Arsenate Adsorption on Fly Ash, Chitosan and Their Composites and Its Relations with Surface, Charge and Pore Properties of the Sorbents
Abstract
1. Introduction
2. Materials and Methods
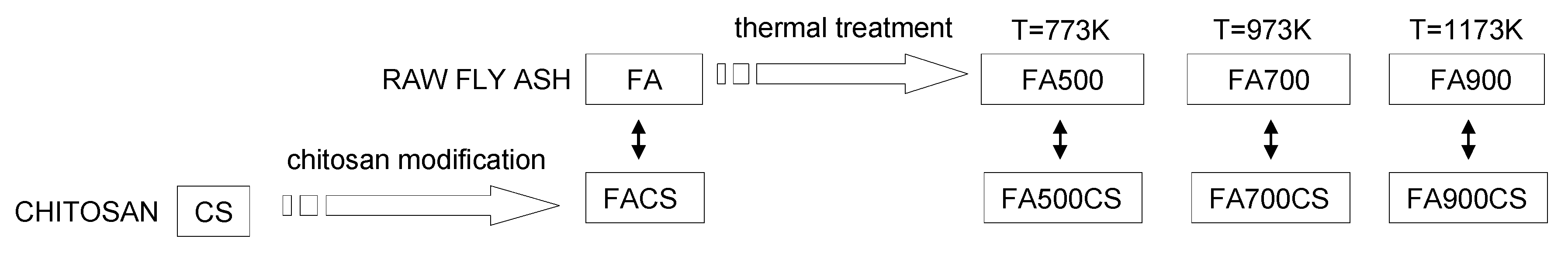
2.1. Preparation of the Adsorbents
2.2. Scanning Electron Microscopy
2.3. Surface and Pore Properties of the Adsorbents
2.3.1. Nitrogen Adsorption
2.3.2. Mercury Intrusion Porosimetry
