Facile Synthesis of g-C3N4/TiO2/Hectorite Z-Scheme Composite and Its Visible Photocatalytic Degradation of Rhodamine B
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Catalyst Preparation
2.2.1. g-C3N4
2.2.2. TiO2/Hectorite Composites
2.2.3. g-C3N4/TiO2/Hectorite Composites
2.3. Characterizations
2.4. Photo-Catalysis
3. Result and Discussion
3.1. Characterization
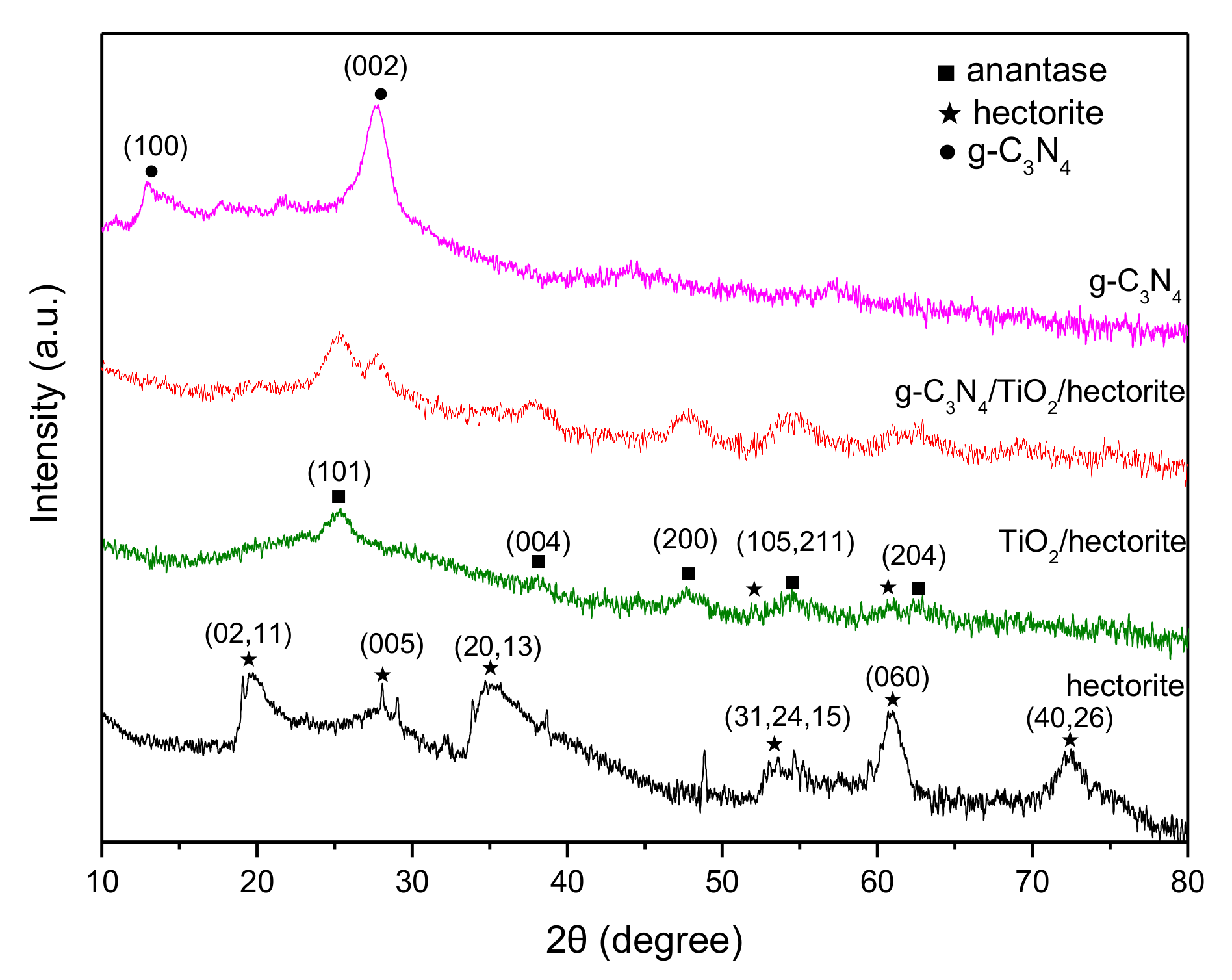
3.1.1. X-ray Diffraction (XRD)
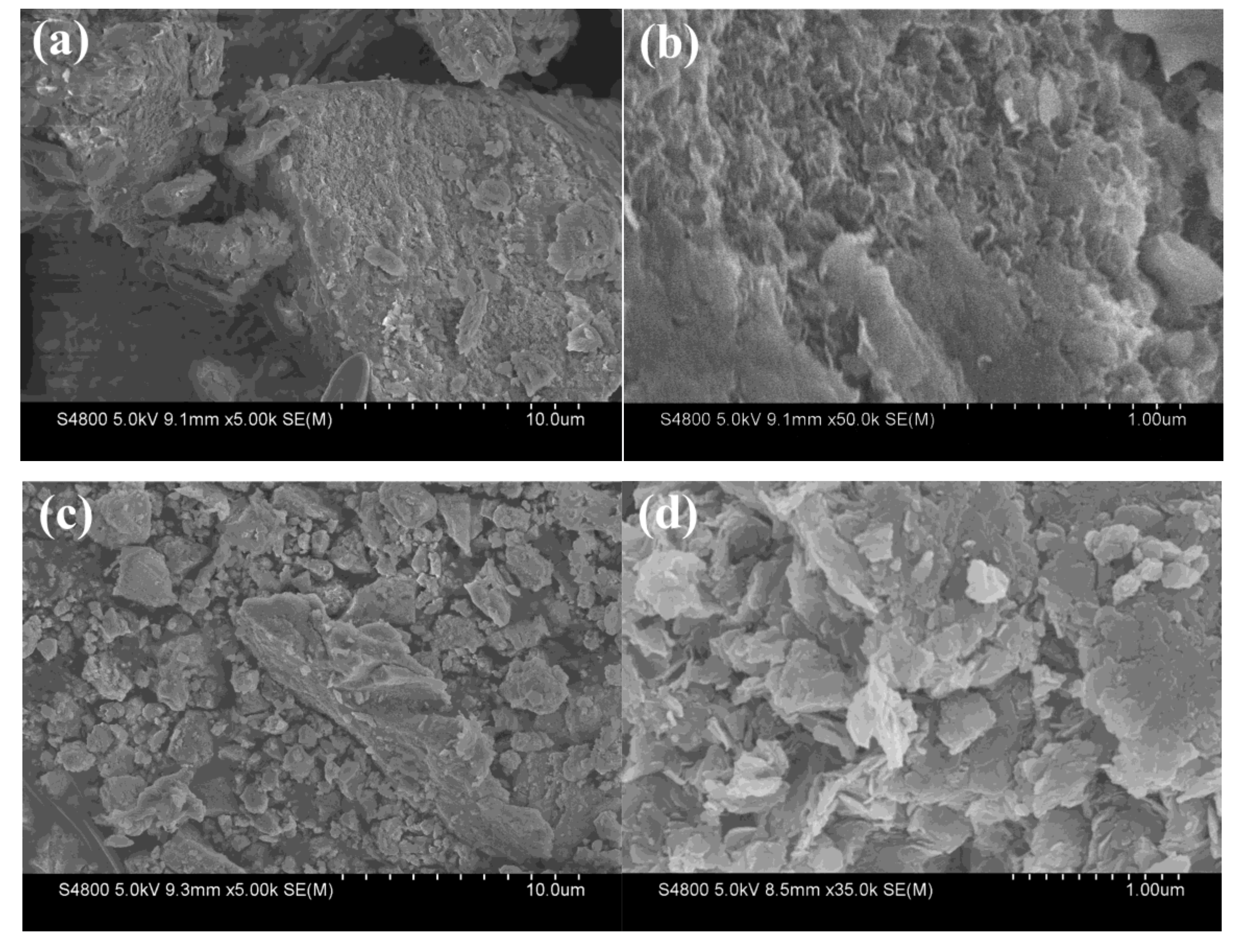
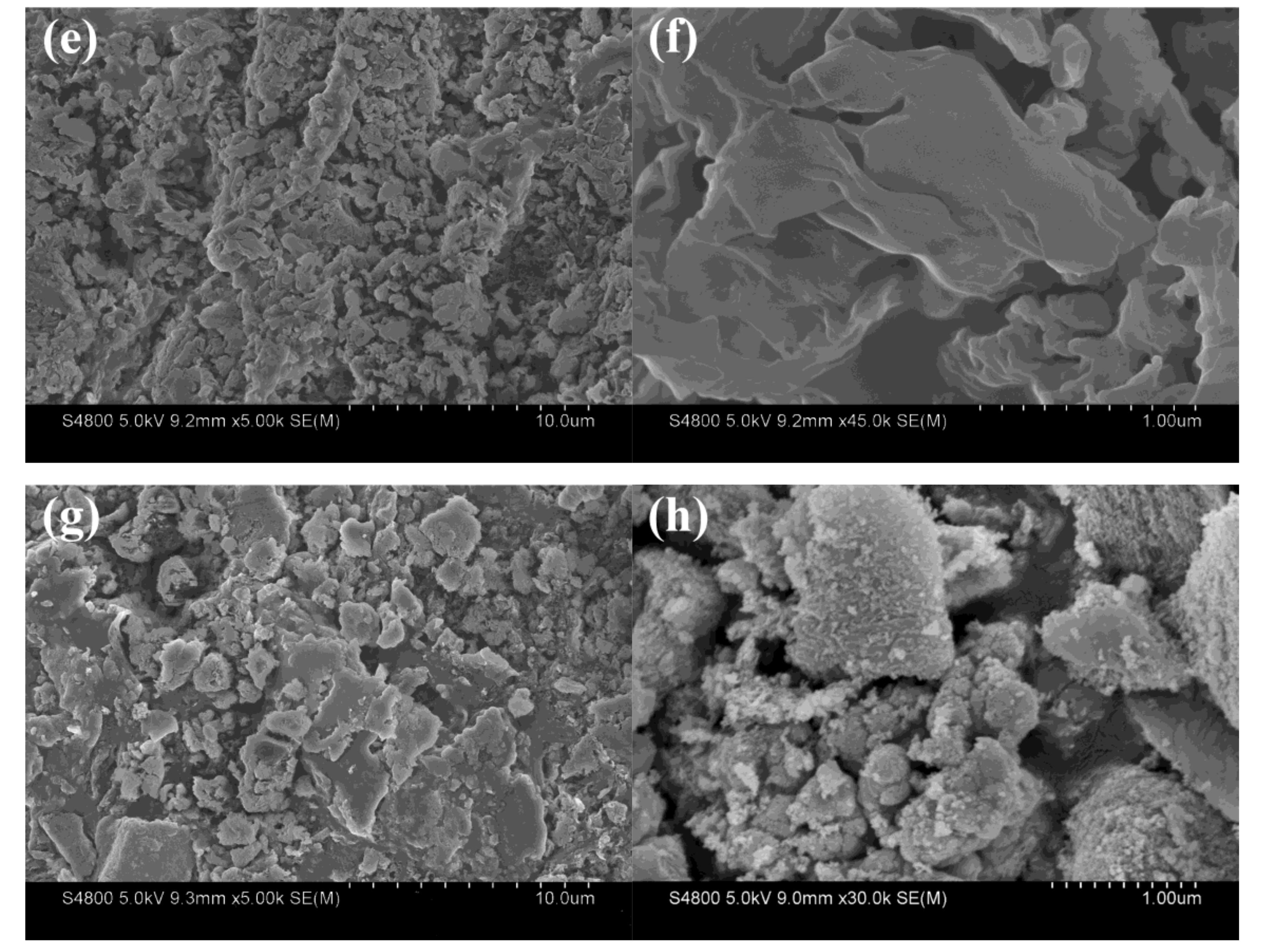
3.1.2. Scanning Electron Microscopy (SEM)
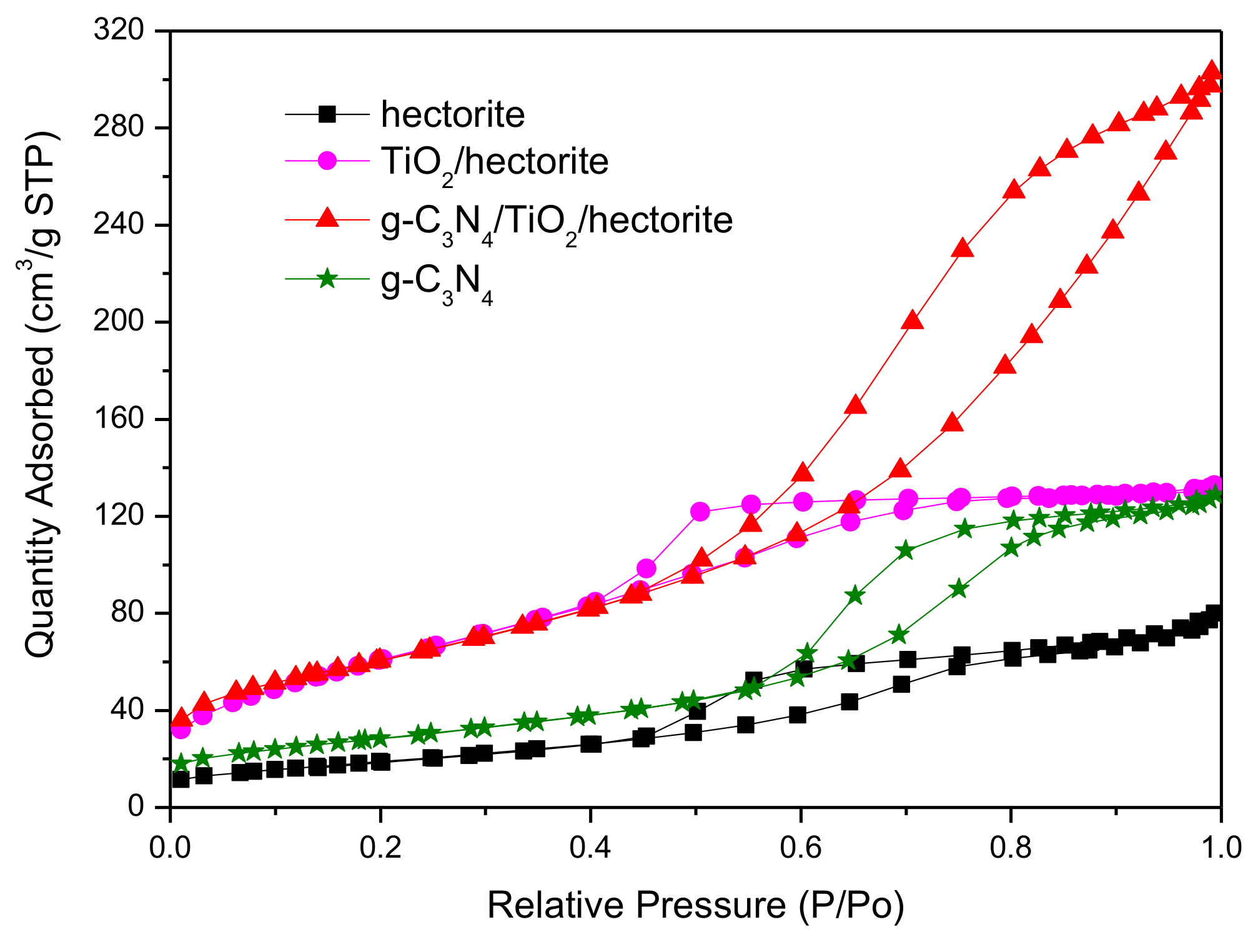
3.1.3. N2 Adsorption-Desorption Isotherms
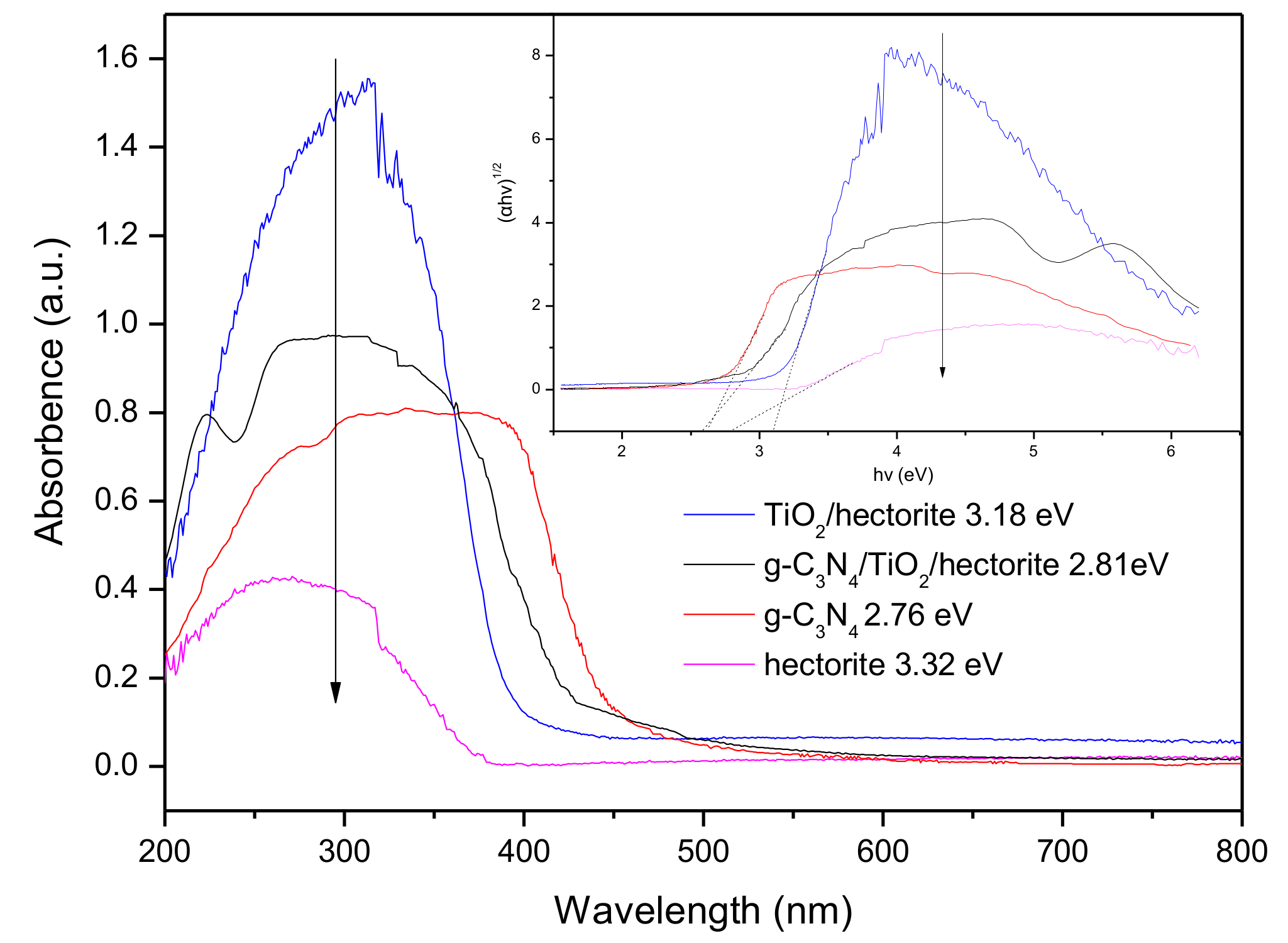
3.1.4. Ultraviolet–Visible Diffuse Reflectance Spectra(UV-Vis DRS)
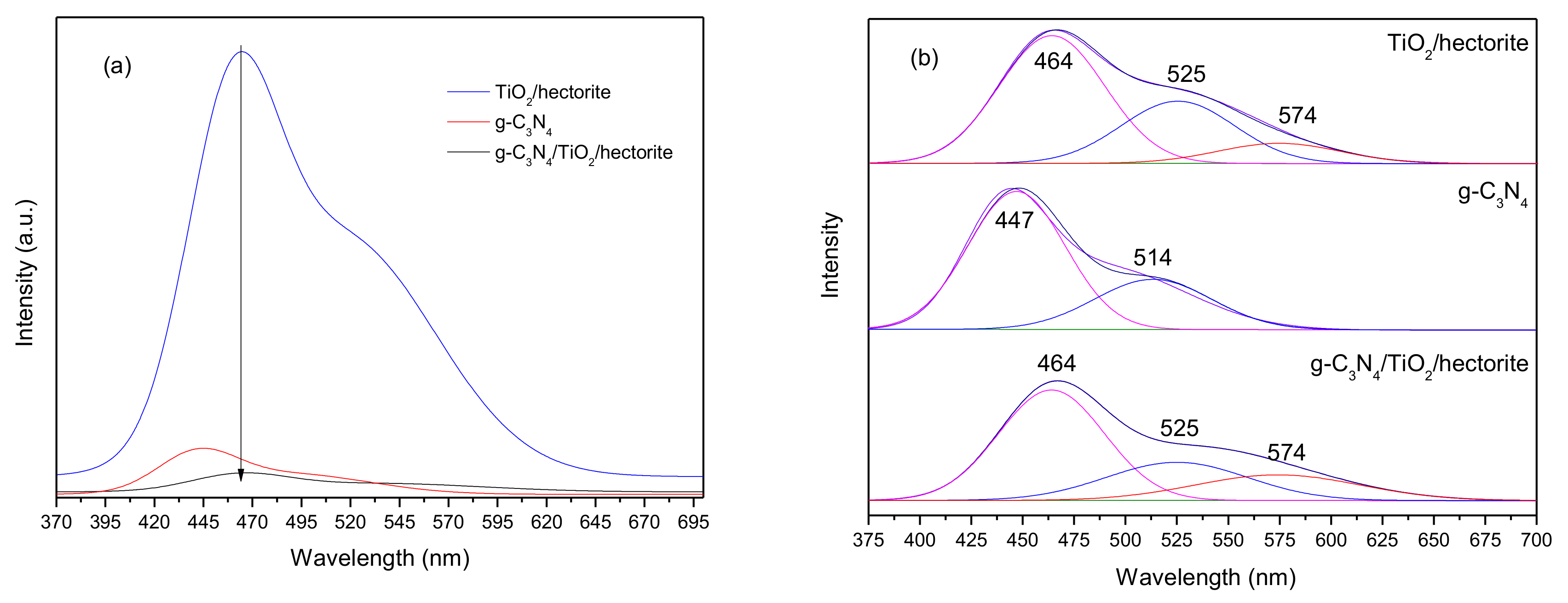
3.1.5. Photoluminescence (PL)
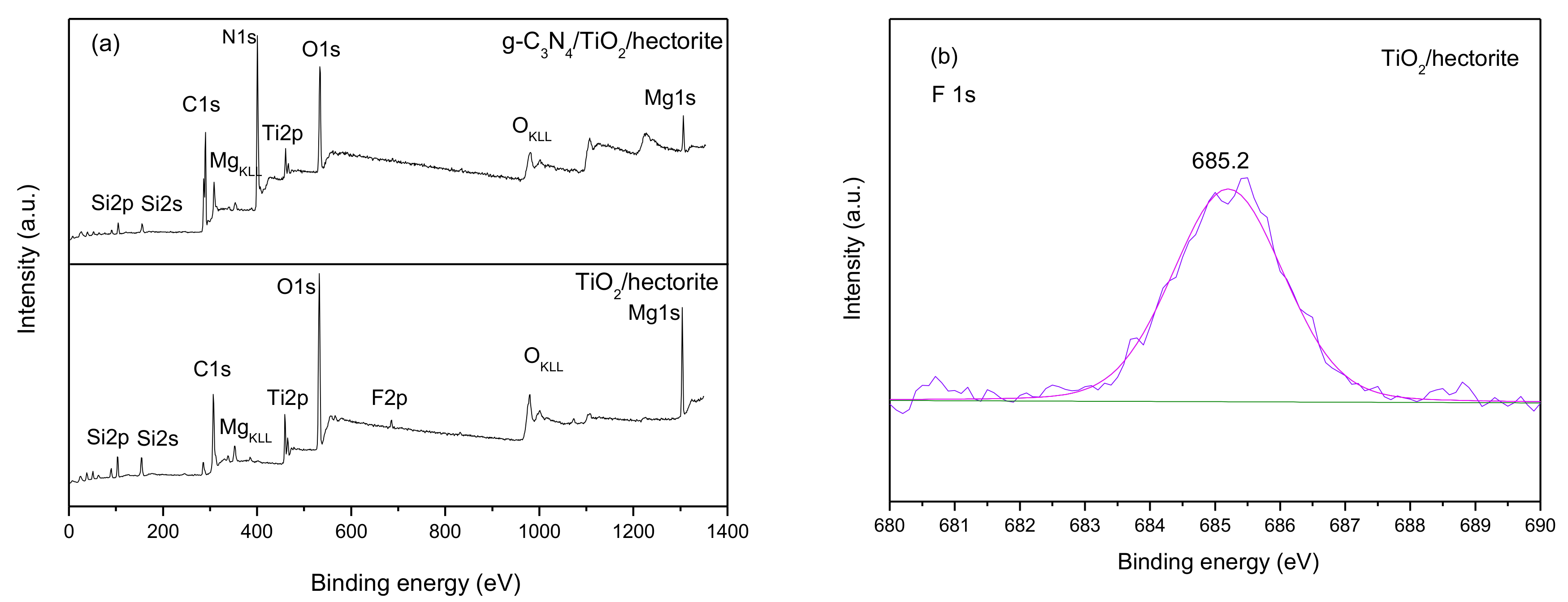
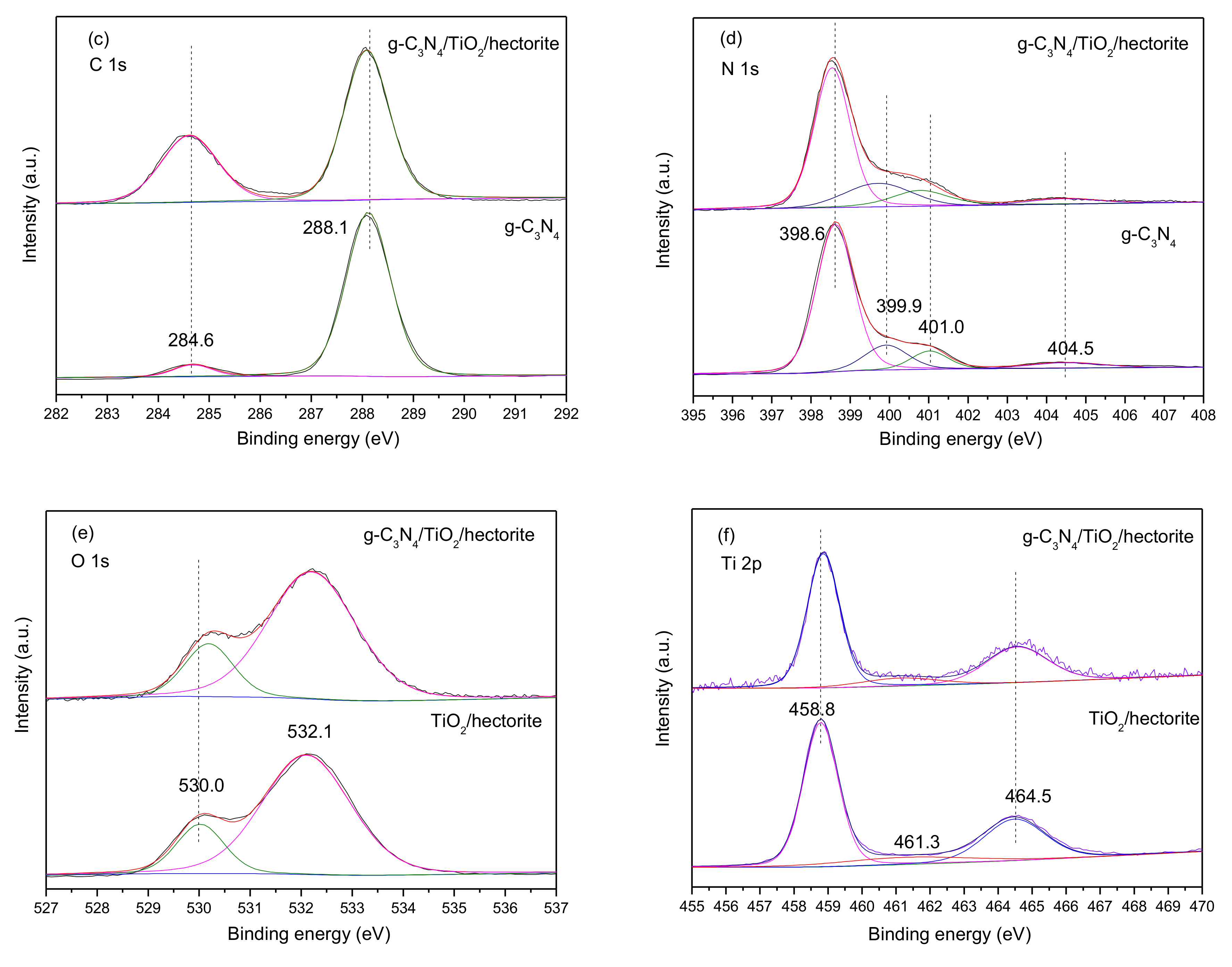
3.1.6. X-ray Photoelectron Spectroscopy (XPS)
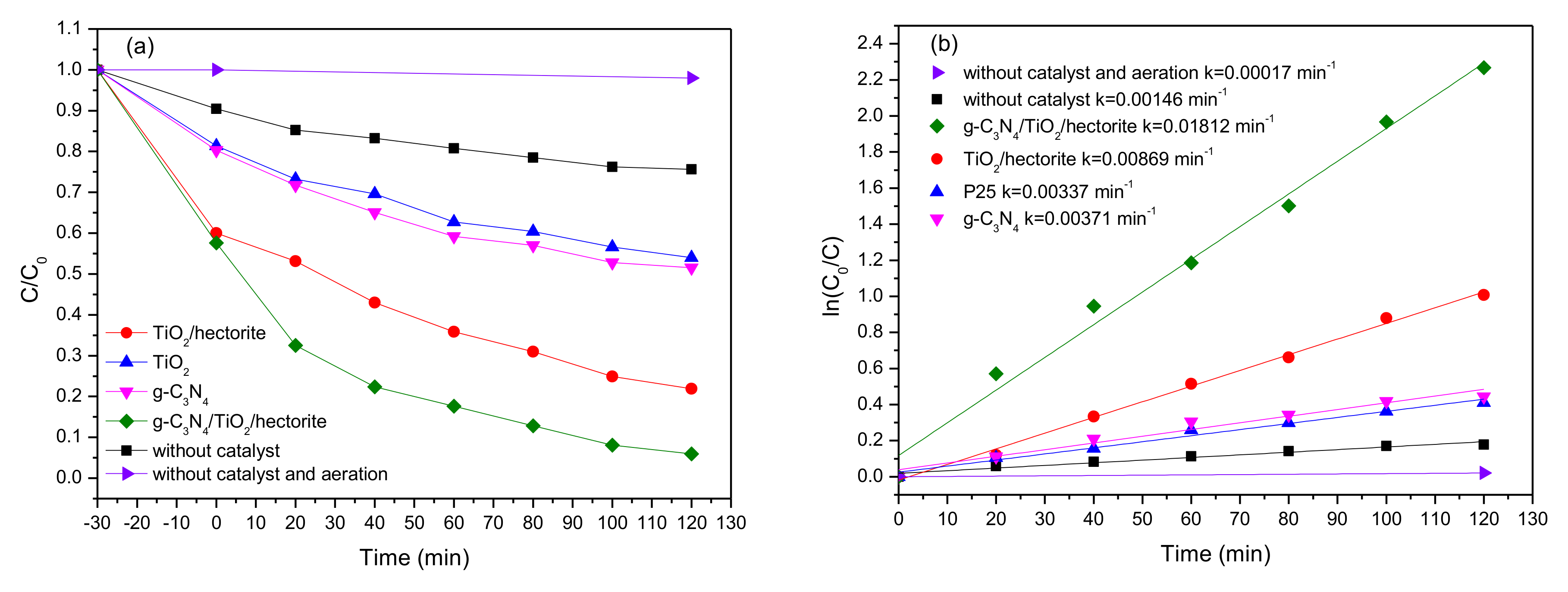
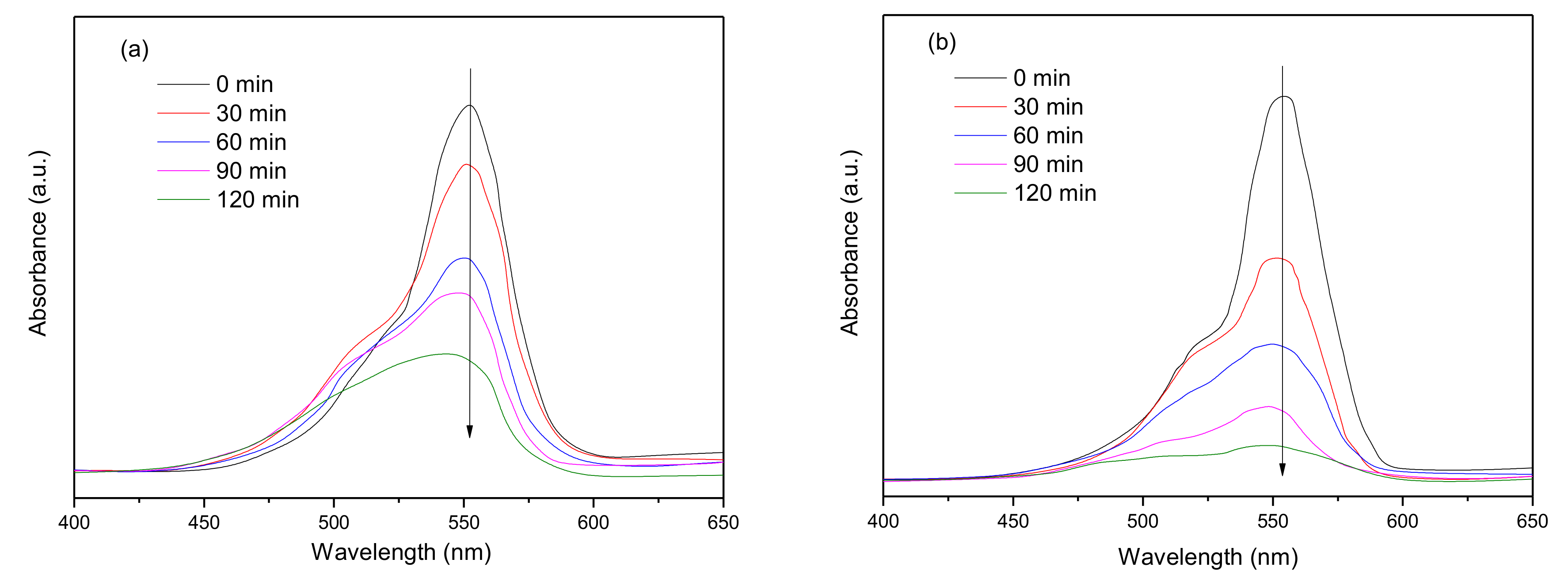
3.2. Photo-Catalytic Activity
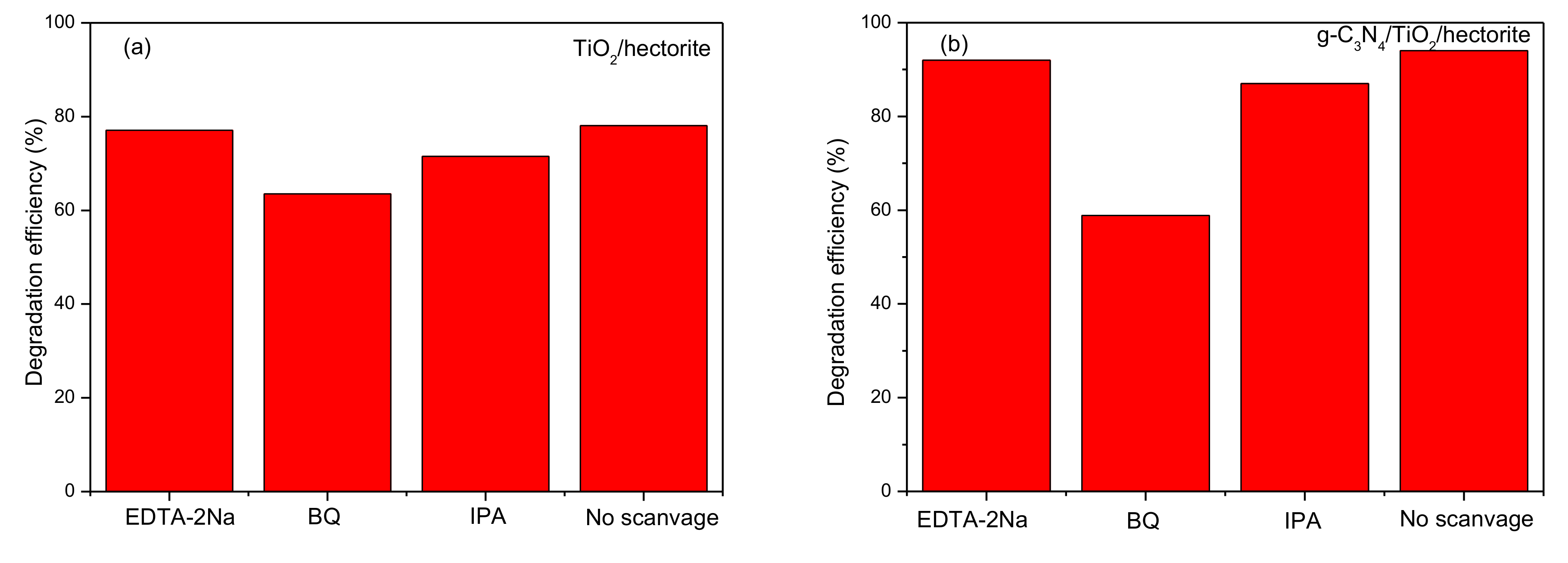
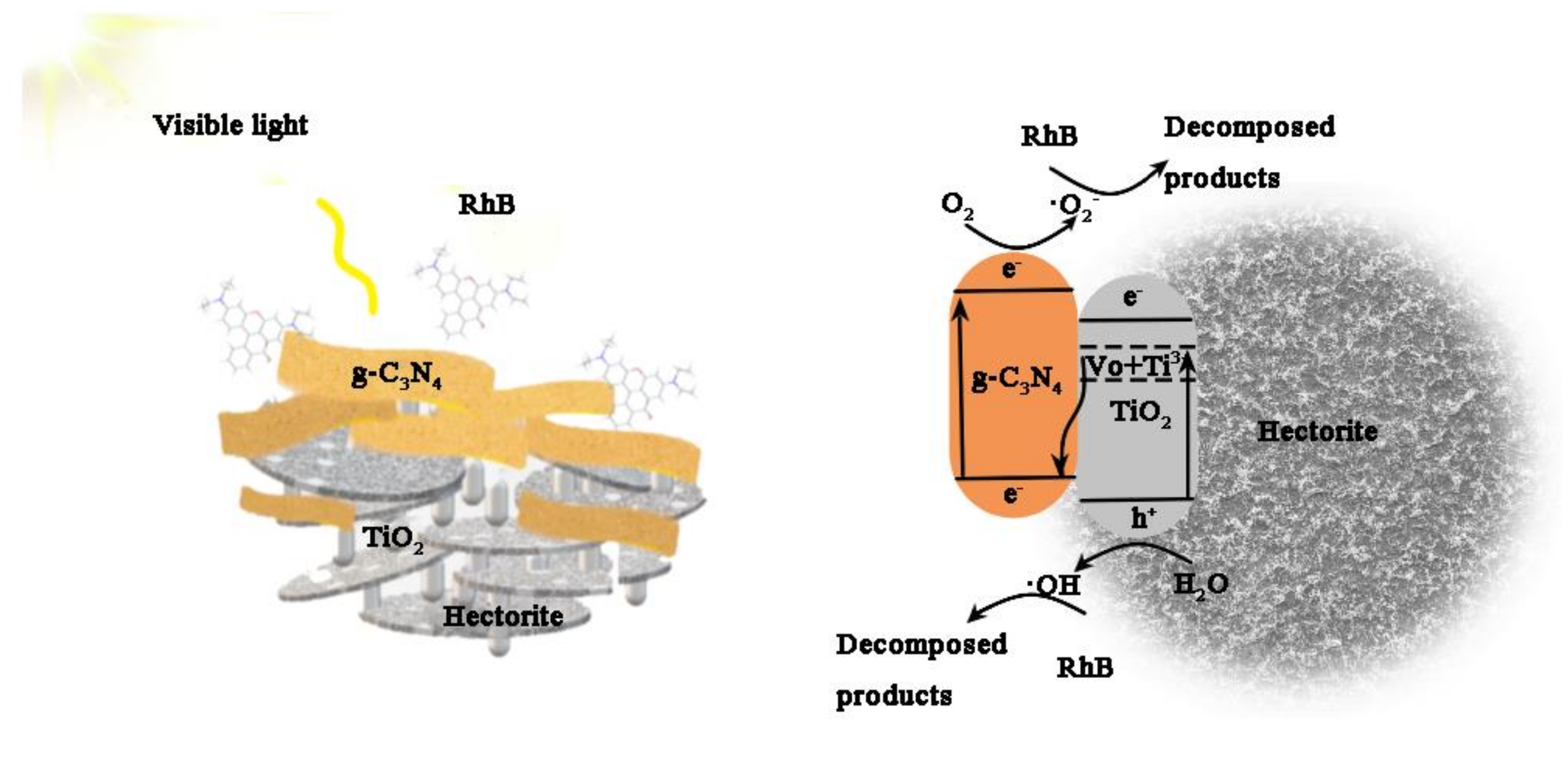
3.3. Photocatalytic Mechanism
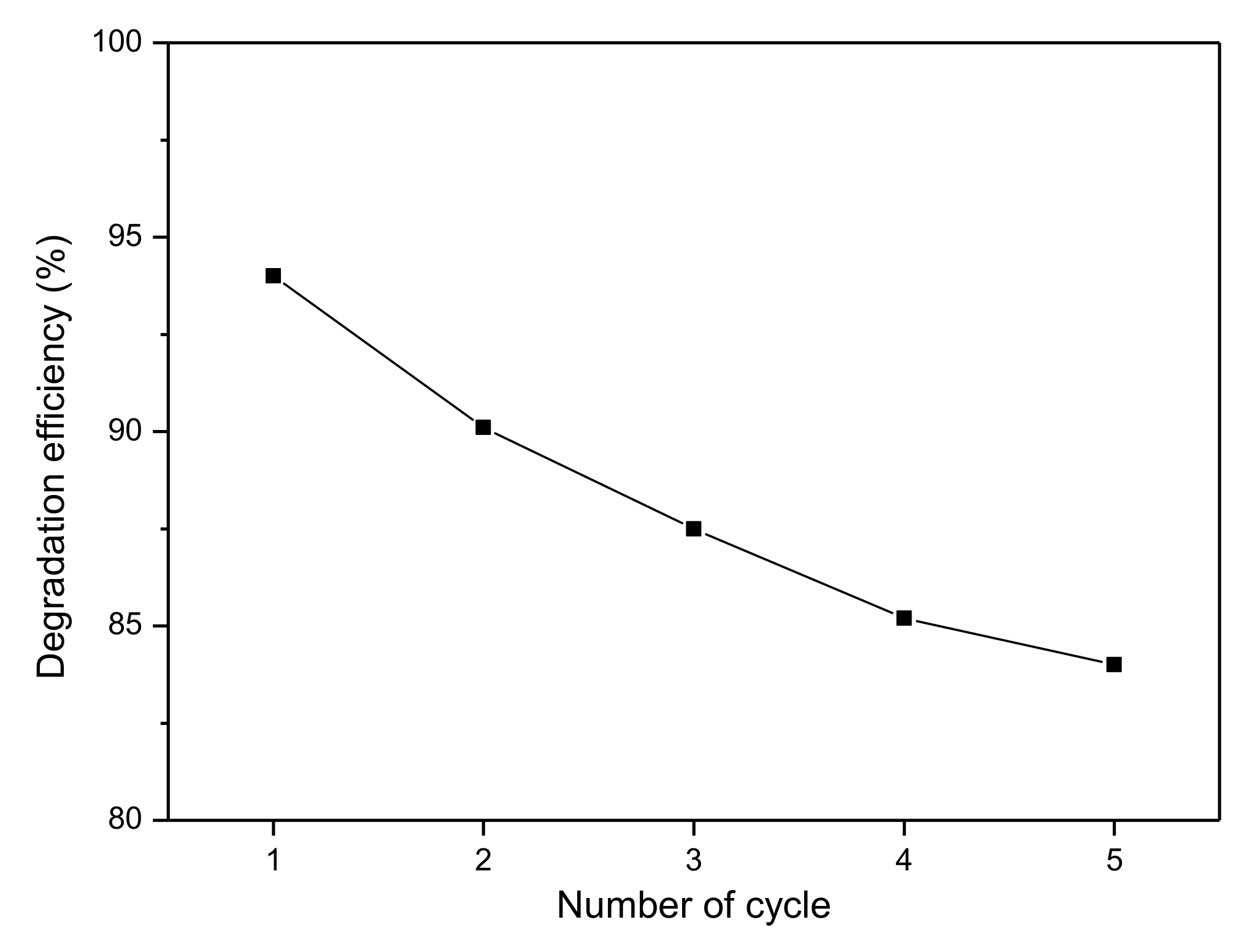
3.4. Reusability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, X.; Sun, J.; Duan, S.; Wang, Y.; Hayat, T.; Alsaedi, A.; Wang, C.; Li, J. A valuable biochar from poplar catkins with high adsorption capacity for both organic pollutants and inorganic heavy metal ions. Sci. Rep. 2017, 7, 10033. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Fatima, R.; Afridi, M.N.; Kumar, V.; Lee, J.; Ali, I.; Kim, K.H.; Kim, J.O. Photocatalytic degradation performance of various types of modified TiO2 against nitrophenols in aqueous systems. J. Clean. Prod. 2019, 231, 899–912. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, W.; Zhou, T.; Li, X. Removal of chelated heavy metals from aqueous solution: A review of current methods and mechanisms. Sci. Total Environ. 2019, 678, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S. Clay supported TiO2 nanoparticles for photocatalytic degradation of environmental pollutants: A review. J. Environ. Chem. Eng. 2018, 6, 6088–6107. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, G. Recent advances in synthesis and applications of clay-based photocatalysts: A review. Phys. Chem. Chem. Phys. 2014, 16, 8178–8192. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Lu, G.Q. Molecular engineered porous nanocomposites of metal oxide and clay using surfactants. Mater. Res. Soc. Symp. Proc. 2001, 703. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Orthman, J.A.; Li, J.Y.; Zhao, J.C.; Churchman, G.J.; Vansant, E.F. Novel composites of TiO2 (anatase) and silicate nanoparticles. Chem. Mater. 2002, 14, 5037–5044. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Li, J.Y.; Zhao, J.C.; Churchman, G.J. Photocatalysts prepared from layered clays and titanium hydrate for degradation of organic pollutants in water. Appl. Clay Sci. 2005, 28, 79–88. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Zhao, J.C.; Liu, J.W.; Yang, X.Z.; Shen, Y.N. General synthesis of a mesoporous composite of metal oxide and silicate nanoparticles from a metal salt and Laponite suspension for catalysis. Chem. Mater. 2006, 18, 3993–4001. [Google Scholar] [CrossRef]
- Belessi, V.; Lambropoulou, D.; Konstantinou, I.; Katsoulidis, A.; Pomonis, P.; Petridis, D.; Albanis, T. Structure and photocatalytic performance of TiO2/clay nanocomposites for the degradation of dimethachlor. Appl. Catal. B Environ. 2007, 73, 292–299. [Google Scholar] [CrossRef]
