Analysis of γ′ Precipitates, Carbides and Nano-Borides in Heat-Treated Ni-Based Superalloy Using SEM, STEM-EDX, and HRSTEM
Abstract
:1. Introduction
2. Experimental Procedure
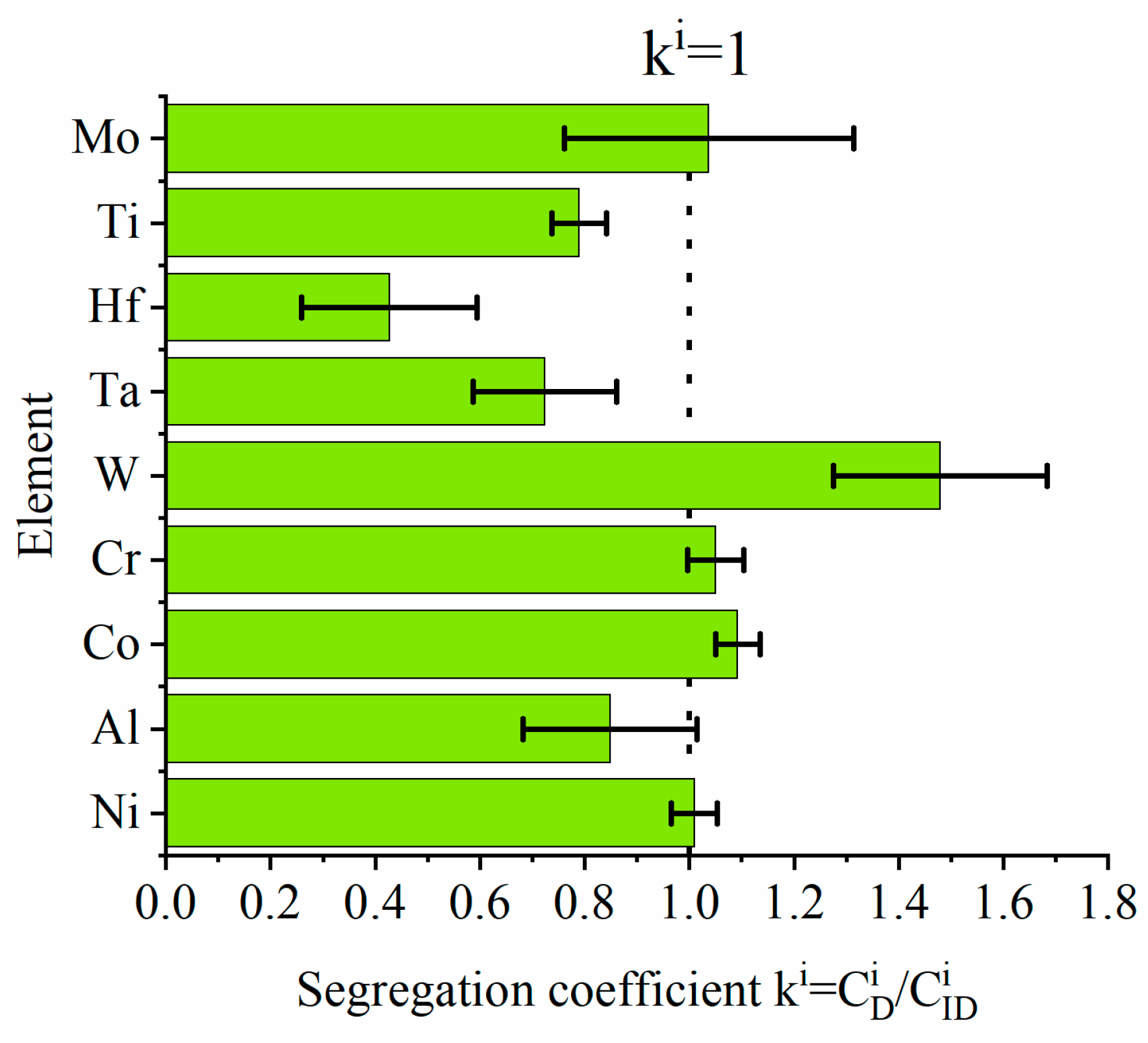
- —concentration of “i” alloying element in dendritic region, at%
