Synthesis and Characterization of Hybrid Metal Zeolitic Imidazolate Framework Membrane for Efficient H2/CO2 Gas Separation
Abstract
1. Introduction
2. Experimental Methods
2.1. Synthesis of ZIF-8-67 Nanocrystals
2.2. Synthesis of ZIF-8, ZIF-67, and ZIF-8-67 Hybrid Membrane
2.3. Characterization
3. Results and Discussion
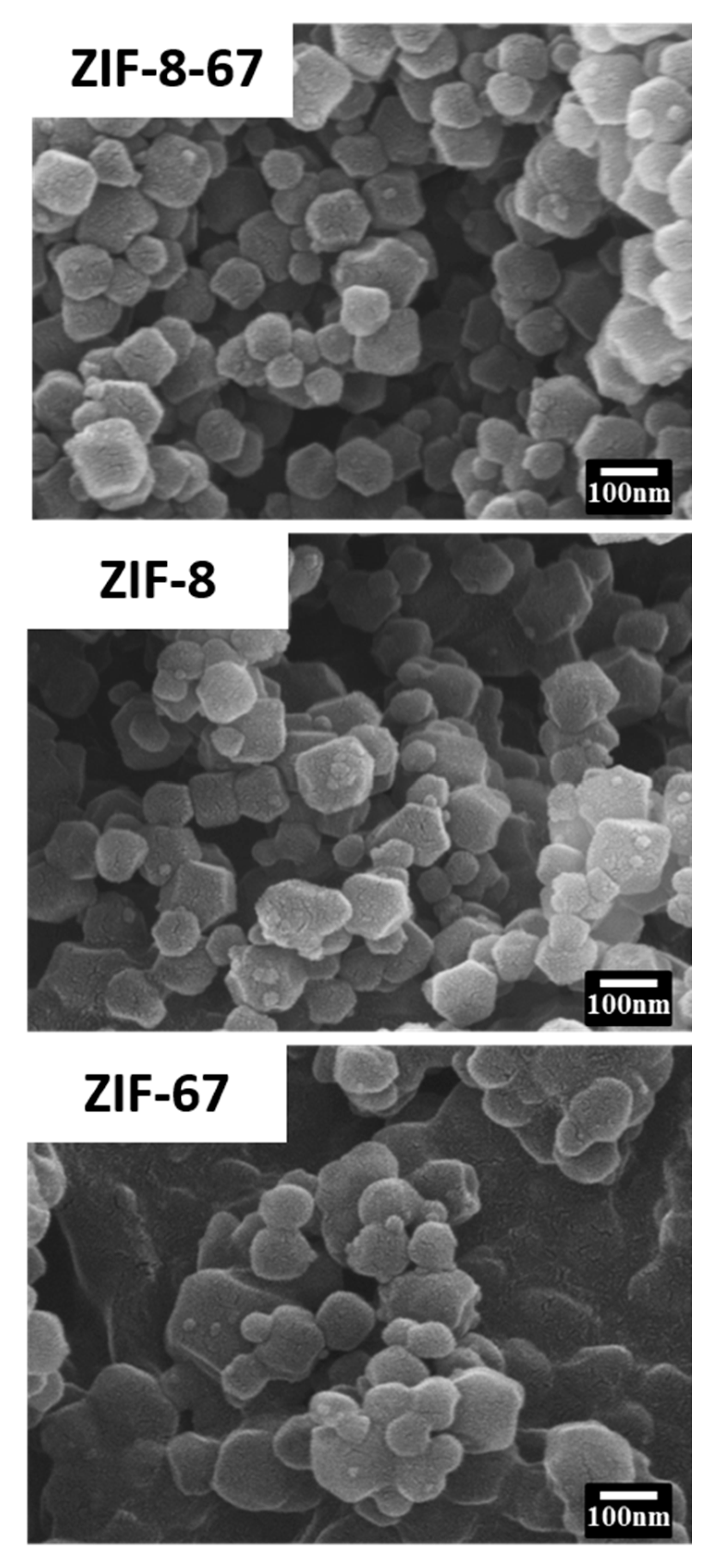
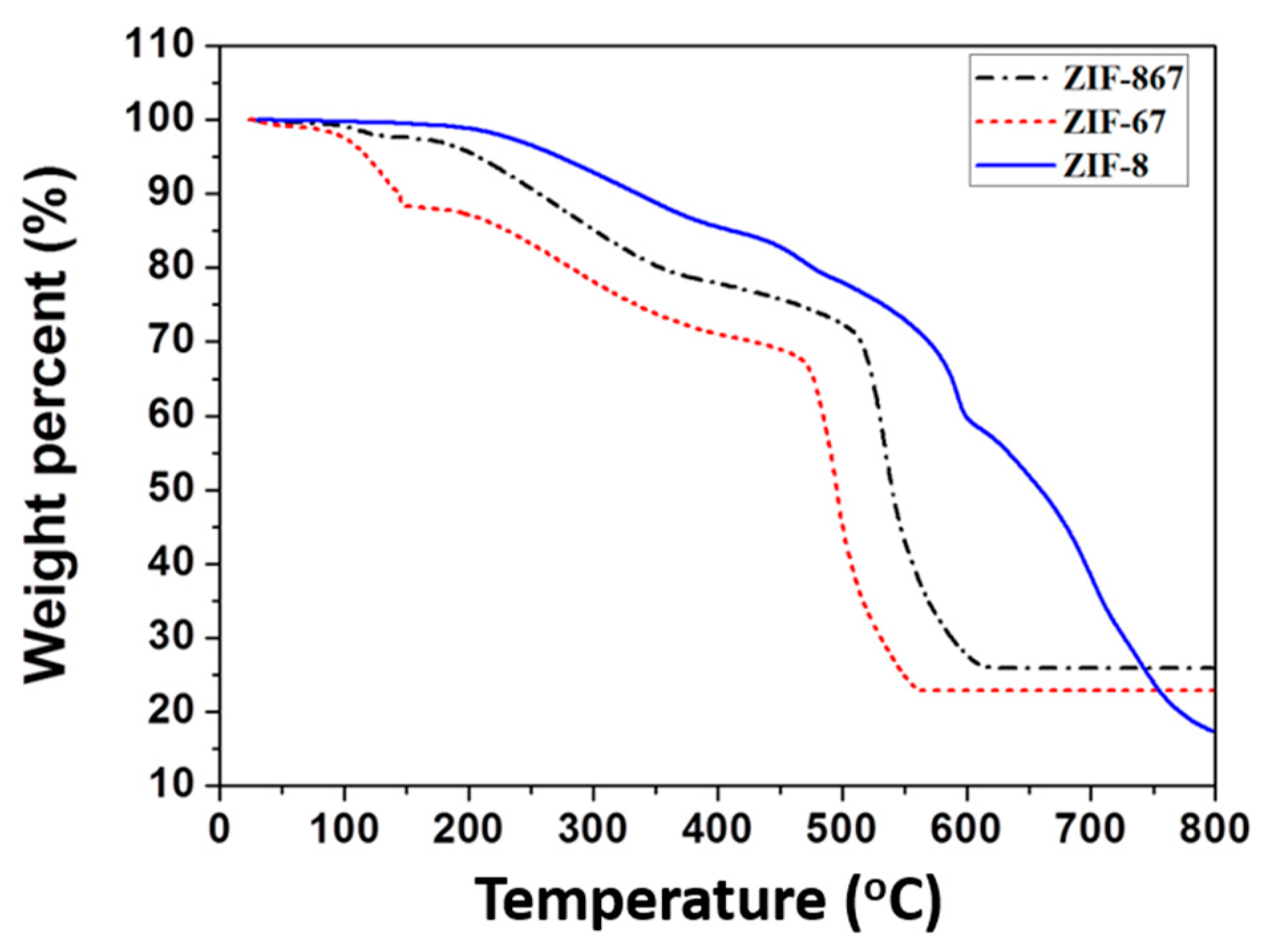
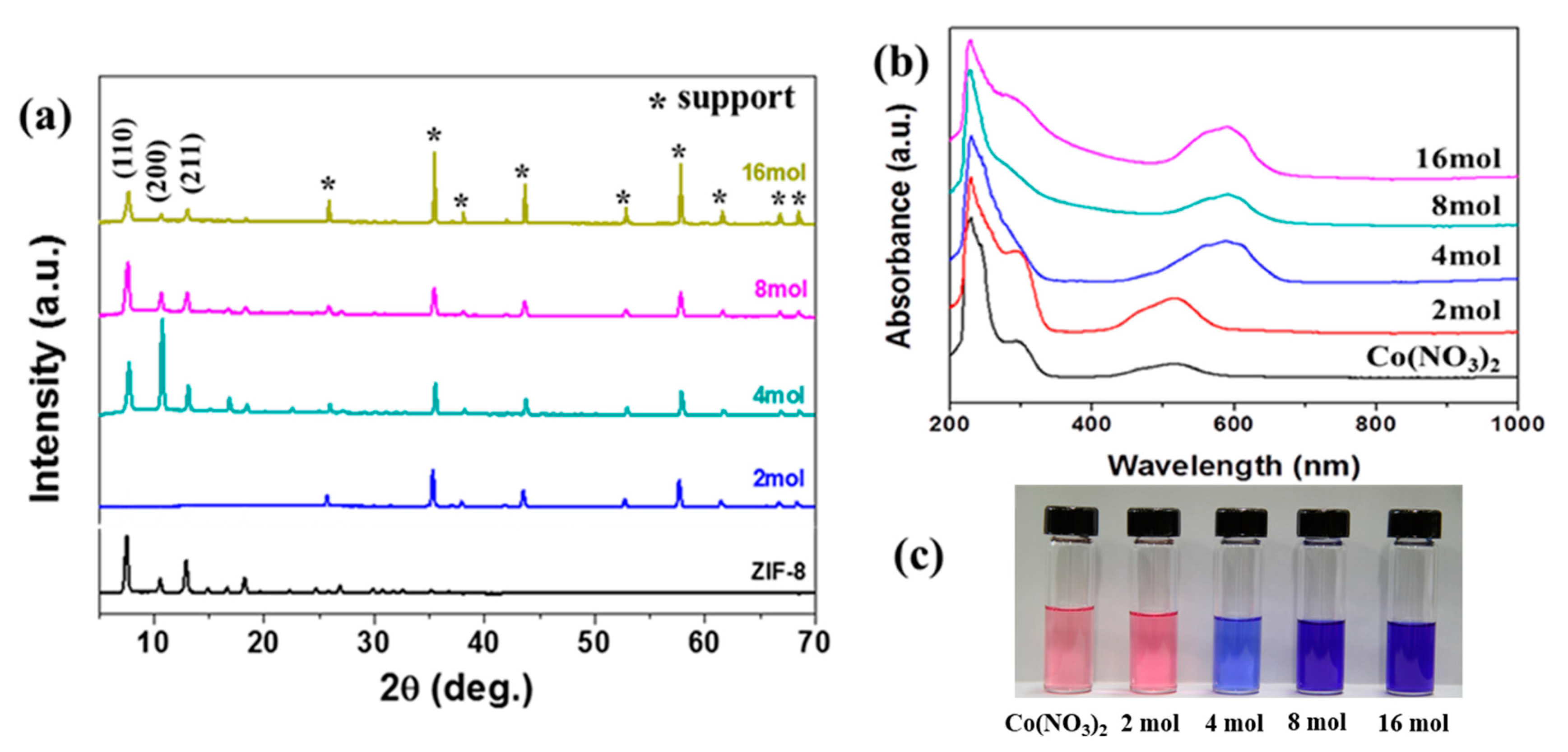
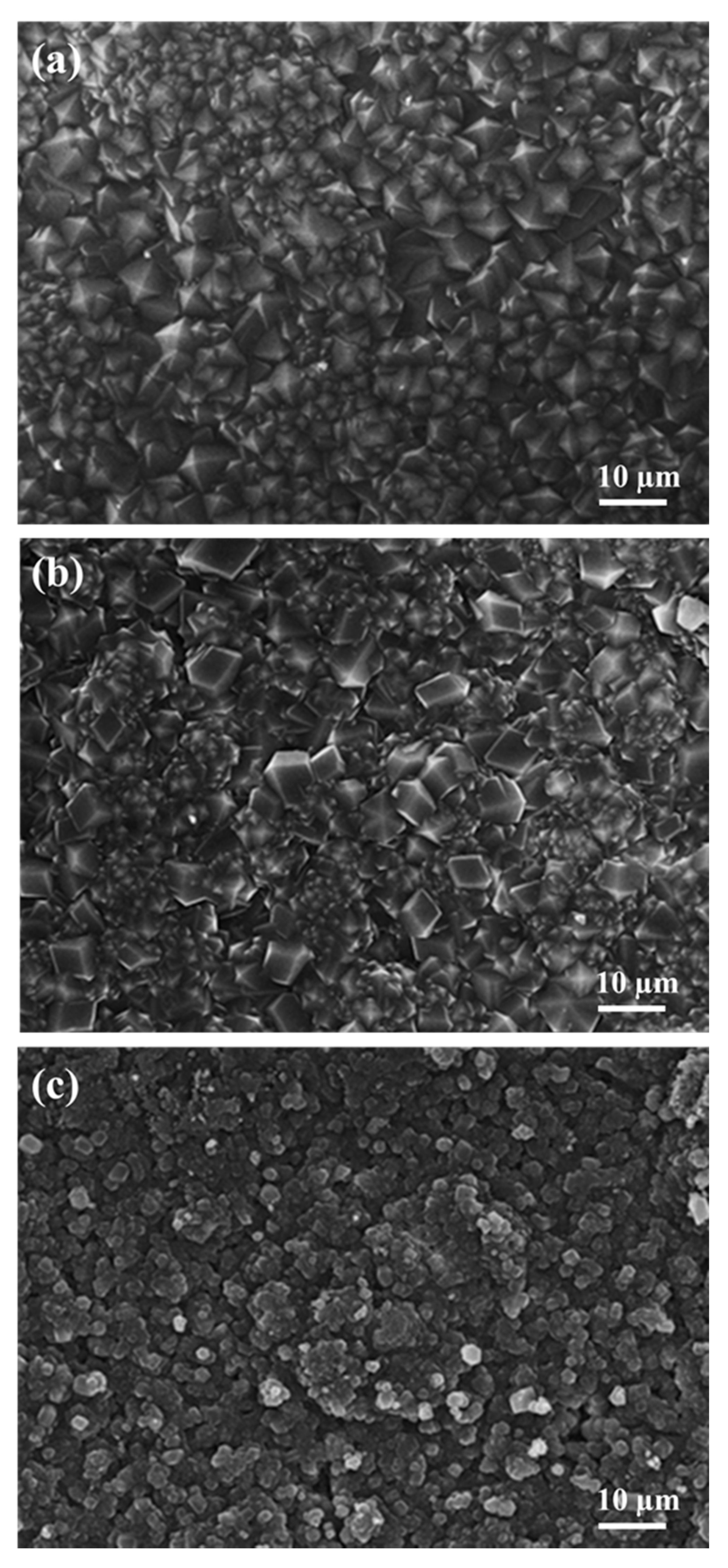
3.1. Characteristics of Nano ZIF-8-67 Seeds
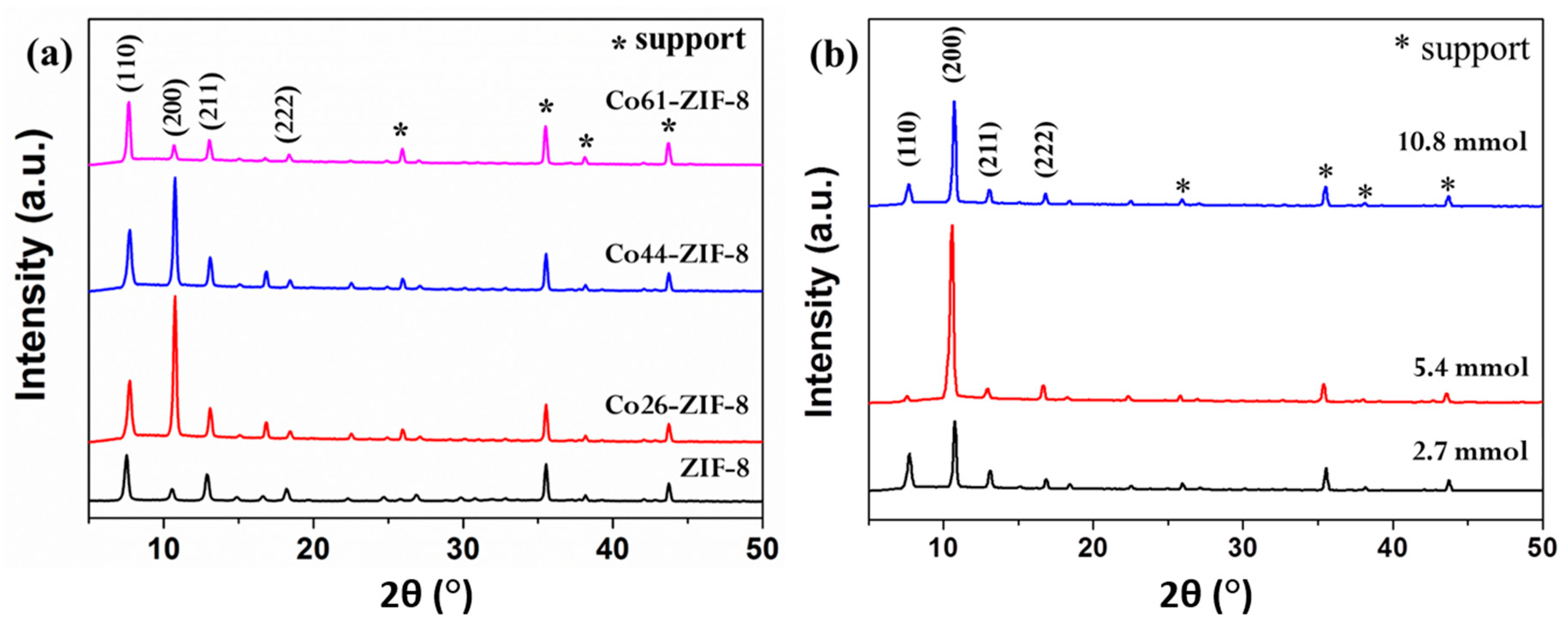
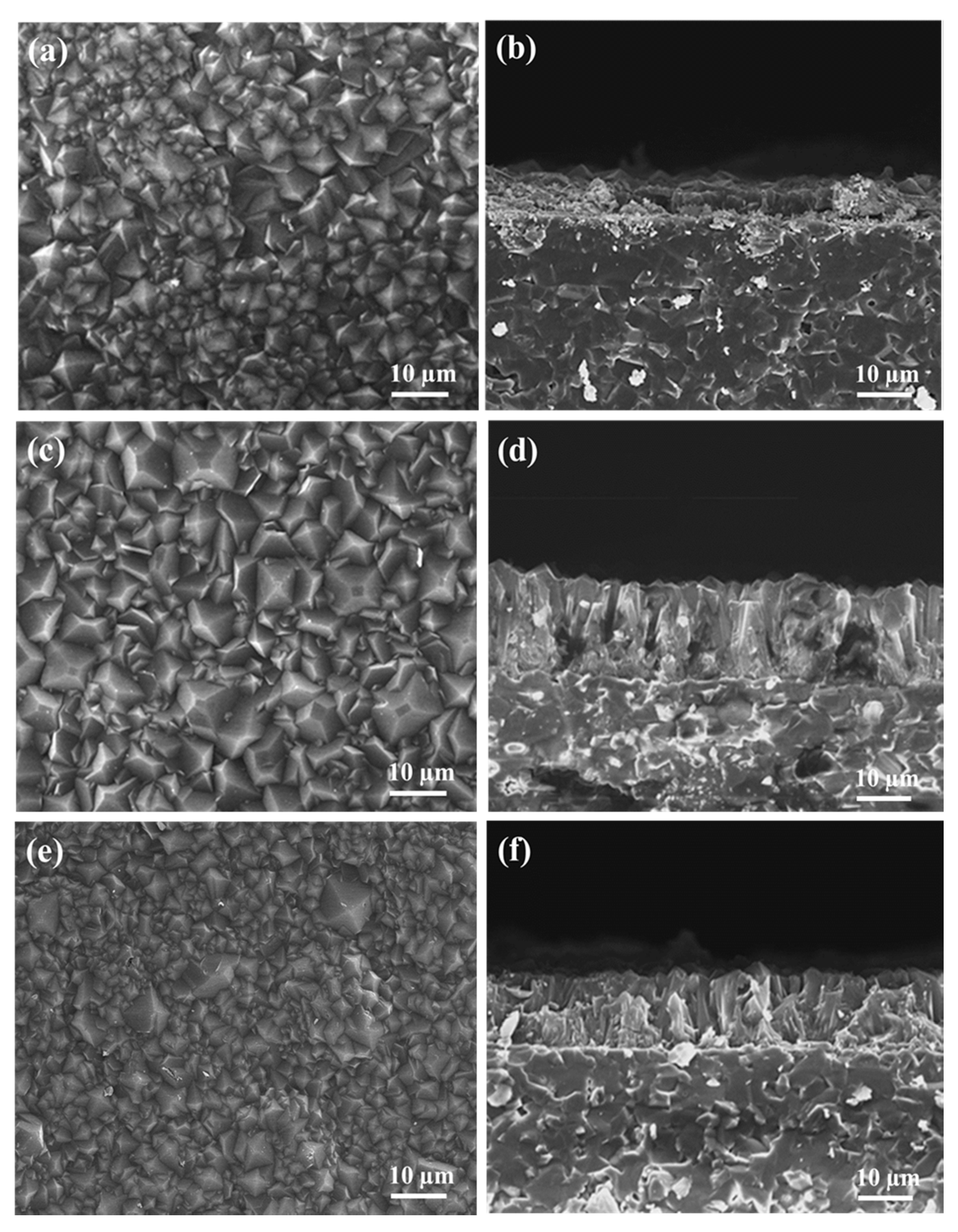
3.2. Characterization of ZIF-8-67 Membrane
3.2.1. Membrane Characterization and Effect of Sodium Formate
3.2.2. Effect of Ligand
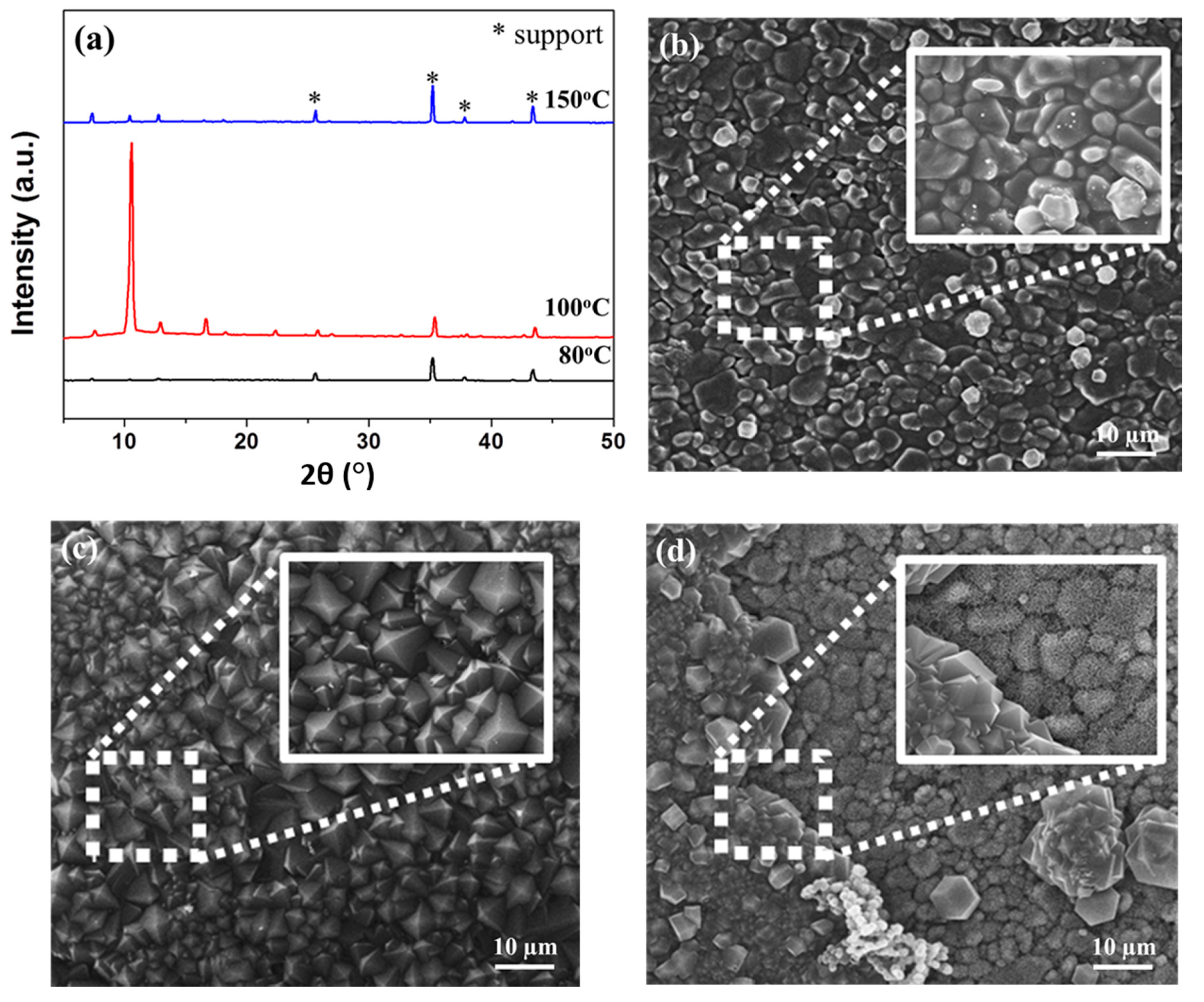
3.2.3. Effect of Microwave Solvothermal Temperatures
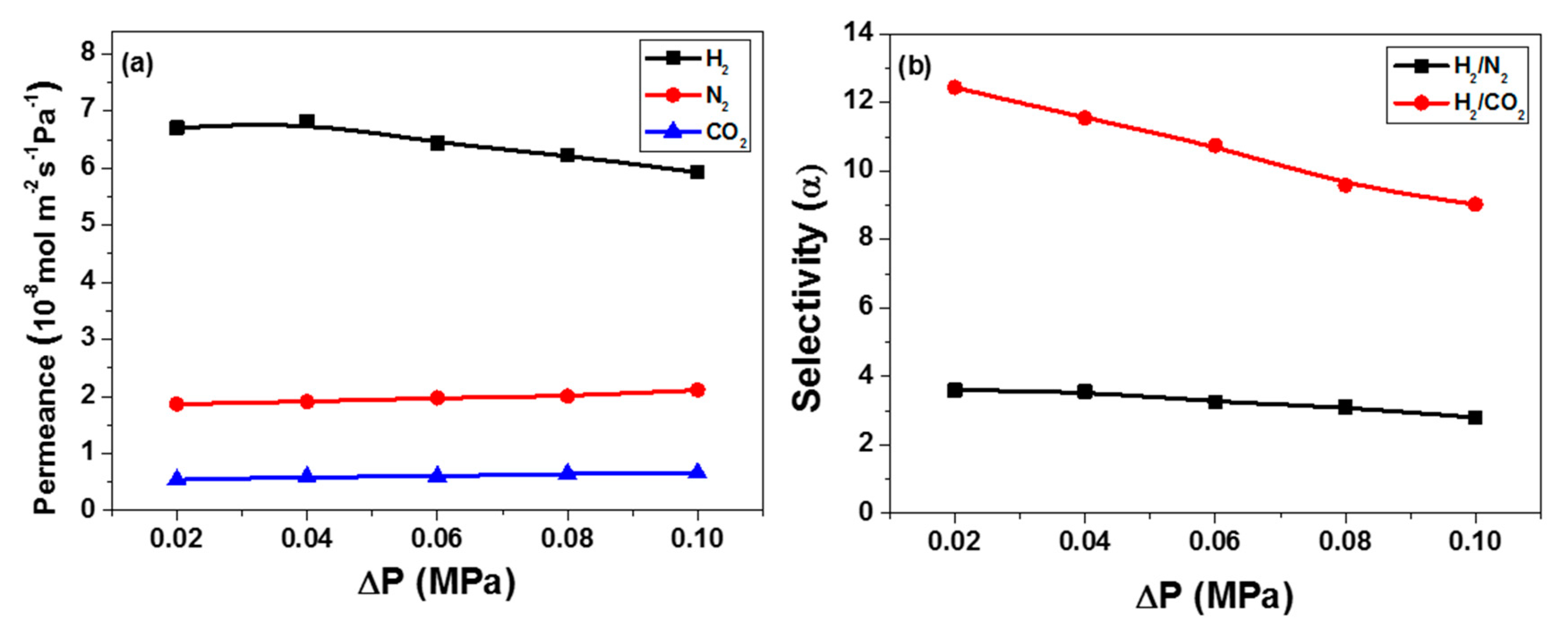
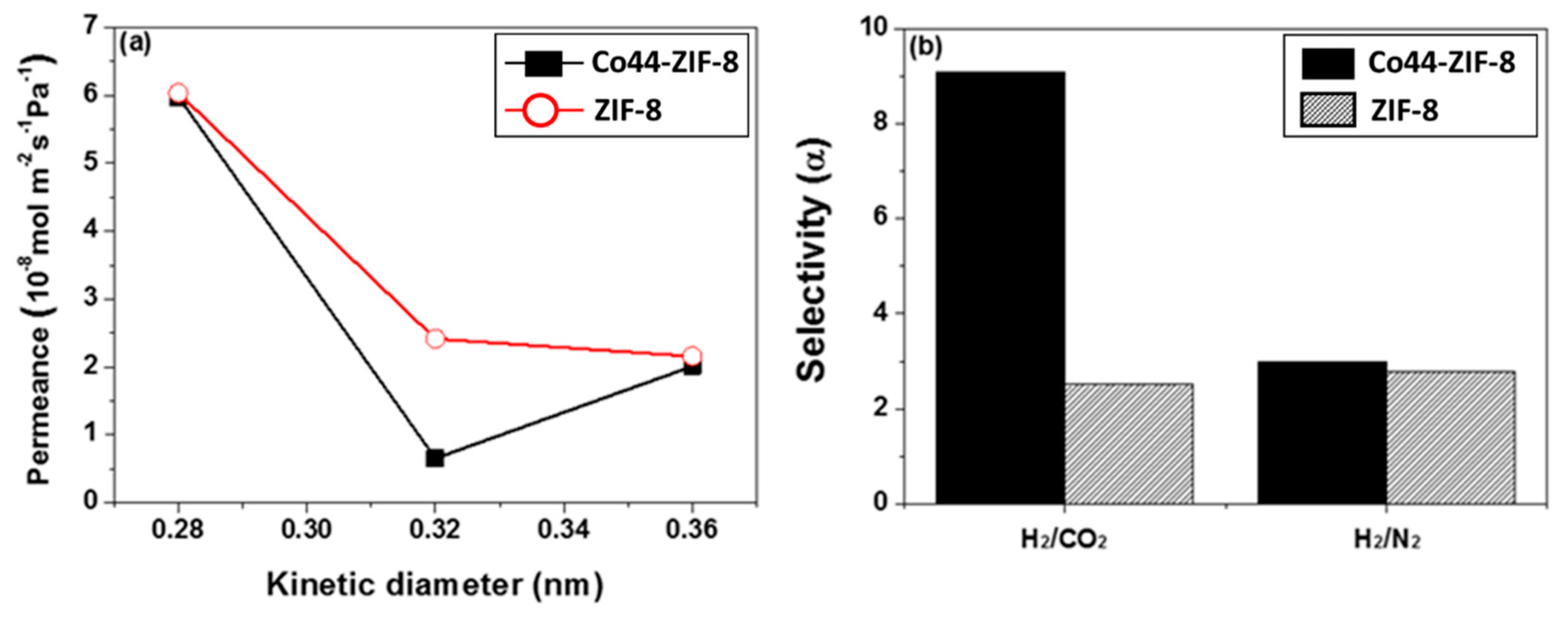
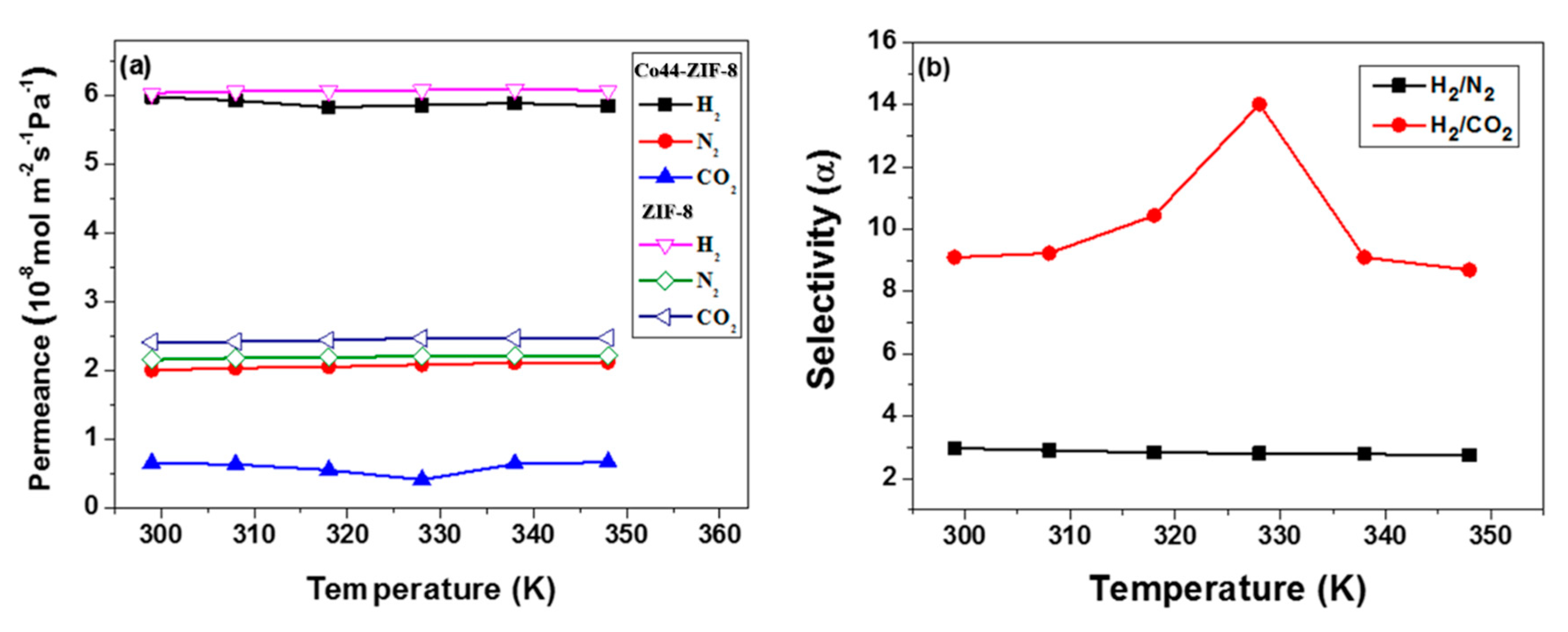
