Microstructural Assessment of a Multiple-Intermetallic-Strengthened Aluminum Alloy Produced from Gas-Atomized Powder by Hot Extrusion and Friction Extrusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Base Materials
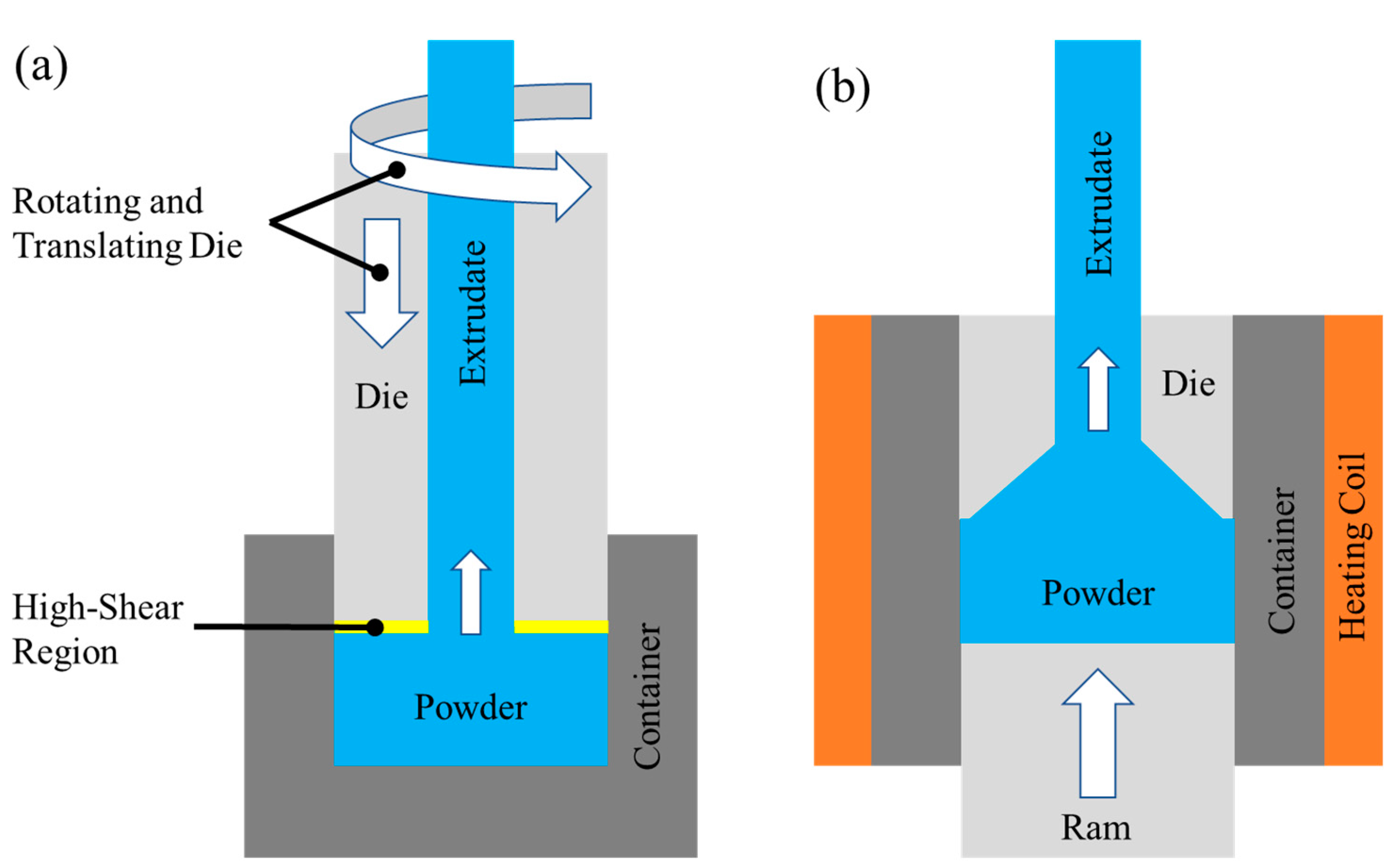
2.2. Friction Extrusion and Hot Extrusion Processes