2.3.3. Potentiometric Titration
2.4. As(V) Adsorption Tests
2.4.1. Adsorption Equilibrium
2.4.2. Adsorption Thermodynamics
2.4.3. Adsorption Kinetics
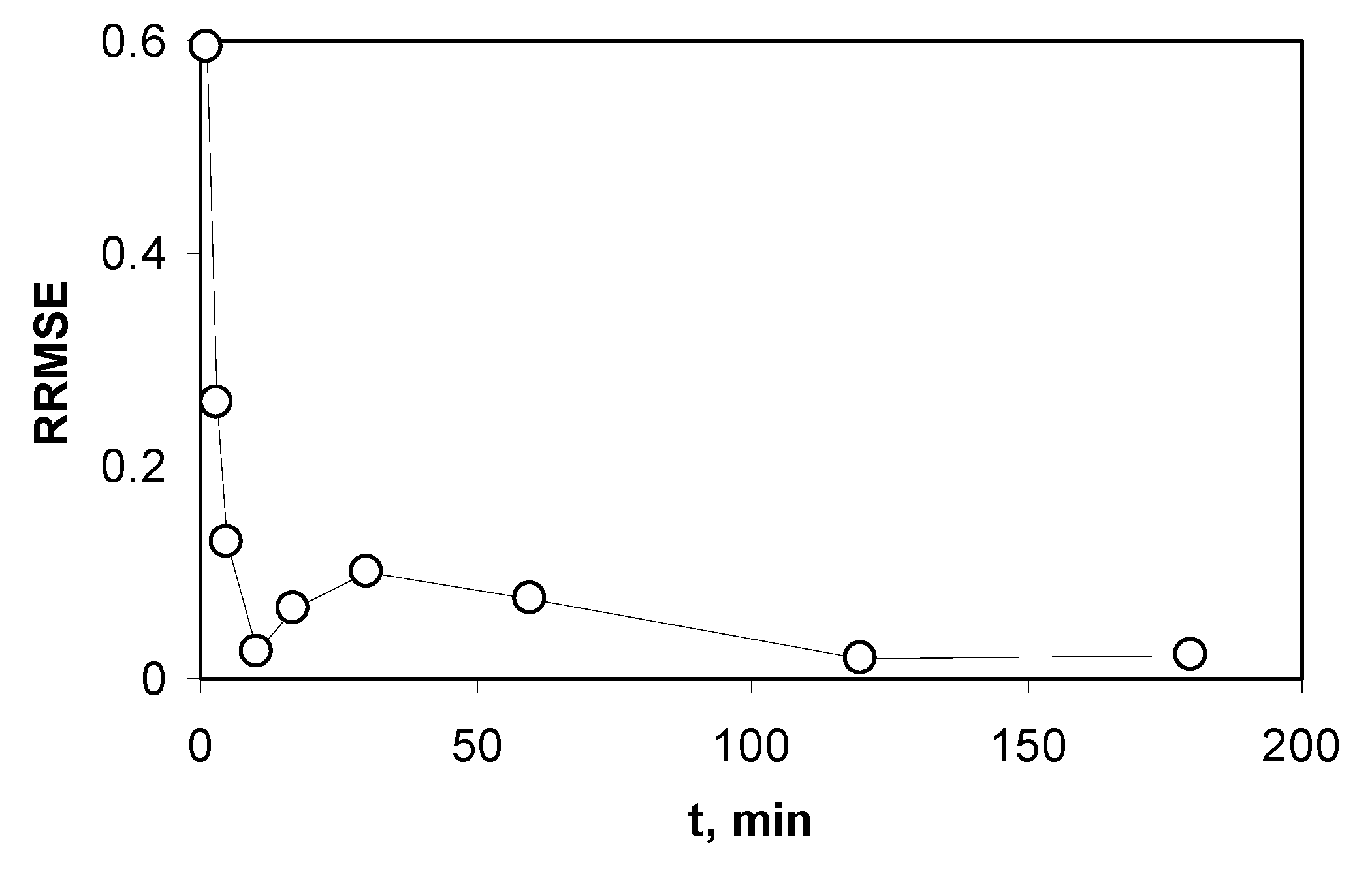
2.5. Analysis of Models Validity
3. Results
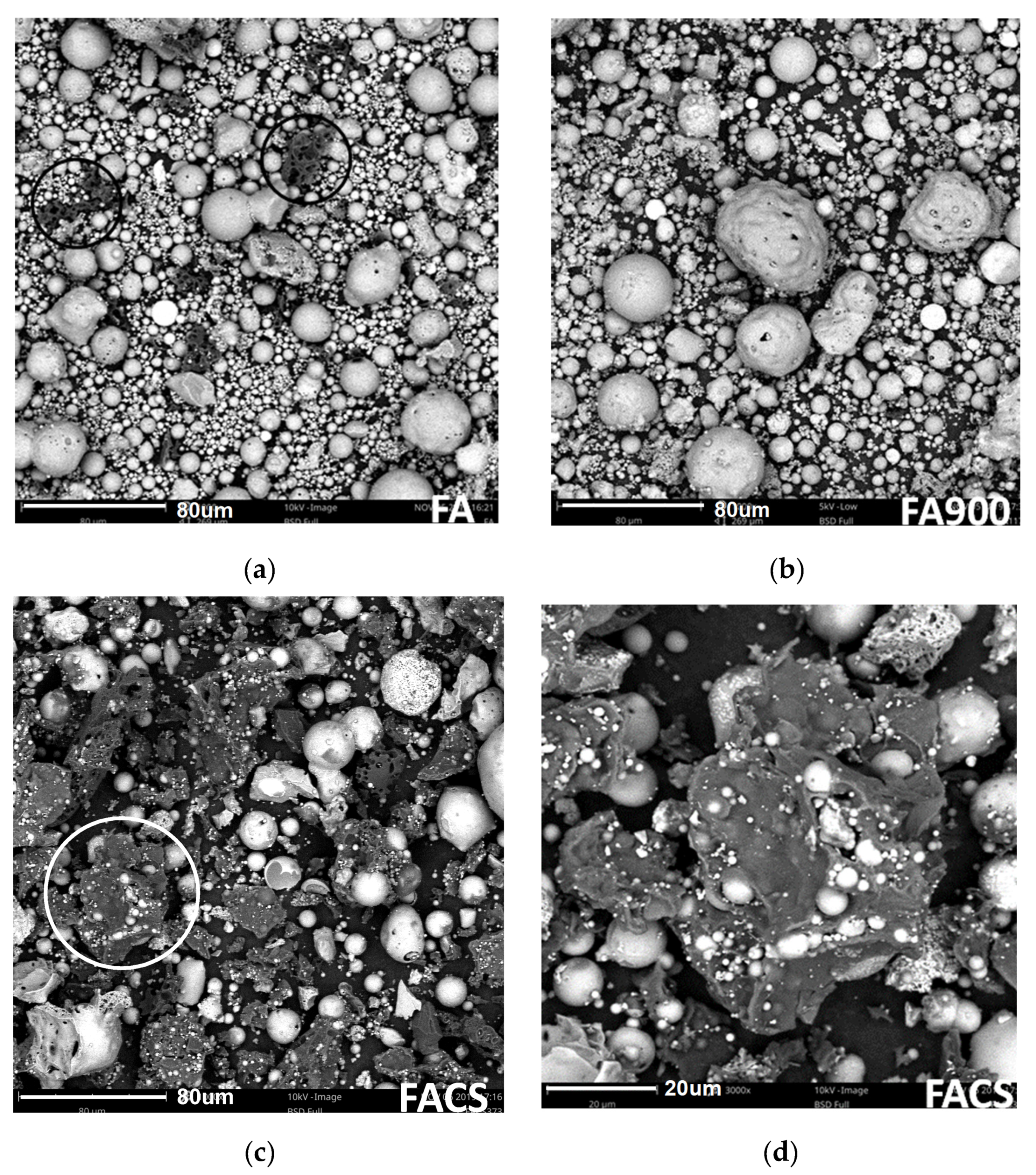
3.1. Solid Phase Characteristics from SEM Images
3.2. Surface and Pore Properties of the Adsorbents
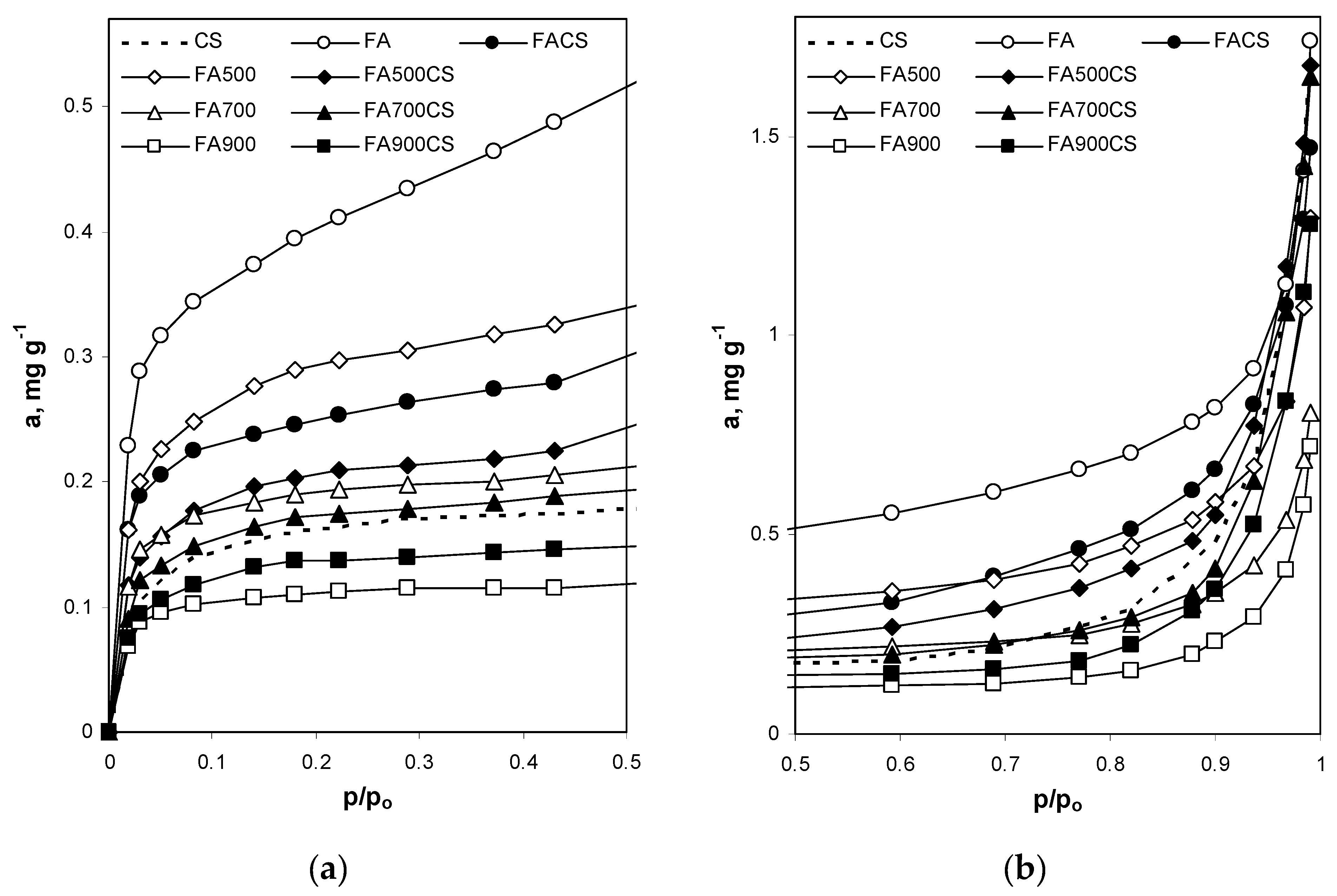
