel Slag. Materials 2020, 13, 5450. https://doi.org/10.3390/ma13235450
Toda K, Kikuchi R, Otake T, Nishimura S, Akashi Y, Aimoto M, Kokado T, Sato T. Effect of Soil Organic Matters in Dredged Soils to Utilization of their Mixtures Made with a Steel Slag. Materials. 2020; 13(23):5450. https://doi.org/10.3390/ma13235450
Chicago/Turabian StyleToda, Kanako, Ryosuke Kikuchi, Tsubasa Otake, Satoshi Nishimura, Yuzoh Akashi, Michihiro Aimoto, Takeshi Kokado, and Tsutomu Sato. 2020. "Effect of Soil Organic Matters in Dredged Soils to Utilization of their Mixtures Made with a Steel Slag" Materials 13, no. 23: 5450. https://doi.org/10.3390/ma13235450
APA StyleToda, K., Kikuchi, R., Otake, T., Nishimura, S., Akashi, Y., Aimoto, M., Kokado, T., & Sato, T. (2020). Effect of Soil Organic Matters in Dredged Soils to Utilization of their Mixtures Made with a Steel Slag. Materials, 13(23), 5450. https://doi.org/10.3390/ma13235450