2.3. Sample Preparation and Characterization Methods
2.4. Equilibrium Thermodynamic Calculation
3. Results and Discussion
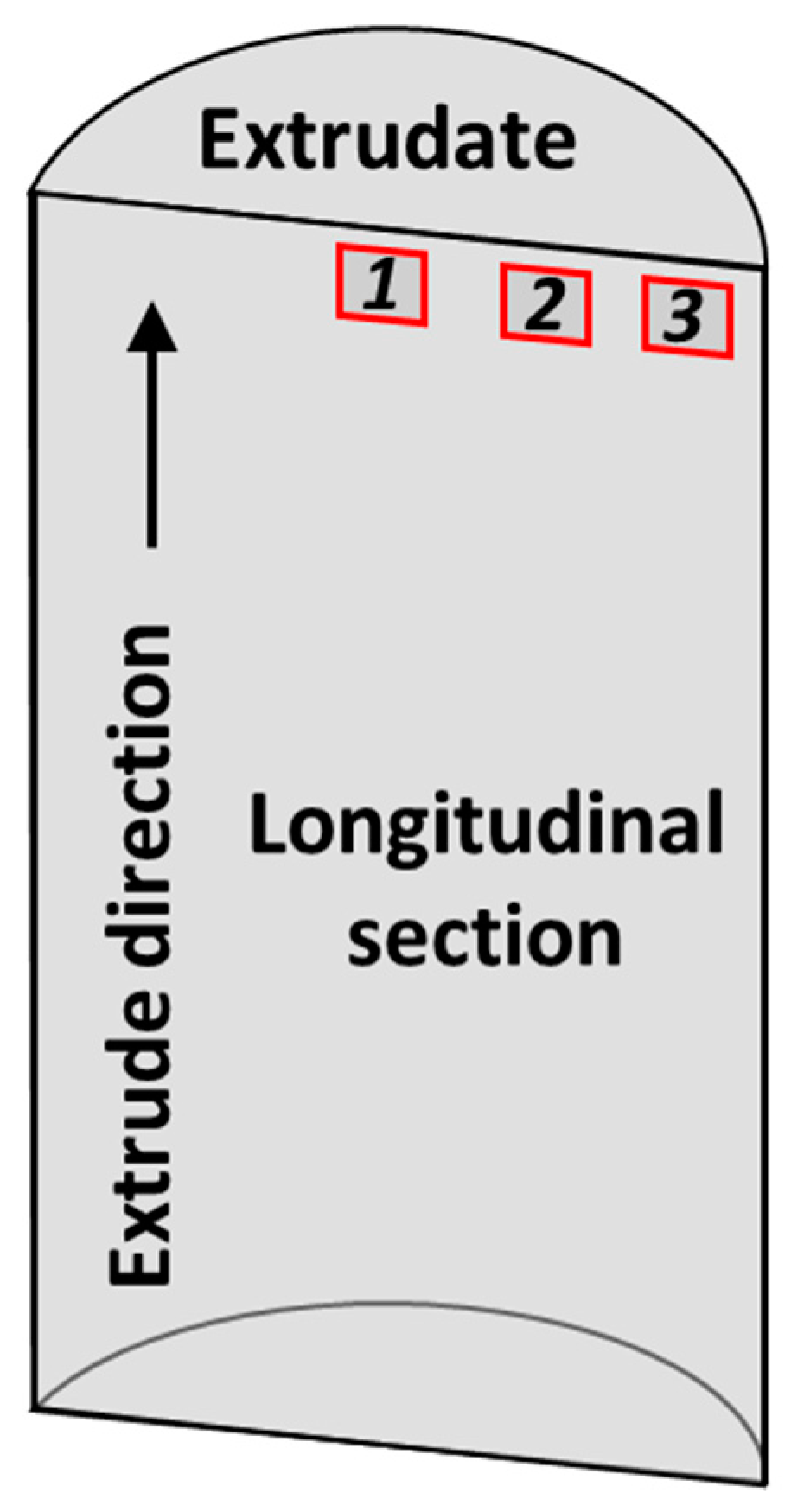
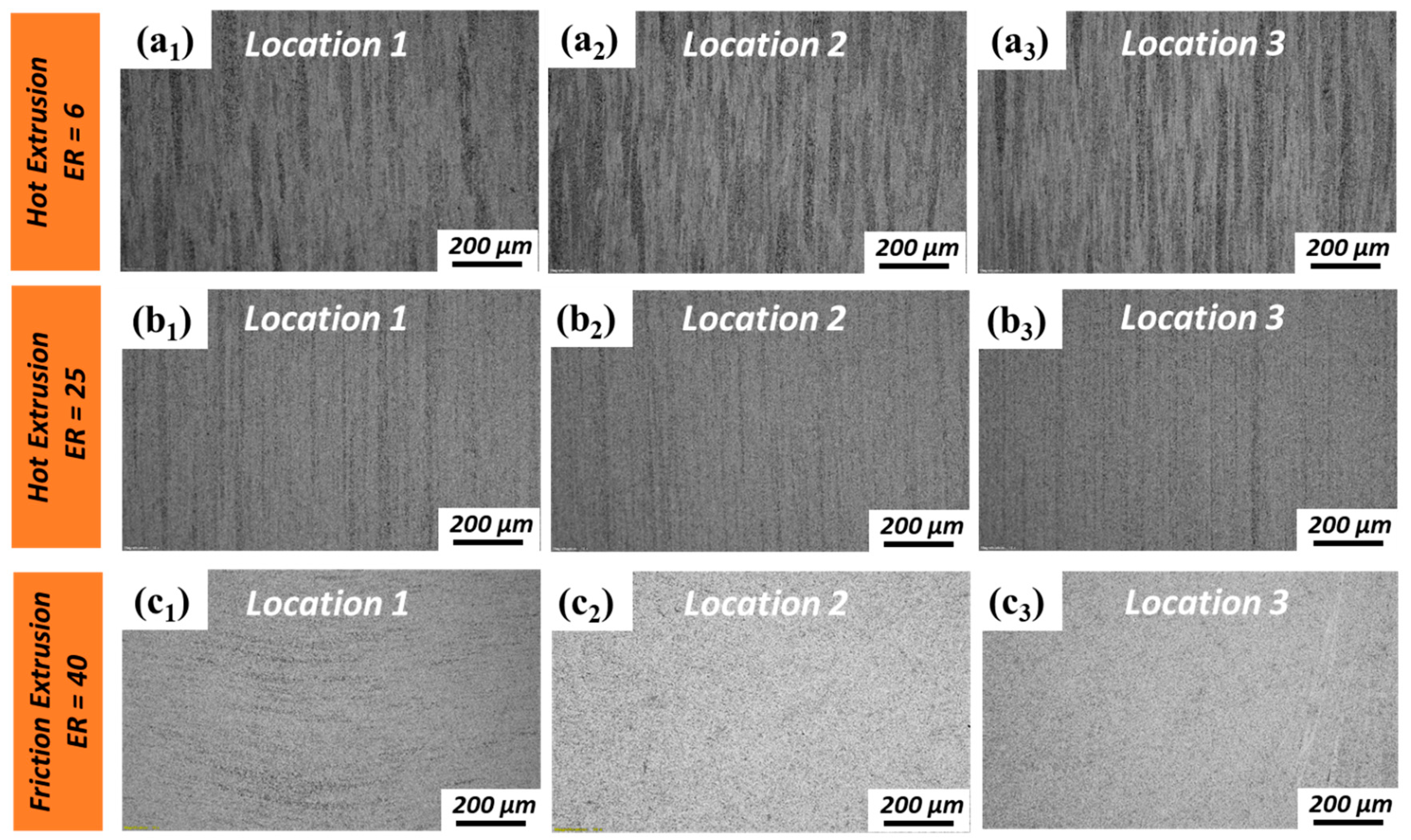
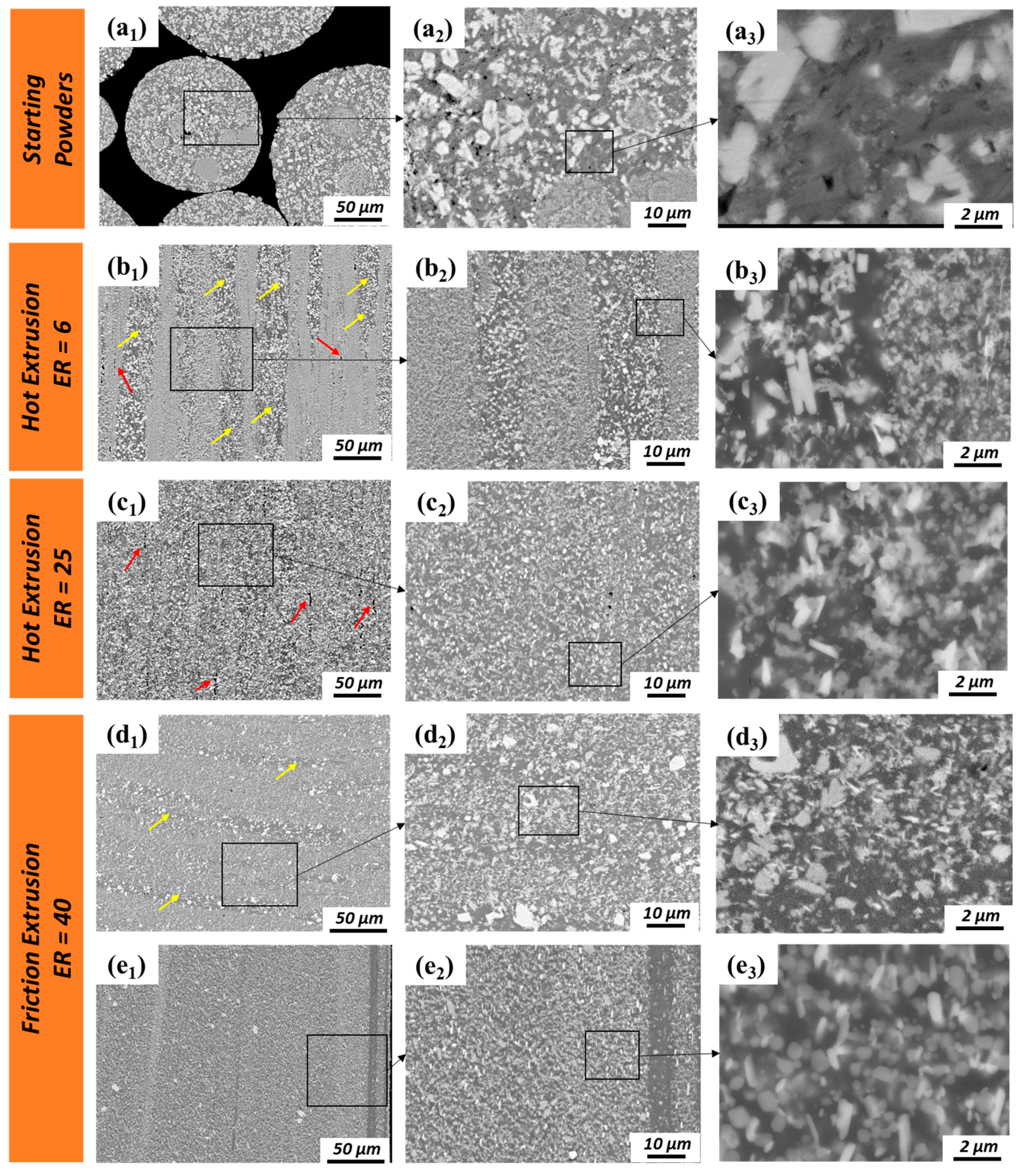
3.1. Microstructural Investigation
3.2. Mechanical Response Characterization
4. Conclusions
- For hot extrusion, deformation at the extrusion periphery region is more severe than at the extrusion center region. While for friction extrusion, material flow perpendicular to the extrusion direction is dominant at the center region, and material flow parallel to the extrusion direction is dominant at the extrusion periphery region.