- —concentration of “i” alloying element in interdendritic space, at%
- —total volume of the phase object per unit volume of the alloy, μm3/μm3
- —total field flat sections on the individual phase of the image per unit area, μm2/μm2
- —total field of flat sections on the individual i-phase, μm2
- A—total image area, μm2
3. Results and Discussion
3.1. Calculation of the Alloying Elements Solubility in Nickel by Thermo-Calc
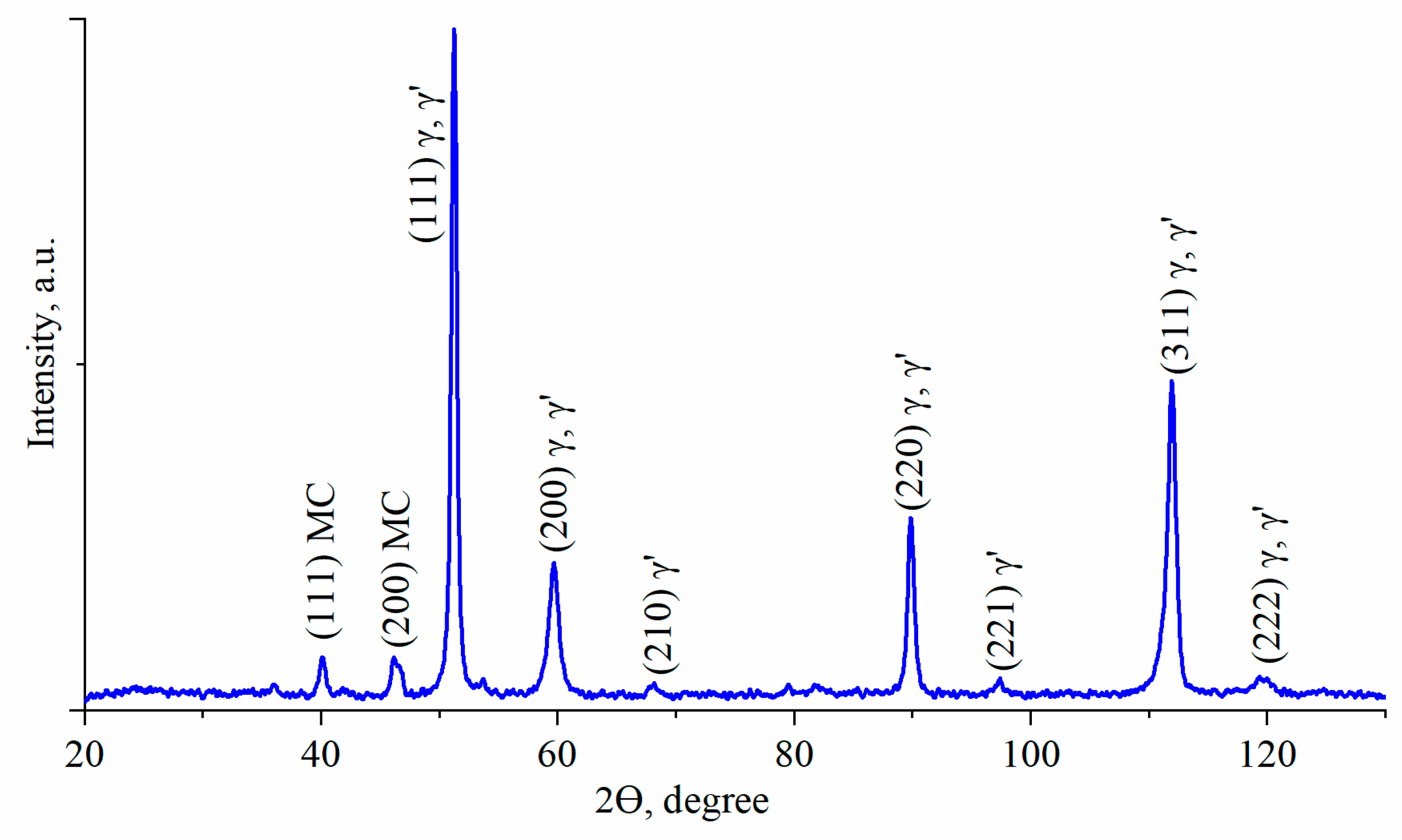
3.2. Characterization of Phase Compositions and γ/γ′ Lattice Misfit by XRD
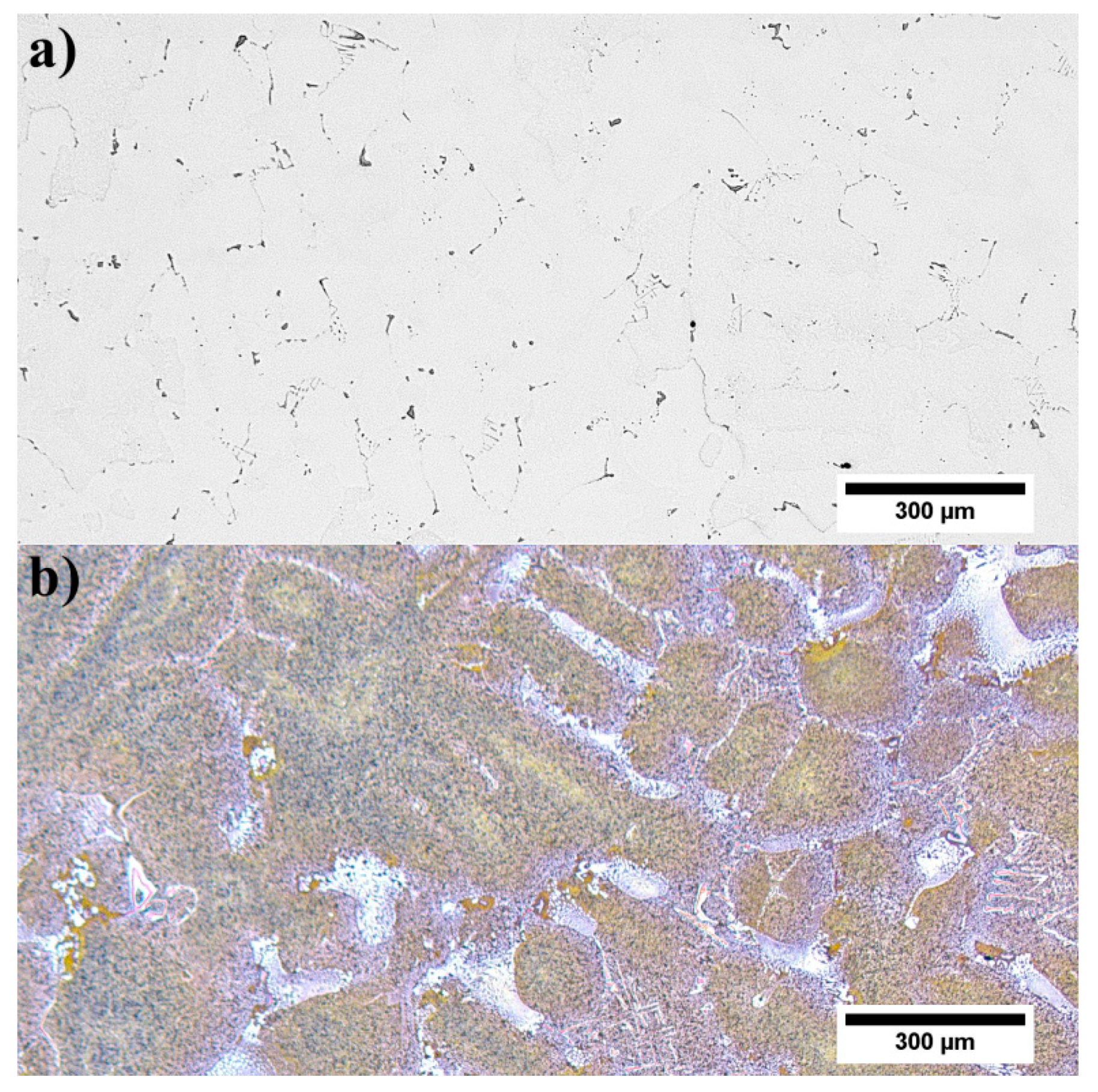
