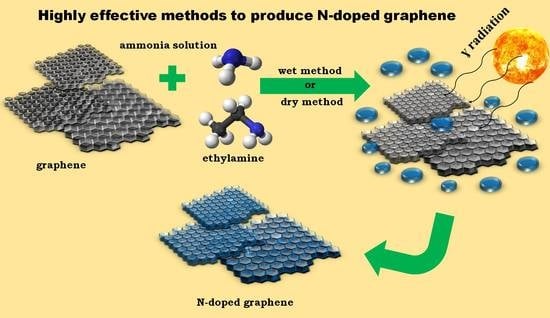
Highly Effective Methods of Obtaining N-Doped Graphene by Gamma Irradiation
Abstract
1. Introduction
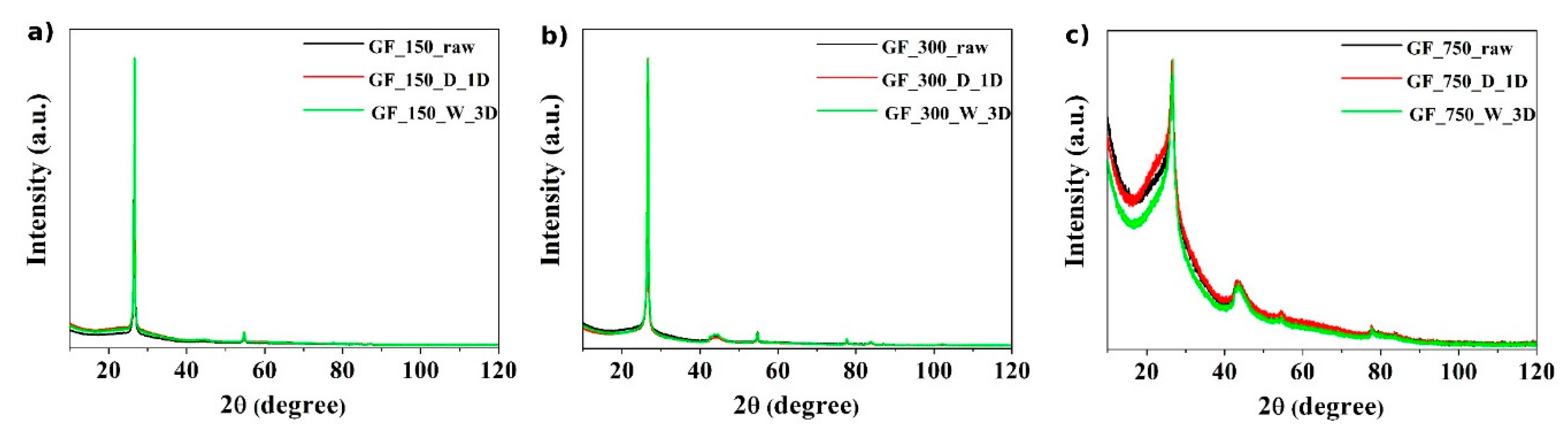
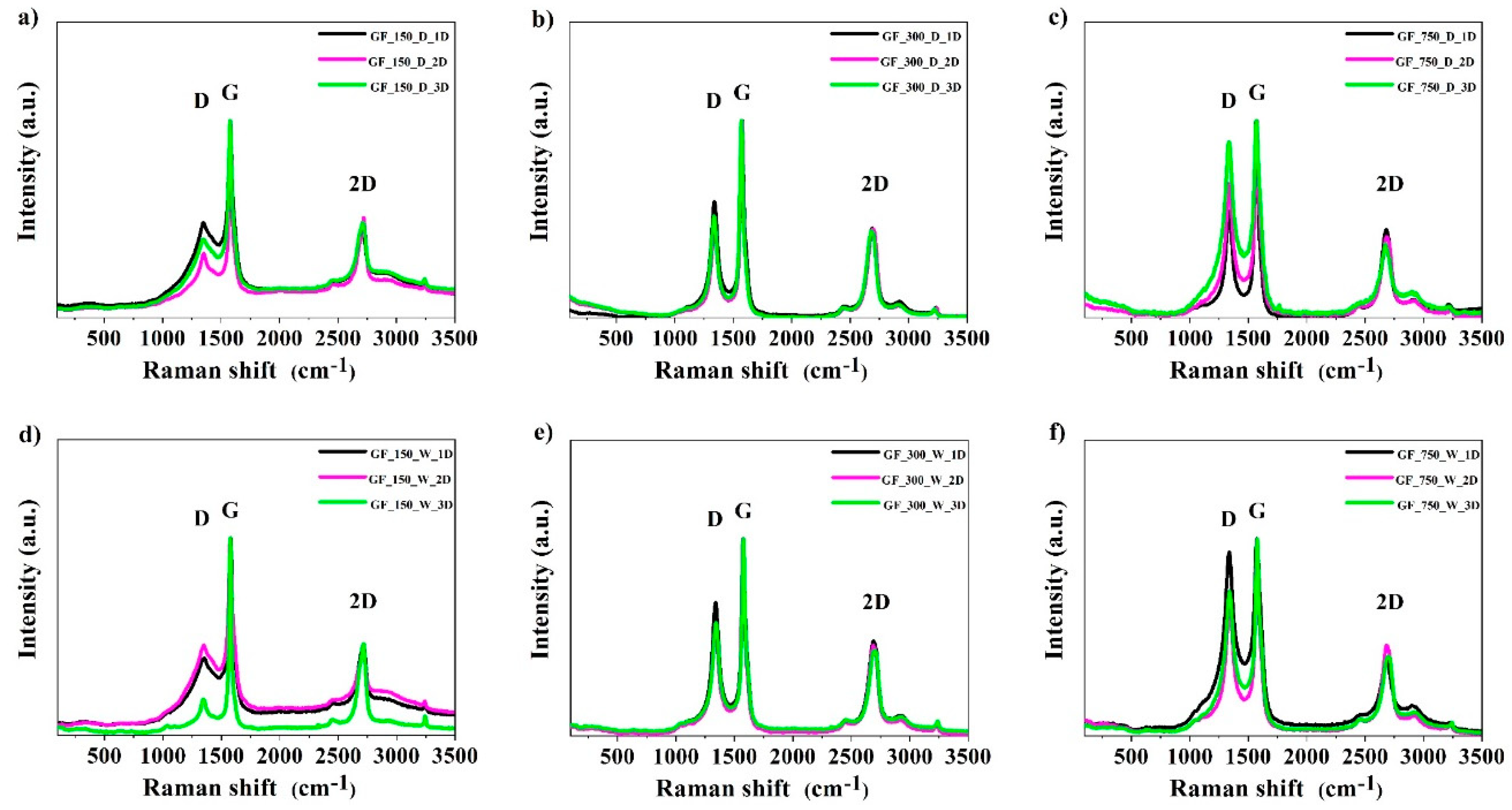
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wolf, E.L. Graphene. A New Paradigm in Condensed Matter and Device Physics; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- McNaught, A.D.; Wilkinson, A.; IUPAC. Compendium of Chemical Terminology; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar] [CrossRef]
- Guirguis, A.; Maina, J.W.; Zhang, X.; Henderson, L.C.; Kong, L.; Shon, H.; Dumee, L.F. Applications of nano-porous graphene materials-critical review on performance and challenges. Mater. Horiz. 2020, 7, 1218–1245. [Google Scholar] [CrossRef]
- Kamedulski, P.; Ilnicka, A.; Lukaszewicz, J.P. Selected Aspects of Graphene Exfoliation as an Introductory Step Towards 3D Structuring of Graphene Nano-Sheets. Curr. Graphene Sci. 2018, 2, 106–117. [Google Scholar] [CrossRef]
- Ilnicka, A.; Skorupska, M.; Kamedulski, P.; Lukaszewicz, J.P. Electro-Exfoliation of Graphite to Graphene in an Aqueous Solution of Inorganic Salt and the Stabilization of Its Sponge Structure with Poly (Furfuryl Alcohol). Nanomaterials 2019, 9, 971. [Google Scholar] [CrossRef] [PubMed]
- Hensleigh, R.M.; Cui, H.; Oakdale, J.S.; Ye, J.C.; Campbell, P.G.; Duoss, E.B.; Spadaccini, C.M.; Zheng, X.; Worsley, M.A. Additive manufacturing of complex micro-architected graphene aerogels. Mater. Horiz. 2018, 5, 1035–1041. [Google Scholar] [CrossRef]
- Kamedulski, P.; Ilnicka, A.; Lukaszewicz, J.P.; Skorupska, M. Highly effective three-dimensional functionalization of graphite to graphene by wet chemical exfoliation methods. Adsorption 2019, 25, 631–638. [Google Scholar] [CrossRef]
- Granzier-Nakajima, T.; Fujisawa, K.; Anil, V.; Terrones, M.; Yeh, Y.-T. Controlling Nitrogen Doping in Graphene with Atomic Precision: Synthesis and Characterization. Nanomaterials 2019, 9, 425. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, M.; Sharma, V.K. Nitrogen-doped graphene and graphene quantum dots: A review onsynthesis and applications in energy, sensors and environment. Adv. Colloid Interface Sci. 2018, 259, 44–64. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Wang, T.; Jiang, X.; Li, J.; Zhang, T.; Xi, P.; Gao, D.; Xue, D. N+-ion irradiation engineering towards the efficient oxygen evolution reaction on NiO nanosheet arrays. J. Mater. Chem. A 2019, 7, 4729–4733. [Google Scholar] [CrossRef]
