Surface-Initiated Photoinduced Iron-Catalyzed Atom Transfer Radical Polymerization with ppm Concentration of FeBr3 under Visible Light
Abstract
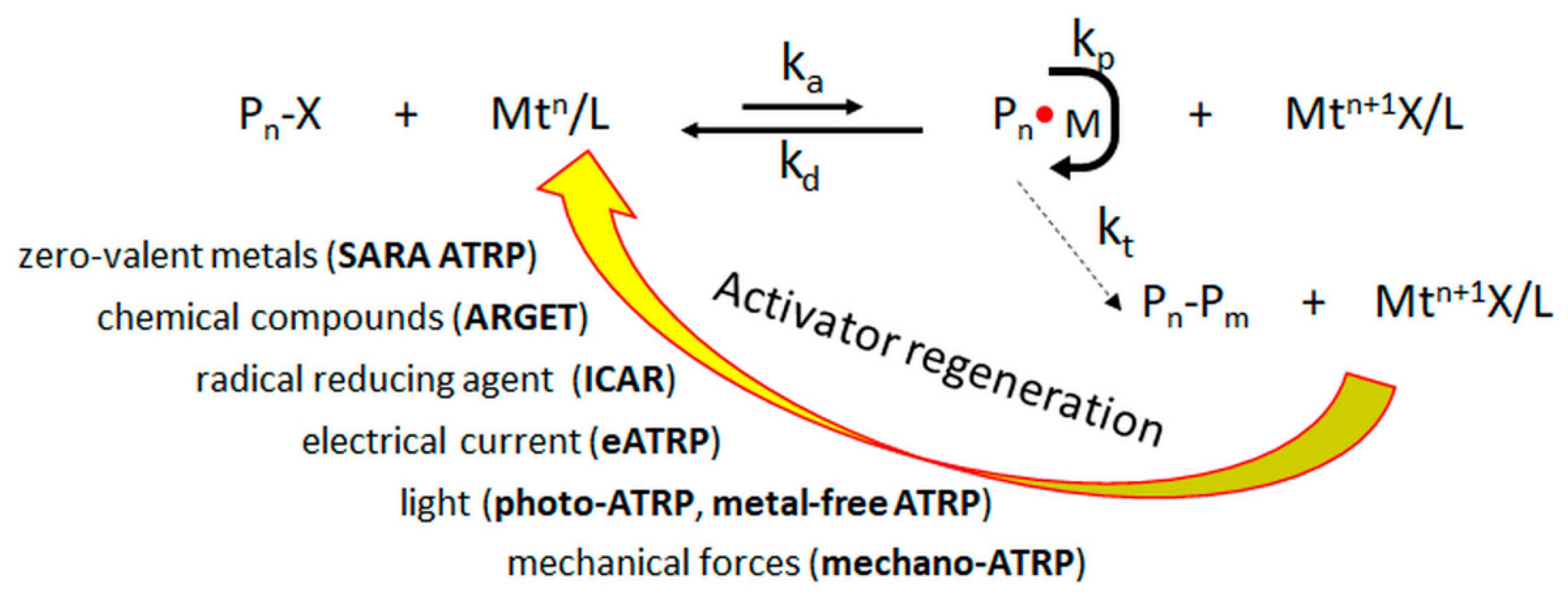
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Procedures
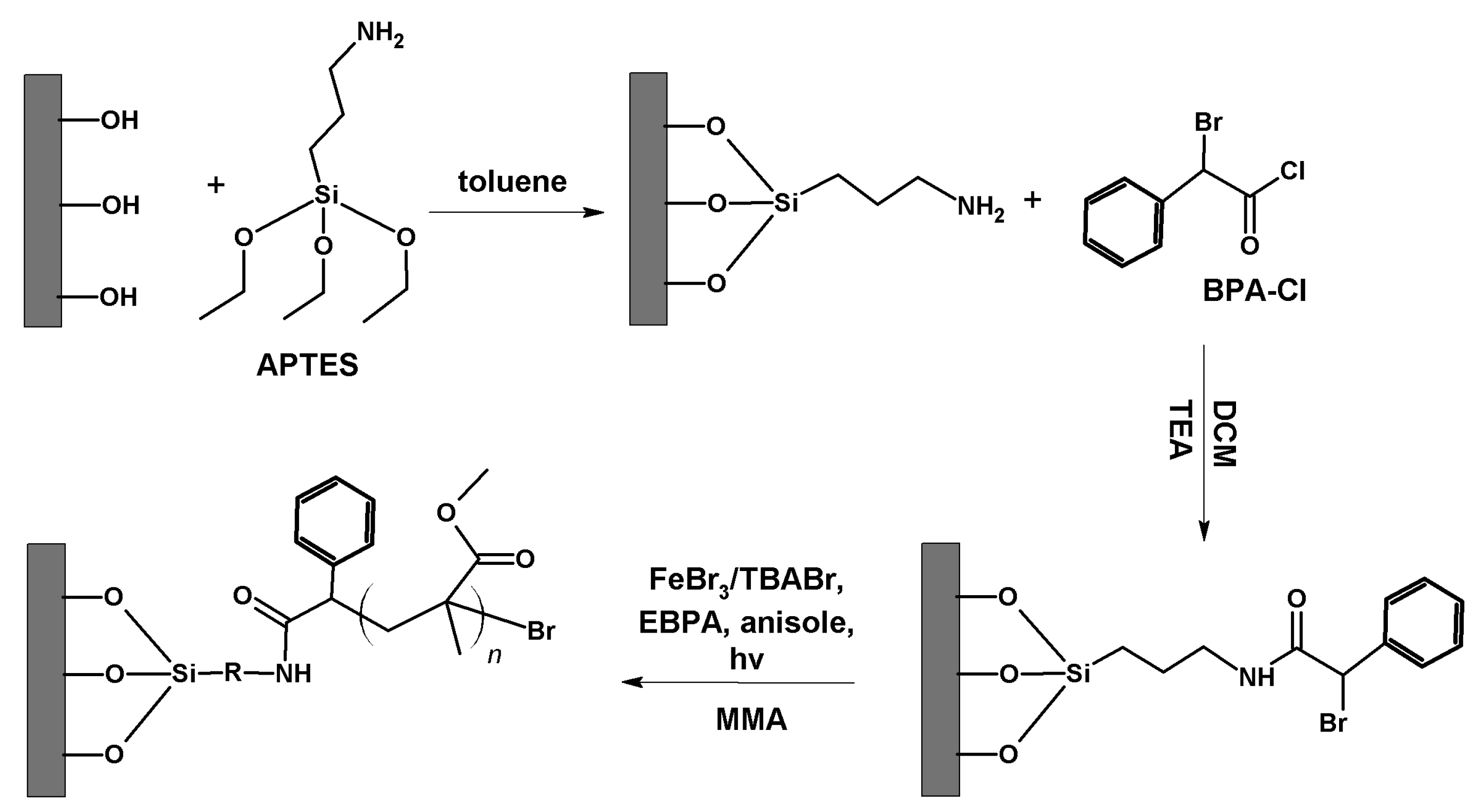
2.3.1. Grafting of APTES-BPA Initiator
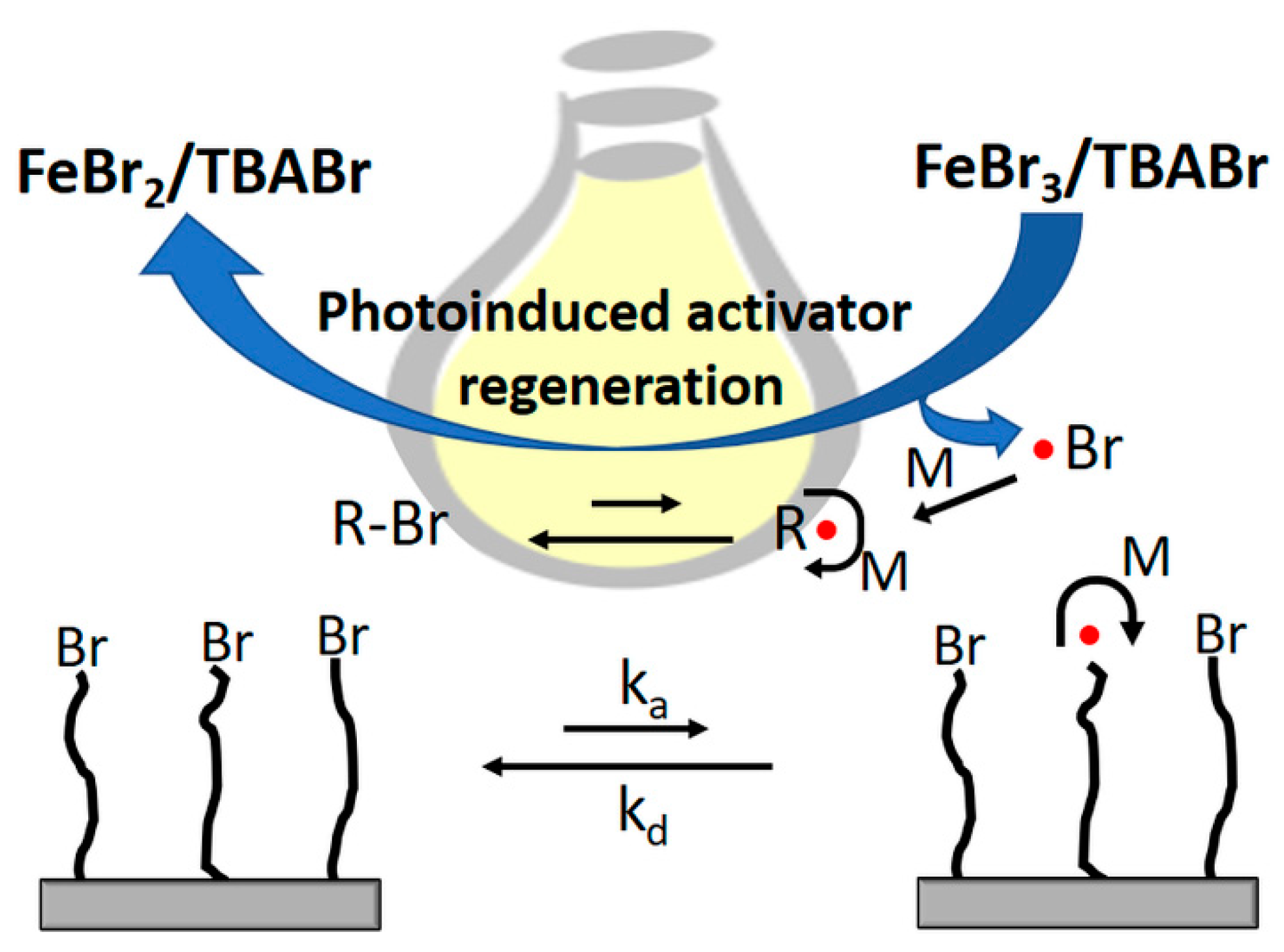
2.3.2. Photoinduced Iron-Catalyzed Surface-Initiated ATRP of MMA
2.3.3. Photoinduced Iron-Catalyzed ATRP of MMA in Solution
2.3.4. Photoreduction Kinetics of FeBr3 Catalyst
3. Results and Discussion
3.1. Grafting of APTES-BPA Initiator
3.2. Surface-Initiated Photoinduced Iron-Catalyzed ATRP of MMA
3.3. Comparison of Kinetics of SI-Photo-Fe-ATRP and Photo-Fe-ATRP
3.4. The Influence of Sacrificial Initiator on SI-Photo-Fe-ATRP Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zaborniak, I.; Chmielarz, P.; Martinez, M.R.; Wolski, K.; Wang, Z.; Matyjaszewski, K. Synthesis of high molecular weight poly(n-butyl acrylate) macromolecules via seATRP: From polymer stars to molecular bottlebrushes. Eur. Polym. J. 2020, 126, 109566. [Google Scholar] [CrossRef]
- Tanaka, J.; Häkkinen, S.; Boeck, P.T.; Cong, Y.; Perrier, S.; Sheiko, S.S.; You, W. Orthogonal Cationic and Radical RAFT Polymerizations to Prepare Bottlebrush Polymers. Angew. Chem. Int. Ed. 2020, 59, 7203–7208. [Google Scholar] [CrossRef] [PubMed]
- Zaborniak, I.; Chmielarz, P.; Wolski, K.; Grześ, G.; Isse, A.A.; Gennaro, A.; Zapotoczny, S.; Sobkowiak, A. Tannic Acid-Inspired Star-Like Macromolecules via Temporally Controlled Multi-Step Potential Electrolysis. Macromol. Chem. Phys. 2019, 220, 1–8. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Sun, Z.; Hao, B.; Lee, T.C.; Feng, A.; Zhang, L.; Thang, S.H. Synthesis of star-shaped polyzwitterions with adjustable UCST and fast responsiveness by a facile RAFT polymerization. Polym. Chem. 2020, 11, 3162–3168. [Google Scholar] [CrossRef]
