Orientation Dependence of Cathodoluminescence and Photoluminescence Spectroscopy of Defects in Chemical-Vapor-Deposited Diamond Microcrystal
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
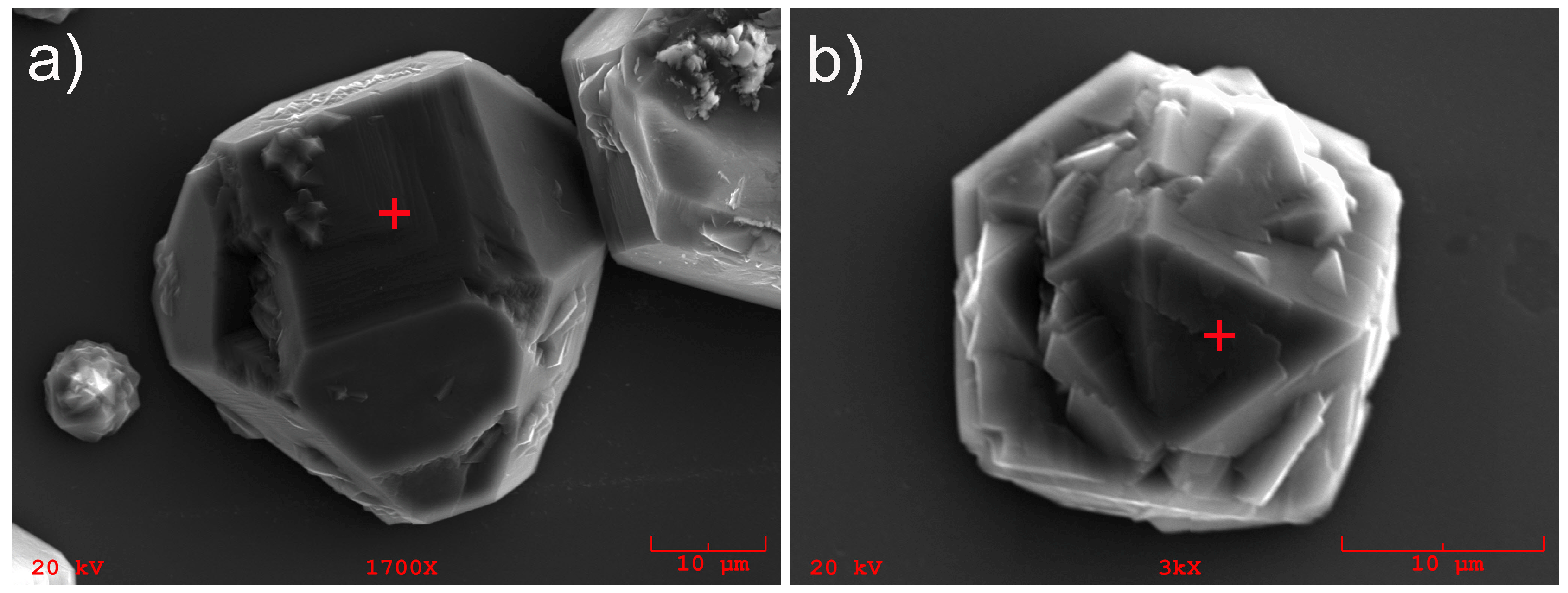
3.1. The SEM Imaging
3.2. The CL and PL Spectroscopies
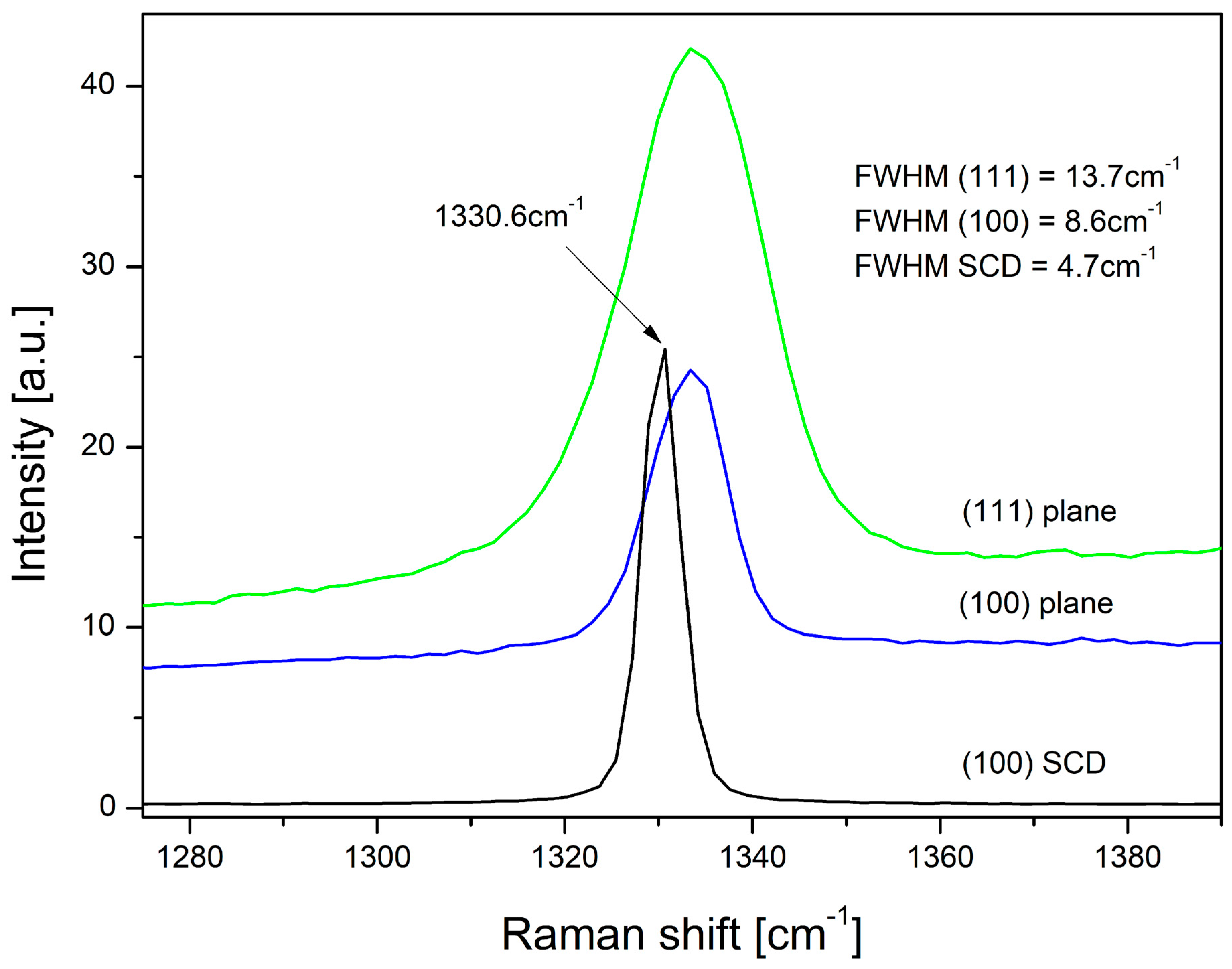
3.3. The Raman Spectroscopy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CVD | chemical vapor deposition |
| HF CVD | hot filament chemical vapor deposition |
| CL | cathodoluminescence |
| SEM | scanning electron microscope |
| PL | photoluminescence |
| RS | Raman spectroscopy |
| NV | nitrogen vacancy centers |
| FWHM | the full width at half maximum |
| MTP | multiply twinned particles |
| TEM | transmission electron microscopy |
References
- Chen, M.; Jian, X.; Sun, F.; Hu, B.; Liu, X. Development of diamond-coated drills and their cutting performance. J. Mater. Process. Technol. 2002, 129, 81–85. [Google Scholar] [CrossRef]
- Sein, H.; Ahmed, W.; Rego, C. Application of diamond coatings onto small dental tools. Diam. Relat. Mater. 2002, 11, 731–735. [Google Scholar] [CrossRef]
- Umezawa, H. Recent advances in diamond power semiconductor devices. Mater. Sci. Semicond. Process. 2018, 78, 147–156. [Google Scholar] [CrossRef]
- Trischuk, W.; Artuso, M.; Bachmair, F.; Bäni, L.; Bartosik, M.; Bellini, V.; Belyaev, V.; Bentele, B.; Berdermann, E.; Bergonzo, P. Diamond particle detectors for high energy physics. Nucl. Part. Phys. Proc. 2016, 273–275, 1023–1028. [Google Scholar] [CrossRef]
- Velardi, L.; Valentini, A.; Cicala, G. Highly efficient and stable ultraviolet photocathode based on nanodiamond particles. Appl. Phys. Lett. 2016, 108, 083503. [Google Scholar] [CrossRef]
- Velardi, L.; Valentini, A.; Cicala, G. UV photocathodes based on nanodiamond particles: Effect of carbon hybridization on the efficiency. Diam. Relat. Mater. 2017, 76, 1–8. [Google Scholar] [CrossRef]
