Laser Surface Texturing and Electropolishing of CoCr and Ti6Al4V-ELI Alloys for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation of CoCr and Ti6Al4V-ELI Alloys
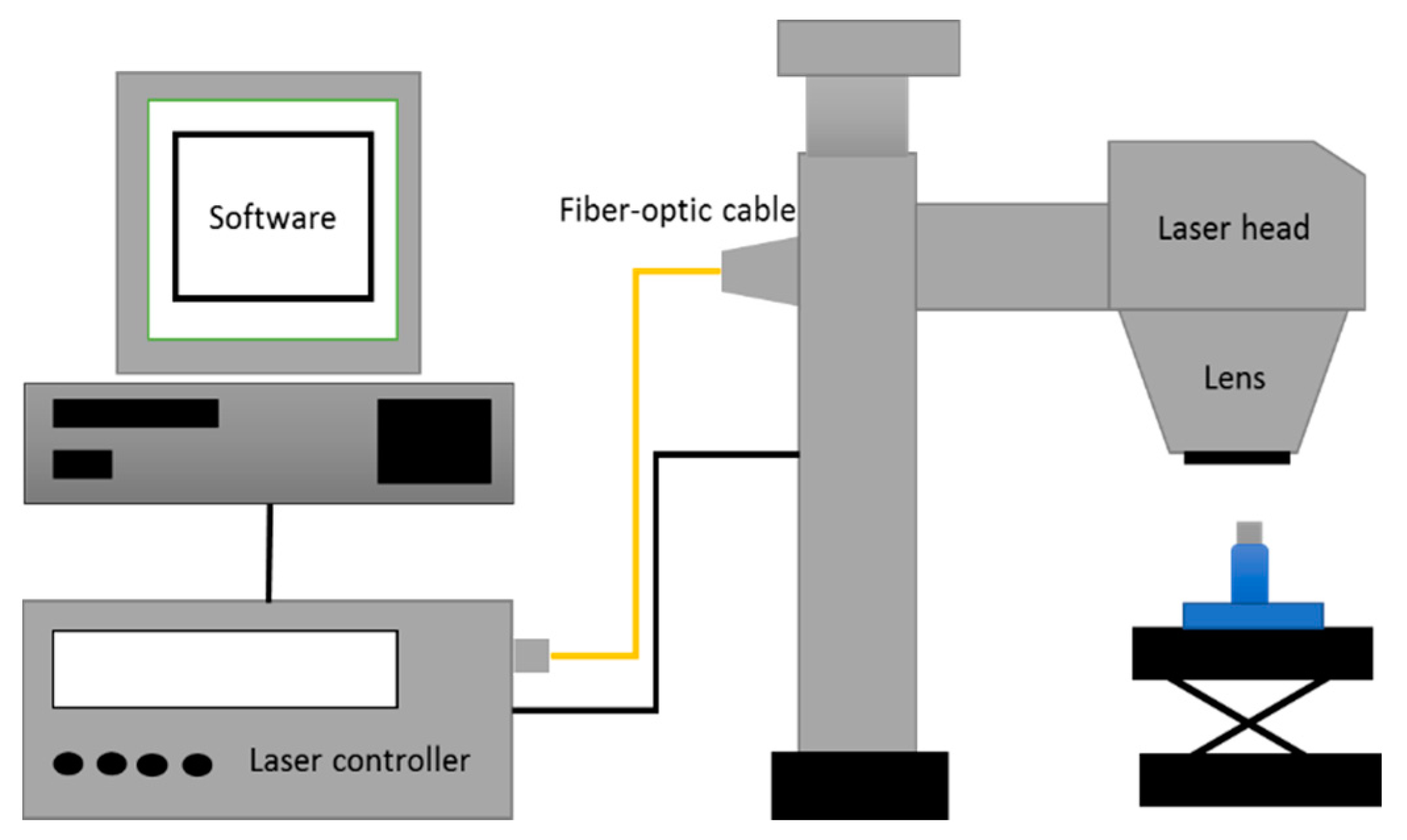
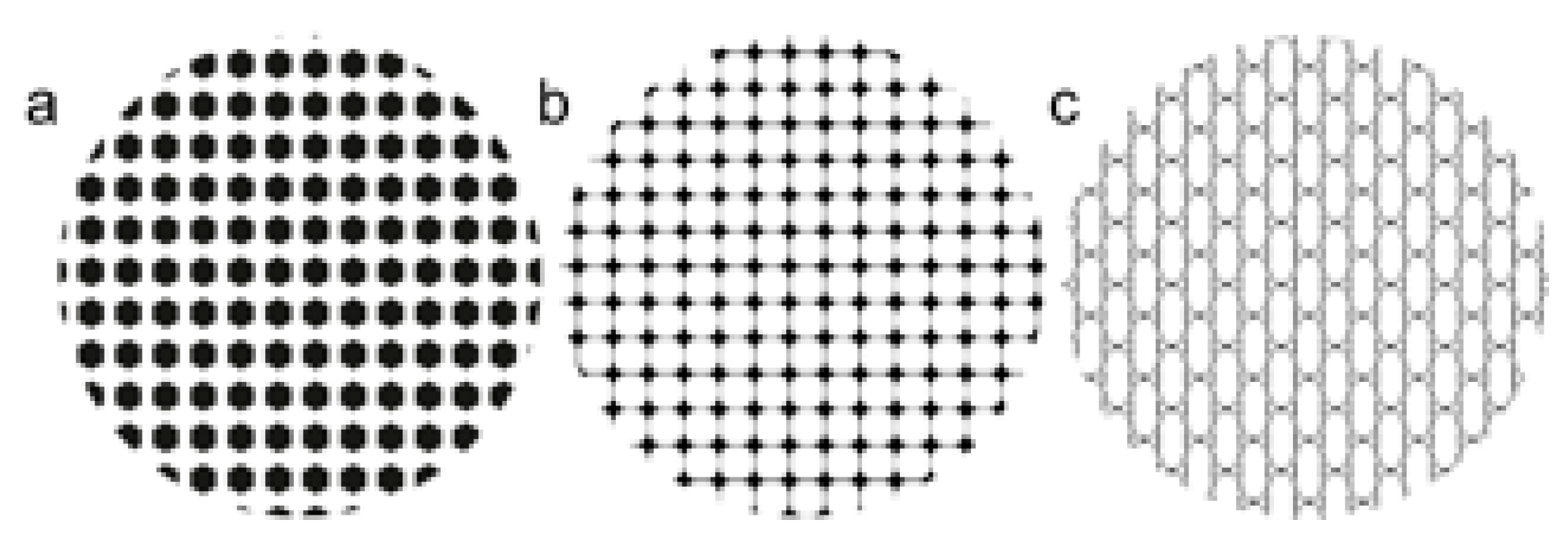