- Liu, P.; Ran, J.; Xia, B.; Xi, S.; Gao, D.; Wang, J. Bifunctional Oxygen Electrocatalyst of Mesoporous Ni/NiO Nanosheets for Flexible Rechargeable Zn-Air Batteries. Nano Micro Lett. 2020, 12, 1–12. [Google Scholar] [CrossRef]
- Xia, B.; Liao, Z.; Liu, Y.; Chi, X.; Xiao, W.; Ding, J.; Wang, T.; Gao, D.; Xue, D. Realization of “single-atom ferromagnetism” in graphene by Cu-N4 moieties anchoring. Appl. Phys. Lett. 2020, 116, 113102. [Google Scholar] [CrossRef]
- Kakaei, K.; Ghadimi, G. A green method for Nitrogen-doped graphene and its application for oxygen reduction reaction in alkaline media. Mater. Technol. 2020. [Google Scholar] [CrossRef]
- Sahoo, M.K.; Gogoi, P.; Rajeshkhanna, G.; Chilukuri, S.V.; Rao, G.R. Significance of optimal N-doping in mesoporous carbon framework to achieve high specific capacitance. Appl. Surf. Sci. 2017, 418, 40–48. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, X.; Mao, S.; Bo, Z.; Kim, H.; Cui, S.; Lu, G.; Feng, X.; Chen, J. Crumpled nitrogen-doped graphene nanosheets with ultrahigh pore volume for high-performance supercapacitor. Adv. Mater. 2012, 24, 5610–5616. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, S.; Engelhard, M.H.; Li, G.; Shao, G.; Wang, Y.; Liu, J.; Aksay, I.A.; Lin, Y. Nitrogen-doped graphene and its electrochemical applications. J. Mater. Chem. 2010, 20, 7491–7496. [Google Scholar] [CrossRef]
- Bourquard, F.; Bleu, Y.; Loir, A.-S.; Caja-Munoz, B.; Avila, J.; Asensio, M.-C.; Raimondi, G.; Shokouhi, M.; Rassas, I.; Farre, C.; et al. Electroanalytical Performance of Nitrogen-Doped Graphene Films Processed in One Step by Pulsed Laser Deposition Directly Coupled with Thermal Annealing. Materials 2019, 12, 666. [Google Scholar] [CrossRef]
- Ilnicka, A.; Kamedulski, P.; Aly, H.M.; Lukaszewicz, J.P. Manufacture of activated carbons using Egyptian wood resources and its application in oligothiophene dye adsorption. Arab. J. Chem. 2020, 13, 5284–5291. [Google Scholar] [CrossRef]
- Choi, W.H.; Choi, M.J.; Bang, J.H. Nitrogen-Doped Carbon Nanocoil Array Integrated on Carbon Nanofiber Paper for Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2015, 7, 19370–19381. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, D.; Sun, Z.; Deng, Y.; Xia, Y.; Zhao, D. A Controllable Synthesis of Rich Nitrogen-Doped Ordered Mesoporous Carbon for CO2 Capture and Supercapacitors. Adv. Funct. Mater. 2013, 23, 2322–2328. [Google Scholar] [CrossRef]