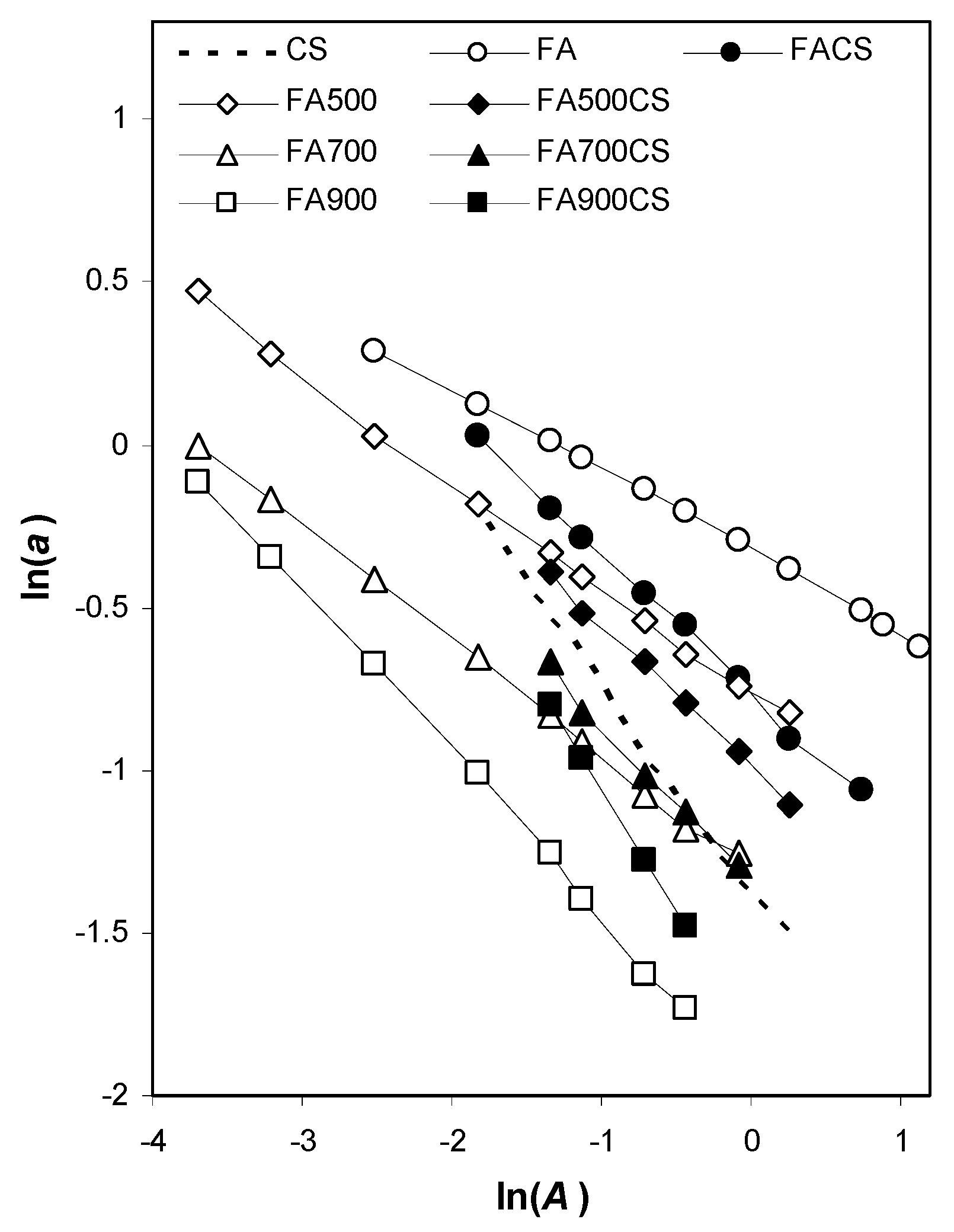
3.2.1. Surface and Nanopore Properties Derived from Nitrogen Adsorption Isotherms
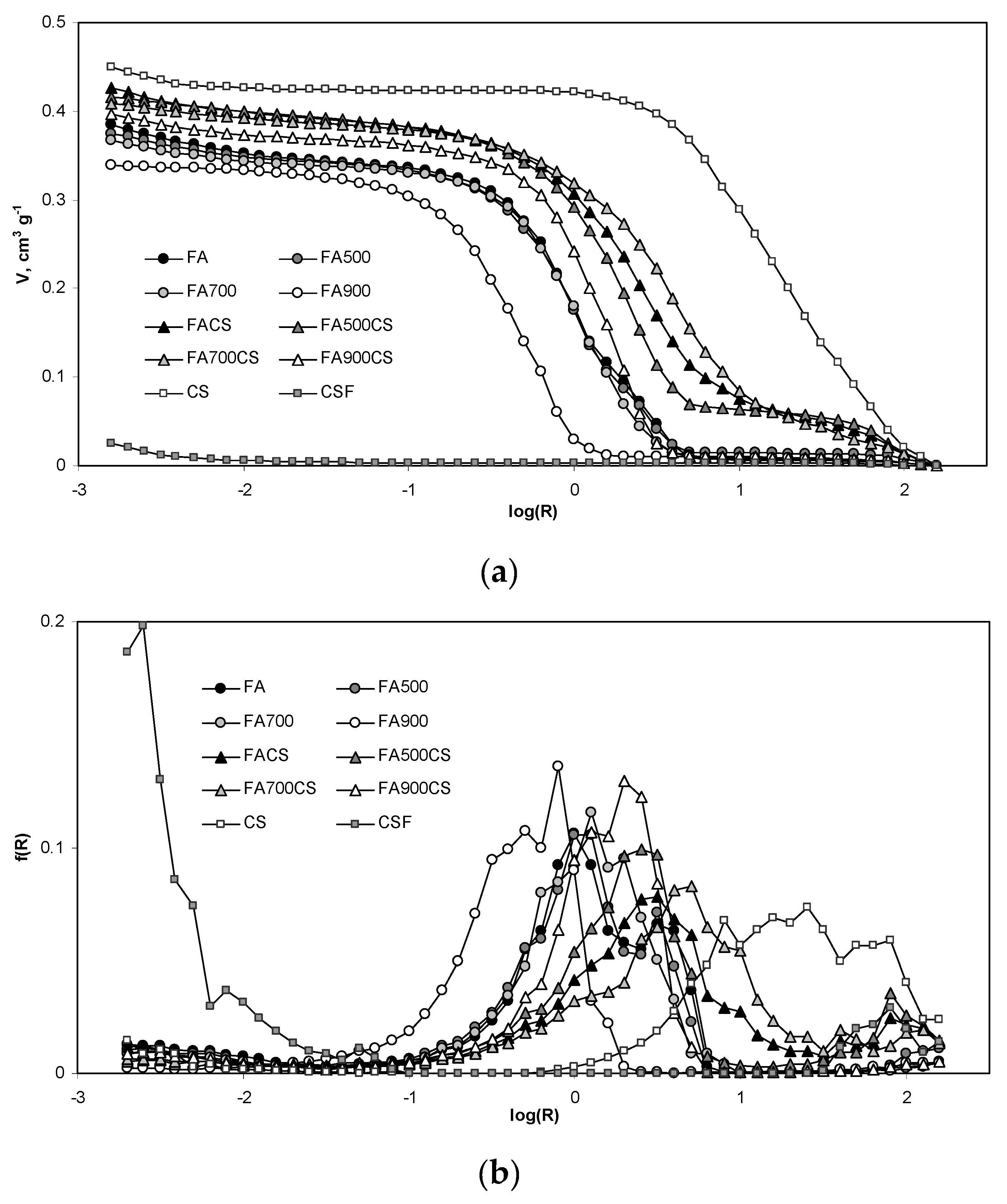
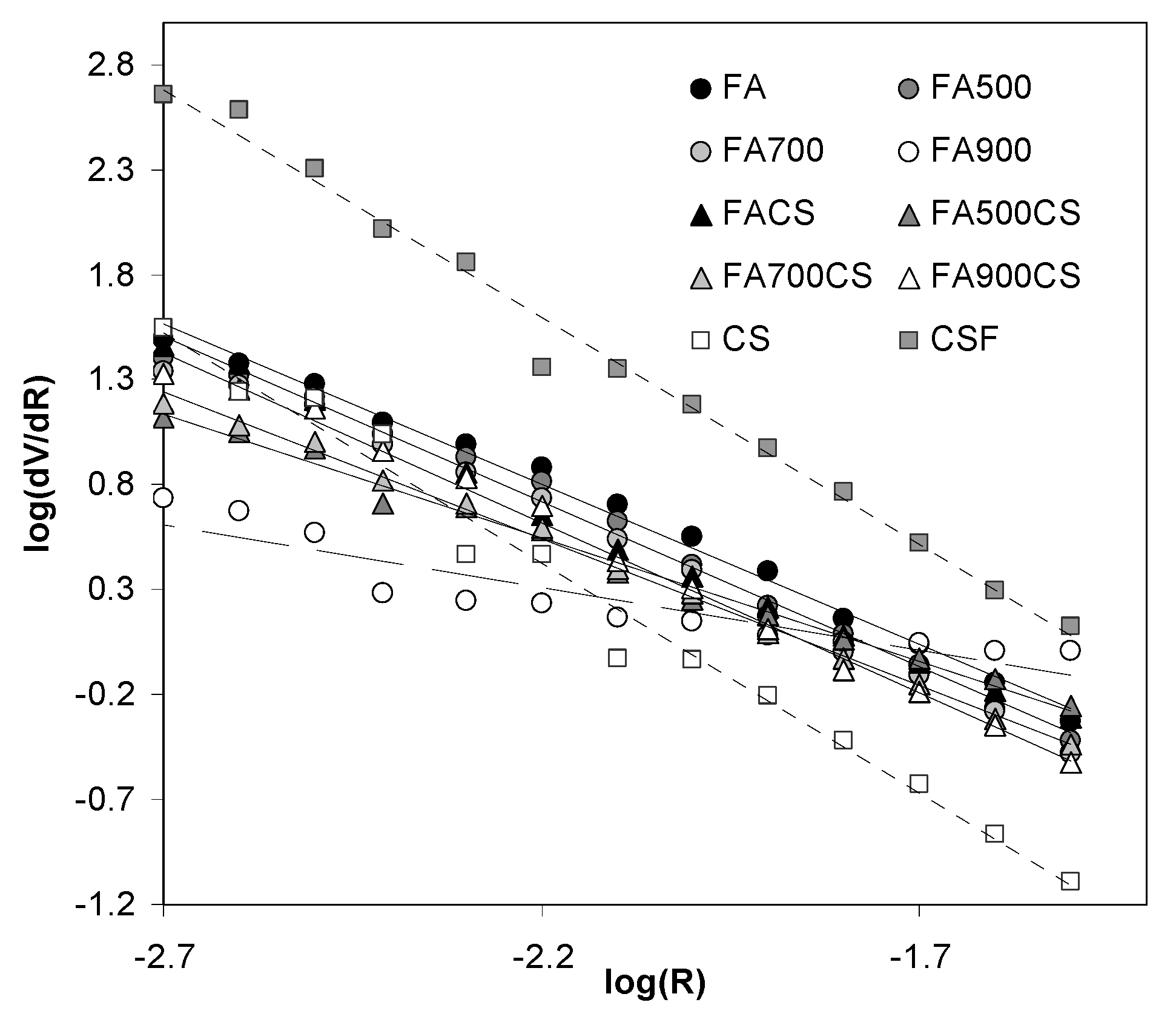
3.2.2. Mesopore Properties of the Adsorbents Derived from Mercury Intrusion Tests
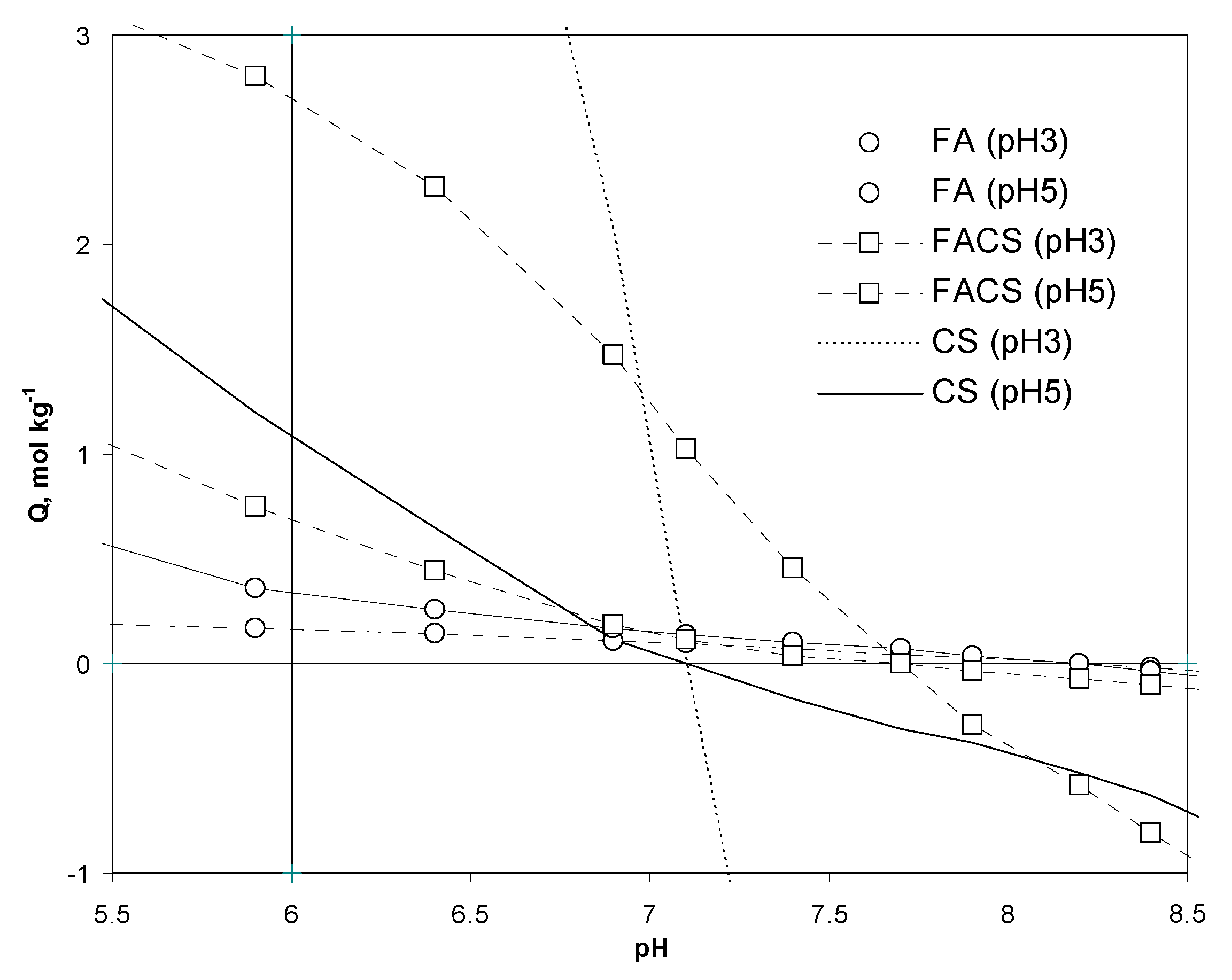
3.2.3. Surface Charge of the Adsorbents Derived from Potentiometric Titration
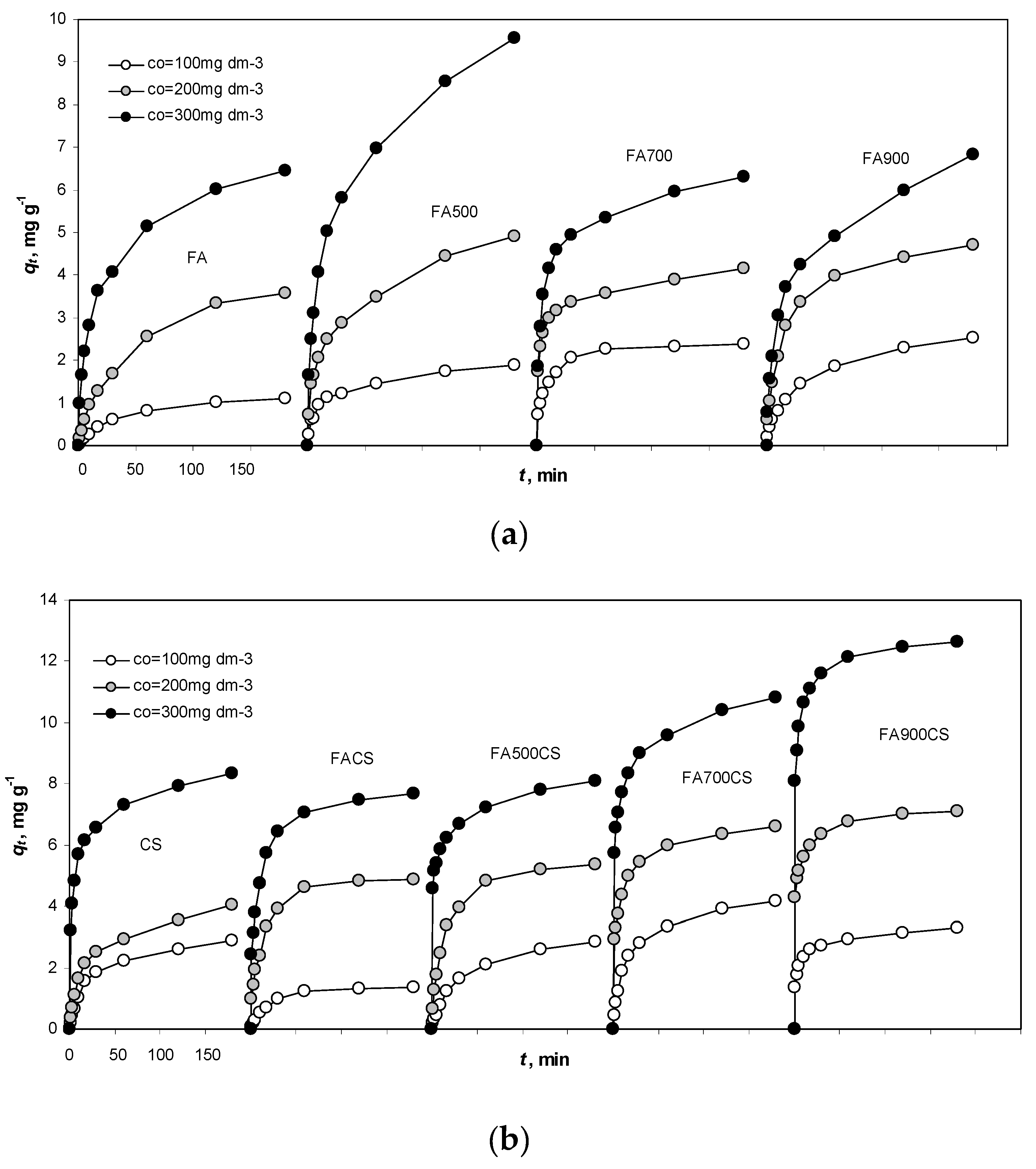