- Wolski, K.; Gruszkiewicz, A.; Wytrwal-Sarna, M.; Bernasik, A.; Zapotoczny, S. The grafting density and thickness of polythiophene-based brushes determine the orientation, conjugation length and stability of the grafted chains. Polym. Chem. 2017, 8, 6250–6262. [Google Scholar] [CrossRef]
- Bayat, H.; Alhmoud, H.; Raoufi, M.; Voelcker, N.H.; Schönherr, H. Geometrical Constraints of Poly(diethylene glycol methyl ether methacrylate) Brushes on Spherical Nanoparticles and Cylindrical Nanowires: Implications for Thermoresponsive Brushes on Nanoobjects. ACS Appl. Nano Mater. 2020, 3, 3693–3705. [Google Scholar] [CrossRef]
- Wolski, K.; Gruszkiewicz, A.; Zapotoczny, S. Conductive polythiophene-based brushes grafted from an ITO surface via a self-templating approach. Polym. Chem. 2015, 6, 7505–7513. [Google Scholar] [CrossRef]
- Słowikowska, M.; Wolski, K.; Wójcik, A.J.; Wesner, D.; Schönherr, H.; Zapotoczny, S. Unraveling the nanomechanical properties of surface-grafted conjugated polymer brushes with ladder-like architecture. Polym. Chem. 2020. [Google Scholar] [CrossRef]
- Wang, J.S.; Matyjaszewski, K. Controlled/“Living” Radical Polymerization. Halogen Atom Transfer Radical Polymerization Promoted by a Cu(I)/Cu(II) Redox Process. Macromolecules 1995, 28, 7901–7910. [Google Scholar] [CrossRef]
- Lamson, M.; Kopec, M.; Ding, H.; Zhong, M.; Matyjaszewski, K. Synthesis of well-defined polyacrylonitrile by ICAR ATRP with low concentrations of catalyst. J. Polym. Sci. Part. A Polym. Chem. 2016, 54, 1961–1968. [Google Scholar] [CrossRef]
- Cordero, R.; Jawaid, A.; Hsiao, M.S.; Lequeux, Z.; Vaia, R.A.; Ober, C.K. Mini monomer encapsulated emulsion polymerization of pmma using aqueous arget atrp. ACS Macro Lett. 2018, 7, 459–463. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P.; Flejszar, M.; Surmacz, K.; Ostatek, R. Preparation of hydrophobic tannins-inspired polymer materials via low-ppm ATRP methods. Polym. Adv. Technol. 2020, 31, 913–921. [Google Scholar] [CrossRef]