3.3. Gas Permeation Performance of Co44-ZIF-8 Membranes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Park, K.S.; Zheng, N.; Côté, A.P.; Choi, J.Y.; Huang, R.D.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Hayashi, H.; Côté, A.P.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. Zeolite A imidazolate frameworks. Nat. Mater. 2007, 6, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, N.A.; Didas, S.A.; Venkatasubbaiah, K.; Jones, C.W. Tuning cooperativity by controlling the linker length of silica-supported amines in catalysis and CO2 capture. J. Am. Chem. Soc. 2012, 134, 13950–13953. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.X.; Dong, X.L.; Nan, J.P.; Jin, W.Q.; Ren, X.M.; Xu, N.P.; Lee, Y.M. Metal–organic framework membranes fabricated via reactive seeding. Chem. Commun. 2011, 47, 737–739. [Google Scholar] [CrossRef]
- Dong, X.L.; Huang, K.; Liu, S.; Ren, R.; Jin, W.; Lin, Y.S. Synthesis of zeolitic imidazolate framework-78 molecular-sieve membrane: Defect formation and elimination. J. Mater. Chem. 2012, 22, 19222–19227. [Google Scholar] [CrossRef]
- Bux, H.; Liang, F.Y.; Li, Y.S.; Cravillon, J.; Wiebcke, M.; Caro, J. Zeolitic imidazolate framework membrane with molecular sieving properties by microwaveassisted solvothermal synthesis. J. Am. Chem. Soc. 2009, 131, 16000–16001. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.C.; Wang, B.; Lai, Z.P. Synthesis of ceramic hollow fiber supported zeolitic imidazolate framework-8 (ZIF-8) membranes with high hydrogen permeability. J. Membr. Sci. 2012, 421–422, 292–298. [Google Scholar] [CrossRef]
- Pimentel, B.R.; Parulkar, A.; Zhou, E.K.; Brunelli, N.A.; Lively, R.P. Zeolitic imidazolate frameworks: Next-generation materials for energy-efficient gas separations. ChemSusChem 2014, 7, 3202–3240. [Google Scholar] [CrossRef]
- Arami-Niya, A.; Birkett, G.; Zhu, Z.; Rufford, T.E. Gate opening effect of zeolitic imidazolate framework ZIF-7 for adsorption of CH4 and CO2 from N2. J. Mater. Chem. A 2017, 5, 21389–21399. [Google Scholar] [CrossRef]