- DiVincenzo, D.P. The Physical Implementation of Quantum Computation. Fortschr. Phys. 2000, 9–11, 771–783. [Google Scholar] [CrossRef]
- Paprocki, K.; Fabisiak, K.; Łoś, S.; Winnicki, J.; Malinowski, P.; Fabisiak, R.; Franków, W. Morphological, cathodoluminescence and thermoluminescence studies of defects in diamond films grown by HF CVD technique. Opt. Mater. 2020, 99, 109506. [Google Scholar] [CrossRef]
- Dychalska, A.; Fabisiak, K.; Paprocki, K.; Makowiecki, J.; Iskaliyeva, A.; Szybowicz, M. A Raman spectroscopy study of the effect of thermal treatment on structural and photoluminescence properties of CVD diamond films. Mater. Des. 2016, 112, 320–327. [Google Scholar] [CrossRef]
- Ščajev, P.; Trinkler, L.; Berzina, B.; Ivakin, E.; Jarašiūnas, K. Influence of boron on donor–acceptor pair recombination in type IIa HPHT diamonds. Diam. Relat. Mater. 2013, 36, 35–43. [Google Scholar] [CrossRef]
- Takeuchi, D.; Watanabe, H.; Yamanaka, H.O.S.; Sawada, H.; Ichinose, H.; Sekiguchi, T.; Kajimura, K. Origin of band-A emission in diamond thin films. Phys. Rev. B Condens. Matter Mater. Phys. 2001, 63, 245328. [Google Scholar] [CrossRef]
- Łoś, S.; Paprocki, K.; Fabisiak, K.; Szybowicz, M. The influence of the space charge on The Ohm’s law conservation in CVD diamond layers. Carbon 2019, 143, 413–418. [Google Scholar] [CrossRef]
- Hofmeister, H. Shape variations and anisotropic growth of multiply twinned nanoparticles. Z. Krist. Cryst. Mater. 2009, 224, 528–538. [Google Scholar] [CrossRef]
- Hofmeister, H. Fivefold Twinned Nanoparticles, Encyclopedia of Nanoscience and Nanotechnology; American Scientific Publishers: Stevenson Ranch, CA, USA, 2004; Volume X, pp. 431–452. [Google Scholar]
- Matsumoto, S.; Matsui, Y. Electron microscopic observation of diamond particles grown from the vapour phase. J. Mater. Sci. 1983, 18, 1785–1793. [Google Scholar] [CrossRef]
- Marciniak, W.; Fabisiak, K.; Orzeszko, S.; Rozploch, F. Observation of twinning in diamond CVD films. J. Cryst. Growth 1992, 123, 587–593. [Google Scholar] [CrossRef]