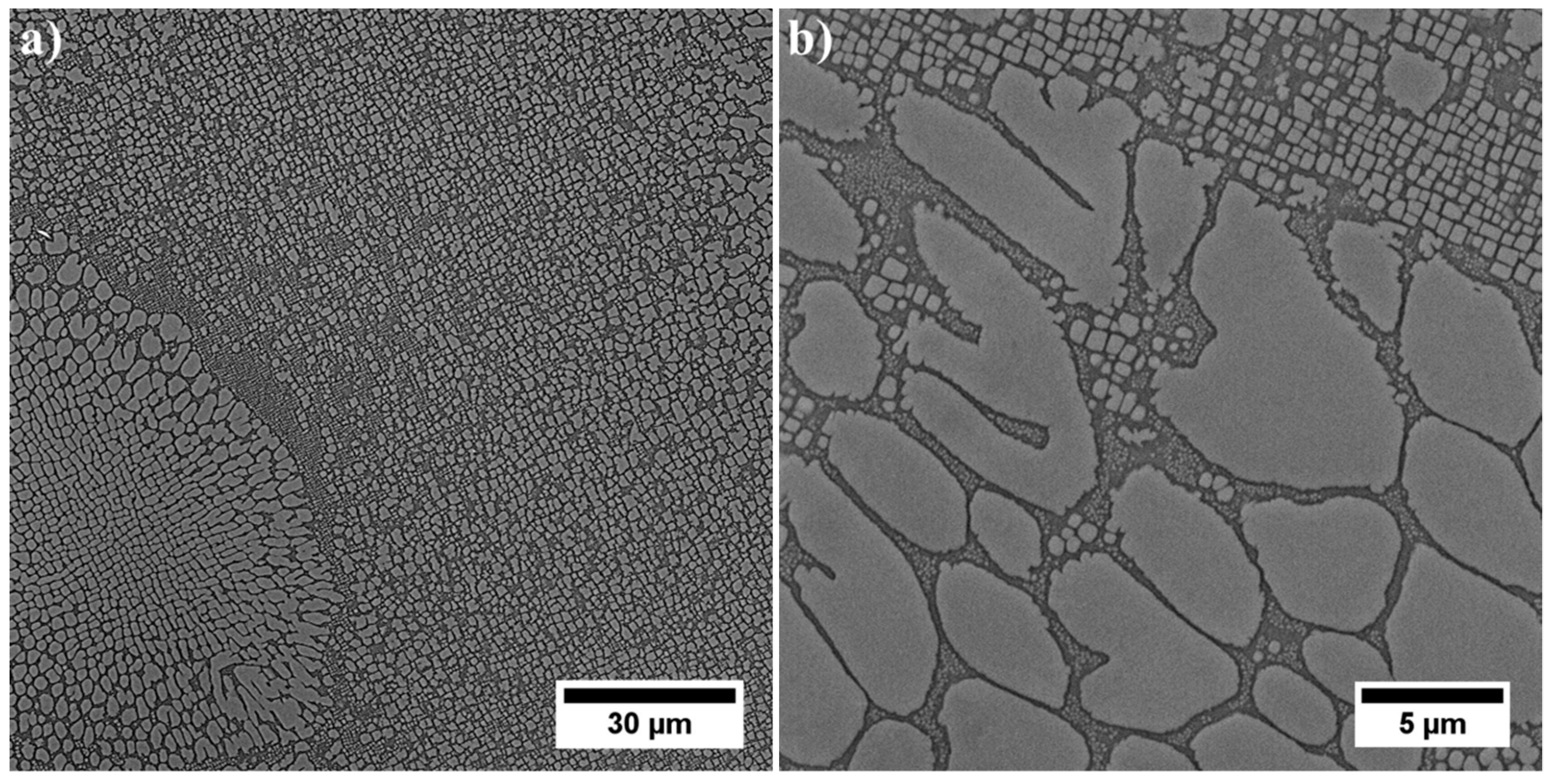
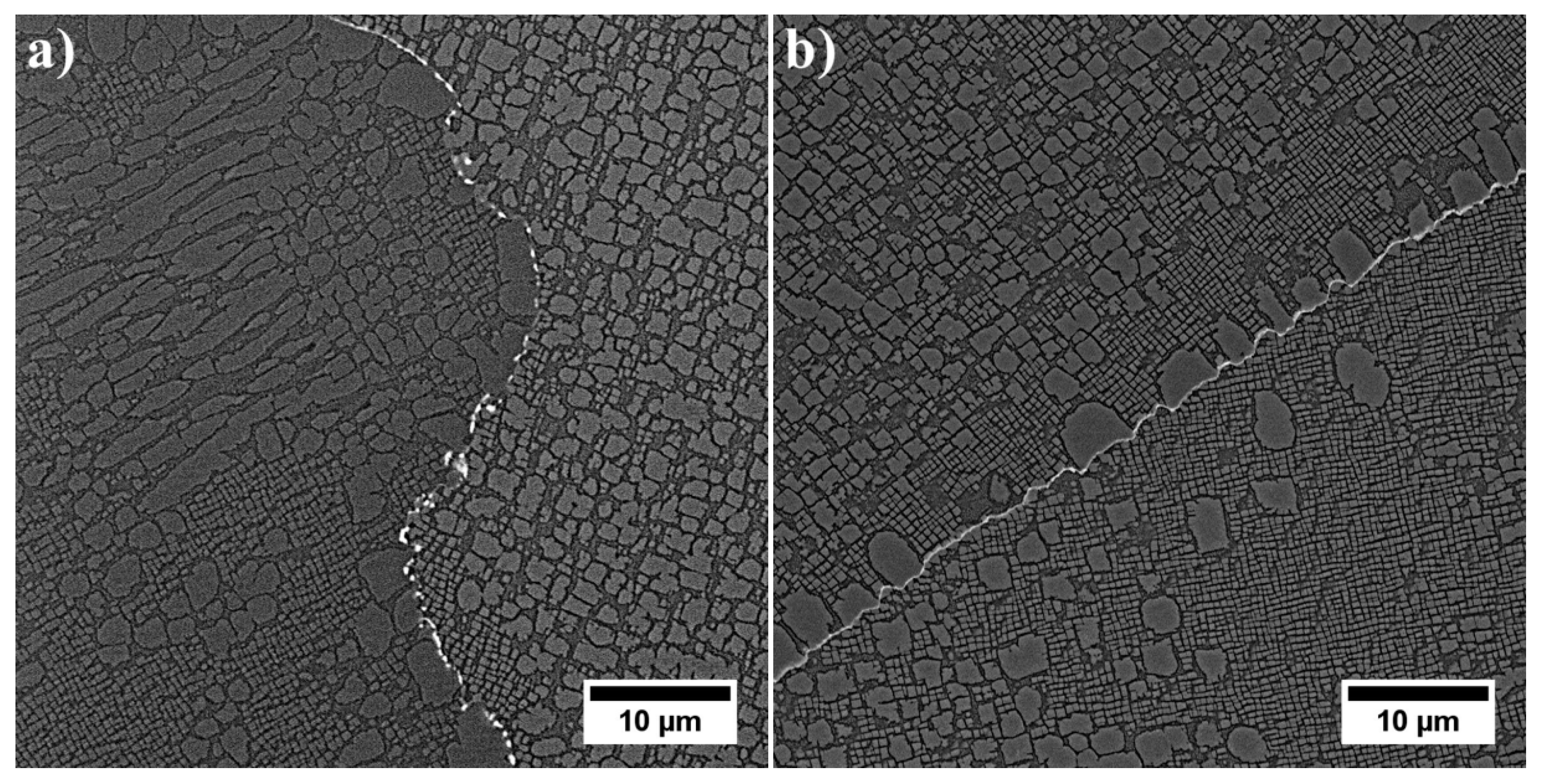
3.3. Microstructure of René 108 Superalloy by LM and SEM
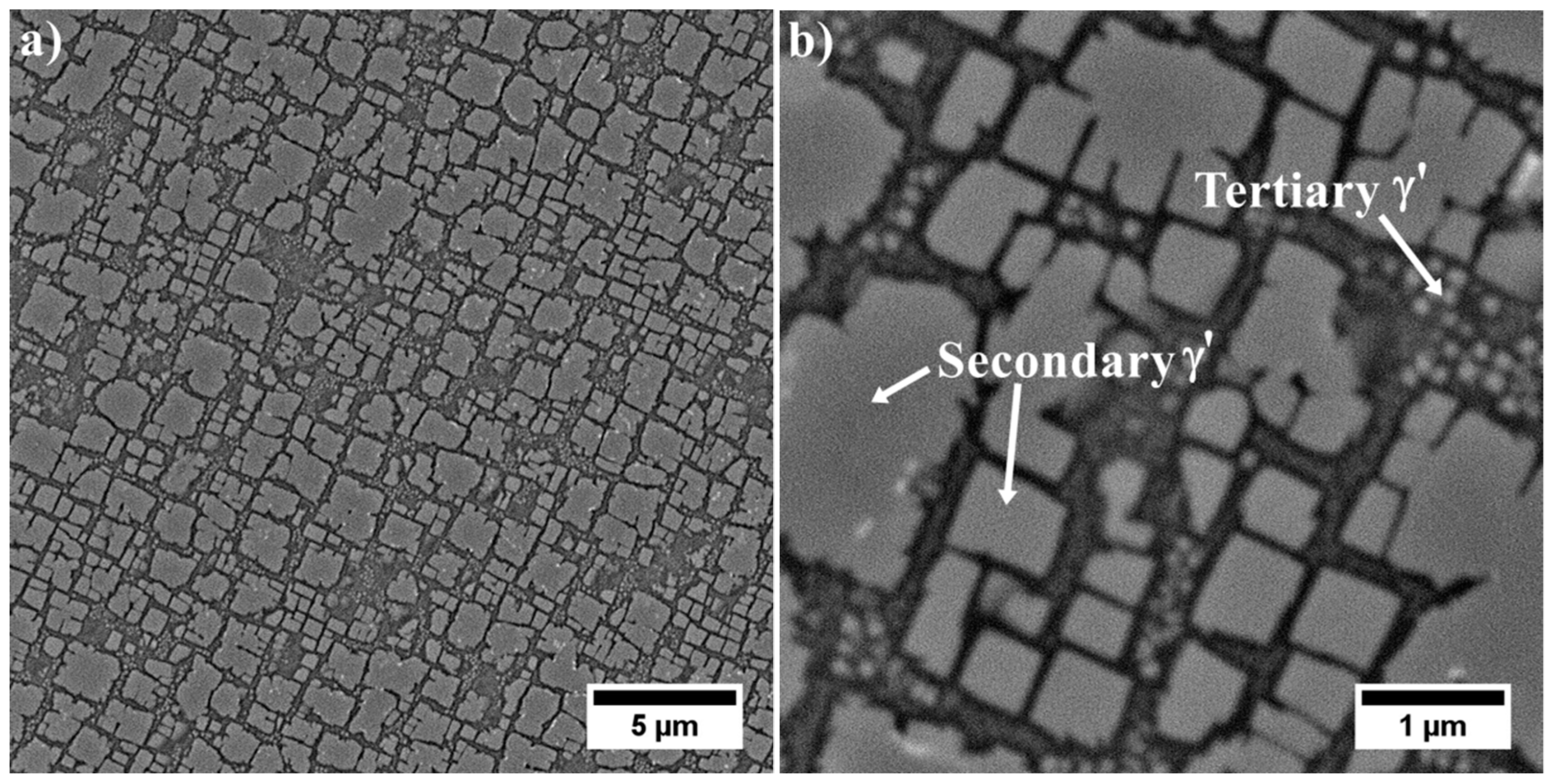
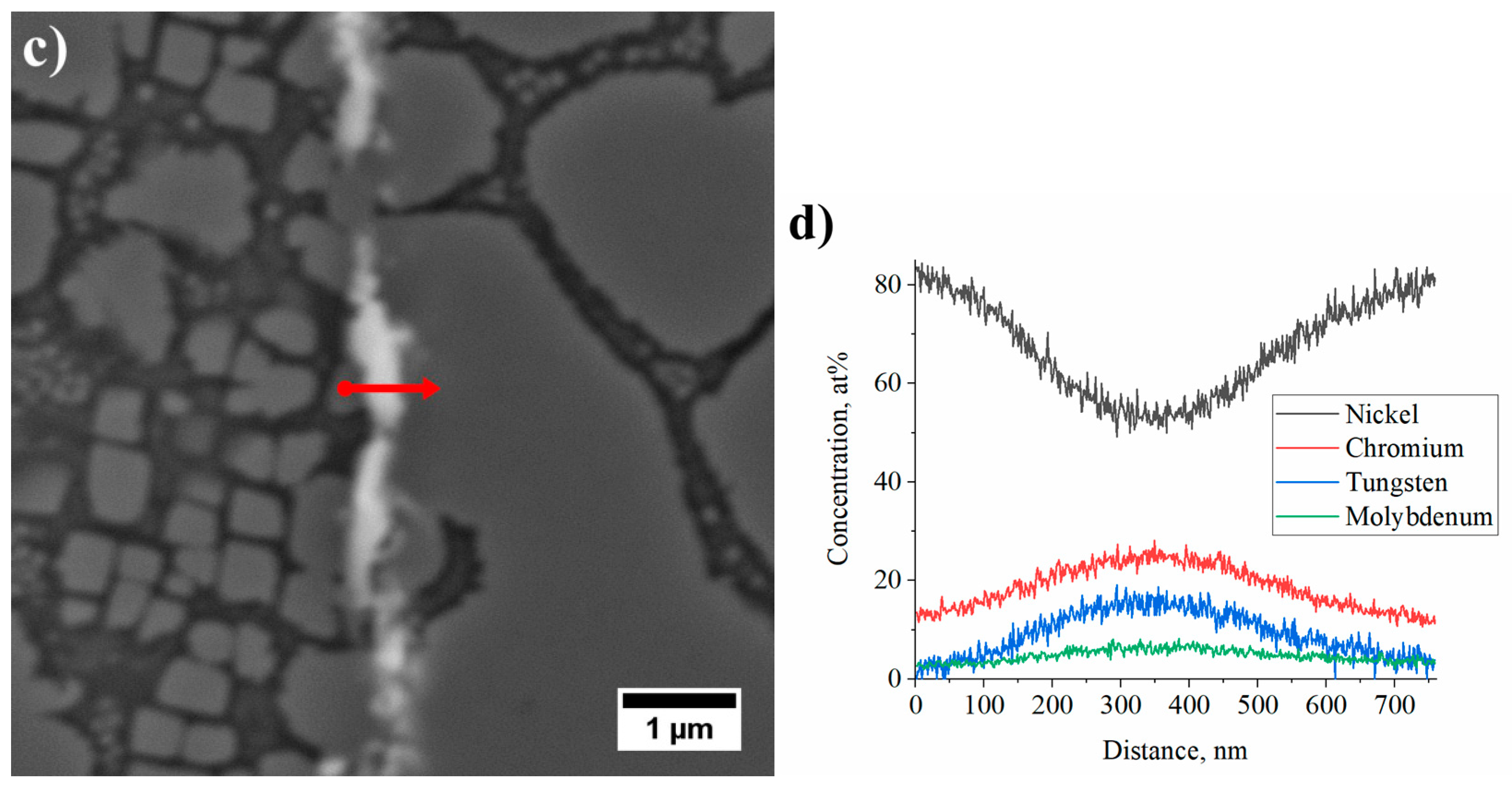
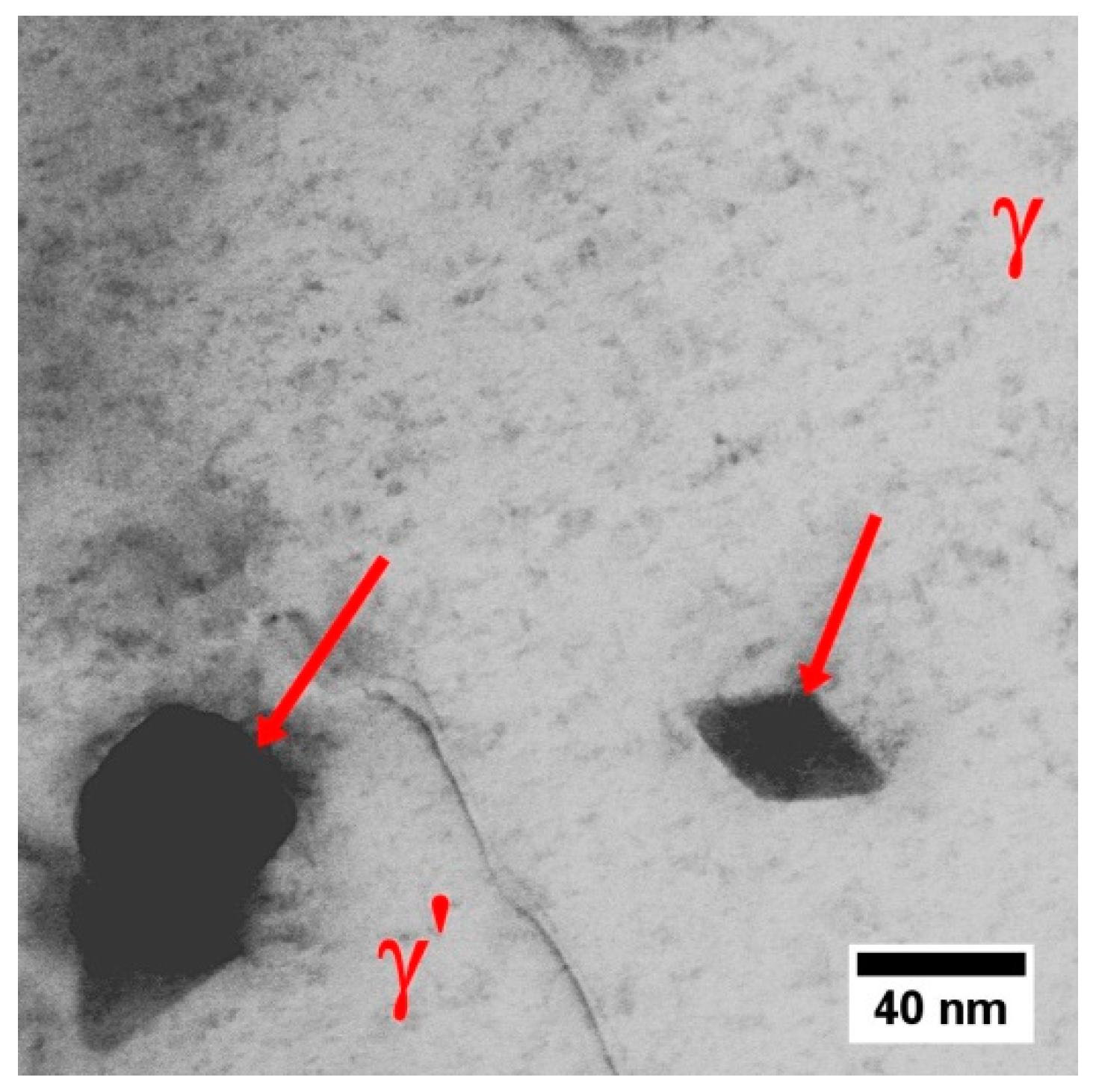
3.4. Characterization of Strengthening Phases by STEM and HRSTEM