- Chmielarz, P.; Fantin, M.; Park, S.; Isse, A.A.; Gennaro, A.; Magenau, A.J.D.; Sobkowiak, A.; Matyjaszewski, K. Electrochemically mediated atom transfer radical polymerization (eATRP). Prog. Polym. Sci. 2017, 69, 47–78. [Google Scholar] [CrossRef]
- Discekici, E.H.; Anastasaki, A.; Read De Alaniz, J.; Hawker, C.J. Evolution and Future Directions of Metal-Free Atom Transfer Radical Polymerization. Macromolecules 2018, 51, 7421–7434. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Lee, I.H.; Anastasaki, A.; Lorandi, F.; Narupai, B.; Dolinski, N.D.; Allegrezza, M.L.; Fantin, M.; Konkolewicz, D.; Hawker, C.J.; et al. Investigating Temporal Control in Photoinduced Atom Transfer Radical Polymerization. Macromolecules 2020, 53, 5280–5288. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, X.; Li, L.; Fantin, M.; Yan, J.; Wang, Z.; Wang, Z.; Xia, H.; Matyjaszewski, K. Enhancing Mechanically Induced ATRP by Promoting Interfacial Electron Transfer from Piezoelectric Nanoparticles to Cu Catalysts. Macromolecules 2017, 50, 7940–7948. [Google Scholar] [CrossRef]
- Pan, X.; Malhotra, N.; Dadashi-Silab, S.; Matyjaszewski, K. A Simplified Fe-Based PhotoATRP Using Only Monomers and Solvent. Macromol. Rapid Commun. 2017, 38, 1–6. [Google Scholar] [CrossRef]
- Pomorska, A.; Wolski, K.; Wytrwal-Sarna, M.; Bernasik, A.; Zapotoczny, S. Polymer brushes grafted from nanostructured zinc oxide layers—Spatially controlled decoration of nanorods. Eur. Polym. J. 2019, 112, 186–194. [Google Scholar] [CrossRef]
- Poręba, R.; de los Santos Pereira, A.; Pola, R.; Jiang, S.; Pop-Georgievski, O.; Sedláková, Z.; Schönherr, H. “Clickable” and Antifouling Block Copolymer Brushes as a Versatile Platform for Peptide-Specific Cell Attachment. Macromol. Biosci. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Mocny, P.; Menétrey, M.; Klok, H.A. Synthesis of Loop Poly(Methyl Methacrylate) Brushes via Chain-End Postpolymerization Modification. Macromolecules 2019, 52, 8394–8403. [Google Scholar] [CrossRef]