- Clausing, R.; Heatherly, L.; Horton, L.; Specht, E.; Begun, G.; Wang, Z. Textures and morphologies of chemical vapor deposited (CVD) diamond. Diam. Relat. Mater. 1992, 1, 411–415. [Google Scholar] [CrossRef]
- Haque, A.; Sumaiya, S. An overview on the formation and processing of nitrogen-vacancy photonic centers in diamond by ion implantation. J. Manuf. Mater. Process. 2017, 1, 6. [Google Scholar] [CrossRef]
- Collins, A.; Davies, G.; Kanda, H.; Woods, G. Spectroscopic studies of carbon-13 synthetic diamond. J. Phys. C Solid State Phys. 1988, 21, 1363. [Google Scholar] [CrossRef]
- Rabeau, J.; Stacey, A.; Rabeau, A.; Prawer, S.; Jelezko, F.; Mirza, I.; Wrachtrup, J. Single nitrogen vacancy centers in chemical vapor deposited diamond nanocrystals. Nano Lett. 2007, 7, 3433–3437. [Google Scholar] [CrossRef]
- Fisher, D.; Evans, D.; Glover, C.; Kelly, C.; Sheehy, M.; Summerton, G. The vacancy as a probe of the strain in type IIa diamonds. Diam. Relat. Mater. 2006, 15, 1636–1642. [Google Scholar] [CrossRef]
- Yelisseyev, A.; Nadolinny, V.; Feigelson, B.; Terentyev, S.; Nosukhin, S. Spatial distribution of impurity defects in synthetic diamonds obtained by the BARS technology. Diam. Relat. Mater. 1996, 5, 1113–1117. [Google Scholar] [CrossRef]
- Field, J. The Properties of Natural and Synthetic Diamond; Academic Press: New York, NY, USA, 1992. [Google Scholar] [CrossRef]
- Solà-Garcia, M.; Meuret, S.; Coenen, T.; Polman, A. Electron-Induced State Conversion in Diamond NV Centers Measured with Pump—Probe Cathodoluminescence Spectroscopy. ACS Photonics 2019, 7, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Heiderhoff, R. Analyse der Elektronischen Eigenschaften von Diamant Mittels Rastermikroskopischer Methoden. Ph.D. Thesis, University of Wuppertal, Wuppertal, Germany, 1997. [Google Scholar]
- Robins, L.; Black, D. Defect mapping of a synthetic diamond single crystal by cathodoluminescence spectroscopy. J. Mater. Res. 1994, 9, 1298–1307. [Google Scholar] [CrossRef]