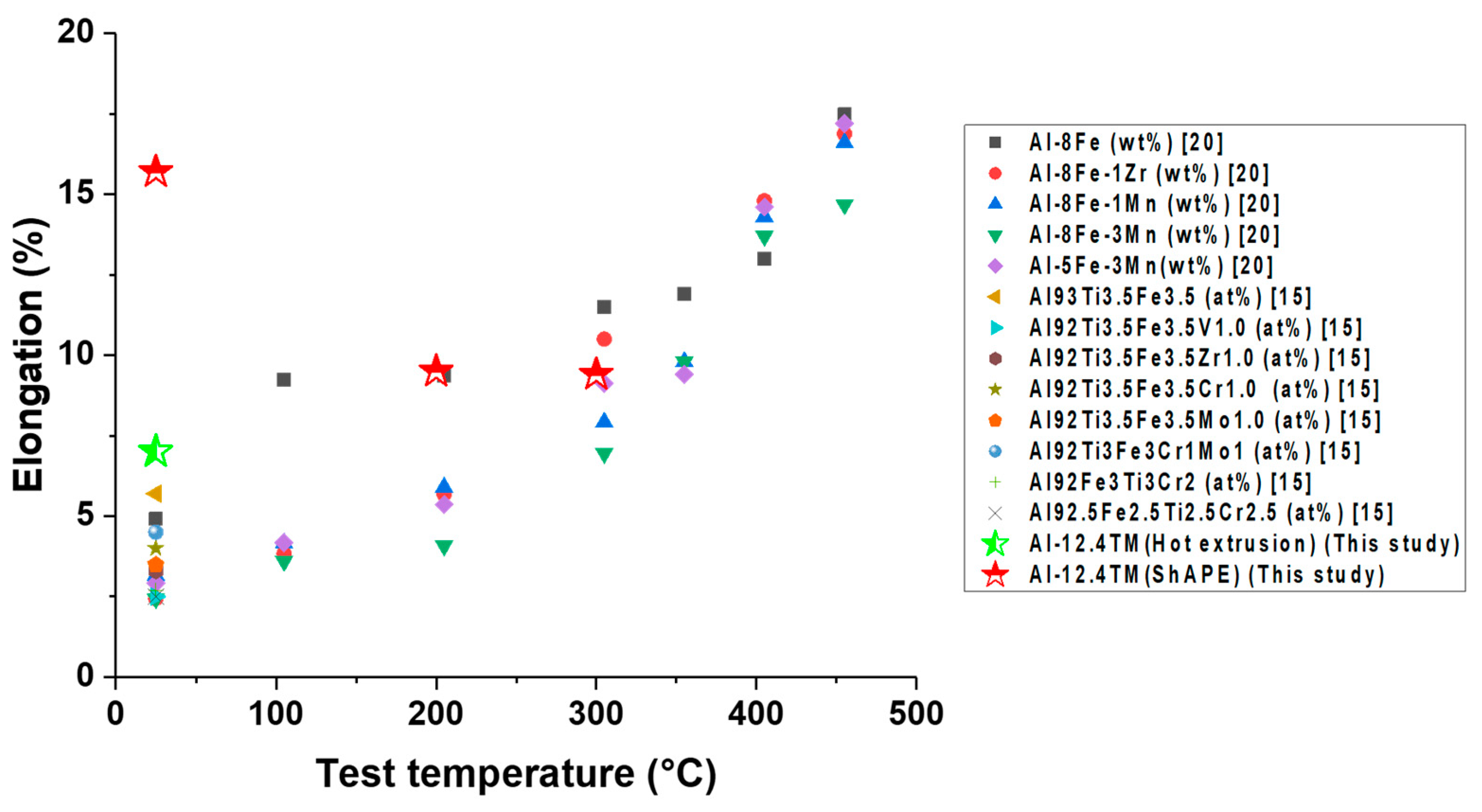
- Friction-extruded Al–12.4TM has a comparable strength (>200 MPa at 300 °C) but a higher ductility (>15% at ambient temperature) than hot-extruded Al–12.4TM. This is likely due to the elimination of voiding and finer second phases, which is enabled by the additional high-shear component introduced during friction extrusion.