- Fantin, M.; Ramakrishna, S.N.; Yan, J.; Yan, W.; Divandari, M.; Spencer, N.D.; Matyjaszewski, K.; Benetti, E.M. The Role of Cu0 in Surface-Initiated Atom Transfer Radical Polymerization: Tuning Catalyst Dissolution for Tailoring Polymer Interfaces. Macromolecules 2018, 51, 6825–6835. [Google Scholar] [CrossRef]
- Raj, W.; Russo, A.; Zhang, Y.; Chapelat, J.; Pietrasik, J. Renewable fabric surface-initiated ATRP polymerizations: Towards mixed polymer brushes. Nanomaterials 2020, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K.; Hongchen, D.; Jakubowski, W.; Pietrasik, J.; Kusumo, A. Grafting from surfaces for “everyone”: ARGET ATRP in the presence of air. Langmuir 2007, 23, 4528–4531. [Google Scholar] [CrossRef] [PubMed]
- Flejszar, M.; Chmielarz, P.; Wolski, K.; Grześ, G.; Zapotoczny, S. Polymer Brushes via Surface-Initiated Electrochemically Mediated ATRP: Role of a Sacrificial Initiator in Polymerization of Acrylates on Silicon Substrates. Materials 2020, 13, 3559. [Google Scholar] [CrossRef]
- Jeyaprakash, J.D.; Samuel, S.; Dhamodharan, R.; Rühe, J. Polymer brushes via ATRP: Role of activator and deactivator in the surface-initiated ATRP of styrene on planar substrates. Macromol. Rapid Commun. 2002, 23, 277–281. [Google Scholar] [CrossRef]
- Cheng, N.; Azzaroni, O.; Moya, S.; Huck, W.T.S. The effect of [CuI]/[CuII] ratio on the kinetics and conformation of polyelectrolyte brushes by atom transfer radical polymerization. Macromol. Rapid Commun. 2006, 27, 1632–1636. [Google Scholar] [CrossRef]
- Discekici, E.H.; Pester, C.W.; Treat, N.J.; Lawrence, J.; Mattson, K.M.; Narupai, B.; Toumayan, E.P.; Luo, Y.; McGrath, A.J.; Clark, P.G.; et al. Simple Benchtop Approach to Polymer Brush Nanostructures Using Visible-Light-Mediated Metal-Free Atom Transfer Radical Polymerization. ACS Macro Lett. 2016, 5, 258–262. [Google Scholar] [CrossRef]