2.2. Laser Surface Texturing Experiments
2.3. Electropolishing Experiments
2.4. Surface Characterization
3. Results
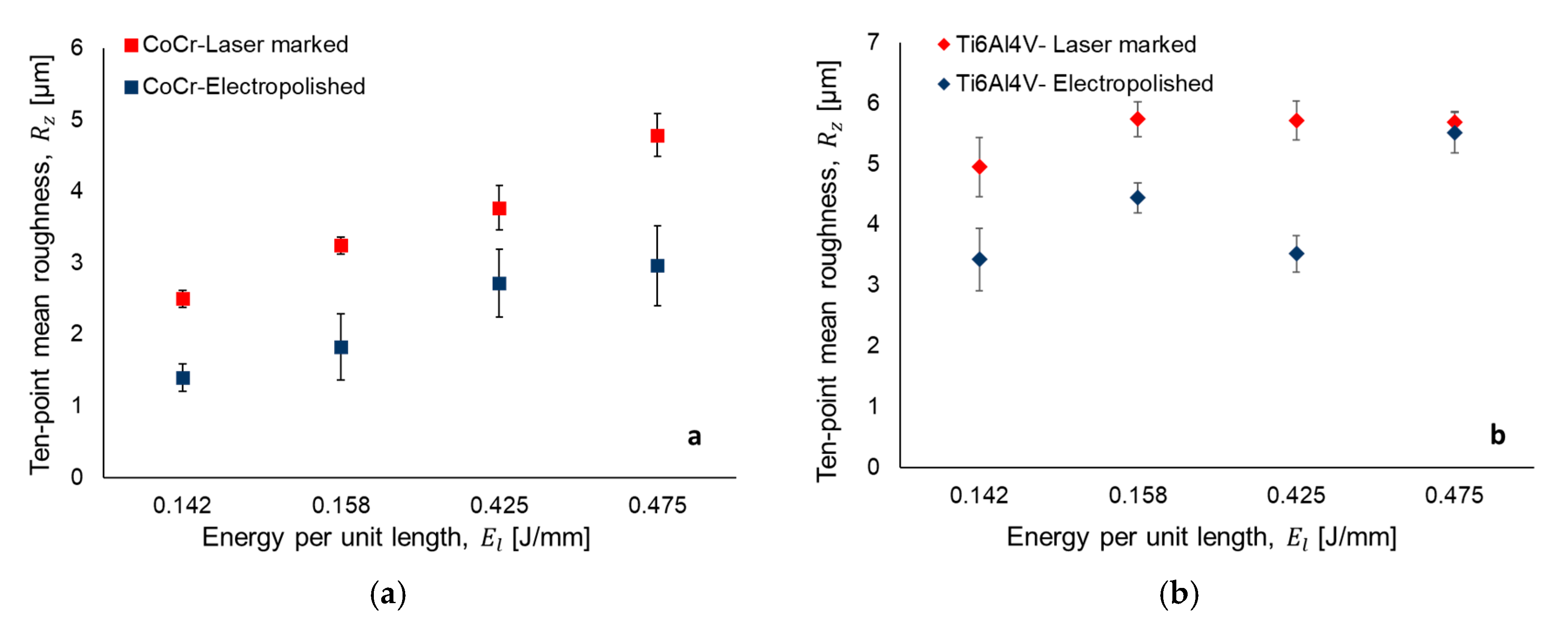
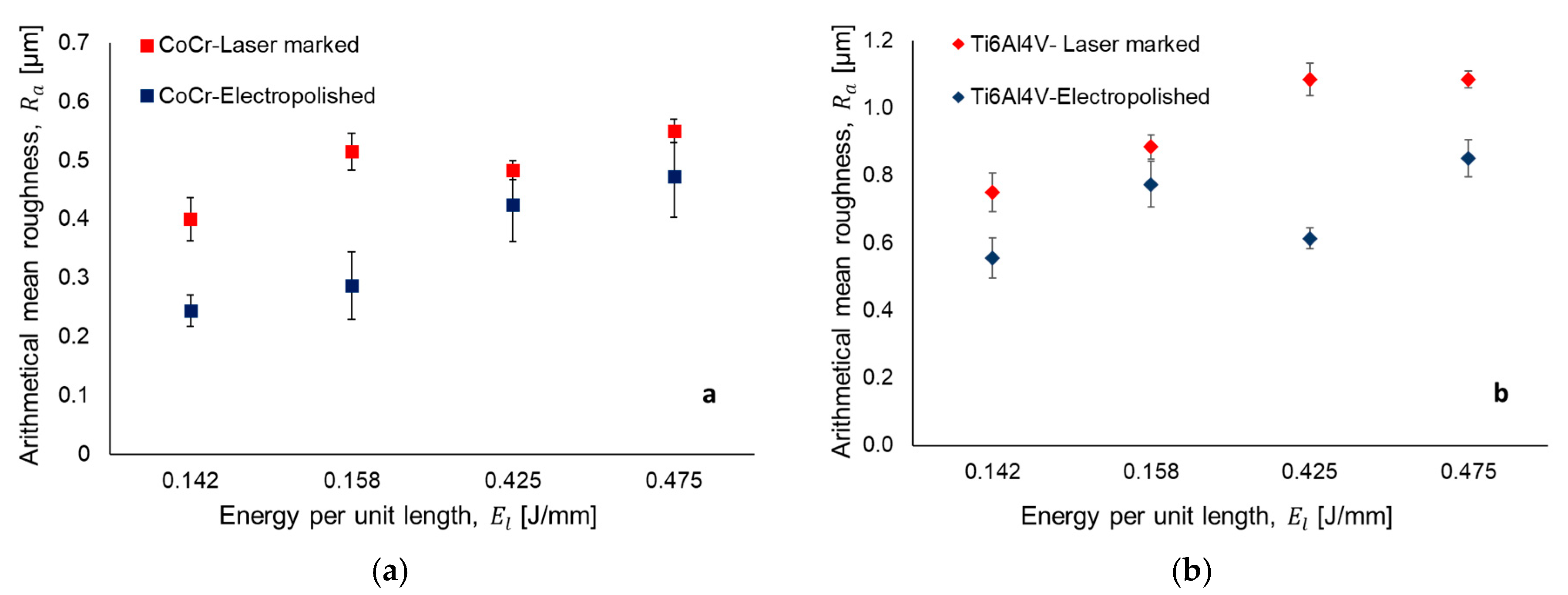
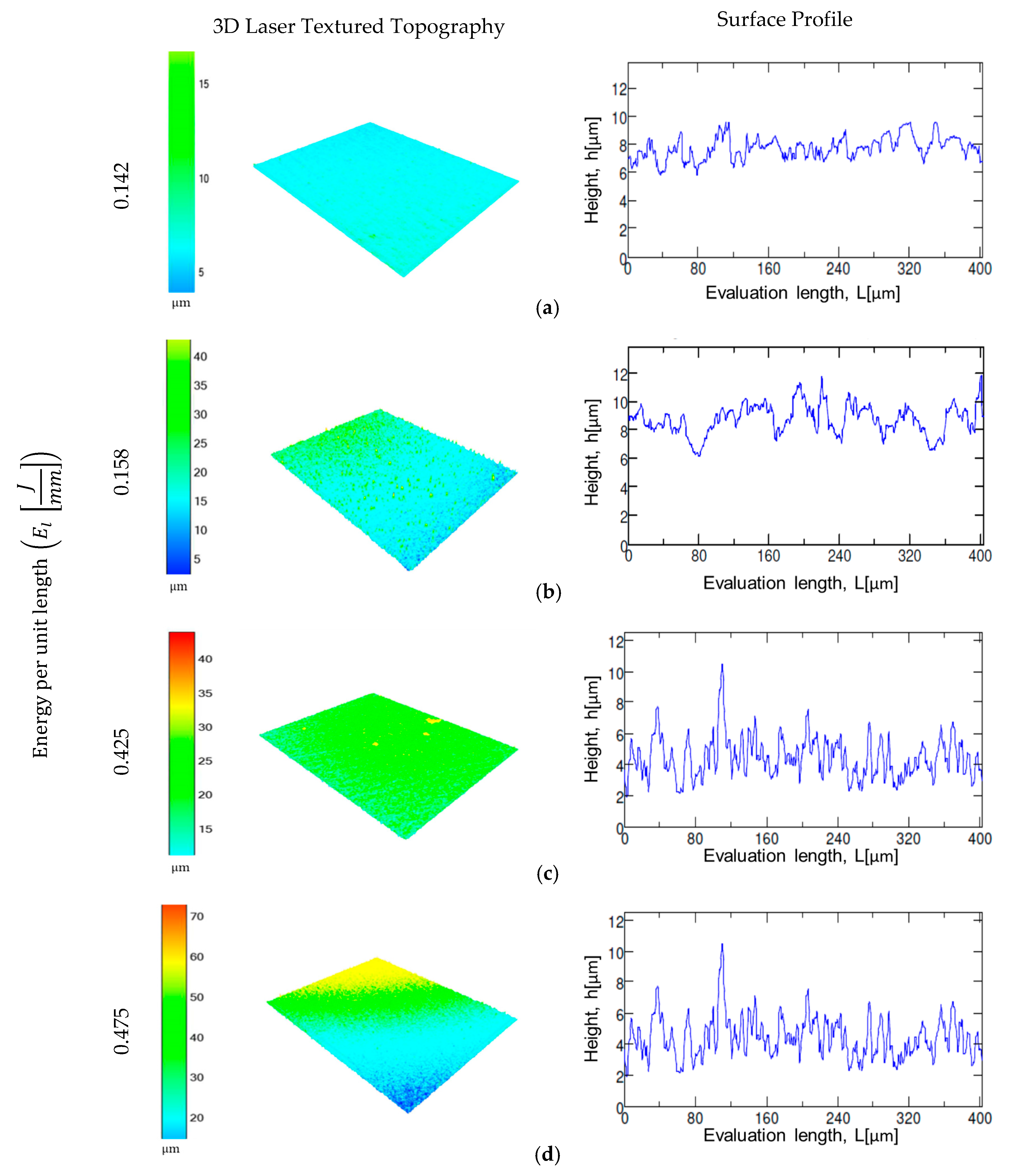