3.3. Adsorption of As(V) at Different Temperatures
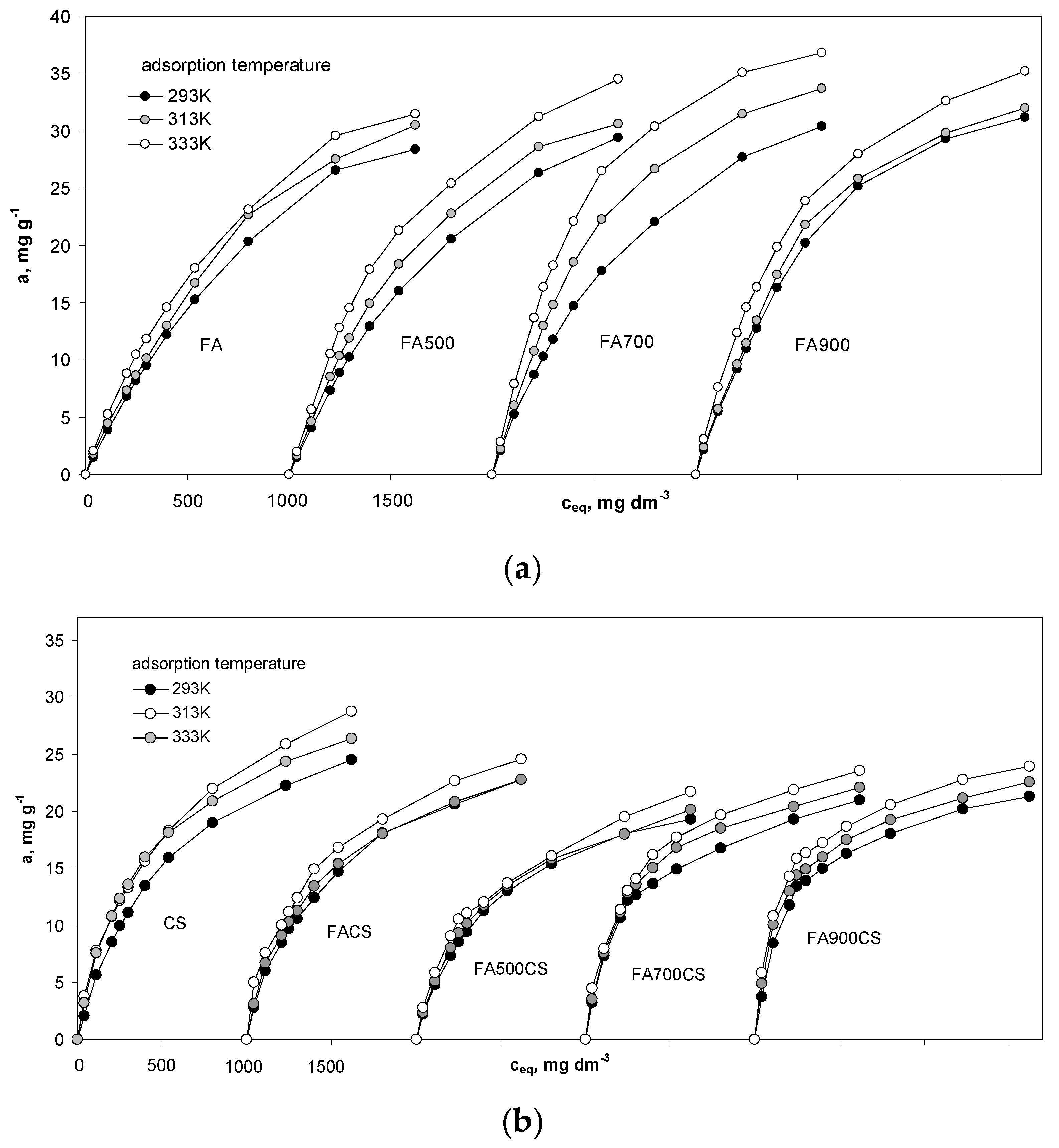
3.3.1. Adsorption Equilibrium
3.3.2. Adsorption Kinetics
4. Discussion
4.1. Physicochemical Parameters of the Sorbents
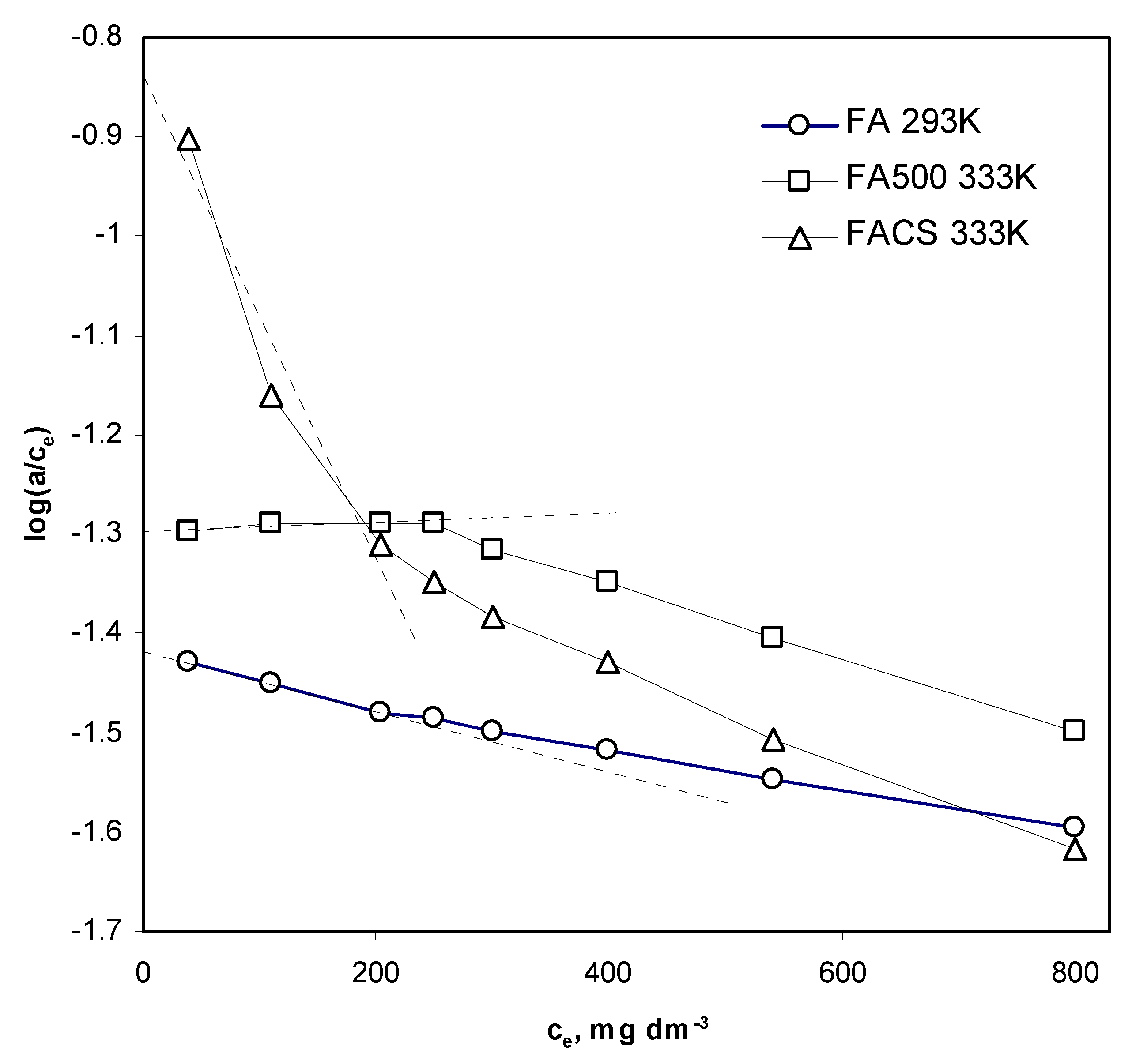
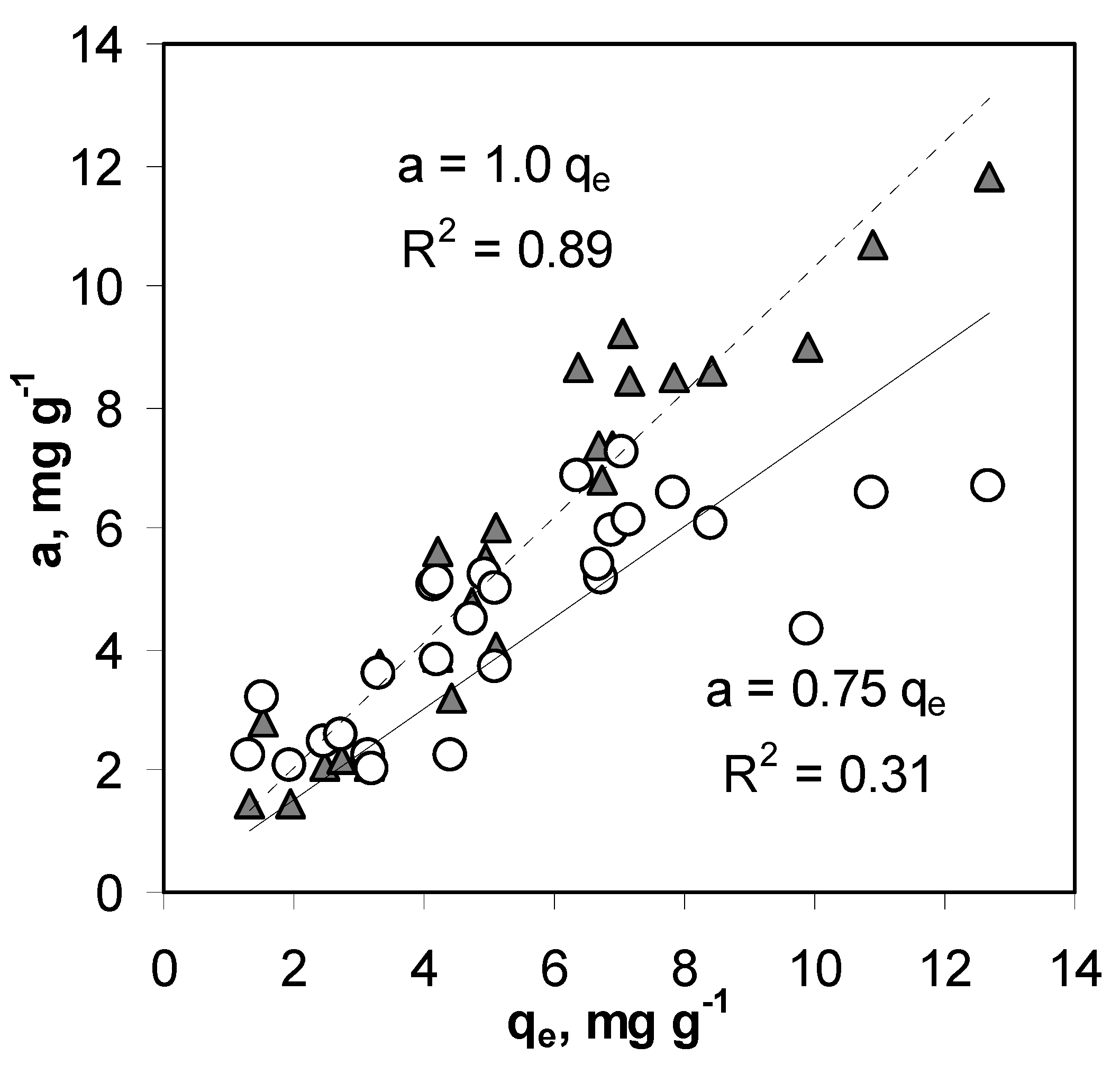
4.2. Anion Adsorption Characteristics
- The a values were linearly interpolated from the respective isotherms at a given ce.