- Daniel, L.M.; Frost, R.L.; Zhu, H.Y. Synthesis and characterisation of clay-supported titania photocatalysts. J. Colloid Interface Sci. 2007, 316, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; Bandosz, T.J. Photooxidation of dibenzothiophene on TiO2/hectorite thin films layered catalyst. J. Colloid Interface Sci. 2006, 299, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Rhimi, B.; Wang, H.; Wang, C. Efficient photocatalytic degradation of gaseous toluene over F-doped TiO2/exfoliated bentonite. Appl. Surf. Sci. 2020, 530, 147286. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Labhsetwar, N.K.; Hishita, S.; Ohashi, N. Visible-light-driven photocatalysis on fluorine-doped TiO2 powders by the creation of surface oxygen vacancies. Chem. Phys. Lett. 2005, 401, 579–584. [Google Scholar] [CrossRef]
- Park, H.; Choi, W. Effects of TiO2 surface fluorination on photocatalytic reactions and photoelectrochemical behaviors. J. Phys. Chem. B 2004, 108, 4086–4093. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.Q.; Fu, X.; Zhang, N.; Xu, Y.J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601. [Google Scholar] [CrossRef]
- Yang, P.; Ou, H.; Fang, Y.; Wang, X. A facile steam reforming strategy to delaminate layered carbon nitride semiconductors for photoredox catalysis. Angew. Chem. 2017, 129, 4050–4054. [Google Scholar] [CrossRef]
- Ji, C.; Yin, S.N.; Sun, S.; Yang, S. An in situ mediator-free route to fabricate Cu2O/g-C3N4 type-II heterojunctions for enhanced visible-light photocatalytic H2 generation. Appl. Surf. Sci. 2018, 434, 1224–1231. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, J.; Fang, W.; Xu, M.; Qin, Z.; Jiang, Z.; Shangguan, W. Photocatalytic hydrogen evolution with simultaneous antibiotic wastewater degradation via the visible-light-responsive bismuth spheres-g-C3N4 nanohybrid: Waste to energy insight. Chem. Eng. J. 2019, 358, 944–954. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Q.; Chai, G.; Liang, M.; Dong, G.; Zhang, Q.; Qiu, J. Synthesis and luminescence mechanism of multicolor-emitting g-C3N4 nanopowders by low temperature thermal condensation of melamine. Sci. Rep. 2013, 3, 1943. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Mater. Sustain. Energy 2010, 271–275. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4 -based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, L.; Zhang, J.; Lei, J.; Liu, Y. The preparation, and applications of g-C3N4/TiO2 heterojunction catalysts—A review. Res. Chem. Intermed. 2017, 43, 2081–2101. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Zhang, W.; Yu, C.; Zheng, S. Highly efficient g-C3N4/TiO2/kaolinite composite with novel three-dimensional structure and enhanced visible light responding ability towards ciprofloxacin and S. aureus. Appl. Catal. B Environ. 2018, 220, 272–282. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. 2D-montmorillonite-dispersed g-C3N4/TiO2 2D/0Dnanocomposite for enhanced photo-induced H2 evolution from glycerol-water mixture. Appl. Surf. Sci. 2019, 471, 1053–1064. [Google Scholar] [CrossRef]
- Dong, H.; Guo, X.; Yang, C.; Ouyang, Z. Synthesis of g-C3N4 by different precursors under burning explosion effect and its photocatalytic degradation for tylosin. Appl. Catal. B Environ. 2018, 230, 65–76. [Google Scholar] [CrossRef]
- Grlich, P.; Karras, H.; Ktitz, G.; Lehmann, R. Spectroscopic properties of activated laser crystals (i). Phys. Status Solidi B 1964, 5, 437. [Google Scholar] [CrossRef]