- Wu, K.; Liu, Q. Nitrogen-doped mesoporous carbons for high performance supercapacitors. Appl. Surf. Sci. 2016, 379, 132–139. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Xu, H.; Ma, L. Nitrogen-doped graphene: Synthesis, characterizations and energy applications. J. Energy Chem. 2018, 27, 146–160. [Google Scholar] [CrossRef]
- Yadav, R.; Dixit, C.K. Synthesis, characterization and prospective applications of nitrogen-doped graphene: A short review. J. Sci. Adv. Mater. Devices 2017, 2, 141–149. [Google Scholar] [CrossRef]
- Zhuang, S.; Nunna, B.B.; Mandal, D.; Lee, E.S. A review of nitrogen-doped graphene catalysts for proton exchange membrane fuel cells-synthesis, characterization, and improvement. Nano Struct. Nano Objects 2018, 15, 140–152. [Google Scholar] [CrossRef]
- Luo, Z.; Lim, S.; Tian, Z.; Shang, J.; Lai, L.; MacDonald, B.; Fu, C.; Shen, Z.; Yu, T.; Lin, J. Pyridinic N doped graphene: Synthesis, electronic structure, and electrocatalytic property. J. Mater. Chem. 2011, 21, 8038–8044. [Google Scholar] [CrossRef]
- Lu, Y.-F.; Lo, S.-T.; Lin, J.-C.; Zhang, W.; Lu, J.-Y.; Liu, F.-H.; Tseng, C.-M.; Lee, Y.-H.; Liang, C.-T.; Li, L.-J. Nitrogen-Doped Graphene Sheets Grown by Chemical Vapor Deposition: Synthesis and Influence of Nitrogen Impurities on Carrier Transport. ACS Nano 2013, 7, 6522–6532. [Google Scholar] [CrossRef]
- Panchakarla, L.S.; Subrahmanyam, K.S.; Saha, S.K.; Govindaraj, A.; Krishnamurthy, H.R.; Waghmare, U.V.; Rao, C.N.R. Synthesis, Structure, and Properties of Boron- and Nitrogen-Doped Graphene. Adv. Mater. 2009, 21, 4726–4730. [Google Scholar] [CrossRef]
- Lin, Z.; Waller, G.; Liu, Y.; Liu, M.; Wong, C.-P. Facile synthesis of nitrogen-doped graphene via pyrolysis of graphene oxide and urea and its electrocatalytic activity toward oxygen reduction reaction. Adv. Energy Mater. 2012, 2, 884–888. [Google Scholar] [CrossRef]
- Sheng, Z.-H.; Shao, L.; Chen, J.-J.; Bao, W.-J.; Wang, F.-B.; Xia, X.-H. Catalyst-Free Synthesis of Nitrogen-Doped Graphene via Thermal Annealing Graphite Oxide with Melamine and Its Excellent Electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, B.; Rao, Z.; Zhang, B.; Gong, J.R. Strong two-photon-induced fluorescence from photostable, biocompatible nitrogen-doped graphene quantum dots for cellular and deep-tissue imaging. Nano Lett. 2013, 13, 2436–2441. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Robinson, J.T.; Sanchez, H.; Diankov, G.; Dai, H. Simultaneous Nitrogen Doping and Reduction of Graphene Oxide. J. Am. Chem. Soc. 2009, 131, 15939–15944. [Google Scholar] [CrossRef]