- The a values for a given ce were calculated from the Langmuir adsorption equation using adsorption capacity (am) and K from Table 5.
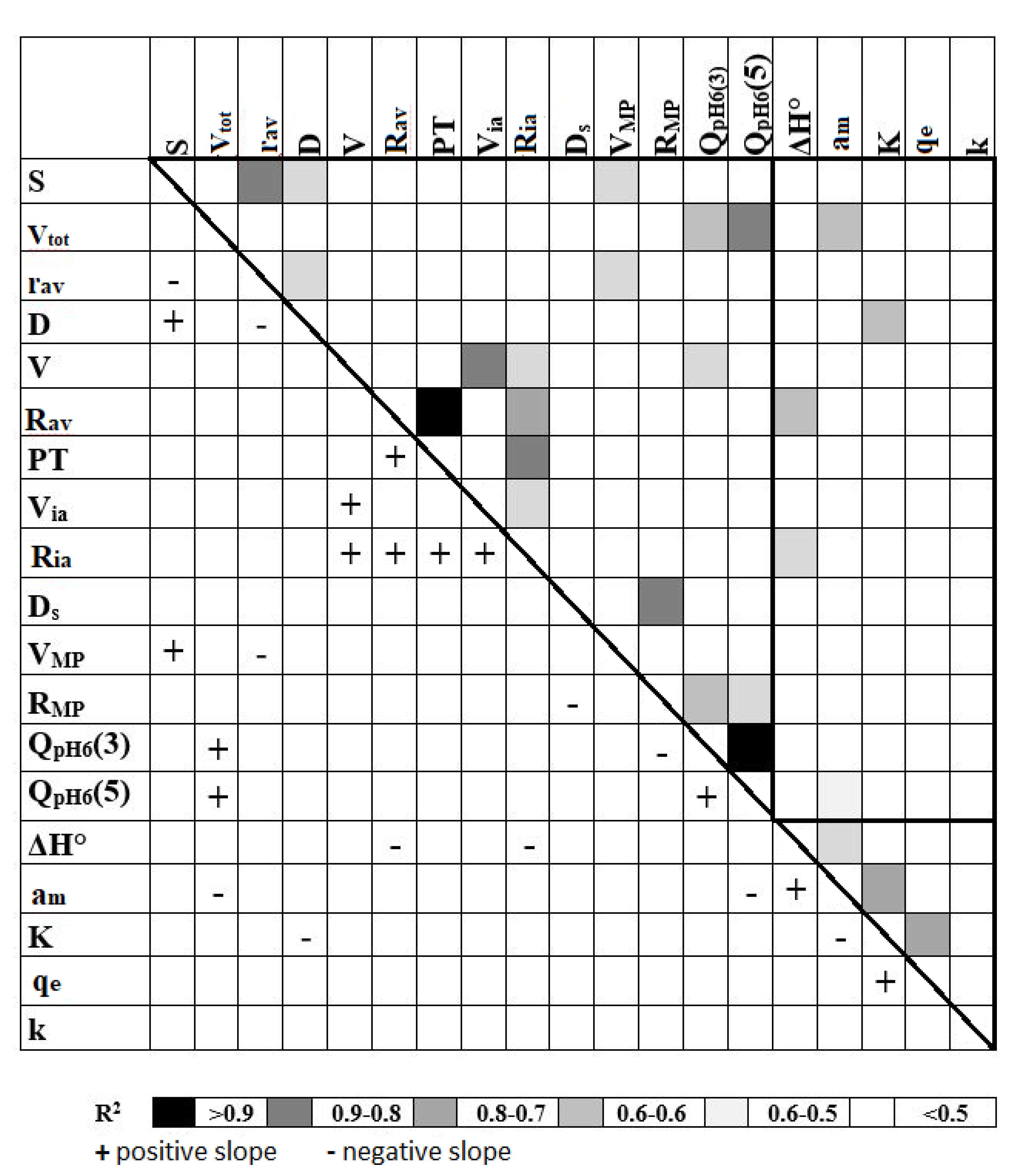
4.3. Relations between Physicochemical Parameters of the Sorbents and Anion Adsorption Characteristics
5. General Comment
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chaparro-Garnica, J.; Navlani-García, M.; Salinas-Torres, D.; Morallon, E.; Cazorla-Amoros, D. Highly stable N-doped carbon-supported Pd-based catalysts prepared from biomass waste for H2 production from formic acid. ACS Sustain. Chem. Eng. 2020, 39, 15030–15043. [Google Scholar] [CrossRef]
- Zhang, X.; Temeche, E.; Laine, R.M. LixSiON (x = 2, 4, 6): A novel solid electrolyte system derived from agricultural waste. Green Chem. 2020, 22, 7491–7505. [Google Scholar] [CrossRef]
- Simonic, M.; Fras Zemljic, L. Functionalized wool as an efficient and sustainable adsorbent for removal of Zn(II) from an aqueous solution. Materials 2020, 13, 3208. [Google Scholar] [CrossRef] [PubMed]
- Jakobik-Kolon, A.; Mitko, K.; Bok-Badura, J. Zinc sorption studies on pectin-based biosorbents. Materials 2017, 10, 844. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.F.; Aly, M.M.; Abdel-Rahman, A.A.; Ramadan, S.; Raslan, H.; Wang, S.; Vincent, T.; Guibal, E. Functionalization of magnetic chitosan particles for the sorption of U(VI), Cu(II) and Zn(II)-hydrazide derivative of glycine-grafted chitosan. Materials 2017, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Voros, V.; Drioli, E.; Fonte, C.; Szekely, G. Process Intensification via Continuous and Simultaneous Isolation of Antioxidants: An Upcycling Approach for Olive Leaf Waste. ACS Sustain. Chem. Ing. 2019, 22, 18444–18452. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- European Coal Combustion Products Association. Available online: www.ecoba.com (accessed on 14 June 2020).
- Buragohain, P.; Sreedeep, S.; Peng, L.; Garg, A. Investigation of fly ash for sorption of copper ions in multiple contaminant system: An implication on re-use of waste material under concept of sponge city. Measurement 2018, 124, 522–528. [Google Scholar] [CrossRef]
- Karanac, M.; Dolic, M.; Veljovic, D.; Rajakovic-Ognjanovic, V.; Velickovic, Z.; Pavicevic, V.; Marinkovic, A. The removal of Zn2+, Pb2+, and As(V) ions by lime activated fly ash and valorization of the exhausted adsorbent. Waste Manag. 2018, 78, 366–378. [Google Scholar] [CrossRef]
- Oliveira, J.A.; Cunha, F.A.; Ruotolo, L.A.M. Synthesis of zeolite from sugarcane bagasse fly ash and its application as a low-cost adsorbent to remove heavy metals. J. Clean. Prod. 2019, 229, 956–963. [Google Scholar] [CrossRef]
- Zhou, H.; Bhattarai, R.; Li, Y.; Li, S.; Fan, Y. Utilization of coal fly and bottom ash pellet for phosphorus adsorption: Sustainable management and evaluation. Resour. Conserv. Recycl. 2019, 149, 372–380. [Google Scholar] [CrossRef]
- Ragheb, S.M. Phosphate removal from aqueous solution using slag and fly ash. HBNRC 2013, 9, 270–275. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Z.H. Humic acid adsorption on fly ash and its derived unburned carbon. J. Colloid Interface Sci. 2007, 315, 41–46. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Yang, S.; Huang, G.; Zhao, S.; Zhang, P.; Yao, Y. Removal of sulfonated humic acid from aqueous phase by modified coal fly ash waste: Equilibrium and kinetic adsorption studies. Fuel 2016, 165, 164–271. [Google Scholar] [CrossRef]
- Majumdar, K.; Singh, N. Effect of soil amendments on sorption and mobility of metribuzin in soils. Chemosphere 2007, 66, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tiana, D.; Hu, J.; Shen, F.; Long, L.; Zhang, Y.; Yang, G.; Zeng, Y.; Zhang, J.; He, J.; et al. Functionalizing bottom ash from biomass power plant for removing methylene blue from aqueous solution. Sci. Total Environ. 2018, 634, 760–768. [Google Scholar] [CrossRef]
- Xu, X.; Li, Q.; Cui, H.; Pang, J.; Sun, L.; An, H.; Zhai, J. Adsorption of fluoride from aqueous solution on magnesia-loaded fly ash cenospheres. Desalination 2011, 272, 233–239. [Google Scholar] [CrossRef]
- Hollis, J.F.; Keren, R.; Gal, M. Boron release and sorption by fly ash as affected by pH and particle size. J. Environ. Qual. 1988, 17, 181–184. [Google Scholar] [CrossRef]
- Franus, M.; Wdowin, M.; Bandura, L.; Franus, W. Removal of environmental pollutions using zeolites from fly ash: A review. Fresenius Environ. Bull. 2015, 24, 854–866. [Google Scholar]
- Agarwal, S.; Rajoria, P.; Rani, A. Adsorption of tannic acid from aqueous solution onto chitosan/NaOH/fly ash composites: Equilibrium, kinetics, thermodynamics and modeling. J. Environ. Chem. Eng. 2018, 6, 1486–1499. [Google Scholar] [CrossRef]
- Bandura, L.; Franus, M.; Jozefaciuk, G.; Franus, W. Synthetic zeolites from fly ash as effective mineral sorbents for land-based petroleum spills cleanup. Fuel 2015, 147, 100–107. [Google Scholar] [CrossRef]
- Bandura, L.; Kołodyńska, D.; Franus, W. Adsorption of BTX from aqueous solutions by Na-P1 zeolite obtained from fly ash. Process Saf. Environ. Prot. 2017, 109, 214–223. [Google Scholar] [CrossRef]
- Fungaro, D.A.; Yamaura, M.; Craesmeyer, G.R. Uranium removal from aqueous solution by zeolite from fly ash-iron oxide magnetic nanocomposite. IRECHE 2012, 4, 353–358. [Google Scholar]
- Mostafa Hosseini Asl, S.; Javadian, H.; Khavarpour, M.; Belviso, C.; Taghavi, M.; Maghsudi, M. Porous adsorbents derived from coal fly ash as cost-effective and environmentally-friendly sources of aluminosilicate for sequestration of aqueous and gaseous pollutants: A review. J. Clean. Prod. 2019, 208, 1131–1147. [Google Scholar] [CrossRef]
- Katara, S.; Kabra, S.; Sharma, A.; Hada, R.; Rani, A. Surface modification of fly ash by thermal activation: A DR/FTIR study. Int. Res. J. Pure Appl. Chem. 2013, 3, 299–307. [Google Scholar] [CrossRef]
- Han, F.; Wang, L.; Li, Y.; Di, S. Application of thermally modified fly ash for adsorption of Ni(II) and Cr(III) from aqueous solution: Equilibrium, kinetic, and thermodynamic studies. Environ. Eng. Sci. 2017, 34, 508–515. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan- based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Varma, A.J.; Deshpande, S.V.; Kennedy, J.F. Metal complexation by chitosan and its derivatives: A review. Carbohydr. Polym. 2004, 55, 77–93. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Fan, L.; Liu, H.; Hu, Y. Chitosan—Metal complexes as antimicrobial agent: Synthesis, characterization and structure-activity study. Polym. Bull. 2005, 55, 105–113. [Google Scholar] [CrossRef]
- Cervera, M.F.; Heinämäki, J.; Räsänen, M.; Maunu, S.L.; Karjalainen, M.; Nieto Acosta, O.M.; Iraizoz Colartea, A.; Yliruusi, J. Solid-state characterization of chitosans derived from lobster chitin. Carbohydr. Polym. 2004, 58, 401–408. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Xie, J.; Li, C.; Chi, L.; Wu, D. Chitosan modified zeolite as a versatile adsorbent for the removal of different pollutants from water. Fuel 2013, 103, 480–485. [Google Scholar] [CrossRef]
- Ocinski, D.; Mazur, P. Highly efficient arsenic sorbent based on residual from water deironing—Sorption mechanisms and column studies. J. Hazard. Mater 2020, 382, 1–10. [Google Scholar] [CrossRef]
- Adamczuk, A.; Kołodynska, D. Removal of chromium, copper, zinc and arsenic from aqueous solutions onto fly ash coated chitosan. Equilibrium, thermodynamic and kinetic studies. Chem. Eng. J. 2015, 274, 200–212. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Fijalkowska, G.; Wisniewska, M.; Wojcik, G. Chromium(VI) reduction and accumulation on the kaolinite surface in the presence of cationic soil flocculant. J. Soils Sediments 2020. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Nowicki, P.; Sokolowska, Z.; Pietrzak, R. Hay-based activated biochars obtained using two different heating methods as effective low-cost sorbents: Solid surface characteristics, adsorptive properties and aggregation in the mixed Cu(II)/PAM system. Chemosphere 2020. [Google Scholar] [CrossRef]