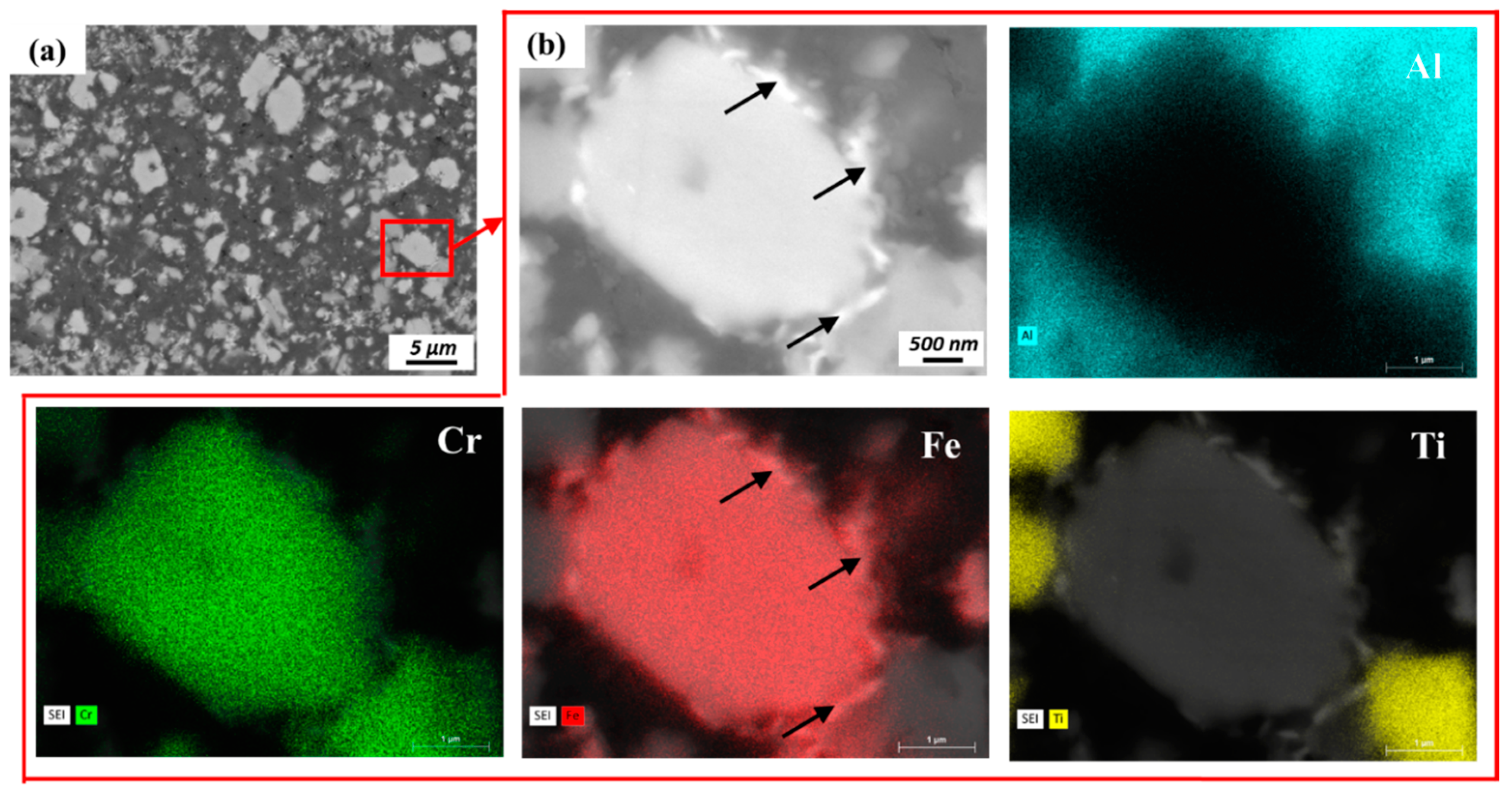
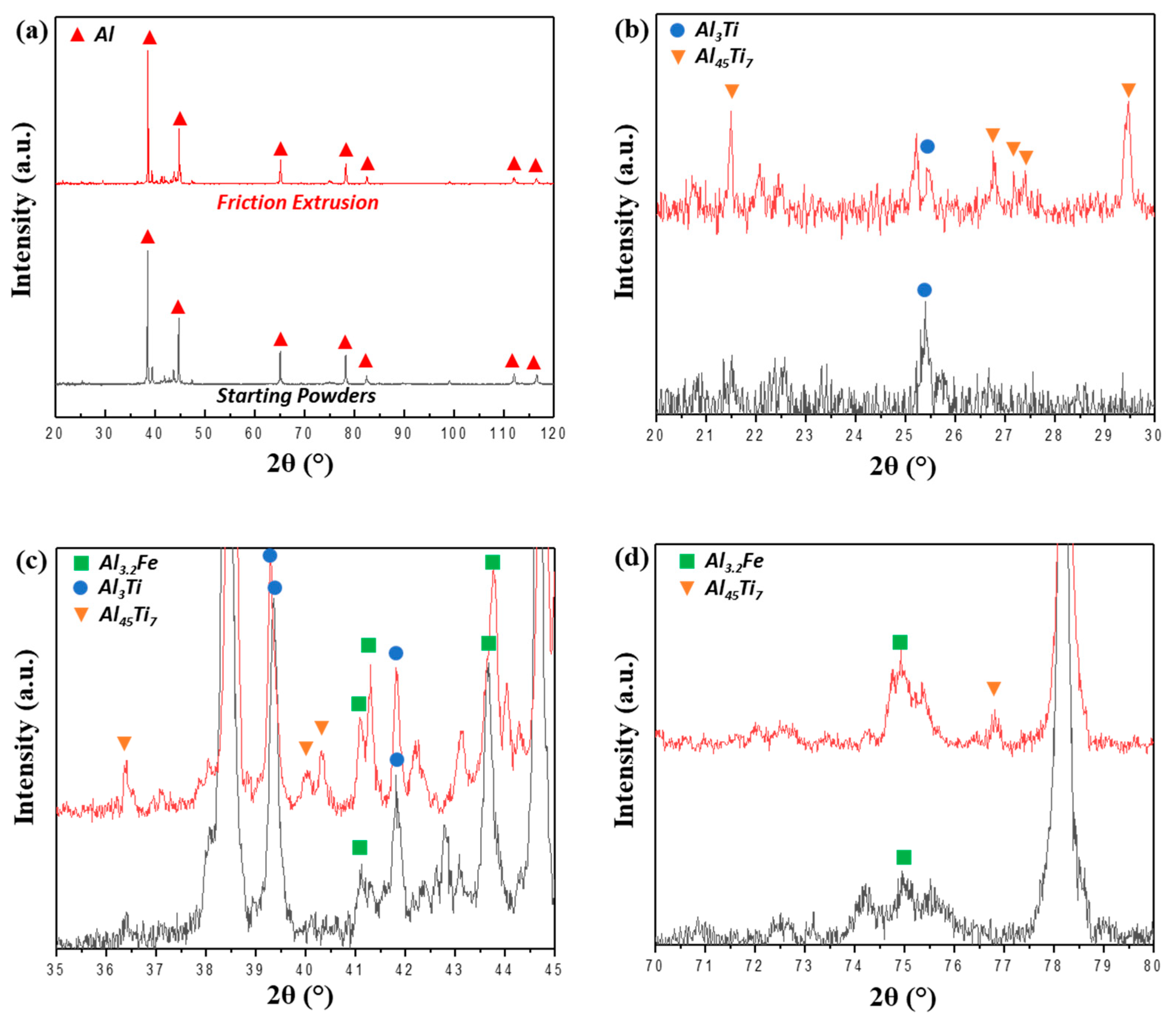
- During extrusion, the quasicrystal approximant IMC phase (70.4 wt % Al, 20.4 wt % Fe, 8.7 wt % Cr, 0.6 wt % Ti) was broken into small pieces. Fe and Cr tend to separate from each other, forming new IMC phases of the Al3.2Fe–type (65 wt % Al, 35 wt % Fe) and non-stoichiometric Al45Cr7-type structures (78 wt % Al, 11 wt % Cr, 9 wt % Fe, 2 wt % Ti).
- The rectangular IMC phases (66.4 wt % Al, 28.8 wt % Ti, 4.7 wt % Cr) with Al3Ti-type structures in the powder precursor break into small pieces and are dissolved into the Al matrix during hot extrusion and friction extrusion processes.
- A fine-scale distribution of multiple intermetallic phases is achieved in this material using friction extrusion. The competition of coarsening at the interfaced between these precipitates pins them and restricts their extent of coarsening even at high temperature.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Davis, J.R. Aluminum and Aluminum Alloys; ASM International: Cleveland, OH, USA, 1993; pp. 351–416. [Google Scholar]
- Miller, W.S.; Zhuang, L.; Bottema, J.; Wittebrood, A.; De Smet, P.; Haszler, A.; Vieregge, A.J.M.S. Recent development in aluminium alloys for the automotive industry. Mater. Sci. Eng. A 2000, 280, 37–49. [Google Scholar] [CrossRef]
- Rambabu, P.P.N.K.V.; Prasad, N.E.; Kutumbarao, V.V.; Wanhill, R.J.H. Aluminium alloys for aerospace applications. In Aerospace Materials and Material Technologies; Springer: Berlin, German, 2017; pp. 29–52. [Google Scholar]
- Kaufman, J.G. Properties and Characteristics of Aluminum and Aluminum Alloys; ASM International: Geauga, OH, USA, 2016; pp. 1–9. [Google Scholar]
- Mishra, R.S.; Ma, Z.Y. Friction stir welding and processing. Mater. Sci. Eng. R 2005, 50, 1–78. [Google Scholar]
- Shyam, A.; Roy, S.; Shin, D.; Poplawsky, J.D.; Allard, L.F.; Yamamoto, Y.; Morris, J.R.; Mazumder, B.; Idrobo, J.C.; Rodriguez, A.; et al. Elevated temperature microstructural stability in cast AlCuMnZr alloys through solute segregation. Mater. Sci. Eng. A 2019, 765, 138279. [Google Scholar] [CrossRef]
- Harada, Y.; Dunand, D.C. Thermal expansion of Al3Sc and Al3(Sc0.75X0.25). Scr. Materialia 2003, 48, 219–222. [Google Scholar] [CrossRef]
- Blake, N.; Hopkins, M.A. Constitution and age hardening of Al-Sc alloys. J. Mater. Sci. 1985, 20, 2861–2867. [Google Scholar] [CrossRef]
- Norman, A.F.; Prangnell, P.B.; McEwen, R.S. The solidification behaviour of dilute aluminium-scandium alloys. Acta Mater. 1998, 46, 5715–5732. [Google Scholar] [CrossRef]