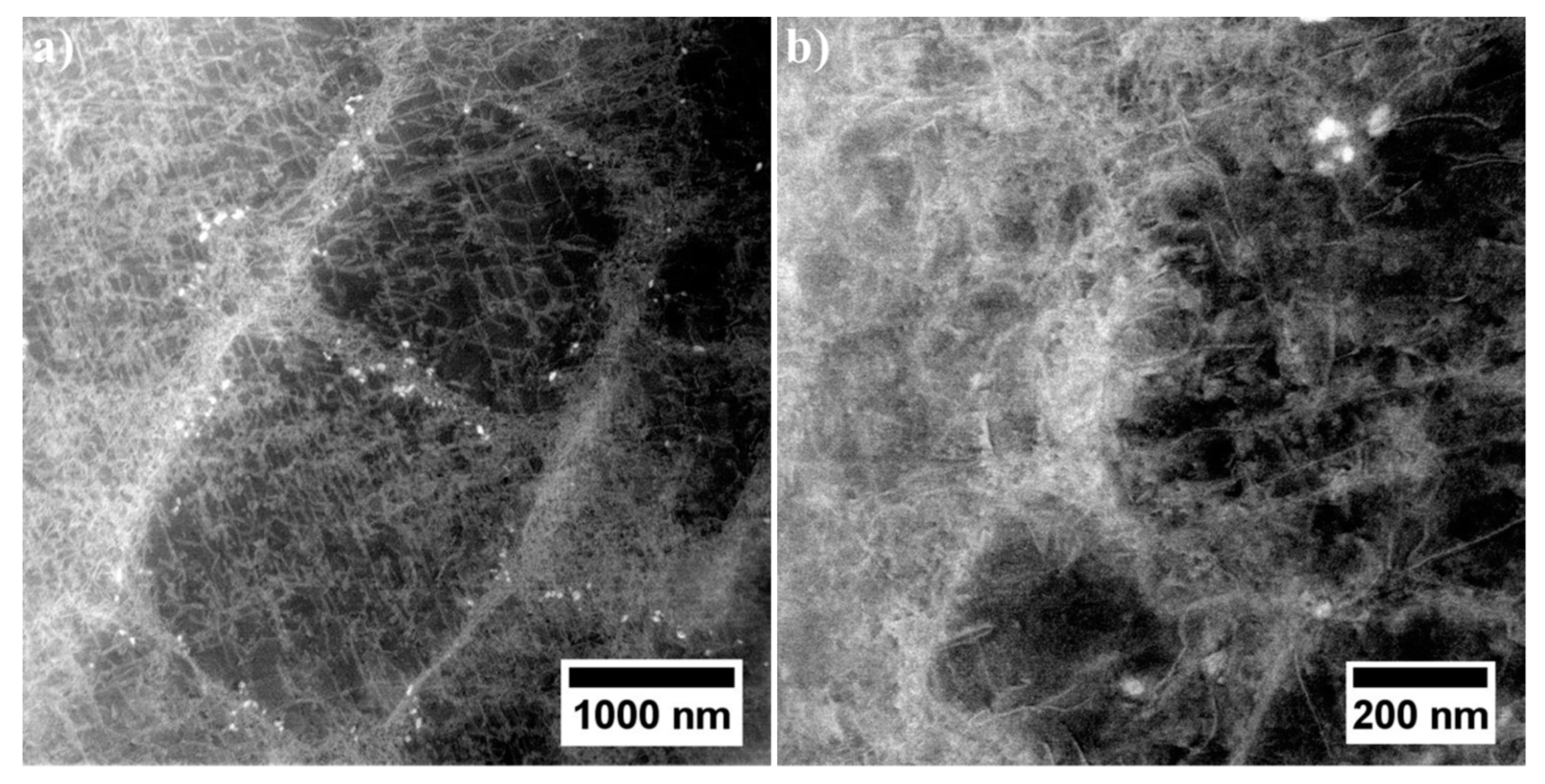
3.4.1. Dendritic Regions
3.4.2. Interdendritic Spaces
4. Conclusions
- The FCC γ matrix is strengthened mainly by coherent precipitates characterised by ordered L12 crystal structure. The mean misfit coefficient between matrix and precipitates is δ = +0.6%.