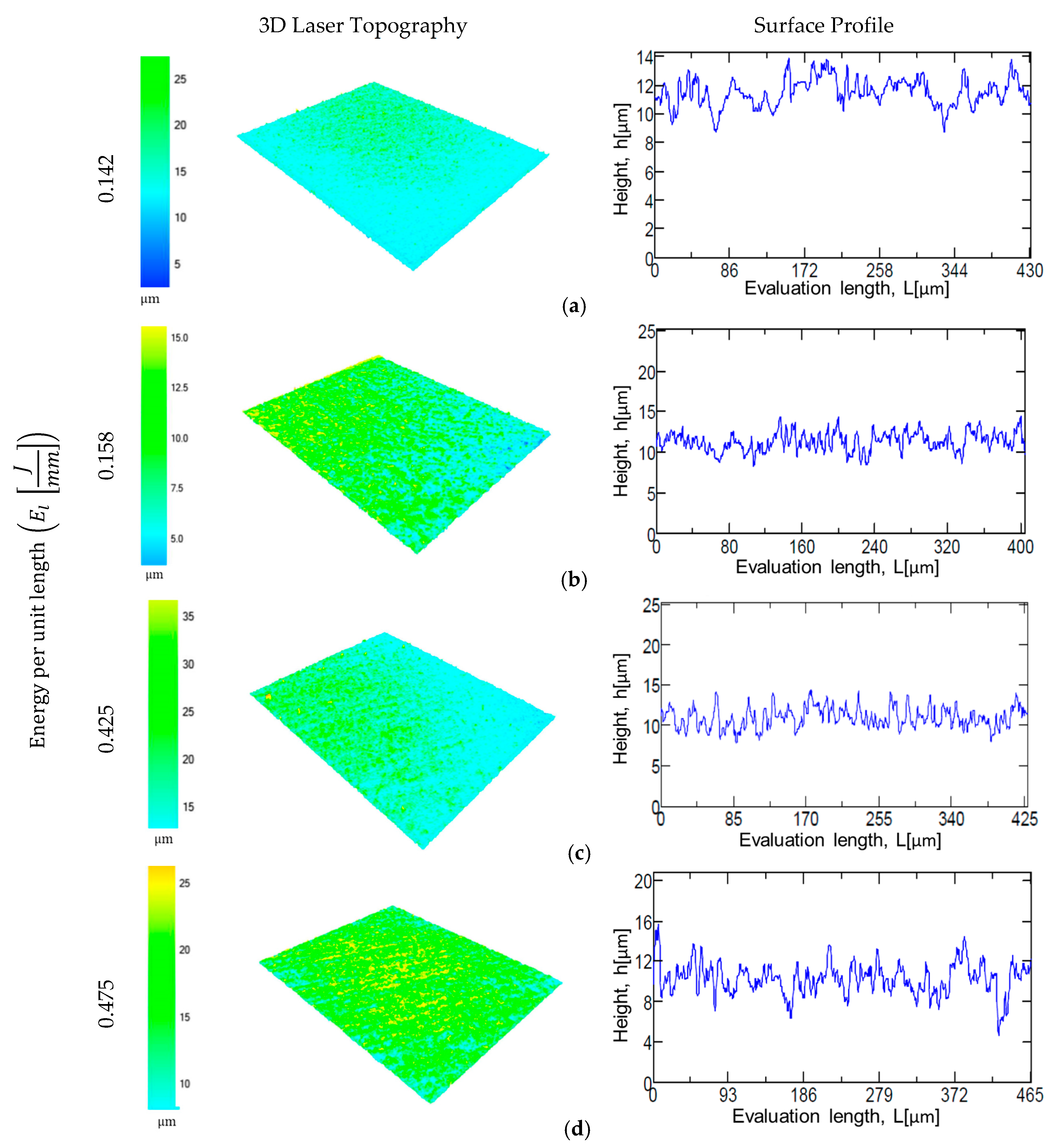
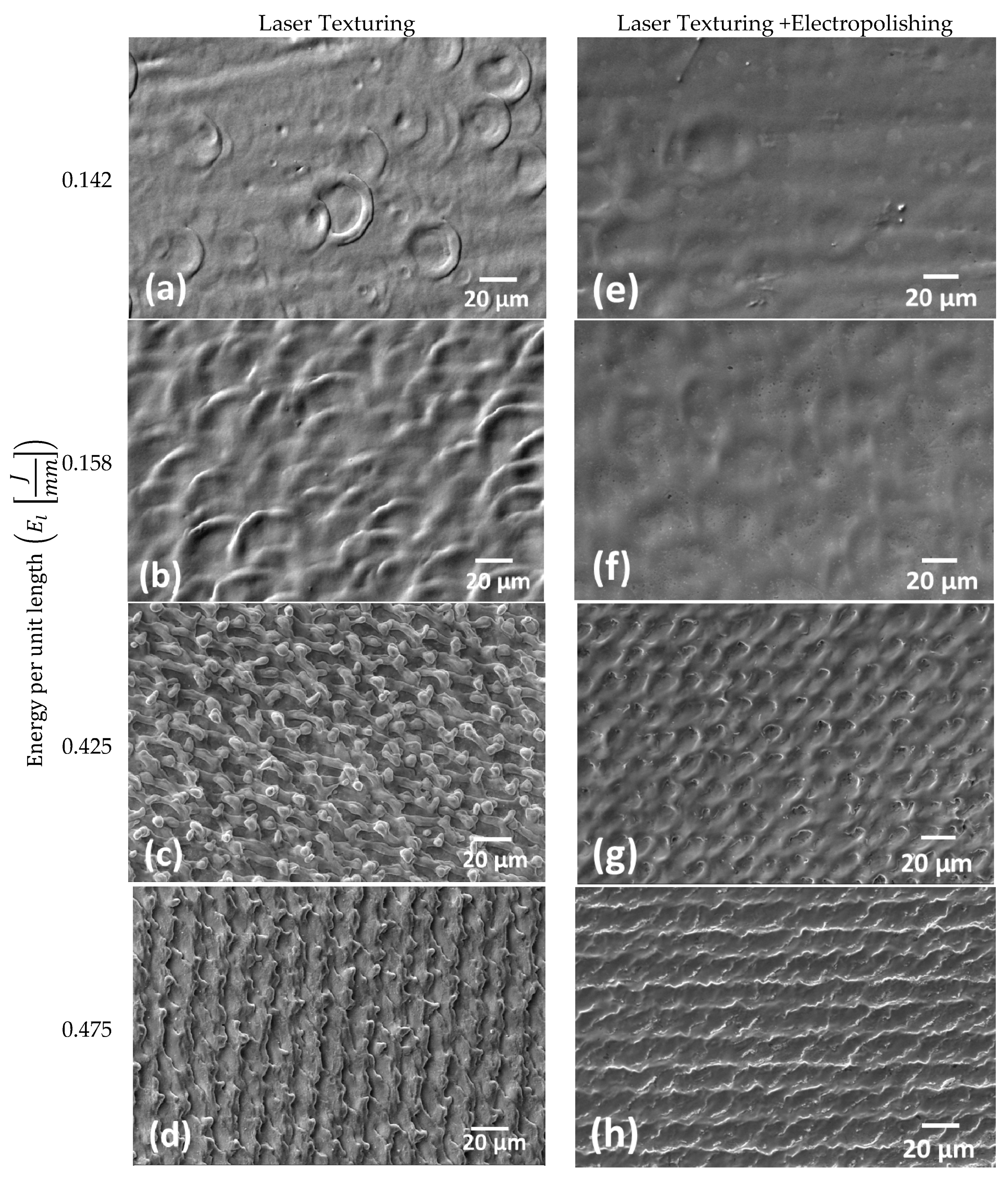
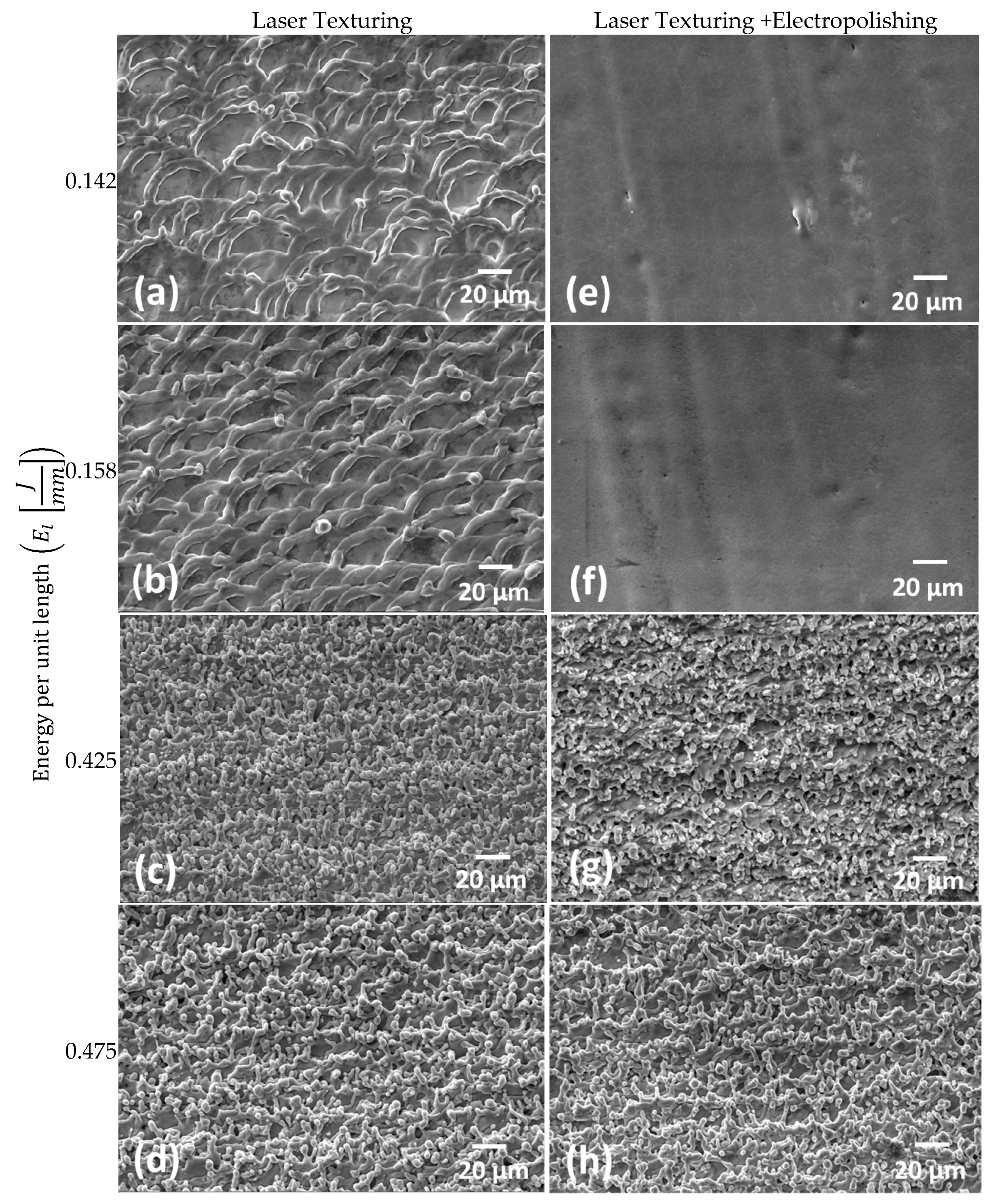
3.1. Laser Texturing and Electropolishing Tests
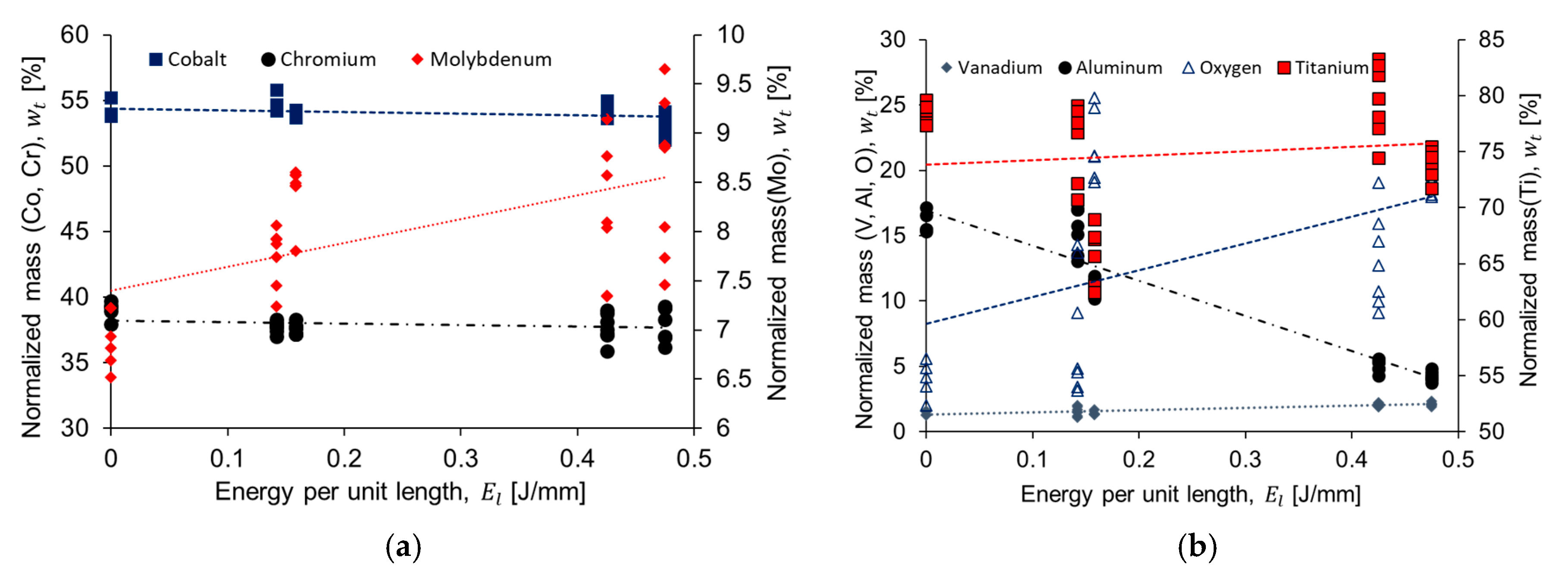
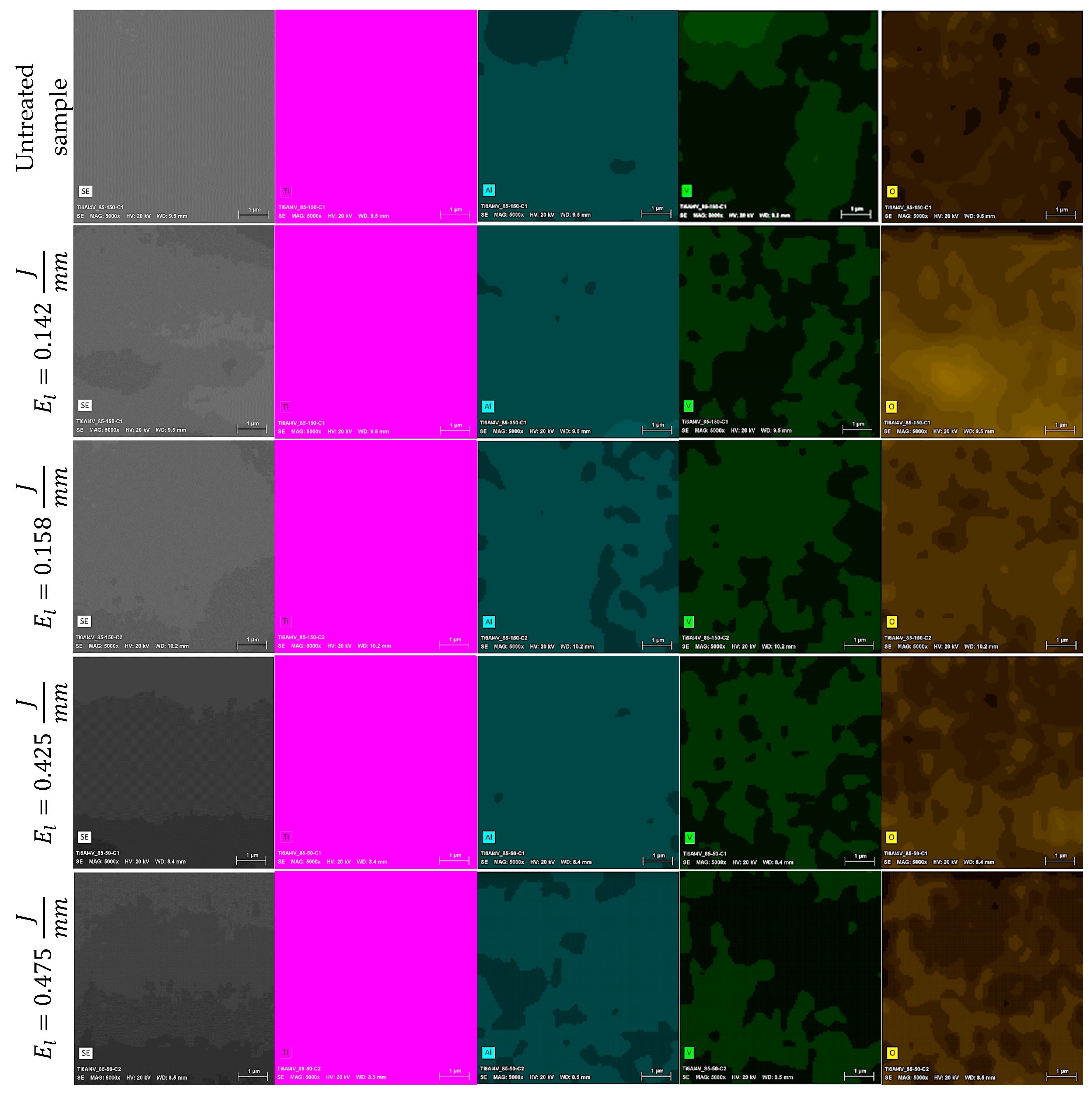