- Røyset, J.; Ryum, N. Kinetics and mechanisms of precipitation in an Al-0.2 wt.% Sc alloy. Mater. Sci. Eng. A 2005, 396, 409–422. [Google Scholar]
- Seibold, A. Phase equilibria in the ternary system Ti-Fe-O and Ti-Al-Fe. Z. Met. 1981, 72, 712–719. [Google Scholar]
- Wagner, C. Theory of precipitate change by redissolution. Z. Elektrochem. 1961, 65, 581–591. [Google Scholar]
- Skinner, D.J.; Bye, R.L.; Raybould, D.; Brown, A.M. Dispersion strengthened Al-Fe-V-Si alloys. Scr. Metall. 1986, 20, 867–872. [Google Scholar] [CrossRef]
- Kimura, H.; Sasamori, K.; Inoue, A. High strength Al-Ti-Fe alloys consisting of amorphous and fcc-Al phases prepared by rapid solidification. Mater. Trans. JIM 1996, 37, 1722–1725. [Google Scholar] [CrossRef][Green Version]
- Kawamura, Y.; Inoue, A.; Takagi, M.; Ohta, H.; Imura, T.; Masumoto, T. Rapidly solidified powder metallurgy of Al-Ti-Fe-X alloys. Scr. Mater. 1999, 40, 1131–1137. [Google Scholar]
- Zawrah, M.; Shaw, L. Microstructure and hardness of nanostructured Al-Fe-Cr-Ti alloys through mechanical alloying. Mater. Sci. Eng. A 2003, 355, 37–49. [Google Scholar] [CrossRef]
- Daye, W.K.; Pelletiers, I.; Thomas, W. Property development of new generation pm aluminum materials via innovative processing. Int. J. Powder Metall. 2018, 54, 35–51. [Google Scholar]
- Whalen, S.; Olszta, M.; Roach, C.; Darsell, J.; Graff, D.; Reza-E-Rabby, M.; Roosendaal, T.; Daye, W.; Pelletiers, T.; Mathaudhu, S.; et al. High ductility aluminum alloy made from powder by friction extrusion. Materialia 2019, 6, 100260. [Google Scholar] [CrossRef]
- Ellis, M.B.D.; Strangwood, M. Welding of rapidly solidified Alloy 8009 (Al-8·5Fe-1·7Si-1·3V): Preliminary study. Mater. Sci. Tech. Lond. 1996, 12, 970–977. [Google Scholar] [CrossRef]
- Thursfield, G.; Stowell, M.J. Mechanical properties of Al-8 wt.% Fe-based alloys prepared by rapid quenching from the liquid state. J. Mater. Sci. 1974, 9, 1644–1660. [Google Scholar] [CrossRef]
- Galano, M.; Audebert, F.; Escorial, A.G.; Stone, I.C.; Cantor, B. Nanoquasicrystalline Al-Fe-Cr-based alloys. Part II. Mechanical properties. Acta Mater. 2009, 57, 5120–5130. [Google Scholar] [CrossRef]
- Overmana, N.R.; Whalena, S.A.; Bowdenab, M.E.; Olsztaa, M.J.; Kruskaa, K.; Clarkc, T.; Stevensd, E.L.; Darsella, J.T.; Joshia, V.V.; Jianga, X.; et al. Homogenization and texture development in rapidly solidified AZ91E consolidated by Shear Assisted Processing and Extrusion (ShAPE). Mater. Sci. Eng. A 2017, 701, 56–68. [Google Scholar] [CrossRef]
- Darsell, J.T.; Overman, N.R.; Joshi, V.V.; Whalen, S.A.; Mathaudhu, S.N. Shear Assisted Processing and Extrusion (ShAPE™) of AZ91E Flake: A study of tooling features and processing effects. J. Mater. Eng. Perform. 2018, 27, 4150–4161. [Google Scholar] [CrossRef]