- Li, X.; Geng, D.; Zhang, Y.; Meng, X.; Li, R.; Sun, X. Superior cycle stability of nitrogen-doped graphene nanosheets as anodes for lithium ion batteries. Electrochem. Commun. 2011, 13, 822–825. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, Y.; Matson, D.W.; Li, J.; Lin, Y. Nitrogen-Doped Graphene and Its Application in Electrochemical Biosensing. ACS Nano 2010, 4, 1790–1798. [Google Scholar] [CrossRef]
- Ju, M.J.; Jeon, I.Y.; Kim, J.C.; Lim, K.; Choi, H.J.; Jung, S.M.; Choi, I.T.; Eom, Y.K.; Kwon, Y.J.; Ko, J. Graphene Nanoplatelets Doped with N at its Edges as Metal-Free Cathodes for Organic Dye-Sensitized Solar Cells. Adv. Mater. 2014, 26, 3055–3062. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Nunna, B.B.; Boscoboinik, J.A.; Lee, E.S. Nitrogen-doped graphene catalysts: High energy wet ball milling synthesis and characterizations of functional groups and particle size variation with time and speed. Int. J. Energy Res. 2017, 41, 2535–2554. [Google Scholar] [CrossRef]
- Aujara, K.M.; Chieng, B.W.; Ibrahim, N.A.; Zainuddin, N.; Ratnam, C.T. Gamma-Irradiation Induced Functionalization of Graphene Oxide with Organosilanes. Int. J. Mol. Sci. 2019, 20, 1910. [Google Scholar] [CrossRef]
- Shlimak, I.; Zion, E.; Butenko, A.; Kaganovskii, Y.; Richter, V.; Sharoni, A.; Kogan, E.; Kaveh, M. Irradiation-induced metal-insulator transition in monolayer graphene. FlatChem 2019, 14, 100084. [Google Scholar] [CrossRef]
- Velo-Gala, I.; López-Peñalver, J.J.; Sánchez-Polo, M.; Rivera-Utrilla, J. Surface modifications of activated carbon by gamma irradiation. Carbon 2014, 67, 236–249. [Google Scholar] [CrossRef]
- Ansón-Casaos, A.; Puértolas, J.A.; Pascual, F.J.; Hernández-Ferrer, J.; Castell, P.; Benito, A.M.; Maser, W.K.; Martínez, M.T. The effect of gamma-irradiation on few-layered graphene materials. Appl. Surf. Sci. 2014, 301, 264–272. [Google Scholar] [CrossRef]
- Vega, E.; Sánchez-Polo, M.; Gonzalez-Olmos, R.; Martin, M.J. Adsorption of odorous sulfur compounds onto activated carbons modified by gamma irradiation. J. Colloid Interface Sci. 2015, 457, 78–85. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Le, H.D.; Nguyen, V.C.; Ngo, T.T.T.; Le, D.Q.; Nguyen, X.N.; Phan, N.M. Synthesis of multi-layer graphene films on copper tape by atmospheric pressure chemical vapor deposition method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 035012. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database. J. Chem. Educ. 1993, 70, A25. [Google Scholar]
- Rouxhet, P.; Genet, M. XPS analysis of bio-organic systems. Surf. Interface Anal. 2011, 43, 1453–1470. [Google Scholar] [CrossRef]