- Karger, J.; Ruthven, D.M. Diffusion in nanoporous materials: Fundamental principles, insights and challenges. New J. Chem. 2016, 40, 4027–4048. [Google Scholar] [CrossRef]
- Avci, C.; Ariñez-Soriano, J.; Carné-Sánchez, A.; Guillerm, C.; Carbonell, C.; Imaz, I.; Maspoch, D. Post-synthetic anisotropic wet-chemical etching of colloidal sodalite ZIF crystals. Angew. Chem. Int. Ed. 2015, 54, 14417–14421. [Google Scholar] [CrossRef]
- Li, Q.; Guo, J.N.; Zhu, H.; Yan, F. Space-confined synthesis of ZIF-67 nanoparticles in hollow carbon nanospheres for CO2 adsorption. Small 2019, 15, 1804874. [Google Scholar] [CrossRef]
- Zhou, Z.M.; Wu, C.; Zhang, B.Q. ZIF-67 membranes synthesized on α-Al2O3-plate-supported cobalt nanosheets with amine modification for enhanced H2/CO2 permselectivity. Ind. Eng. Chem. Res. 2020, 59, 3182–3188. [Google Scholar] [CrossRef]
- Thompson, J.A.; Blad, C.R.; Brunelli, N.A.; Lydon, M.E.; Lively, R.P.; Jones, C.W.; Nair, S. Hybrid zeolitic imidazolate frameworks: Controlling framework porosity and functionality by mixed-linker synthesis. Chem. Mater. 2012, 24, 1930–1936. [Google Scholar] [CrossRef]
- Kahr, J.; Mowat, J.P.S.; Slawin, A.M.Z.; Morris, R.E.; Fairen-Jimenez, D.; Wright, P.A. Synthetic control of framework zinc purinate crystallisation and properties of a large pore, decorated, mixed-linker RHO-type ZIF. Chem. Commun. 2012, 48, 6690–6692. [Google Scholar] [CrossRef] [PubMed]
- Botas, J.A.; Calleja, G.; Sánchez-Sánchez, M.; Orcajo, M.G. Cobalt doping of the MOF-5 framework and its effect on gas-adsorption properties. Langmuir 2010, 26, 5300–5303. [Google Scholar] [CrossRef]
- Zhang, X.R.; Zhang, T.; Wang, Y.H.; Li, J.P.; Liu, C.C.; Li, N.W.; Liao, J.Y. Mixed-matrix membranes based on Zn/Ni-ZIF-8-PEBA for high performance CO2 separation. J. Membr. Sci. 2018, 560, 38–46. [Google Scholar] [CrossRef]