- Pinto, H.; Jones, R.; Goss, J.; Briddon, P. Point and extended defects in chemical vapour deposited diamond. J. Phys. Conf. Ser. 2011, 281, 012023. [Google Scholar] [CrossRef]
- Collins, A.; Kanda, H.; Kitawaki, H. Colour changes produced in natural brown diamonds by high-pressure, high-temperature treatment. Diam. Relat. Mater. 2000, 9, 113–122. [Google Scholar] [CrossRef]
- Ruan, J.; Kobashi, K.; Choyke, W. On the “band-A” emission and boron related luminescence in diamond. Appl. Phys. Lett. 1992, 60, 3138–3140. [Google Scholar] [CrossRef]
- Benabdesselam, M.; Iacconi, P.; Butler, J.; Briand, D. Thermoluminescence properties of nitrogen containing chemical vapour deposited diamond films. Diam. Relat. Mater. 2001, 10, 2084–2091. [Google Scholar] [CrossRef]
- Bąk, G.; Fabisiak, K.; Klimek, L.; Kozanecki, M.; Staryga, E. Investigation of biaxial stresses in diamond films deposited on a silicon substrate by the HF CVD method. Opt. Mater. 2008, 30, 770–773. [Google Scholar] [CrossRef]
- Łoś, S.; Paprocki, K.; Szybowicz, M.; Fabisiak, K. The n–Si/p–CVD Diamond Heterojunction. Materials 2020, 13, 3530. [Google Scholar] [CrossRef]
- Butler, J.; Oleynik, I. A mechanism for crystal twinning in the growth of diamond by chemical vapour deposition. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2008, 366, 295–311. [Google Scholar] [CrossRef]

| Peak Assignment | Energy [eV] | Element Six with (100) Plane | HF CVD with (100) Plane | HF CVD with (111) Plane |
|---|---|---|---|---|
| GR1 is thought to consist of a vacancy in a neutral charge state (V) [21] | 1.675 | No | Yes (PL) | Yes (PL) |
| Center observed in synthetic diamonds. It relates to nitrogen and is especially strong in deep yellow nitrogen- containing sectors of diamonds [22] | 1.797 | No | Yes (PL) | No |
| This defect can strongly affects the thermal conductivity of synthetic diamonds [22,23] | 1.881 | Yes (PL) | Yes (PL) | Yes (PL) |
| Nitrogen-linked centers can exist in two configurations with negative vacancy (NV) at 1.945 eV and neutral one (NV) at 2.156 eV [24] | 1.947 | Yes (PL) | Yes (PL) | Yes (PL) |