3.2. Chemical Composition
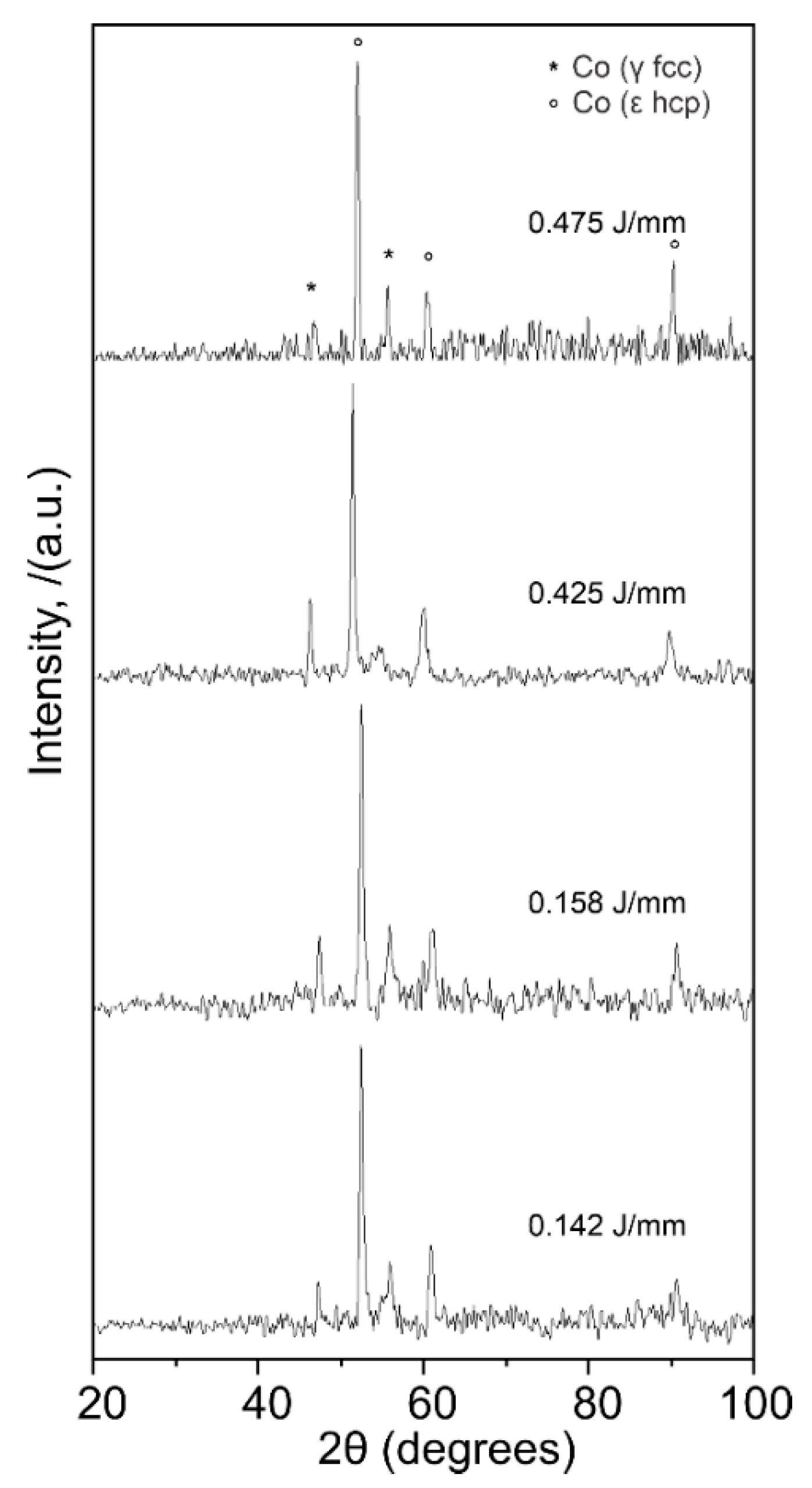
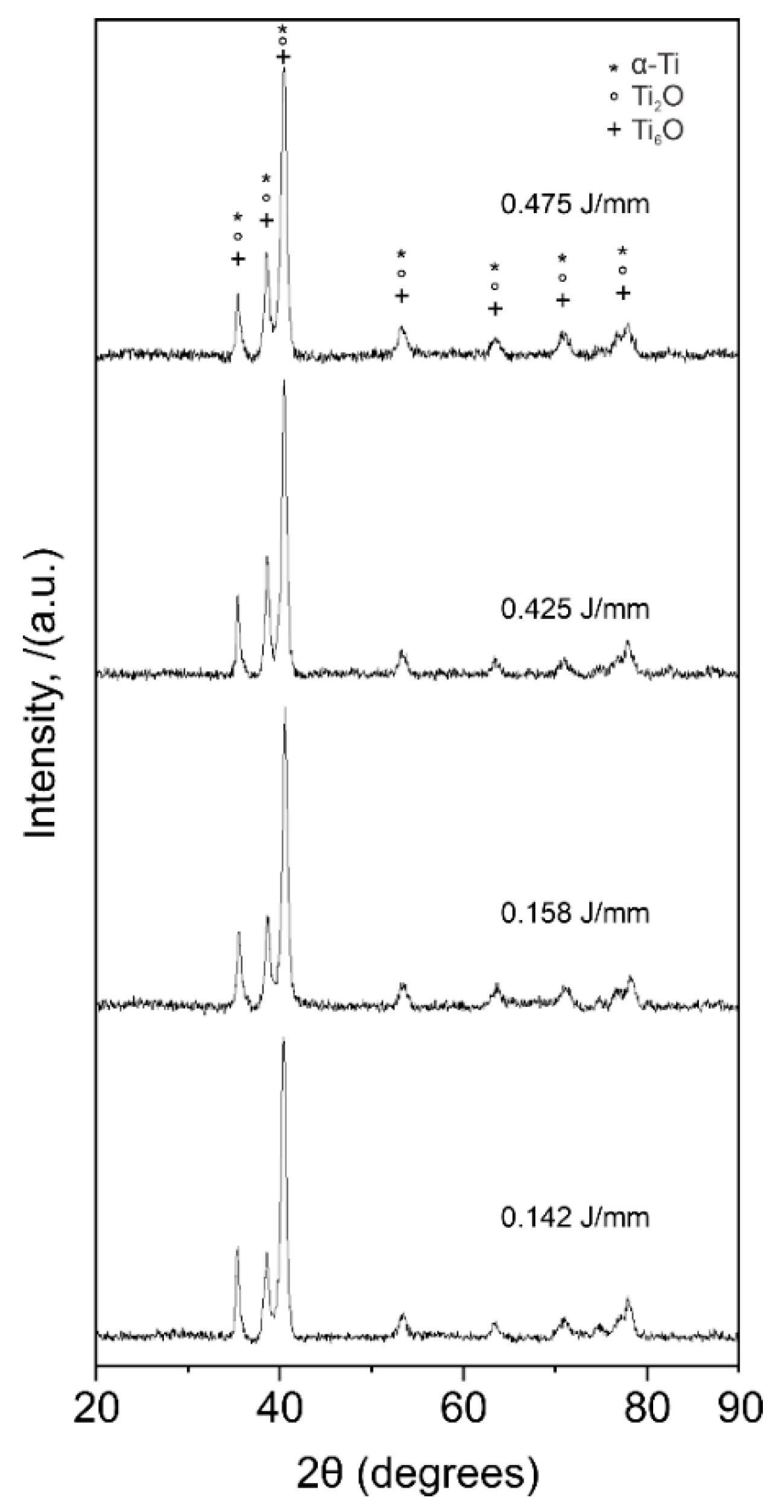
3.3. X-ray Diffraction
4. Discussion
5. Conclusions
- The surface topography modification was related to the HAZ by means of the energy per unit length during laser texturing. For CoCr alloy, an increase in the energy per unit length (El) shows a linear increment of surface roughness parameters, whilst for the Ti6AL4V-ELI alloy, the laser effect at high El remained constant.