- Narupai, B.; Page, Z.A.; Treat, N.J.; McGrath, A.J.; Pester, C.W.; Discekici, E.H.; Dolinski, N.D.; Meyers, G.F.; Read de Alaniz, J.; Hawker, C.J. Simultaneous Preparation of Multiple Polymer Brushes under Ambient Conditions using Microliter Volumes. Angew. Chemie Int. Ed. 2018, 57, 13433–13438. [Google Scholar] [CrossRef]
- Kopeć, M.; Pikiel, M.; Vancso, G.J. Surface-grafted polyacrylonitrile brushes with aggregation-induced emission properties. Polym. Chem. 2020, 11, 669–674. [Google Scholar] [CrossRef]
- Yan, W.; Dadashi-Silab, S.; Matyjaszewski, K.; Spencer, N.D.; Benetti, E.M. Surface-Initiated Photoinduced ATRP: Mechanism, Oxygen Tolerance, and Temporal Control during the Synthesis of Polymer Brushes. Macromolecules 2020, 53, 2801–2810. [Google Scholar] [CrossRef]
- Zhao, H.; Sha, J.; Wu, T.; Chen, T.; Chen, X.; Ji, H.; Wang, Y.; Zhu, H.; Xie, L.; Ma, Y. Spatial modulation of biomolecules immobilization by fabrication of hierarchically structured PEG-derived brush micropatterns: An versatile cellular microarray platform. Appl. Surf. Sci. 2020, 529, 147056. [Google Scholar] [CrossRef]
- Page, Z.A.; Narupai, B.; Pester, C.W.; Bou Zerdan, R.; Sokolov, A.; Laitar, D.S.; Mukhopadhyay, S.; Sprague, S.; McGrath, A.J.; Kramer, J.W.; et al. Novel Strategy for Photopatterning Emissive Polymer Brushes for Organic Light Emitting Diode Applications. ACS Cent. Sci. 2017, 3, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.N.; Guo, J.K.; Li, J.J.; Luo, Z.H. Photoinduced Iron(III)-Mediated Atom Transfer Radical Polymerization with in Situ Generated Initiator: Mechanism and Kinetics Studies. Ind. Eng. Chem. Res. 2016, 55, 10235–10242. [Google Scholar] [CrossRef]
- Pan, X.; Malhotra, N.; Zhang, J.; Matyjaszewski, K. Photoinduced Fe-Based Atom Transfer Radical Polymerization in the Absence of Additional Ligands, Reducing Agents, and Radical Initiators. Macromolecules 2015, 48, 6948–6954. [Google Scholar] [CrossRef]