| H3 (NVN) and possibly N3 (3NV) centers observed in natural type I diamonds [25] | 2.001 | Yes (PL) | No | No |
| This center is attributed generally to N-aggregates in A centers [8,25] | 2.055 | Yes (PL, CL) | Yes (CL) | Yes (CL) |
| Nitrogen with neutral vacancy (NV) [26] | 2.156 | Yes (PL, CL) | Yes (PL, CL) | Yes (PL, CL) |
| In CVD diamond it results in pink coloration [27] | 2.418 | Yes (CL) | No | No |
| Defect of uncertain structure (some- times attributed to substitutional oxygen) in type I diamonds [28] | 2.551 | No | No | Yes (CL) |
| A-band attributed to lattice disorder such as dislocations [11,29] | 2.815 | No | Yes (CL) | Yes (CL) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabisiak, K.; Łoś, S.; Paprocki, K.; Szybowicz, M.; Winiecki, J.; Dychalska, A. Orientation Dependence of Cathodoluminescence and Photoluminescence Spectroscopy of Defects in Chemical-Vapor-Deposited Diamond Microcrystal. Materials 2020, 13, 5446. https://doi.org/10.3390/ma13235446
Fabisiak K, Łoś S, Paprocki K, Szybowicz M, Winiecki J, Dychalska A. Orientation Dependence of Cathodoluminescence and Photoluminescence Spectroscopy of Defects in Chemical-Vapor-Deposited Diamond Microcrystal. Materials. 2020; 13(23):5446. https://doi.org/10.3390/ma13235446
Chicago/Turabian StyleFabisiak, Kazimierz, Szymon Łoś, Kazimierz Paprocki, Mirosław Szybowicz, Janusz Winiecki, and Anna Dychalska. 2020. "Orientation Dependence of Cathodoluminescence and Photoluminescence Spectroscopy of Defects in Chemical-Vapor-Deposited Diamond Microcrystal" Materials 13, no. 23: 5446. https://doi.org/10.3390/ma13235446
APA StyleFabisiak, K., Łoś, S., Paprocki, K., Szybowicz, M., Winiecki, J., & Dychalska, A. (2020). Orientation Dependence of Cathodoluminescence and Photoluminescence Spectroscopy of Defects in Chemical-Vapor-Deposited Diamond Microcrystal. Materials, 13(23), 5446. https://doi.org/10.3390/ma13235446