- Fan, Y.F.; Huiya Yu, H.Y.; Xu, S.; Shen, Q.C.; Ye, H.M.; Li, N.W. Zn(II)-modified imidazole containing polyimide/ZIF-8 mixed matrix membranes for gas separations. J. Membr. Sci. 2020, 597, 117775–117785. [Google Scholar] [CrossRef]
- McCarthy, M.C.; Varela-Guerrero, V.; Barnett, G.V.; Jeong, H.-K. Synthesis of zeolitic imidazolate framework films and membranes with controlled microstructures. Langmuir 2010, 26, 14636–14641. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, E.; Khan, E.A.; Lai, Z. Synthesis and characterization of ZIF-69 membranes and separation for CO2/CO mixture. J. Membr. Sci. 2010, 353, 36–40. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Xian, S.K.; Xia, Q.B.; Wang, H.H.; Zhong, L.; Li, J. Enhancement of CO2 Adsorption and CO2/N2 Selectivity on ZIF-8 via Postsynthetic Modification. AIChE J. 2013, 59, 2195–2206. [Google Scholar] [CrossRef]
- Liu, B.; Smit, B. Molecular Simulation Studies of Separation of CO2/N2, CO2/CH4, and CH4/N2 by ZIFs. J. Phys. Chem. C 2010, 114, 8515–8522. [Google Scholar] [CrossRef]