- Whalen, S.; Joshi, V.; Overman, N.; Caldwell, D.; Lavender, C.; Skszek, T. Scaled-up fabrication of thin-walled ZK60 tubing using shear assisted processing and extrusion (ShAPE). In Magnesium Technology; Springer: Berlin, German, 2017; pp. 315–321. [Google Scholar]
- Whalen, S.; Overman, N.; Joshi, V.; Varga, T.; Graff, D.; Lavender, C. Magnesium alloy ZK60 tubing made by Shear Assisted Processing and Extrusion (ShAPE). Mater. Sci. Eng. A 2019, 755, 278–288. [Google Scholar] [CrossRef]
- Krishnan, K.N. On the formation of onion rings in friction stir welds. Mater. Sci. Eng. A 2002, 327, 246–251. [Google Scholar] [CrossRef]
- Capdevila, C.; Miller, M.K.; Russell, K.F. Aluminum partitioning during phase separation in Fe-20% Cr-6% Al ODS alloy. J. Mater. Sci. 2008, 43, 3889–3893. [Google Scholar] [CrossRef]
- Kuronen, A.; Granroth, S.; Heinonen, M.H.; Perälä, R.E.; Kilpi, T.; Laukkanen, P.; Lång, J.; Dahl, J.; Punkkinen, M.P.J.; Kokko, K.; et al. Segregation, precipitation, and α-α′ phase separation in Fe-Cr alloys. Phys. Rev. B 2015, 92, 214113. [Google Scholar]
- Airiskallio, E.; Nurmi, E.; Heinonen, M.H.; Väyrynen, I.J.; Kokko, K.; Ropo, M.; Punkkinen, M.P.J.; Pitkänen, H.; Alatalo, M.; Kollár, J.; et al. Third element effect in the surface zone of FeCr-Al alloys. Phys. Rev. B 2010, 81, 033105. [Google Scholar]
- Terentyev, D.; Bergner, F.; Osetsky, Y. Cr segregation on dislocation loops enhances hardening in ferritic Fe-Cr alloys. Acta Mater. 2013, 61, 1444–1453. [Google Scholar] [CrossRef]
- Choudhury, S.; Barnard, L.; Tucker, J.D.; Allen, T.R.; Wirth, B.D.; Asta, M.; Morgan, D. Ab-initio based modeling of diffusion in dilute bcc Fe-Ni and Fe-Cr alloys and implications for radiation induced segregation. J. Nucl. Mater. 2011, 411, 1–14. [Google Scholar] [CrossRef]




| Elements | Al | Fe | Si | V | Zr | Cr | Mo | Mn | Ti | Nb | Ta | Methods | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Composition (atomic%) | Balance | 8.5 | 1.7 | 1.3 | – | – | – | – | – | – | – | RS | [19] |
| Balance | 8 | – | – | – | – | – | – | – | – | – | RS | [20] | |
| Balance | 8 | – | – | 1 | – | – | – | – | – | – | RS | [20] | |
| Balance | 8 | – | – | – | – | – | 1 | – | – | – | RS | [20] | |
| Balance | 8 | – | – | – | – | – | 3 | – | – | – | RS | [20] | |
| Balance | 5 | – | – | – | – | – | 3 | – | – | – | RS | [20] | |
| Balance | 3.5 | – | – | – | – | – | – | 3.5 | – | – | RS | [15] | |
| Balance | 3.5 | – | 1 | – | – | – | – | 3.5 | – | – | RS | [15] | |