- Briggs, D. Surface Analysis of Polymers by XPS and Static SIMS; Cambridge University Press: New York, NY, USA, 2005. [Google Scholar]
- Genet, M.J.; Dupont-Gillain, C.C.; Rouxhet, P.G. XPS Analysis of Biosystems and Biomaterials. In Medical Applications of Colloids; Matijevic, E., Ed.; Springer Science Business Media LLC: New York, NY, USA, 2008. [Google Scholar]
- Hulicova-Jurcakova, D.; Seredych, M.; Lu, G.Q.; Bandosz, T.J. Combined Effect of Nitrogen- and Oxygen-Containing Functional Groups of Microporous Activated Carbon on its Electrochemical Performance in Supercapacitors. Adv. Funct. Mater. 2009, 19, 438–447. [Google Scholar] [CrossRef]
- Park, G.S.; Lee, J.-S.; Kim, S.T.; Park, S.; Cho, J. Porous nitrogen doped carbon fiber with churros morphology derived from electrospun bicomponent polymer as highly efficient electrocatalyst for Zn-air batteries. J. Power Sources 2013, 243, 267–273. [Google Scholar] [CrossRef]
- Ilnicka, A.; Skorupska, M.; Romanowski, P.; Kamedulski, P.; Lukaszewicz, J.P. Improving the Performance of Zn-Air Batteries with N-Doped Electroexfoliated Graphene. Materials 2020, 13, 2115. [Google Scholar] [CrossRef] [PubMed]
- Khamsanga, S.; Nguyen, M.T.; Yonezawa, T.; Thamyongkit, P.; Pornprasertsuk, R.; Pattananuwat, P.; Tuantranont, A.; Siwamogsatham, S.; Kheawhom, S. MnO2 Heterostructure on Carbon Nanotubes as Cathode Material for Aqueous Zinc-Ion Batteries. Int. J. Mol. Sci. 2020, 21, 4689. [Google Scholar] [CrossRef] [PubMed]
- Kamedulski, P.; Kaczmarek-Kedziera, A.; Lukaszewicz, J.P. Influence of intermolecular interactions on the properties of carbon nanotubes. Bull. Mater. Sci. 2018, 41, 76. [Google Scholar] [CrossRef]
- Sliwak, A.; Diez, N.; Miniach, E.; Gryglewicz, G. Nitrogen-containing chitosan-based carbon as an electrode material for high-performance supercapacitors. J. Appl. Electrochem. 2016, 46, 667–677. [Google Scholar] [CrossRef]



| Method | Description | Drawbacks | Example Study |
|---|---|---|---|
| CVD | High temperature furnace up to 1000 °C, vacuum 1 Torr, catalyst, NH3 as nitrogen source, He as shielding gas | Complex instrumentation, very low yield | [27,28] |
| Arc Discharge | Electric arc discharge conditions, pyridine and ammonia as a nitrogen carrier | Complex instrumentation, difficult to control, very low yield | [29] |
| Pyrolysis | High temperature pyrolysis of a stolid mixture of GO—urea lattice, respectively | Limited yield, long time high temperature synthesis, application of GO instead of pure graphene | [30] |