- Ojang, L.; Zhang, L.; Zhang, Z.; Zhou, N.; Cheng, Z.; Xiulin, Z. Air-tolerantly surface-initiated AGET ATRP mediated by iron catalyst from silica nanoparticles. J. Polym. Sci. Part. A Polym. Chem. 2010, 48, 2006–2015. [Google Scholar] [CrossRef]
- Layadi, A.; Kessel, B.; Yan, W.; Romio, M.; Spencer, N.D.; Zenobi-Wong, M.; Matyjaszewski, K.; Benetti, E.M. Oxygen Tolerant and Cytocompatible Iron(0)-Mediated ATRP Enables the Controlled Growth of Polymer Brushes from Mammalian Cell Cultures. J. Am. Chem. Soc. 2020, 142, 3158–3164. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Pan, X.; Matyjaszewski, K. Photoinduced Iron-Catalyzed Atom Transfer Radical Polymerization with ppm Levels of Iron Catalyst under Blue Light Irradiation. Macromolecules 2017, 50, 7967–7977. [Google Scholar] [CrossRef]
- Gruszkiewicz, A.; Słowikowska, M.; Grześ, G.; Wójcik, A.; Rokita, J.; Fiocco, A.; Wytrwal-Sarna, M.; Marzec, M.; Trzebicka, B.; Kopeć, M.; et al. Enhancement of the growth of polymer brushes via ATRP initiated from ions-releasing indium tin oxide substrates. Eur. Polym. J. 2019, 112, 817–821. [Google Scholar] [CrossRef]
- Eckenhoff, W.T.; Biernesser, A.B.; Pintauer, T. Structural characterization and investigation of iron(III) complexes with nitrogen and phosphorus based ligands in atom transfer radical addition (ATRA). Inorg. Chim. Acta 2012, 382, 84–95. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Matyjaszewski, K. Iron catalysts in atom transfer radical polymerization. Molecules 2020, 25, 1648. [Google Scholar] [CrossRef]
- Schroeder, H.; Buback, J.; Demeshko, S.; Matyjaszewski, K.; Meyer, F.; Buback, M. Speciation analysis in iron-mediated ATRP studied via FT-Near-IR and mössbauer spectroscopy. Macromolecules 2015, 48, 1981–1990. [Google Scholar] [CrossRef]