- Bernal, V.; Giraldo, L.; Moreno-Pirajan, J.C. Physicochemical Properties of Activated Carbon: Their Effect on the Adsorption of Pharmaceutical Compounds and Adsorbate-Adsorbent Interactions. J. Carbon Res. 2018, 4, 62. [Google Scholar] [CrossRef]
- Bharat, G.K.; Yenkie, M.K.N.; Natarajan, G.S. Influence of physico-chemical characteristics of adsorbent and adsorbate on competitive adsorption equilibrium and kinetics. In Fundamentals of Adsorption; The Kluwer International Series in Engineering and Computer Science; LeVan, M.D., Ed.; Springer Kluwer: Boston, MA, USA, 1996; Volume 356, pp. 91–99. [Google Scholar]
- Gokce, Y.; Aktas, Z. Nitric acid modification of activated carbon produced from waste tea and adsorption of methylene blue and phenol. Appl. Surf. Sci. 2014, 313, 352–359. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Arsenic, metals, fibres, and dusts. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100C, 1–526. [Google Scholar]
- Mucha, S.; Berezowski, M.; Markowska, K. Mechanisms of arsenic toxicity and transport in microorganisms. Postępy Mikrobiol. 2017, 56, 88–99. (In Polish) [Google Scholar] [CrossRef]
- Li, W.G.; Gong, X.J.; Wang, K.; Zhang, X.R.; Fan, W.B. Adsorption characteristics of arsenic from micro-polluted water by an innovative coal-based mesoporous activated carbon. Bioresour. Technol. 2014, 165, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents-a critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Di Natale, F.; Erto, A.; Lancia, A.; Musmarra, D. Experimental and modelling analysis of As(V) ions adsorption on granular activated carbon. Water Res. 2008, 42, 2007–2016. [Google Scholar] [CrossRef]
- Gong, X.J.; Li, W.G.; Zhang, D.Y.; Fan, W.B.; Zhang, X.R. Adsorption of arsenic from micro-polluted water by an innovative coal-based mesoporous activated carbon in the presence of co-existing ions. Int. Biodeterior. Biodegrad. 2015, 102, 256–264. [Google Scholar] [CrossRef]
- Hassan, A.F.; Abdel-Mohsen, A.M.; Elhadidy, H. Adsorption of arsenic by activated carbon, calcium alginate and their composite beads. Int. J. Biol. Macromol. 2014, 68, 125–130. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, G.; Li, H. Efficient removal of arsenic from water using a granular adsorbent: Fe-Mn binary oxide impregnated chitosan bead. Bioresour. Technol. 2015, 193, 243–249. [Google Scholar] [CrossRef]
- Yu, C.; Peltola, P.; Nystrand, M.I.; Virtasalo, J.J.; Österholm, P.; Ojala, A.E.K.; Hogmalm, J.K.; Åström, M.E. Arsenic removal from contaminated brackish sea water by sorption onto Al hydroxides and Fe phases mobilized by land-use. Sci. Total Environ. 2016, 542, 923–934. [Google Scholar] [CrossRef]
- Ashraf, S.; Siddiqa, A.; Shahida, S.; Qaisar, S. Titanium-based nanocomposite materials for arsenic removal from water: A review. Heliyon 2019, 5, e01577. [Google Scholar] [CrossRef]
- Karanac, M.; Dolic, M.; Velićkovic, Z.; Kapidzic, A.; Ivanovski, V.; Mitric, M.; Marinkovic, A. Efficient multistep arsenate removal onto magnetite modified fly ash. J. Environ. Manag. 2018, 224, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Active Standard ASTM C618. ASTM standard specification for coal fly ash and raw or calcined natural pozzolan for use in concrete (C618). In Annual Book of ASTM Standards, Concrete and Aggregates; American Society for Testing Materials: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Pasiecznik, I.; Szczepaniak, W. Arsenic ion removal from water solutions using magnesium-aluminium layered double hydroxides. Ochr. Środowiska 2014, 36, 33–37. (In Polish) [Google Scholar]
- Aranovich, G.L. The theory of polymolecular adsorption. Langmuir 1992, 8, 736–739. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmet, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–314. [Google Scholar] [CrossRef]
- Neimark, A.V. Calculating fractal dimension of adsorbents. Adsorp. Sci. Technol. 1990, 7, 210–219. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with the special reference to the determination of surface area and porosity. Pure Appl. Chem. 1982, 54, 2201–2218. [Google Scholar] [CrossRef]
- Jozefaciuk, G. Effect of the size of aggregates on pore characteristics of minerals measured by mercury intrusion and water-vapor desorption techniques. Clays Clay Miner. 2009, 57, 586–601. [Google Scholar] [CrossRef]
- Washburn, E.W. The Dynamics of Capillary Flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Sridharan, A.; Venkatappa Rao, G. Pore size distribution of soils from mercury intrusion porosimetry data. Soil Sci. Soc. Am. Proc. 1972, 36, 980–981. [Google Scholar] [CrossRef]
- Pachepsky, Y.A.; Polubesova, T.A.; Hajnos, M.; Sokolowska, Z.; Jozefaciuk, G. Fractal parameters of pore surface area as influenced by simulated soil degradation. Soil Sci. Soc. Am. J. 1995, 59, 68–75. [Google Scholar] [CrossRef]
- Nederlof, M.M.; De Wit, J.C.; Riemsdijk, W.H.; Koopal, L.K. Determination of proton affinity distributions for humic substances. Environ. Sci. Technol. 1993, 27, 846–856. [Google Scholar] [CrossRef]
- Jozefaciuk, G.; Szatanik-Kloc, A.; Lukowska, M.; Szerement, J. Pitfalls and uncertainties of using potentiometric titration for estimation of plant roots surface charge and acid-base properties. Am. J. Plant Sci. 2014, 5, 1862–1876. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, T.; Chen, C.; Feng, L.; Su, X.; Zhou, L.; Chen, Y.; Xia, A.; Wang, X. Spent substrate of Ganodorma lucidum as a new bio-adsorbent for adsorption of three typical dyes. Bioresour. Technol. 2018, 266, 134–138. [Google Scholar] [CrossRef]
- Franus, M.; Jozefaciuk, G.; Bandura, L.; Lamorski, K.; Hajnos, M.; Franus, W. Modification of lightweight aggregates’ microstructure by used motor oil addition. Materials 2016, 9, 845. [Google Scholar] [CrossRef]
- Pakde, P.M.; Peighambardoust, S.J. Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohydr. Polym. 2018, 201, 264–279. [Google Scholar] [CrossRef]
- Allen, S.J.; Mckay, G.; Porter, J.F. Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. J. Colloid Interface Sci. 2004, 280, 322–333. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Aber, S. Chromium adsorption and Cr(VI) reduction to trivalent chromium in aqueous solutions by soya cake. J. Hazard. Mater. 2002, 94, 49–61. [Google Scholar] [CrossRef]
- Huang, C.P.; Rhoads, E.A. The adsorption of Zn(II) onto hydrous aluminosilicates. J. Colloid Interface Sci. 1989, 131, 289–306. [Google Scholar] [CrossRef]
- Tofan, L.; Paduraru, C.; Bilba, D.; Rotariu, M. Thermal power plants ash as sorbent for the removal of Cu(II) and Zn(II) ions from wastewaters. J. Hazard. Mater. 2008, 156, 1–8. [Google Scholar] [CrossRef]
- Zubair, A.; Bhatti, N.N.; Hanif, M.A.F. Shafqat, Kinetic and equilibrium modeling for Cr(III) and Cr(VI) removal from aqueous solutions by Citrus Reticulata waste biomass. Water Air Soil Polution 2008, 191, 305–318. [Google Scholar] [CrossRef]
- Khan, A.; Singh, R. Adsorption thermodynamics of carbofuran on Sn(IV) arsenosilicate in H+, Na+ and Ca2+ forms. Colloids Surf. 1987, 24, 33–42. [Google Scholar] [CrossRef]
- Lyubchik, S.; Lyubchik, A.; Lygina, O.; Lyubchik, S.; Fonseca, I. Comparison of the Thermodynamic Parameters Estimation for the Adsorption Process of the Metals from Liquid Phase on Activated Carbons. In Thermodynamics—Interaction Studies—Solids, Liquids and Gases; Moreno Pirajan, J.C., Ed.; IntechOpen: London, UK, 2011; ISBN 978-953-307-563-1. Available online: https://www.intechopen.com/books/thermodynamics-interaction-studies-solids-liquids-and-gases/comparison-of-the-thermodynamic-parameters-estimation-for-the-adsorption-process-of-the-metals-from- (accessed on 25 September 2020).