- Jang, E.H.; Kim, E.J.; Kim, H.J.; Lee, T.H.; Yeom, H.J.; Kim, Y.K.; Choi, J.K. Formation of ZIF-8 membranes inside porous supports for improving both their H2/CO2 separation performance and thermal/mechanical stability. J. Membr. Sci. 2017, 540, 430–439. [Google Scholar] [CrossRef]
- Xu, X.L.; Zhao, X.X.; Sun, L.B.; Liu, X.Q. Adsorption separation of carbon dioxide, methane, and nitrogen on Hb and Na-exchanged b-zeolite. J. Nat. Gas Chem. 2008, 17, 391–396. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Ma, X.L.; Li, Z.; Lin, Y.S. Synthesis, characterization and gas transport properties of MOF-5 membranes. J. Membr. Sci. 2011, 382, 82–90. [Google Scholar] [CrossRef]
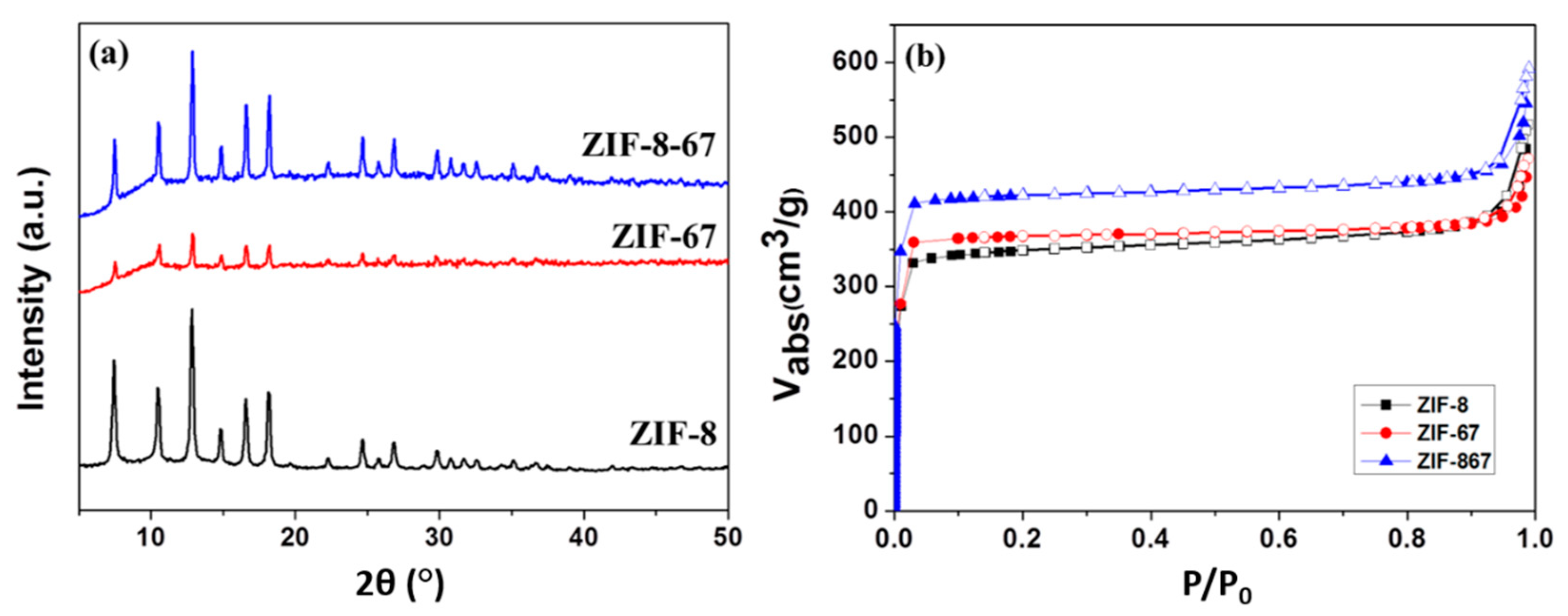

| Material | BET Surface Area (m2 g−1) | Micropore Volume (cm3 g−1) |
|---|---|---|
| ZIF-8 | 1048.33 | 0.485 |
| ZIF-67 | 1067.82 | 0.542 |