- Miranda, C.; Mansilla, H.; Yáñez, J.; Obregón, S.; Colón, G. Improved photocatalytic activity of g-C3N4/TiO2 composites prepared by a simple impregnation method. J. Photochem. Photobiol. A Chem. 2013, 253, 16–21. [Google Scholar] [CrossRef]
- Qi, Y.; Al-Mukhtar, M.; Alcover, J.F.; Bergaya, F. Coupling analysis of macroscopic and microscopic behaviour in highly consolidated Na-laponite clays. Appl. Clay Sci. 1996, 11, 185–197. [Google Scholar] [CrossRef]
- Décsiné Gombos, E.; Krakkó, D.; Záray, G.; Illés, Á.; Dóbé, S.; Szegedi, Á. Laponite immobilized catalysts for photocatalytic degradation of phenols. J. Photochem. Photobiol. A Chem. 2020, 387, 112045. [Google Scholar] [CrossRef]
- Xuzhuang, Y.; Yang, D.; Huaiyong, Z.; Jiangwen, L.; Martins, W.N.; Frost, R.; Daniel, L.; Yuenian, S. Mesoporous structure with size controllable anatase attached on silicate layers for efficient photocatalysis. J. Phys. Chem. C 2009, 113, 8243–8248. [Google Scholar] [CrossRef]
- Komadel, P. Dissolution of hectorite in inorganic acids. Clays Clay Miner. 1996, 44, 228–236. [Google Scholar] [CrossRef]
- Bahranowski, K.; Gaweł, A.; Klimek, A.; Michalik-Zym, A.; Napruszewska, B.D.; Nattich-Rak, M.; Rogowska, M.; Serwicka, E.M. Influence of purification method of Na-montmorillonite on textural properties of clay mineral composites with TiO2 nanoparticles. Appl. Clay Sci. 2017, 140, 75–80. [Google Scholar] [CrossRef]
- Ren, J.; Liu, X.; Zhang, L.; Liu, Q.; Gao, R.; Dai, W.L. Thermal oxidative etching method derived graphitic C3N4: Highly efficient metal-free catalyst in the selective epoxidation of styrene. RSC Adv. 2017, 7, 5340–5348. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Kainth, S.; Basu, S. Effect of g-C3N4 loading on TiO2/bentonite nanocomposites for efficient heterogeneous photocatalytic degradation of industrial dye under visible light. J. Alloy. Compd. 2018, 764, 406–415. [Google Scholar] [CrossRef]