| Heat treating | Heating to 800–1000 °C a solid mixture of GO-nitrogen source, neutral atmosphere, melamine as a potential nitrogen source | [31] | |
| Solvothermal | 200–300 °C, 4–5 h duration, dimethylformamide as a solvent and nitrogen source | Yield limited by the experimental vessel volume, use environmentally and health unfriendly reagents | [32] |
| Gas Annealing | High temperature of 500–1000 °C during electrical annealing of GO in nitrogen atmosphere, ammonia gas (NH3) as a nitrogen source | GO applied instead of pure graphene/graphite, low yield, a high temperature method | [33,34] |
| N2 Plasma Treatment | Nitrogen content controlled by the plasma strength and exposure time, example plasma generator parameters 40–200 W, 900 V DC bias, high vacuum 200 mTorr, 20–80 min treatment, graphene or GO as a key precursor, N2 and NH3 as nitrogen source | Sophisticated instrumentation and challenging synthesis conditions, low yield | [35] |
| Dry Ball Milling | Mechanochemical process, room temperature direct grinding of dry powdered graphite in the N2 or NH3 atmosphere, nitrogen content controllable by changing milling parameters | Unwanted insertion of impurities from the grinding setup, which must be removed by additional treatment, laboratory scale process | [36] |
| Nanoscale High Energy Wet Ball Milling | Mechanochemical process, room to 80 °C wet milling; gas, liquid and solid nitrogen carriers permittable, GO advised as carbon precursor | Complex manufacturing pathway including frequent rising, laboratory scale process | [37] |
| Experiment Description | Example Study |
|---|---|
| Gamma-ray radiation for altering of physicochemical properties of graphene oxide (GO) by insertion of aminosilanes | [38] |
| Metal-to-insulator transition of monolayered graphene | [39] |
| Changing activated carbon surface upon gamma irradiation in the presence of pure and contaminated water | [40] |
| The effect of gamma irradiation on the structure and composition of chemically synthesized few-layered graphene-based materials | [41] |
| Surface activation of commercial activated carbon induced by gamma radiation and the applicability of modified carbon for the removal of odors | [42] |
| Method | Sample | Elemental Analysis (wt.%) | |||
|---|---|---|---|---|---|
| N | C | H | Residuals (Oxygen) | ||
| GF_150_raw | 0.61 | 90.77 | 0.90 | 7.72 | |
| GF_300_raw | 0.34 | 98.04 | 0.47 | 1.15 | |
| GF_750_raw | 0.72 | 87.32 | 0.87 | 11.09 | |