- Machesky, M.L. Influence of temperature on ion adsorption by hydrous metal oxides, in: Chemical modeling of aqueous systems II. ACS Symp. Ser. 1990, 416, 282–292. [Google Scholar] [CrossRef]
- Sahai, N. Estimating adsorption enthalpies and affinity sequences of monovalent electrolyte ions on oxide surfaces in aqueous solution. Geochim. Cosmochim. Acta. 2000, 64, 3629–3641. [Google Scholar] [CrossRef]
- Rehab, M.; Hesham, A.; Hamad, M.M.; Hussein, G.F. Malash. Potential of using green adsorbent of heavy metal removal from aqueous solutions: Adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol. Eng. 2016, 91, 317–332. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L. Removal of phosphate anions using the modified chitosan beads: Adsorption kinetic, isotherm and mechanism studies. Powder Technol. 2015, 277, 112–119. [Google Scholar] [CrossRef]
- Adegoke, H.I.; Adekola, F.A.; Abdulraheem, M.N. Kinetic and thermodynamic studies on adsorption of sulphate from aqueous solution by magnetite, activated carbon and magnetite-activated carbon composites. Nig. J. Chem. Res. 2017, 22, 39–69. [Google Scholar]
- Morghi, M.; Abidar, F.; Soudani, A.; Zerbet, M.; Chiban, M.; Kabli, H.; Sinan, F. Removal of nitrate ions from aqueous solution using chitin as natural adsorbent. Int. J. Res. Environ. Stud. 2015, 2, 8–20. [Google Scholar]
- Barakan, S.; Aghazadeh, V.; Beyragh, A.S.; Mohammadi, S. Thermodynamic, kinetic and equilibrium isotherm studies of As(V) adsorption by Fe(III)-impregnated bentonite. Environ. Dev. Sustain. 2020, 22, 5273–5295. [Google Scholar] [CrossRef]
- Chiban, M.; Soudani, A.; Kabli, H.; Sinan, F.; Persin, M. Mineral ions adsorption at lowand middle concentrations onto grinded and dried of Carpobrotus edulis plant as new adsorbent. In Proceedings of the XIII IWRA World Water Congress, Montpellier, France, 1–4 September 2008; Available online: https://iwra.org/member/congress/resource/abs377_article.pdf (accessed on 10 September 2020).
- Wang, J.; Huang, H. Thermodynamics of NO3-adsorption on different kinds of anion exchange resins. Bulg. Chem. Commun. 2016, 48, 73–79. Available online: http://bcc.bas.bg/BCC_Volumes/Volume_48_Special_D_2016/BCC-48-D-2016-11-Jiping%20Wang-73-79.pdf (accessed on 12 September 2020).
- Mustafa, S.; Hussain, S.Y.; Rehana, N.; Alamzeb, N. Temperature effect on ion exchange sorption of phosphate. Solvent Extr. Ion Exch. 1989, 7, 705–720. [Google Scholar] [CrossRef]
- Humelnicu, D.; Dragan, E.S. Evaluation of phosphate adsorption by porous strong base anion exchangers having hydroxyethyl substituents: Kinetics, equilibrium, and thermodynamics. Environ. Sci. Pollut. Res. Int. 2020. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; He, M.; Lin, C. Adsorption of antimony(III) and antimony(V) on bentonite: Kinetics, thermodynamics and anion competition. Microchem. J. 2011, 97, 85–91. [Google Scholar] [CrossRef]
- Ali, M.H.; Hussian, A.E.M.; Abdel-Satar, A.M.; Goher, M.E.; Napiorkowska-Krzebietke, A.; Abd El-Monem, A.M. The isotherm and kinetic studies of the biosorption of heavy metals by non-living cells of Chlorella vulgaris. J. Elem. 2016, 21, 1263–1276. [Google Scholar] [CrossRef]
- Huang, Y.; Farooq, M.U.; Lai, S.; Sampranpiboon, P.; Wang, X.; Huang, W.; Feng, X. Model fitting of sorption kinetics data: Rectification of misapplications overlooked. Aiche J. 2018, 64, 1793. [Google Scholar] [CrossRef]
- Chiban, M.; Zerbet, M.; Carja, G.; Sinan, F. Application of low-cost adsorbents for arsenic removal: A review. J. Environ. Chem. Ecotoxicol. 2012, 4, 91–102. [Google Scholar] [CrossRef]
- Baig, S.A.; Sheng, T.; Hu, Y.; Xu, J.; Xu, X. Arsenic Removal from Natural Water Using Low Cost Granulated Adsorbents: A Review. Clean Soil Air Water 2014, 42, 1–14. [Google Scholar] [CrossRef]
- Jovanovic, B.M.; Vukasinovic-Pesic, V.L.; Veljovic, D.N.; Rajakovic, L.V. Arsenic removal from water using low-cost adsorbents—A comparative study. J. Serb. Chem. Soc. 2011, 76, 1437–1452. [Google Scholar] [CrossRef]
- Diamaddopoulos, E.; Loannidis, S.; Sakellaropoulos, G.P. As(V) removal from aqueous solutions by fly ash. Water Res. 1993, 27, 1773–1777. [Google Scholar] [CrossRef]
- Vivek, C.; Nalini, S. Preparation and Evaluation of Iron-Chitosan Composites for Removal of As(III) and As(V) from Arsenic Contaminated Real Life Groundwater. Water Res. 2009, 43, 3862–3870. [Google Scholar] [CrossRef]
- Gupta, A.; Yunus, M.; Sankararamakrishnan, N. Zero-valent iron encapsulated chitosan nanospheres—A novel adsorbent for the removal of total inorganic arsenic from aqueous systems. Chemosphere 2011, 86, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, I.; Memona, S.; Yilmaz, M. An excellent arsenic(V) sorption behavior of p-tert-butylcalix[8]areneoctamide impregnated resin. C. R. Chim. 2010, 13, 1416–1423. [Google Scholar] [CrossRef]

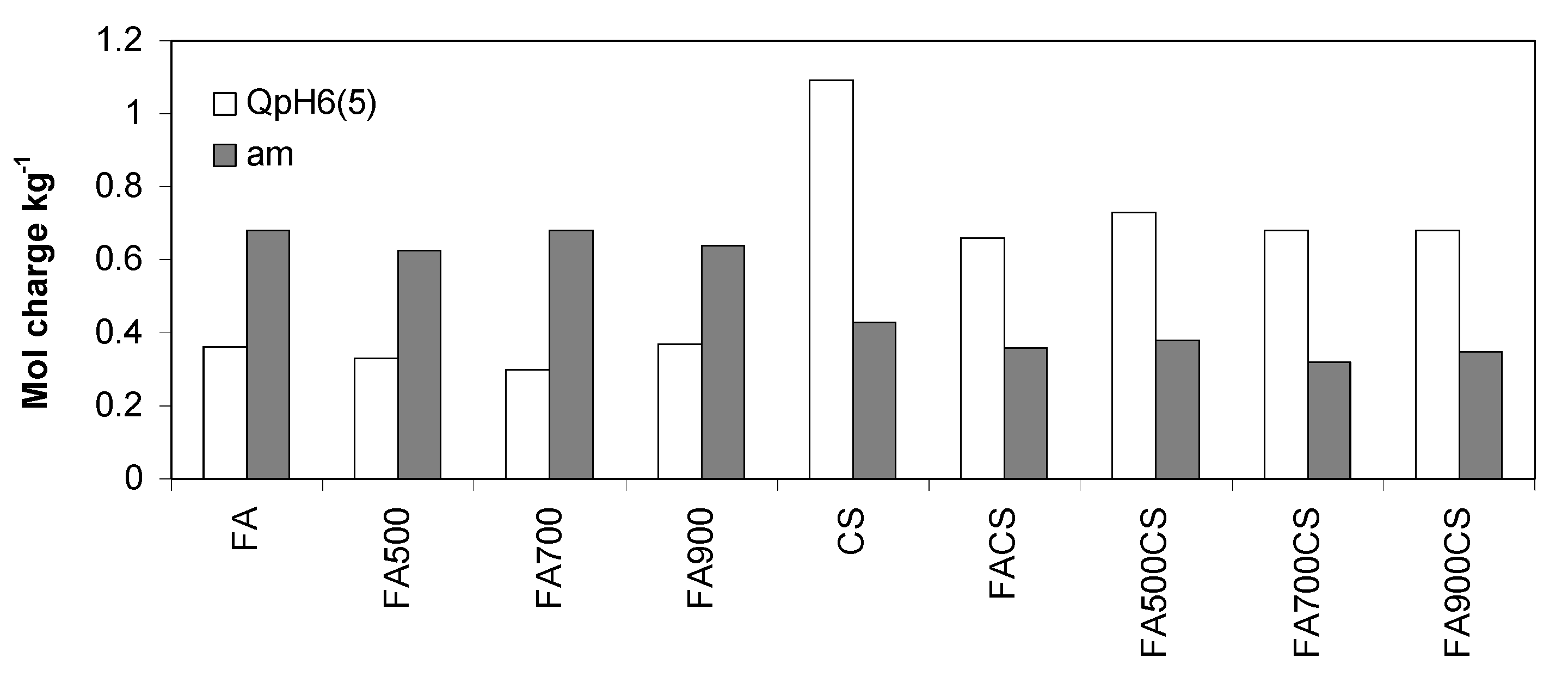
| Parameter | FA | FA500 | FA700 | FA900 | CS | FACS | FA500CS | FA700CS | FA900CS |
|---|---|---|---|---|---|---|---|---|---|
| S, m2g−1 | 12.1 | 7.6 | 4.5 | 2.5 | 4.1 | 7.4 | 6.0 | 4.3 | 3.1 |
| vtot, mm3g−1 | 0.85 | 0.80 | 0.52 | 0.49 | 1.46 | 1.19 | 1.45 | 1.41 | 1.09 |
| rav, nm | 143 | 174 | 179 | 198 | 201 | 163 | 186 | 209 | 201 |
| D | 2.75 | 2.67 | 2.64 | 2.50 | 2.37 | 2.57 | 2.56 | 2.52 | 2.26 |
| Parameter | FA | FA500 | FA700 | FA900 | CS | CSF | FACS | FA500CS | FA700CS | FA900CS |
|---|---|---|---|---|---|---|---|---|---|---|
| V, mm3g−1 | 384 | 375 | 367 | 339 | 448 | 25 | 427 | 417 | 409 | 396 |
| Rav, μm | 0.62 | 0.69 | 0.68 | 0.38 | 10.86 | 0.01 | 1.82 | 1.73 | 2.68 | 0.87 |
| PT, μm | 0.89 | 0.89 | 1.12 | 0.63 | 8.91 | 0.01 | 2.82 | 1.78 | 3.55 | 1.78 |
| Via, mm3g−1 | 188 | 180 | 207 | 233 | 146 | 20 | 241 | 202 | 203 | 263 |