- Dendritic structure with significant segregation of alloying elements and microstructural constituents is observed.
- The mean volume fraction of MC carbides is around 0.8% (LM), while γ′ precipitates in dendritic regions around 54.99% (SEM-BSE).
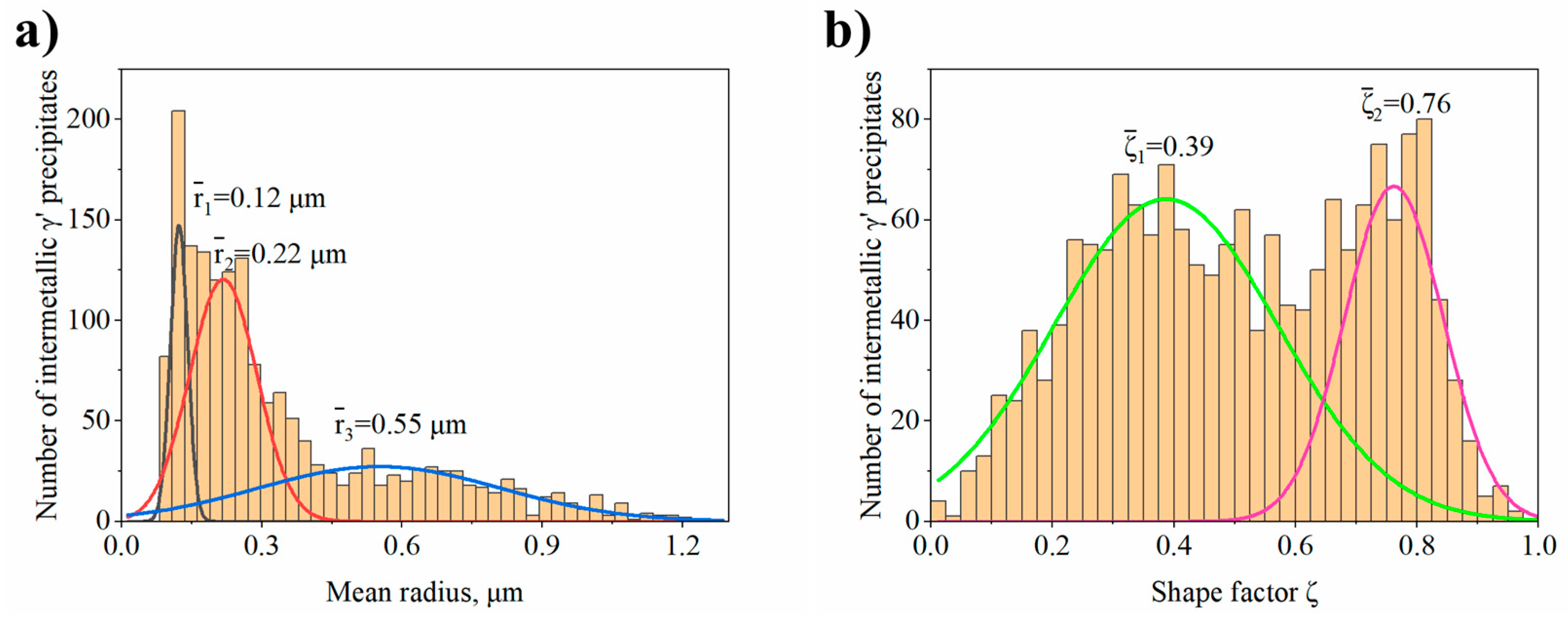
- In dendritic regions the γ′ precipitates have a complex morphology with two classes of shape factor for which the mean ξ values are 0.39 and 0.76. Too high diversity in size and morphology of precipitates in interdendritic spaces did not allow their effective comparison.