| dry | GF_150_D_1D | 2.19 | 90.79 | 1.55 | 5.47 |
| GF_150_D_2D | 2.14 | 91.88 | 1.63 | 4.35 | |
| GF_150_D_3D | 2.09 | 94.92 | 1.83 | 1.16 | |
| GF_300_D_1D | 1.66 | 93.68 | 0.88 | 3.78 | |
| GF_300_D_2D | 1.66 | 92.44 | 0.85 | 5.05 | |
| GF_300_D_3D | 1.66 | 96.35 | 0.96 | 1.03 | |
| GF_750_D_1D | 4.19 | 83.95 | 1.64 | 10.22 | |
| GF_750_D_2D | 4.03 | 84.66 | 1.55 | 9.76 | |
| GF_750_D_3D | 4.01 | 83.42 | 1.71 | 10.86 | |
| wet | GF_150_W_1D | 0.62 | 90.52 | 1.15 | 7.71 |
| GF_150_W_2D | 1.29 | 90.77 | 1.36 | 6.58 | |
| GF_150_W_3D | 1.38 | 89.84 | 1.11 | 7.67 | |
| GF_300_W_1D | 0.77 | 91.85 | 0.68 | 6.70 | |
| GF_300_W_2D | 0.98 | 95.70 | 0.72 | 2.60 | |
| GF_300_W_3D | 1.17 | 96.13 | 0.54 | 2.16 | |
| GF_750_W_1D | 1.52 | 82.85 | 0.91 | 14.72 | |
| GF_750_W_2D | 1.98 | 84.12 | 1.51 | 12.39 | |
| GF_750_W_3D | 2.00 | 84.27 | 1.23 | 12.50 | |
| Element | C | O | N | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Binding Energy (eV) | 284.6 | 285.0 | 286.3 | 287.7 | 288.6 | 289.6 | 292.1 | % of Total | 532.0 | 533.3 | % of Total | 400.5 |
| Sample | Content (at.%) | Content (at.%) | Content (at.%) | |||||||||
| GF_150_D_1D | 52.8 | 23.9 | 7.4 | 2.8 | 1.2 | 5.1 | 3.4 | 96.6 | 1.0 | 1.4 | 2.4 | 1.0 |
| GF_150_W_3D | 60.8 | 13.9 | 6.5 | 2.7 | 0.3 | 6.7 | 4.8 | 95,7 | 1.6 | 1.5 | 3.1 | 1.2 |
| GF_300_D_1D | 45.5 | 23.2 | 9.8 | 3.8 | 2.0 | 6.7 | 4.5 | 95.5 | 1.6 | 1.5 | 3.1 | 1.4 |
| GF_300_W_3D | 54.1 | 17.3 | 7.8 | 3.5 | 0.1 | 8.0 | 5.3 | 96.1 | 1.0 | 1.5 | 2.5 | 1.4 |
| GF_750_D_1D | 28.3 | 34.5 | 10.8 | 4.8 | 3.2 | 5.6 | 3.7 | 90.9 | 2.8 | 2.5 | 5.3 | 3.8 |
| GF_750_W_3D | 52.8 | 12.4 | 9.3 | 3.7 | 0.8 | 8.9 | 4.3 | 92.2 | 2.0 | 3.0 | 5.0 | 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamedulski, P.; Truszkowski, S.; Lukaszewicz, J.P. Highly Effective Methods of Obtaining N-Doped Graphene by Gamma Irradiation. Materials 2020, 13, 4975. https://doi.org/10.3390/ma13214975
Kamedulski P, Truszkowski S, Lukaszewicz JP. Highly Effective Methods of Obtaining N-Doped Graphene by Gamma Irradiation. Materials. 2020; 13(21):4975. https://doi.org/10.3390/ma13214975
Chicago/Turabian StyleKamedulski, Piotr, Stanislaw Truszkowski, and Jerzy P. Lukaszewicz. 2020. "Highly Effective Methods of Obtaining N-Doped Graphene by Gamma Irradiation" Materials 13, no. 21: 4975. https://doi.org/10.3390/ma13214975
APA StyleKamedulski, P., Truszkowski, S., & Lukaszewicz, J. P. (2020). Highly Effective Methods of Obtaining N-Doped Graphene by Gamma Irradiation. Materials, 13(21), 4975. https://doi.org/10.3390/ma13214975