- The electropolishing test showed that the final surface roughness could be controlled to comply with different medical applications.
- The laser texturing test showed a surface modification in terms of the chemical composition, and hence the phases present on the treated surface.
- The chemical analysis of the CoCr alloys provided indications of the formation of intermetallic σ phase (Co(Cr,Mo)) or the M23C6 precipitate. More studies are needed to fully characterize this precipitates morphology as they are related with wear and corrosion resistant in implants.
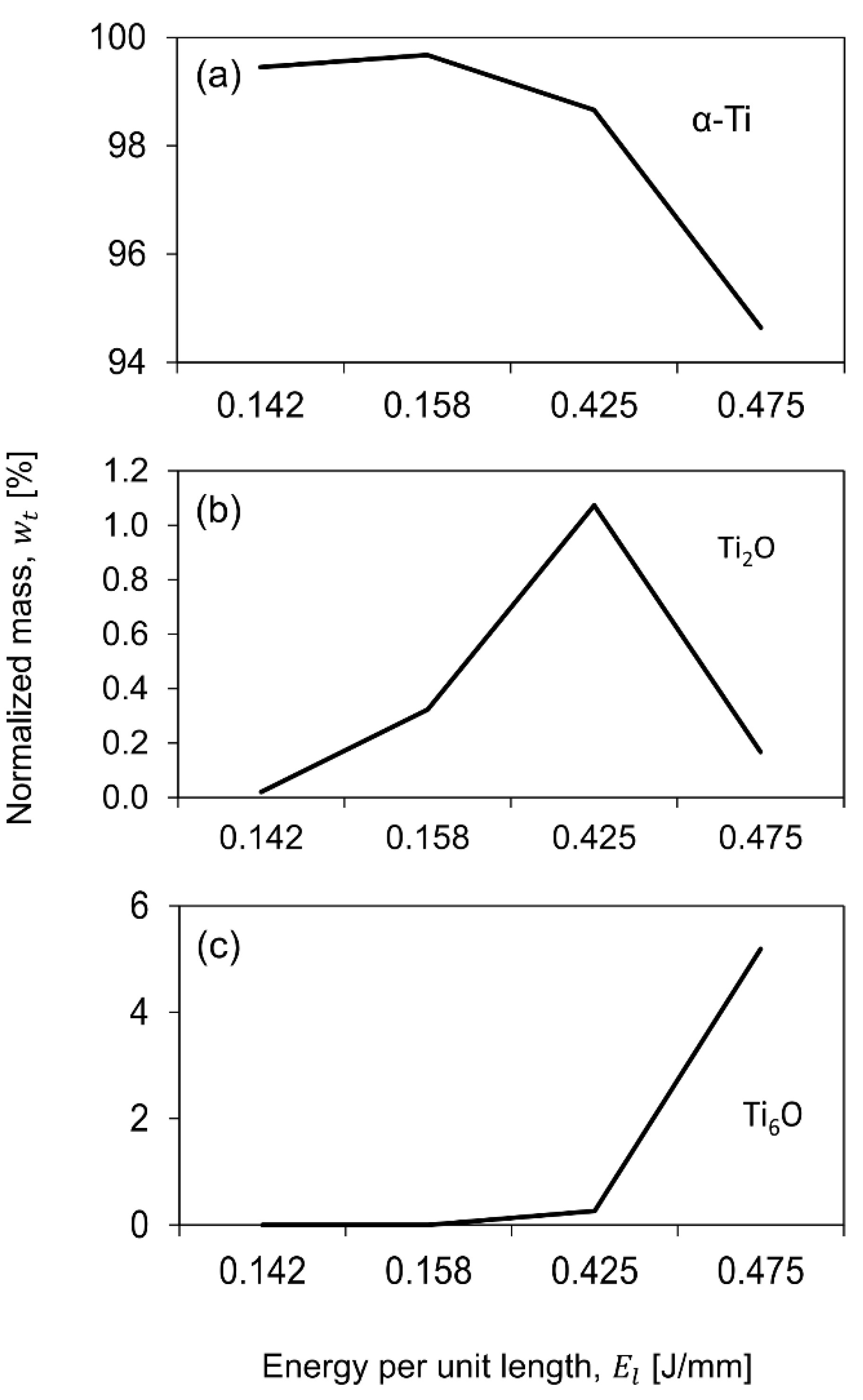
- A reduction in the α-Ti and an increase in the formation of Ti oxides (which have a sterilization effect enhancing biocompatibility) was observed with increasing energy per unit length. This was also achieved due to the chemical composition of the Ti6AL4V-ELI alloy and the high heating/cooling rate of the laser process.