- Nesheva, D. Photoluminescence from SiOx layers containing amorphous silicon nanoparticles. Phys. Status Solidi A 2012, 209, 746–751. [Google Scholar] [CrossRef]
- Rebohle, L.; von Borany, J.; Fröb, H.; Skorupa, W. Blue photo-and electroluminescence of silicon dioxide layers ion-implanted with group IV elements. Appl. Phys. B 2000, 71, 131–151. [Google Scholar] [CrossRef]
- Song, C.; Lv, M.; Yang, P.; Xu, D.; Yuan, D. Structure and photoluminescence properties of sol–gel TiO2–SiO2 films. Thin Solid Films 2002, 413, 155–159. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Oxygen vacancy and dopant concentration dependent magnetic properties of Mn doped TiO2 nanoparticle. Curr. Appl. Phys. 2013, 13, 1025–1031. [Google Scholar] [CrossRef]
- Mochizuki, S.; Shimizu, T.; Fujishiro, F. Photoluminescence study on defects in pristine anatase and anatase-based composites. Phys. B Condens. Matter. 2003, 340–342, 956–959. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Guan, Z.; Li, Q.; He, C.; Yang, J. Synergistic effect of surface and bulk single-electron-trapped oxygen vacancy of TiO2 in the photocatalytic reduction of CO2. Appl. Catal. B Environ. 2017, 206, 300–307. [Google Scholar] [CrossRef]
- Huang, J.; Cao, Y.; Wang, H.; Yu, H.; Peng, F.; Zou, H.; Liu, Z. Revealing active-site structure of porous nitrogen-defected carbon nitride for highly effective photocatalytic hydrogen evolution. Chem. Eng. J. 2019, 373, 687–699. [Google Scholar] [CrossRef]