- Tranchida, D.; Piccarolo, S.; Soliman, M. Nanoscale mechanical characterization of polymers by AFM nanoindentations: Criticalr approach to the elastic characterization. Macromolecules 2006, 39, 4547–4556. [Google Scholar] [CrossRef]
- Tranchida, D.; Sperotto, E.; Chateauminois, A.; Schönherr, H. Entropic effects on the mechanical behavior of dry polymer brushes during nanoindentation by atomic force microscopy. Macromolecules 2011, 44, 368–374. [Google Scholar] [CrossRef]
- Wang, H.; Black, C.T.; Akcora, P. Elastic Properties of Protein Functionalized Nanoporous Polymer Films. Langmuir 2016, 32, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Rolland, M.; Truong, N.P.; Whitfield, R.; Anastasaki, A. Tailoring Polymer Dispersity in Photoinduced Iron-Catalyzed ATRP. ACS Macro Lett. 2020, 9, 459–463. [Google Scholar] [CrossRef]
- Tang, W.; Matyjaszewski, K. Effects of initiator structure on activation rate constants in ATRP. Macromolecules 2007, 40, 1858–1863. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, T.; Lin, K.C.; Li, S.; Yan, J.; Olszewski, M.; Sobieski, J.; Pietrasik, J.; Bockstaller, M.R.; Matyjaszewski, K. Synthesis of Ultra-high Molecular Weight SiO2-g-PMMA Particle Brushes. J. Inorg. Organomet. Polym. Mater. 2020, 30, 174–181. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, J.; Liu, T.; Wei, Q.; Li, S.; Olszewski, M.; Wu, J.; Sobieski, J.; Fantin, M.; Bockstaller, M.R.; et al. Control of Dispersity and Grafting Density of Particle Brushes by Variation of ATRP Catalyst Concentration. ACS Macro Lett. 2019, 8, 859–864. [Google Scholar] [CrossRef]
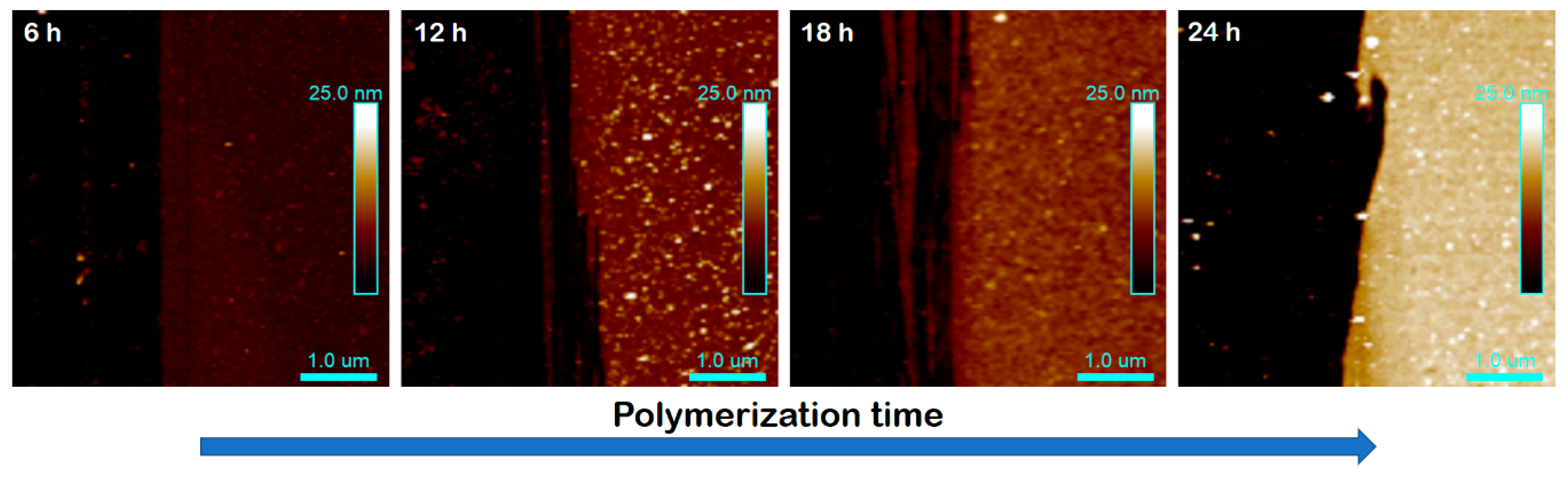
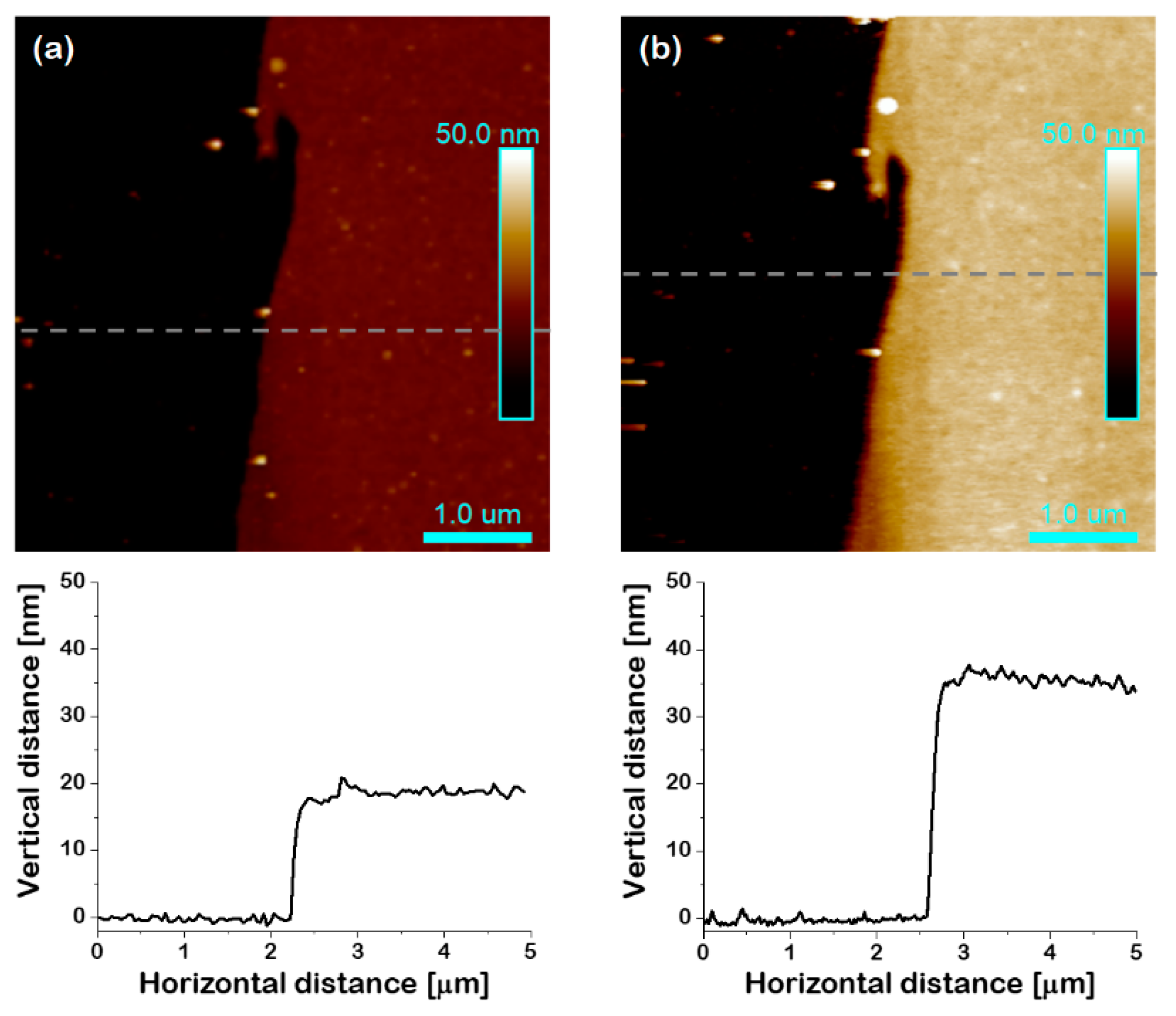
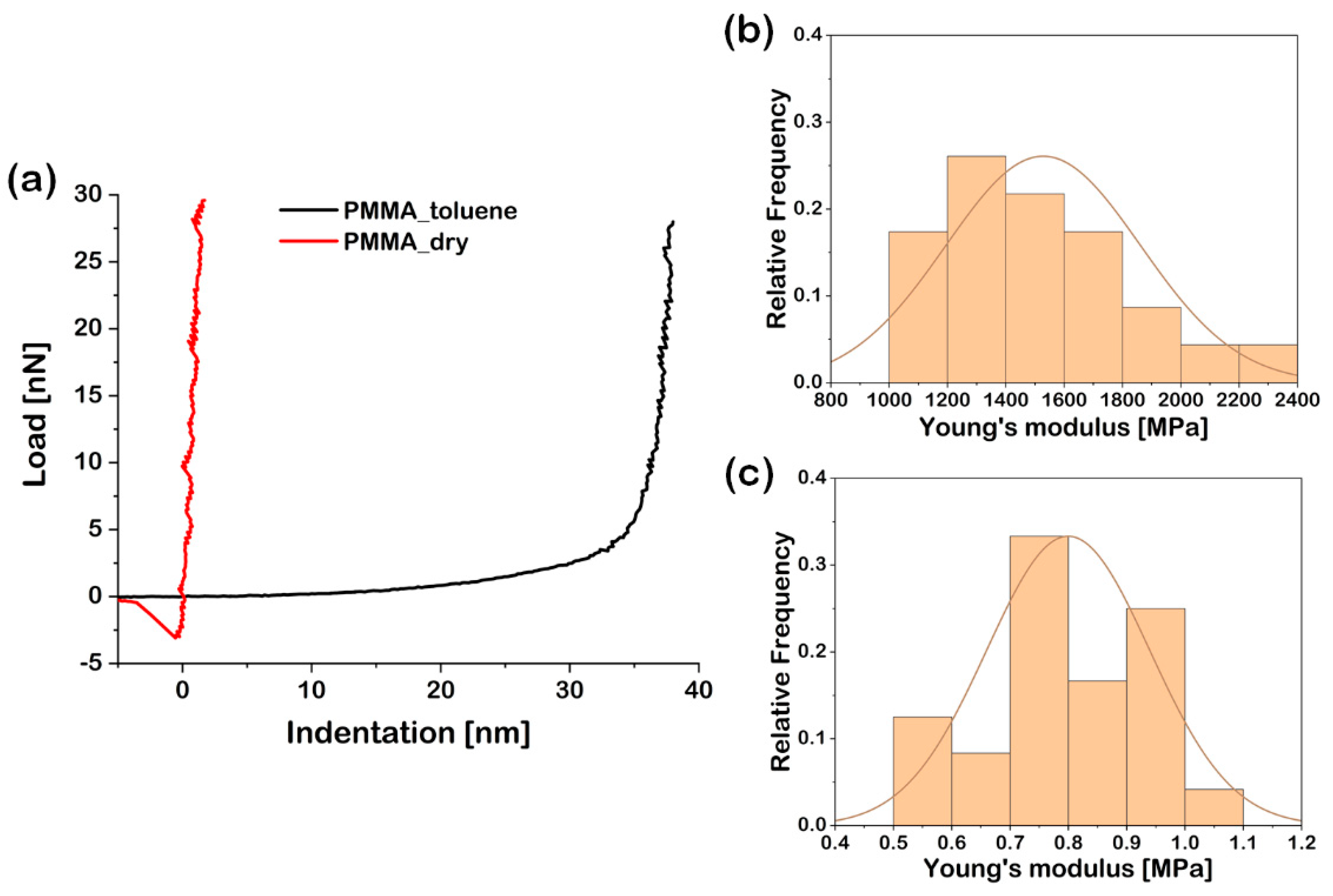


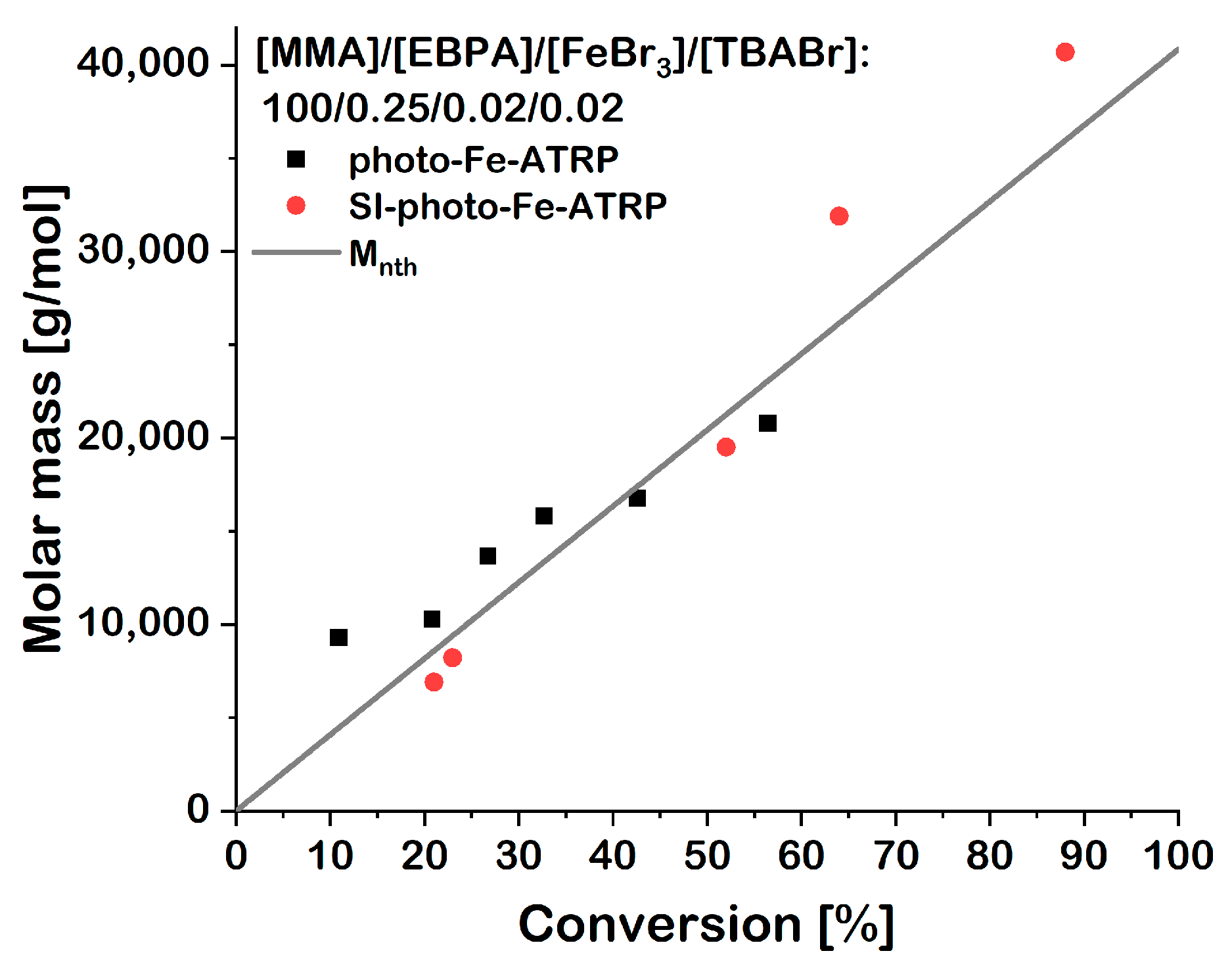
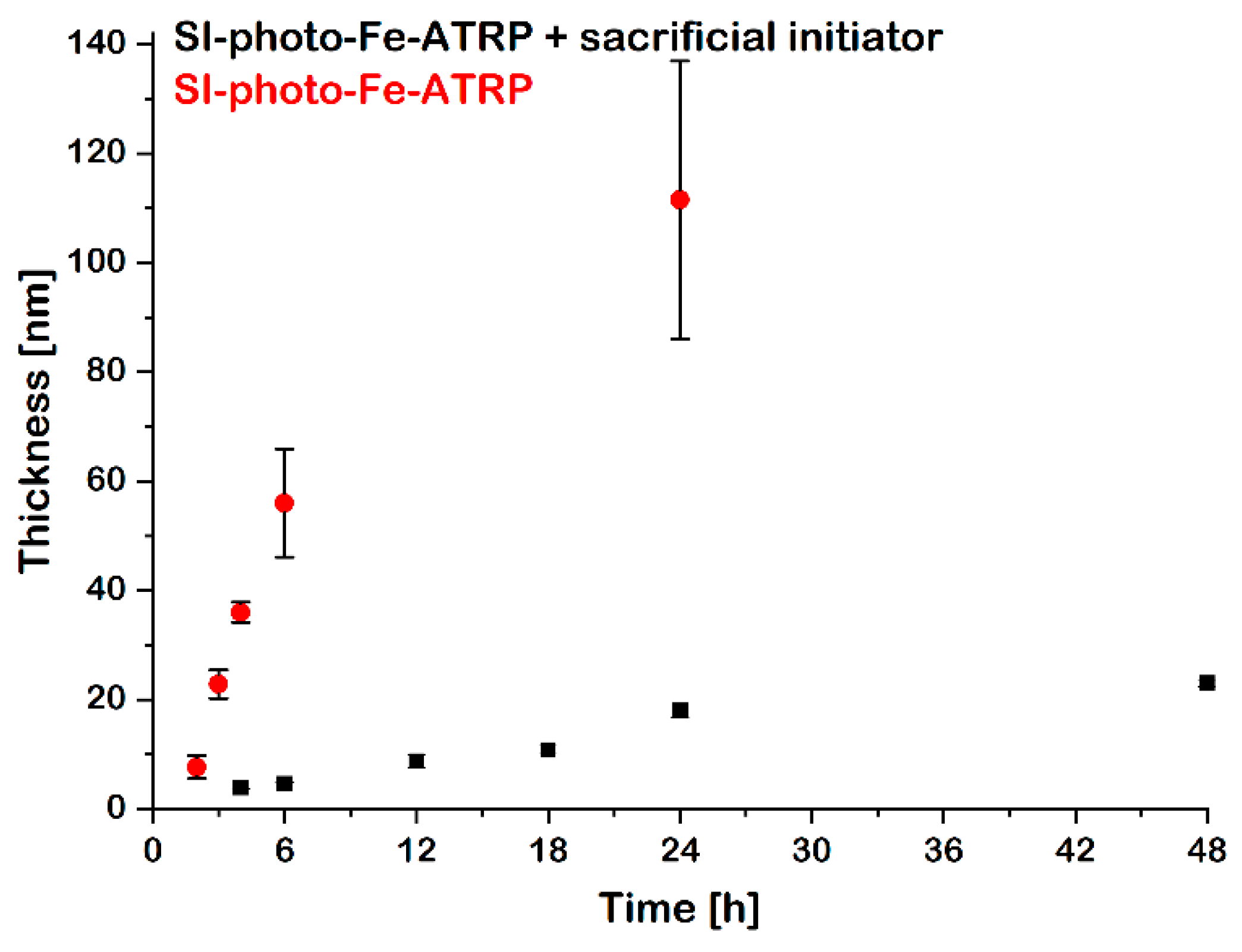
| Entry | Time (h) | hdry (nm) | Mn | Mw/Mn | Conversion (%) | Mnth | Mα |
|---|---|---|---|---|---|---|---|
| 1 | 4 | 3.9 ± 0.3 | – | – | 21 | 8600 | 6900 |
| 2 | 6 | 4.6 ± 0.4 | – | – | 23 | 9400 | 8200 |
| 3 | 12 | 8.7 ± 1.2 | – | – | – | – | – |