| Balance | 3.5 | – | – | 1 | – | – | – | 3.5 | – | – | RS | [15] | |
| Balance | 3.5 | – | – | – | 1 | – | – | 3.5 | – | – | RS | [15] | |
| Balance | 3.5 | – | – | – | – | 1 | – | 3.5 | – | – | RS | [15] | |
| Balance | 3 | – | – | – | 1 | 1 | – | 3 | – | – | RS | [15] | |
| Balance | 3 | – | – | – | 2 | – | – | 3 | – | – | RS | [15] | |
| Balance | 2.5 | – | – | – | 2.5 | – | – | 2.5 | – | – | RS | [15] | |
| Balance | 4.2 | – | – | – | 2.8 | – | – | – | – | – | RS | [21] | |
| Balance | 3 | – | – | – | 2 | – | – | 2 | – | – | RS | [21] | |
| Balance | 3 | – | 2 | – | 2 | – | – | – | – | – | RS | [21] | |
| Balance | 3 | – | – | – | 2 | – | – | – | 2 | – | RS | [21] | |
| Balance | 2 | – | – | – | 2 | – | – | – | – | 2 | RS | [21] | |
| Balance | 2 | – | – | – | – | – | – | 3 | – | – | RS | [14] | |
| Balance | 2 | – | – | – | – | – | – | 4 | – | – | RS | [14] | |
| Balance | 2 | – | – | – | – | – | – | 5 | – | – | RS | [14] | |
| Balance | 2 | – | – | – | – | – | – | 6 | – | – | RS | [14] | |
| Balance | 2 | – | – | – | – | – | – | 8 | – | – | RS | [14] | |
| Balance | 3 | – | – | – | 2 | – | – | 2 | – | – | MA | [16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Gwalani, B.; Silverstein, J.; Darsell, J.; Jana, S.; Roosendaal, T.; Ortiz, A.; Daye, W.; Pelletiers, T.; Whalen, S. Microstructural Assessment of a Multiple-Intermetallic-Strengthened Aluminum Alloy Produced from Gas-Atomized Powder by Hot Extrusion and Friction Extrusion. Materials 2020, 13, 5333. https://doi.org/10.3390/ma13235333
Wang T, Gwalani B, Silverstein J, Darsell J, Jana S, Roosendaal T, Ortiz A, Daye W, Pelletiers T, Whalen S. Microstructural Assessment of a Multiple-Intermetallic-Strengthened Aluminum Alloy Produced from Gas-Atomized Powder by Hot Extrusion and Friction Extrusion. Materials. 2020; 13(23):5333. https://doi.org/10.3390/ma13235333
Chicago/Turabian StyleWang, Tianhao, Bharat Gwalani, Joshua Silverstein, Jens Darsell, Saumyadeep Jana, Timothy Roosendaal, Angel Ortiz, Wayne Daye, Tom Pelletiers, and Scott Whalen. 2020. "Microstructural Assessment of a Multiple-Intermetallic-Strengthened Aluminum Alloy Produced from Gas-Atomized Powder by Hot Extrusion and Friction Extrusion" Materials 13, no. 23: 5333. https://doi.org/10.3390/ma13235333
APA StyleWang, T., Gwalani, B., Silverstein, J., Darsell, J., Jana, S., Roosendaal, T., Ortiz, A., Daye, W., Pelletiers, T., & Whalen, S. (2020). Microstructural Assessment of a Multiple-Intermetallic-Strengthened Aluminum Alloy Produced from Gas-Atomized Powder by Hot Extrusion and Friction Extrusion. Materials, 13(23), 5333. https://doi.org/10.3390/ma13235333