- Kuźniarska-Biernacka, I.; Silva, A.R.; Carvalho, A.P.; Pires, J.; Freire, C. Organo-Laponites as novel mesoporous supports for manganese (III) salen catalysts. Langmuir 2005, 21, 10825–10834. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Meng, X.; Wang, Z.; Zhou, J.; Tang, H. One-step electrospinning synthesis of TiO2/g-C 3N4 nanofibers with enhanced photocatalytic properties. Appl. Surf. Sci. 2018, 430, 253–262. [Google Scholar] [CrossRef]
- Wu, T.; Liu, G.; Zhao, J.; Hidaka, H.; Serpone, N. Photoassisted degradation of dye pollutants. V. Self-photosensitized oxidative transformation of Rhodamine B under visible light irradiation in aqueous TiO2 dispersions. J. Phys. Chem. B 1998, 102, 5845–5851. [Google Scholar] [CrossRef]
- Hu, X.; Mohamood, T.; Ma, W.; Chen, C.; Zhao, J. Oxidative decomposition of rhodamine B dye in the presence of VO2+ and/or Pt(IV) under visible light irradiation: N-deethylation, chromophore cleavage, and mineralization. J. Phys. Chem. B 2006, 110, 26012–26018. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, L.-M.; An, X.; Wei, T. New insights into interfacial photocharge transfer in TiO2/C3N4 heterostructures: Effects of facets and defects. New J. Chem. 2019, 43, 4511–4517. [Google Scholar] [CrossRef]
- Yan, M.-Y.; Jiang, Z.-Y.; Zheng, J.-M.; Lin, Y.-M.; Zhang, Z.-Y. Theoretical study on transport-scheme conversion of g-C3N4/TiO2 heterojunctions by oxygen vacancies. Appl. Surf. Sci. 2020, 531, 147318. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Li, X.; Li, Q.; Yang, J. Effect of the calcination temperature on the visible light photocatalytic activity of direct contact Z-scheme g-C3N4-TiO2 heterojunction. Appl. Catal. B Environ. 2017, 212, 106–114. [Google Scholar] [CrossRef]