| ZIF-8-67 | 1260.40 | 0.616 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, P.-H.; Lee, Y.-T.; Peng, C.-H. Synthesis and Characterization of Hybrid Metal Zeolitic Imidazolate Framework Membrane for Efficient H2/CO2 Gas Separation. Materials 2020, 13, 5009. https://doi.org/10.3390/ma13215009
Chang P-H, Lee Y-T, Peng C-H. Synthesis and Characterization of Hybrid Metal Zeolitic Imidazolate Framework Membrane for Efficient H2/CO2 Gas Separation. Materials. 2020; 13(21):5009. https://doi.org/10.3390/ma13215009
Chicago/Turabian StyleChang, Po-Hsueh, Yuan-Tse Lee, and Cheng-Hsiung Peng. 2020. "Synthesis and Characterization of Hybrid Metal Zeolitic Imidazolate Framework Membrane for Efficient H2/CO2 Gas Separation" Materials 13, no. 21: 5009. https://doi.org/10.3390/ma13215009
APA StyleChang, P.-H., Lee, Y.-T., & Peng, C.-H. (2020). Synthesis and Characterization of Hybrid Metal Zeolitic Imidazolate Framework Membrane for Efficient H2/CO2 Gas Separation. Materials, 13(21), 5009. https://doi.org/10.3390/ma13215009