Author Contributions
Funding
Conflicts of Interest
References
- Lyndon, J.A.; Boyd, B.J.; Birbilis, N. Metallic implant drug/device combinations for controlled drug release in orthopaedic applications. J. Control. Release 2014, 179, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Merritt, K. Detection of antibodies against corrosion products in patients after Co–Cr total joint replacements. J. Biomed. Mater. Res. 1994, 28, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.-H.; Jun, S.-H.; Kim, H.-E.; Koh, Y.-H. Deposition of titanium nitride (TiN) on Co–Cr and their potential application as vascular stent. Appl. Surf. Sci. 2012, 258, 2864–2868. [Google Scholar] [CrossRef]
- Veruva, S.Y.; Steinbeck, M.J.; Toth, J.; Alexander, D.D.; Kurtz, S.M. Which Design and Biomaterial Factors Affect Clinical Wear Performance of Total Disc Replacements? A Systematic Review. Clin. Orthop. Relat. Res. 2014, 472, 3759–3769. [Google Scholar] [CrossRef] [PubMed]
- Çelen, S.; Özden, H. Laser-induced novel patterns: As smart strain actuators for new-age dental implant surfaces. Appl. Surf. Sci. 2012, 263, 579–585. [Google Scholar] [CrossRef]
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef] [PubMed]
- Lewallen, E.A.; Riester, S.M.; Bonin, C.A.; Kremers, H.M.; Dudakovic, A.; Kakar, S.; Cohen, R.C.; Westendorf, J.J.; Lewallen, D.G.; Van Wijnen, A.J. Biological Strategies for Improved Osseointegration and Osteoinduction of Porous Metal Orthopedic Implants. Tissue Eng. Part B Rev. 2014, 21, 218–230. [Google Scholar] [CrossRef]
- Götz, H.; Müller, M.; Emmel, A.; Holzwarth, U.; Erben, R.; Stangl, R. Effect of surface finish on the osseointegration of laser-treated titanium alloy implants. Biomaterials 2004, 25, 4057–4064. [Google Scholar] [CrossRef]
- Aebli, N.; Stich, H.; Schawalder, P.; Walton, M.; Schwenke, D.; Gruner, H.; Gasser, B.; Theis, J.-C. In vivo comparison of the osseointegration of vacuum plasma sprayed titanium- and hydroxyapatite-coated implants. J. Biomed. Mater. Res. 2003, 66, 356–363. [Google Scholar] [CrossRef]
- Wong, M.; Eulenberger, J.; Schenk, R.; Hunziker, E. Effect of surface topology on the osseointegration of implant materials in trabecular bone. J. Biomed. Mater. Res. 1995, 29, 1567–1575. [Google Scholar] [CrossRef]
- Hindy, A.; Farahmand, F.; Tabatabaei, F.S. In vitro biological outcome of laser application for modification or processing of titanium dental implants. Lasers Med. Sci. 2017, 32, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.; Zouani, O.F.; Plawinski, L.; Rego, A.M.B.D.; Almeida, A.; Vilar, R.; Durrieu, M.-C. Human mesenchymal stem cell behavior on femtosecond laser-textured Ti-6Al-4V surfaces. Nanomedicine 2015, 10, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Coathup, M.; Blunn, G.W.; Mirhosseini, N.; Erskine, K.; Liu, Z.; Garrod, D.R.; Li, L. Controlled laser texturing of titanium results in reliable osteointegration. J. Orthop. Res. 2016, 35, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Lin, P.; Zhang, Y.; Dong, G.; Zeng, Q. Influence of surface wettability on the tribological properties of laser textured Co–Cr–Mo alloy in aqueous bovine serum albumin solution. Appl. Surf. Sci. 2013, 268, 79–86. [Google Scholar] [CrossRef]
- Mirhosseini, N.; Crouse, P.L.; Schmidth, M.; Li, L.; Garrod, D. Laser surface micro-texturing of Ti–6Al–4V substrates for improved cell integration. Appl. Surf. Sci. 2007, 253, 7738–7743. [Google Scholar] [CrossRef]
- Soboyejo, W.O.; Nemetski, B.; Allameh, S.; Marcantonio, N.; Mercer, C.; Ricci, J. Interactions between MC3T3-E1 cells and textured Ti6Al4V surfaces. J. Biomed. Mater. Res. 2002, 62, 56–72. [Google Scholar] [CrossRef]
- Nevins, M.; Nevins, M.L.; Camelo, M.; Boyesen, J.L.; Kim, D.M. Human histologic evidence of a connective tissue attachment to a dental implant. Int. J. Periodontics Restor. Dent. 2008, 28, 111–121. [Google Scholar]
- Yuzhakov, V.V.; Chang, H.-C.; Miller, A.E. Pattern formation during electropolishing. Phys. Rev. B 1997, 56, 12608–12624. [Google Scholar] [CrossRef]
- Szmukler-Moncler, S.; Testori, T.; Bernard, J.P. Etched implants: A comparative surface analysis of four implant systems. J. Biomed. Mater. Res. 2004, 69, 46–57. [Google Scholar] [CrossRef]
- Chen, J.; Mwenifumbo, S.; Langhammer, C.; McGovern, J.-P.; Li, M.; Beye, A.; Soboyejo, W.O. Cell/surface interactions and adhesion on Ti-6Al-4V: Effects of surface texture. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2007, 82, 360–373. [Google Scholar] [CrossRef]
- Valle, J.; Burgui, S.; Langheinrich, D.; Gil, C.; Solano, C.; Toledo-Arana, A.; Helbig, R.; Lasagni, A.; Lasa, I. Evaluation of Surface Microtopography Engineered by Direct Laser Interference for Bacterial Anti-Biofouling. Macromol. Biosci. 2015, 15, 1060–1069. [Google Scholar] [CrossRef]
- Çelen, S.; Efeoğlu, C.; Özden, H. Pulsed Laser-Induced Micro-Pits: As Bone Stabilizers. Phys. Procedia 2011, 12, 245–251. [Google Scholar] [CrossRef][Green Version]
- Erdoǧan, M.; Oktem, B.; Kalaycıoǧlu, H.; Yavaş, S.; Mukhopadhyay, P.K.; Eken, K.; Özgören, K.; Aykaç, Y.; Tazebay, U.H.; Ilday, F.; et al. Texturing of titanium (Ti6Al4V) medical implant surfaces with MHz-repetition-rate femtosecond and picosecond Yb-doped fiber lasers. Opt. Express 2011, 19, 10986–10996. [Google Scholar] [CrossRef]
- Cunha, A.; Serro, A.P.; Oliveira, V.; Almeida, A.; Vilar, R.; Durrieu, M.-C. Wetting behaviour of femtosecond laser textured Ti–6Al–4V surfaces. Appl. Surf. Sci. 2013, 265, 688–696. [Google Scholar] [CrossRef]
- Hu, T.; Hu, L.; Ding, Q. The effect of laser surface texturing on the tribological behavior of Ti-6Al-4V. Proc. Inst. Mech. Eng. Part J: J. Eng. Tribol. 2012, 226, 854–863. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, L.; Hua, M.; Dong, G.-N.; Chin, K.-S. A tribological study of the petaloid surface texturing for Co–Cr–Mo alloy artificial joints. Appl. Surf. Sci. 2015, 332, 557–564. [Google Scholar] [CrossRef]
- Baumeister, M.; Dickmann, K.; Hoult, T. Fiber laser micro-cutting of stainless steel sheets. Appl. Phys. A 2006, 85, 121–124. [Google Scholar] [CrossRef]
- Lutterotti, L.; Matthies, S.; Wenk, H.-R. MAUD (material analysis using diffraction): A user friendly Java program for Rietveld texture analysis and more. In Proceedings of the ICOTOM (International Conference on Textures of Materials), Montreal, QC, Canada, 9–13 August 1999. [Google Scholar]
- Akman, E.; Demir, A.; Canel, T.; Sınmazçelik, T. Laser welding of Ti6Al4V titanium alloys. J. Mater. Process. Technol. 2009, 209, 3705–3713. [Google Scholar] [CrossRef]
- Gao, X.-L.; Zhang, L.-J.; Liu, J.; Zhang, J.-X. A comparative study of pulsed Nd:YAG laser welding and TIG welding of thin Ti6Al4V titanium alloy plate. Mater. Sci. Eng. A 2013, 559, 14–21. [Google Scholar] [CrossRef]
- Yang, J.; Yu, H.; Yin, J.; Gao, M.; Wang, Z.; Zeng, X. Formation and control of martensite in Ti-6Al-4V alloy produced by selective laser melting. Mater. Des. 2016, 108, 308–318. [Google Scholar] [CrossRef]
- Ravi, N.; Aliyar, H.A. Tissue Reaction to Prosthetic Materials. Bionic Human 2007, 133–158. [Google Scholar] [CrossRef]
- Leone, C.; Genna, S.; Caprino, G.; De Iorio, I. AISI 304 stainless steel marking by a Q-switched diode pumped Nd:YAG laser. J. Mater. Process. Technol. 2010, 210, 1297–1303. [Google Scholar] [CrossRef]