- Yu, X.; Fan, X.; An, L.; Liu, G.; Li, Z.; Liu, J.; Hu, P. Mesocrystalline Ti3+ TiO2 hybridized g-C3N4 for efficient visible-light photocatalysis. Carbon 2018, 128, 21–30. [Google Scholar] [CrossRef]
| Sample | Ti (%) | Si (%) | Mg (%) | N (%) | O (%) | C (%) |
|---|---|---|---|---|---|---|
| g-C3N4/TiO2/hectorite | 2.07 | 3.70 | 2.09 | 36.52 | 16.89 | 38.74 |
| recycled g-C3N4/TiO2/hectorite 1 | 3.44 | 6.15 | 3.99 | 31.73 | 27.37 | 27.31 |
| Sample | Specific Surface Area 1 (m2/g) | Pore Volume 2 (cm3/g) | Average Pore Radius 3 (nm) | Crystallite Size of TiO2 4 (nm) |
|---|---|---|---|---|
| Hectorite | 70.0005 | 0.102052 | 4.8226 | - |
| TiO2/hectorite | 228.8244 | 0.174202 | 3.2794 | 11.0 |
| g-C3N4/TiO2/hectorite | 219.0311 | 0.360411 | 5.8204 | 11.3 |
| g-C3N4 | 103.0738 | 0.185361 | 5.9241 | - |
| P25 | 50 | - | - | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, R.; Chen, J.; Hong, M.; Li, J.; Hong, X. Facile Synthesis of g-C3N4/TiO2/Hectorite Z-Scheme Composite and Its Visible Photocatalytic Degradation of Rhodamine B. Materials 2020, 13, 5304. https://doi.org/10.3390/ma13225304
You R, Chen J, Hong M, Li J, Hong X. Facile Synthesis of g-C3N4/TiO2/Hectorite Z-Scheme Composite and Its Visible Photocatalytic Degradation of Rhodamine B. Materials. 2020; 13(22):5304. https://doi.org/10.3390/ma13225304
Chicago/Turabian StyleYou, Rong, Jinyang Chen, Menghan Hong, Jinrui Li, and Xiaomin Hong. 2020. "Facile Synthesis of g-C3N4/TiO2/Hectorite Z-Scheme Composite and Its Visible Photocatalytic Degradation of Rhodamine B" Materials 13, no. 22: 5304. https://doi.org/10.3390/ma13225304
APA StyleYou, R., Chen, J., Hong, M., Li, J., & Hong, X. (2020). Facile Synthesis of g-C3N4/TiO2/Hectorite Z-Scheme Composite and Its Visible Photocatalytic Degradation of Rhodamine B. Materials, 13(22), 5304. https://doi.org/10.3390/ma13225304