| Ria, μm | 0.19 | 0.20 | 0.31 | 0.24 | 1.39 | 0.003 | 0.48 | 0.43 | 0.57 | 0.50 |
| Ds | 3.53 | 3.58 | 3.57 | 2.60 | 4.19 | 4.17 | 3.52 | 3.18 | 3.40 | 3.62 |
| Parameter | FA | FA500 | FA700 | FA900 | CS | CSF | FACS | FA500CS | FA700CS | FA900CS |
|---|---|---|---|---|---|---|---|---|---|---|
| VMP, mm3 g−1 | 32.0 | 26.5 | 22.6 | 6.1 | 23.9 | 19.7 | 27.8 | 16.3 | 17.6 | 22.7 |
| RMP, nm | 4.07 | 4.02 | 4.01 | 4.58 | 3.21 | 3.21 | 3.74 | 4.15 | 4.09 | 3.90 |
| pH * | Parameter | FA | FA500 | FA700 | FA900 | CS | FACS | FA500CS | FA700CS | FA900CS |
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | QpH6, mM g−1 | 0.19 | 0.20 | 0.16 | 0.11 | 7.57 | 2.70 | 2.66 | 2.67 | 2.77 |
| 5 | QpH6, mM g−1 | 0.36 | 0.33 | 0.30 | 0.37 | 1.09 | 0.66 | 0.73 | 0.68 | 0.68 |
| 3 | SCD, Cm−2 | 1.5 | 2.5 | 3.4 | 4.2 | 178.2 | 35.2 | 42.8 | 59.9 | 86.2 |
| 5 | SCD, Cm−2 | 2.9 | 4.2 | 6.4 | 14.3 | 25.7 | 8.6 | 11.7 | 15.3 | 21.2 |
| Parameter | Temperature Activated Fly Ashes | |||||||||||
| FA | FA | FA | FA500 | FA500 | FA500 | FA700 | FA700 | FA700 | FA900 | FA900 | FA900 | |
| T, K | 293 | 313 | 333 | 293 | 313 | 333 | 293 | 313 | 333 | 293 | 313 | 333 |
| am, Mol kg−1 | 0.679 | 0.733 | 0.733 | 0.625 | 0.705 | 0.728 | 0.678 | 0.691 | 0.726 | 0.637 | 0.638 | 0.641 |
| KL, m3 Mol−1 | 0.053 | 0.060 | 0.078 | 0.056 | 0.069 | 0.083 | 0.086 | 0.097 | 0.132 | 0.093 | 0.101 | 0.132 |
| R2 | 0.99 | 0.99 | 1.00 | 0.99 | 0.98 | 0.98 | 1.00 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 |
| Chitosan | ||||||||||||
| T, K | 293 | 313 | 333 | |||||||||
| am, Mol kg−1 | 0.428 | 0.439 | 0.471 | |||||||||
| KL, m3 Mol−1 | 0.130 | 0.171 | 0.193 | |||||||||
| R2 | 1.00 | 0.99 | 1.00 | |||||||||
| Chitosan Modified Fly Ashes | ||||||||||||
| FA CS | FA CS | FA CS | FA500 CS | FA500 CS | FA500 CS | FA700 CS | FA700 CS | FA700 CS | FA900 CS | FA900 CS | FA900 CS | |
| T, K | 293 | 313 | 333 | 293 | 313 | 333 | 293 | 313 | 333 | 293 | 313 | 333 |
| am, Mol kg−1 | 0.359 | 0.369 | 0.382 | 0.326 | 0.328 | 0.349 | 0.318 | 0.335 | 0.356 | 0.318 | 0.328 | 0.346 |
| KL, m3 Mol−1 | 0.166 | 0.202 | 0.231 | 0.167 | 0.180 | 0.182 | 0.271 | 0.290 | 0.297 | 0.337 | 0.400 | 0.433 |
| R2 | 0.99 | 0.99 | 0.98 | 1.00 | 1.00 | 0.99 | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Parameter | FA | FA500 | FA700 | FA900 | CS | FACS | FA500CS | FA700CS | FA900CS |
|---|---|---|---|---|---|---|---|---|---|
| ΔH°, kJ mol−1 | 7.73 | 8.02 | 8.76 | 6.97 | 8.03 | 6.65 | 1.83 | 1.93 | 5.09 |
| R2 | 0.94 | 1.00 | 0.92 | 0.89 | 0.97 | 1.00 | 0.89 | 0.95 | 0.97 |
| Parameter | Temperature Activated Fly Ash | |||||||||||
| FA | FA | FA | FA500 | FA | FA500 | FA | FA700 | FA | FA900 | FA | FA900 | |
| c0, mg dm−3 | 100 | 200 | 300 | 100 | 200 | 300 | 100 | 200 | 300 | 100 | 200 | 300 |
| qe, mol kg−1 | 0.018 | 0.056 | 0.090 | 0.026 | 0.068 | 0.132 | 0.033 | 0.055 | 0.085 | 0.037 | 0.066 | 0.094 |
| k, mol kg−1 s−1 | 0.028 | 0.009 | 0.014 | 0.054 | 0.017 | 0.009 | 0.097 | 0.067 | 0.034 | 0.021 | 0.021 | 0.012 |
| R2 | 1.00 | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 0.99 |
| Chitosan | ||||||||||||
| CS | CS | CS | ||||||||||
| c0, mg dm−3 | 100 | 200 | 300 | |||||||||
| qe, mol kg−1 | 0.042 | 0.056 | 0.112 | |||||||||
| k, mol kg−1 s−1 | 0.021 | 0.018 | 0.029 | |||||||||
| R2 | 1.00 | 1.00 | 1.00 | |||||||||
| Chitosan Modified Fly Ash | ||||||||||||
| FA CS | FA CS | FA CS | FA CS | FA CS | FA CS | FA CS | FA CS | FA CS | FA CS | FA CS | FA CS | |
| c0, mg dm−3 | 100 | 200 | 300 | 100 | 200 | 300 | 100 | 200 | 300 | 100 | 200 | 300 |
| qe, mol kg−1 | 0.021 | 0.063 | 0.145 | 0.044 | 0.068 | 0.092 | 0.059 | 0.095 | 0.105 | 0.043 | 0.089 | 0.169 |
| k, mol kg−1 s−1 | 0.042 | 0.021 | 0.029 | 0.088 | 0.031 | 0.028 | 0.021 | 0.074 | 0.030 | 0.014 | 0.041 | 0.053 |
| R2 | 0.99 | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.99 | 1.00 | 1.00 |
| K0 Definition | FA | FA500 | FA700 | FA900 | CS | FACS | FA500CS | FA700CS | FA900CS | |
|---|---|---|---|---|---|---|---|---|---|---|
| K0 = KD = a/ce | ΔH° | 6.67 | 6.13 | 6.48 | 6.76 | 9.43 | 11.57 | 4.41 | 6.68 | 9.12 |
| R2 | 1.00 | 0.98 | 0.87 | 0.90 | 0.54 | 0.85 | 0.95 | 0.91 | 0.99 | |
| K0 = KD(K&S) | ΔH° | 10.8 | 6.98 | 6.02 | 6.30 | 9.27 | 6.88 | 4.30 | 6.17 | 9.24 |
| R2 | 0.96 | 0.88 | 0.98 | 0.81 | 0.75 | 0.96 | 0.94 | 0.89 | 0.98 | |
| K0 = KD(L) = am KL | ΔH° | 9.34 | 11.10 | 9.96 | 7.13 | 9.98 | 7.97 | 3.12 | 4.15 | 6.81 |
| R2 | 0.99 | 0.99 | 0.92 | 0.89 | 0.99 | 1.00 | 1.00 | 1.00 | 0.99 | |
| K0 = KL | ΔH° | 7.73 | 8.02 | 8.76 | 6.97 | 8.03 | 6.65 | 1.83 | 1.93 | 5.09 |
| R2 | 0.94 | 1.00 | 0.92 | 0.89 | 0.97 | 1.00 | 0.89 | 0.95 | 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczuk, A.; Sofinska-Chmiel, W.; Jozefaciuk, G. Arsenate Adsorption on Fly Ash, Chitosan and Their Composites and Its Relations with Surface, Charge and Pore Properties of the Sorbents. Materials 2020, 13, 5381. https://doi.org/10.3390/ma13235381
Adamczuk A, Sofinska-Chmiel W, Jozefaciuk G. Arsenate Adsorption on Fly Ash, Chitosan and Their Composites and Its Relations with Surface, Charge and Pore Properties of the Sorbents. Materials. 2020; 13(23):5381. https://doi.org/10.3390/ma13235381
Chicago/Turabian StyleAdamczuk, Agnieszka, Weronika Sofinska-Chmiel, and Grzegorz Jozefaciuk. 2020. "Arsenate Adsorption on Fly Ash, Chitosan and Their Composites and Its Relations with Surface, Charge and Pore Properties of the Sorbents" Materials 13, no. 23: 5381. https://doi.org/10.3390/ma13235381
APA StyleAdamczuk, A., Sofinska-Chmiel, W., & Jozefaciuk, G. (2020). Arsenate Adsorption on Fly Ash, Chitosan and Their Composites and Its Relations with Surface, Charge and Pore Properties of the Sorbents. Materials, 13(23), 5381. https://doi.org/10.3390/ma13235381