| 4 | 18 | 11.0 ± 0.7 | – | – | 52 | 21,200 | 19,500 |
| 5 | 24 | 18.0 ± 1.9 | 20,500 | 1.55 | 64 | 26,000 | 31,900 |
| 6 | 48 | 23.0 ± 1.6 | 27,500 | 1.54 | 88 | 35,500 | 40,700 |
| Entry | Time (h) | Mn (g/mol) | Mnth (g/mol) | Conversion (%) | Mw/Mn |
|---|---|---|---|---|---|
| [MMA]/[EBPA]/[FeBr3]/[TBABr] = 100/0.25/0.02/0.02 | |||||
| 1 | 2 | 9300 | 4600 | 11 | 1.33 |
| 2 | 4 | 10,300 | 8600 | 21 | 1.63 |
| 3 | 6 | 13,700 | 10,900 | 27 | 1.67 |
| 4 | 8 | 15,800 | 13,300 | 33 | 1.78 |
| 5 | 12 | 16,800 | 17,300 | 43 | 1.90 |
| 6 | 24 | 20,800 | 22,800 | 56 | 1.96 |
| [MMA]/[EBPA]/[FeBr3]/[TBABr] = 100/1/0.02/0.02 | |||||
| 7 | 4 | 5200 | 2900 | 26 | 1.30 |
| 8 | 6 | 5800 | 4200 | 40 | 1.29 |
| 9 | 24 | 7000 | 9000 | 87 | 1.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Słowikowska, M.; Chajec, K.; Michalski, A.; Zapotoczny, S.; Wolski, K. Surface-Initiated Photoinduced Iron-Catalyzed Atom Transfer Radical Polymerization with ppm Concentration of FeBr3 under Visible Light. Materials 2020, 13, 5139. https://doi.org/10.3390/ma13225139
Słowikowska M, Chajec K, Michalski A, Zapotoczny S, Wolski K. Surface-Initiated Photoinduced Iron-Catalyzed Atom Transfer Radical Polymerization with ppm Concentration of FeBr3 under Visible Light. Materials. 2020; 13(22):5139. https://doi.org/10.3390/ma13225139
Chicago/Turabian StyleSłowikowska, Monika, Kamila Chajec, Adam Michalski, Szczepan Zapotoczny, and Karol Wolski. 2020. "Surface-Initiated Photoinduced Iron-Catalyzed Atom Transfer Radical Polymerization with ppm Concentration of FeBr3 under Visible Light" Materials 13, no. 22: 5139. https://doi.org/10.3390/ma13225139
APA StyleSłowikowska, M., Chajec, K., Michalski, A., Zapotoczny, S., & Wolski, K. (2020). Surface-Initiated Photoinduced Iron-Catalyzed Atom Transfer Radical Polymerization with ppm Concentration of FeBr3 under Visible Light. Materials, 13(22), 5139. https://doi.org/10.3390/ma13225139