- Lavvafi, H.; Lewandowski, M.E.; Schwam, D.; Lewandowski, J.J. Effects of surface laser treatments on microstructure, tension, and fatigue behavior of AISI 316LVM biomedical wires. Mater. Sci. Eng. A 2017, 688, 101–113. [Google Scholar] [CrossRef]
- Boyd, R.D.; Verran, J.; Jones, M.V.; Bhakoo, M. Use of the Atomic Force Microscope To Determine the Effect of Substratum Surface Topography on Bacterial Adhesion. Langmuir 2002, 18, 2343–2346. [Google Scholar] [CrossRef]
- Wennerberg, A.; Hallgren, C.; Johansson, C.; Danelli, S. A histomorphometric evaluation of screw-shaped implants each prepared with two surface roughnesses. Clin. Oral Implant. Res. 1998, 9, 11–19. [Google Scholar] [CrossRef]
- Asri, R.; Harun, W.; Samykano, M.; Lah, N.; Ghani, S.; Tarlochan, F.; Raza, M. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C 2017, 77, 1261–1274. [Google Scholar] [CrossRef]
- Sweeney, C.A.; O׳brien, B.; Dunne, F.; McHugh, P.; Leen, S. Micro-scale testing and micromechanical modelling for high cycle fatigue of CoCr stent material. J. Mech. Behav. Biomed. Mater. 2015, 46, 244–260. [Google Scholar] [CrossRef]
- Sojitra, P.; Raval, A.; Kothwala, D.; Jariwala, A.; Kotadia, H.; Adeshara, S.; Mehta, G. Surface enhancement and characterization of L-605 cobalt alloy cardiovascular stent by novel electrochemical treatment. Trends Biomater. Artif. Organs 2009, 23, 55–64. [Google Scholar]
- Mingear, J.; Zhang, B.; Hartl, D.J.; Elwany, A. Effect of process parameters and electropolishing on the surface roughness of interior channels in additively manufactured nickel-titanium shape memory alloy actuators. Addit. Manuf. 2019, 27, 565–575. [Google Scholar] [CrossRef]
- Han, W.; Fang, F. Investigation of electrochemical properties of electropolishing Co–Cr dental alloy. J. Appl. Electrochem. 2020, 50, 367–381. [Google Scholar] [CrossRef]
- Spriano, S.; Verne’, E.; Faga, M.; Bugliosi, S.; Maina, G. Surface treatment on an implant cobalt alloy for high biocompatibility and wear resistance. Wear 2005, 259, 919–925. [Google Scholar] [CrossRef]
- Mineta, S.; Namba, S.; Yoneda, T.; Ueda, K.; Narushima, T. Carbide Formation and Dissolution in Biomedical Co-Cr-Mo Alloys with Different Carbon Contents during Solution Treatment. Met. Mater. Trans. A 2010, 41, 2129–2138. [Google Scholar] [CrossRef]
- Mungole, M.N.; Singh, N.; Mathur, G.N. Oxidation behaviour of Ti6Al4V titanium alloy in oxygen. Mater. Sci. Technol. 2002, 18, 111–114. [Google Scholar] [CrossRef]
- Juechter, V.; Scharowsky, T.; Singer, R.; Korner, C. Processing window and evaporation phenomena for Ti–6Al–4V produced by selective electron beam melting. Acta Mater. 2014, 76, 252–258. [Google Scholar] [CrossRef]
- Murray, J.L.; Wriedt, H.A. The O−Ti (Oxygen-Titanium) system. J. Phase Equilibria Diffus. 1987, 8, 148–165. [Google Scholar] [CrossRef]


| Ref. | Year | Material | Geometry Texturized | Laser Parameters | Results | ||||
|---|---|---|---|---|---|---|---|---|---|
| Chemical Composition | Geometrical/Surface Quality | Cell Attachment | Mechanical Properties/Tribology | ||||||
| [20] | 2006 | Ti6Al4V | Parallel grooves | Laser type | UV | X | X | ||
| Wavelength (nm) | 355 | ||||||||
| Scanning speed (mm/s) | 250 | ||||||||
| Pulse frequency (kHz) | 50 | ||||||||
| Avg. power (W) | 1.65 | ||||||||
| spot size (µm) | 8.5 | ||||||||
| [22] | 2011 | Titanium | Pits | Laser type | Yb fiber | X | |||
| Wavelength (nm) | 1060 | ||||||||
| Avg. power (W) | - | ||||||||
| Scanning speed (mm/s) | 50–150 | ||||||||
| P. frequency (kHz) | - | ||||||||
| [23] | 2011 | Ti6Al4V | Dots and lines | Laser type | Yb Fiber | X | X | X | |
| Wavelength (nm) | 1060 | ||||||||
| Avg. power (W) | 8 | ||||||||
| Pulse duration (ns) | 70 | ||||||||
| P. frequency (kHz) | 20–200 | ||||||||
| [10] | 2012 | Ti6Al4V | Dimples | Laser type | Nd-YAG | X | |||
| Wavelength (nm) | 1064 | ||||||||
| Laser avg. power (W) | 10 | ||||||||
| P. frequency (kHz) | 10 | ||||||||
| Scanning speed (mm/s) | 5 | ||||||||
| [24] | 2013 | Ti6Al4V | Periodic waves | Laser type | Yb:KWY | X | |||
| Wavelength (nm) | 1030 | ||||||||
| Scanning speed (mm/s) | 0.8 | ||||||||
| P. frequency (Hz) | 50 | ||||||||
| [25] | 2013 | Co-Cr-Mo | Square, triangle, and circle | Laser type | DPSS | X | |||
| Wavelength (nm) | 1064 | ||||||||
| Laser power (W) | 50 | ||||||||
| P. frequency (kHz) | 20 | ||||||||
| Spot size (µm) | 15 | ||||||||
| [14] | 2014 | Co-Cr-Mo | Square, triangle, and circle | Laser type | X | X | |||
| Wavelength (nm) | 1064 | ||||||||
| Avg. power (W) | 20 | ||||||||
| Scanning speed (mm/s) | 50–200 | ||||||||
| P. frequency (kHz) | 3–5.5 | ||||||||
| [26] | 2015 | Co-Cr-Mo | Petaloid | Laser type | VAN | X | X | ||
| Wavelength (nm) | 532 | ||||||||
| Laser power (mW) | 50 | ||||||||
| P. frequency (kHz) | 100 | ||||||||
| Pulse duration (ps) | 10 | ||||||||
| Chemical Composition CoCr (wt.%) | |||||||
| Co | 65.45 | Cr | 27.34 | Mo | 5.47 | Mn | 0.69 |
| Si | 0.67 | N | 0.16 | Fe | 0.07 | Ni | 0.06 |
| W | 0.02 | O | 0.02 | Cu | 0.01 | Al | 0.01 |
| Nb | 0.01 | C | 0.037 | Ti | 0.005 | P | 0.004 |
| B | 0.003 | S | 0.002 | ||||
| Chemical Composition Ti6Al4V-ELI (wt.%) | |||||||
| Al | 5.95 | C | 0.015 | Fe | 0.10 | H | 0.003 |
| N | 0.007 | O | 0.104 | V | 4.03 | ||
| Laser Power, P (W) | Marking Speed, v (mm/s) | Energy per Unit Length (J/mm) |
|---|---|---|
| 21.25 | 150 | 0.142 |
| 23.75 | 150 | 0.158 |
| 21.25 | 50 | 0.425 |
| 23.75 | 50 | 0.475 |
| Phase | Crystalline System | Space Group | Cell Parameters (Å) |
|---|---|---|---|
| α-Ti | Hexagonal | P63/mmc | a = b= 2.936, c = 4.679 |
| Ti2O | Trigonal | P-3m1 | a = b = 3.465, c = 4.049 |
| Ti6O | Trigonal | P31c | a = b = 5.14, c = 9.48 |
| Source | Mean ± St. dev. | Range | F-Value | P-Value |
|---|---|---|---|---|
| CoCr alloy | ||||
| Cobalt (Co) | 54.043 ± 0.669 | (51.980, 55.780) | 3.16 | 0.029 |
| Chromium (Cr) | 37.940 ± 0.947 | (35.890,39.690) | 2.66 | 0.053 |
| Molybdenum (Mo) | 8.018 ± 0.773 | (6.520, 9.650) | 9.32 | 0.000 |
| Ti6Al4V alloy | ||||
| Titanium (Ti) | 75.015 ± 5.398 | (62.46,83.29) | 30.12 | 0.000 |
| Aluminum (Al) | 10.213 ± 5.214 | (3.76, 17.16) | 232.67 | 0.000 |
| Vanadium (V) | 1.7394 ± 0.3574 | (1.10, 2.34) | 18.81 | 0.000 |
| Oxygen (O) | 13.18 ± 7.43 | (2.01, 25.54) | 38.13 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandoval-Robles, J.A.; Rodríguez, C.A.; García-López, E. Laser Surface Texturing and Electropolishing of CoCr and Ti6Al4V-ELI Alloys for Biomedical Applications. Materials 2020, 13, 5203. https://doi.org/10.3390/ma13225203
Sandoval-Robles JA, Rodríguez CA, García-López E. Laser Surface Texturing and Electropolishing of CoCr and Ti6Al4V-ELI Alloys for Biomedical Applications. Materials. 2020; 13(22):5203. https://doi.org/10.3390/ma13225203
Chicago/Turabian StyleSandoval-Robles, Jesús A., Ciro A. Rodríguez, and Erika García-López. 2020. "Laser Surface Texturing and Electropolishing of CoCr and Ti6Al4V-ELI Alloys for Biomedical Applications" Materials 13, no. 22: 5203. https://doi.org/10.3390/ma13225203
APA StyleSandoval-Robles, J. A., Rodríguez, C. A., & García-López, E. (2020). Laser Surface Texturing and Electropolishing of CoCr and Ti6Al4V-ELI Alloys for Biomedical Applications. Materials, 13(22), 5203. https://doi.org/10.3390/ma13225203