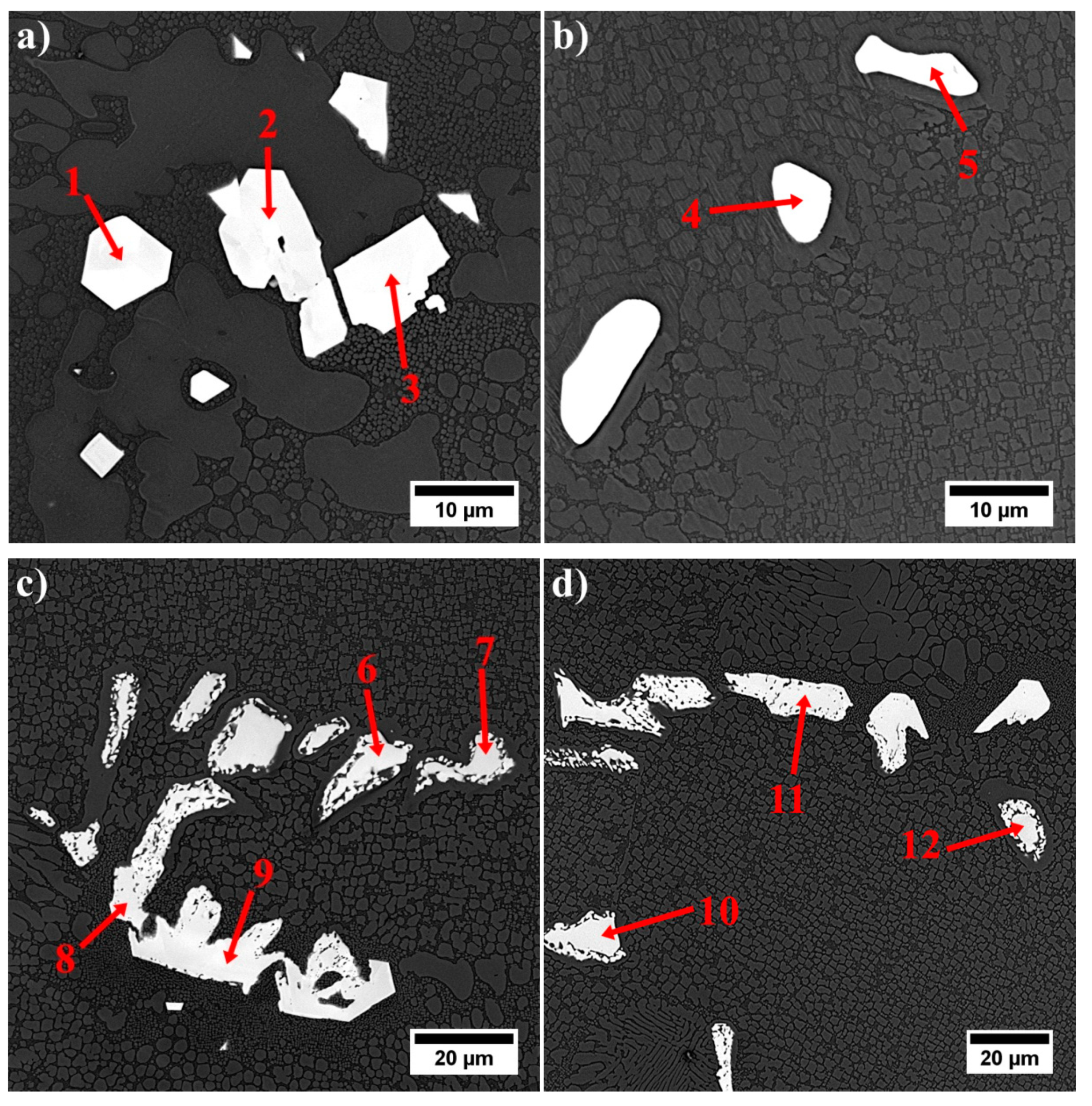
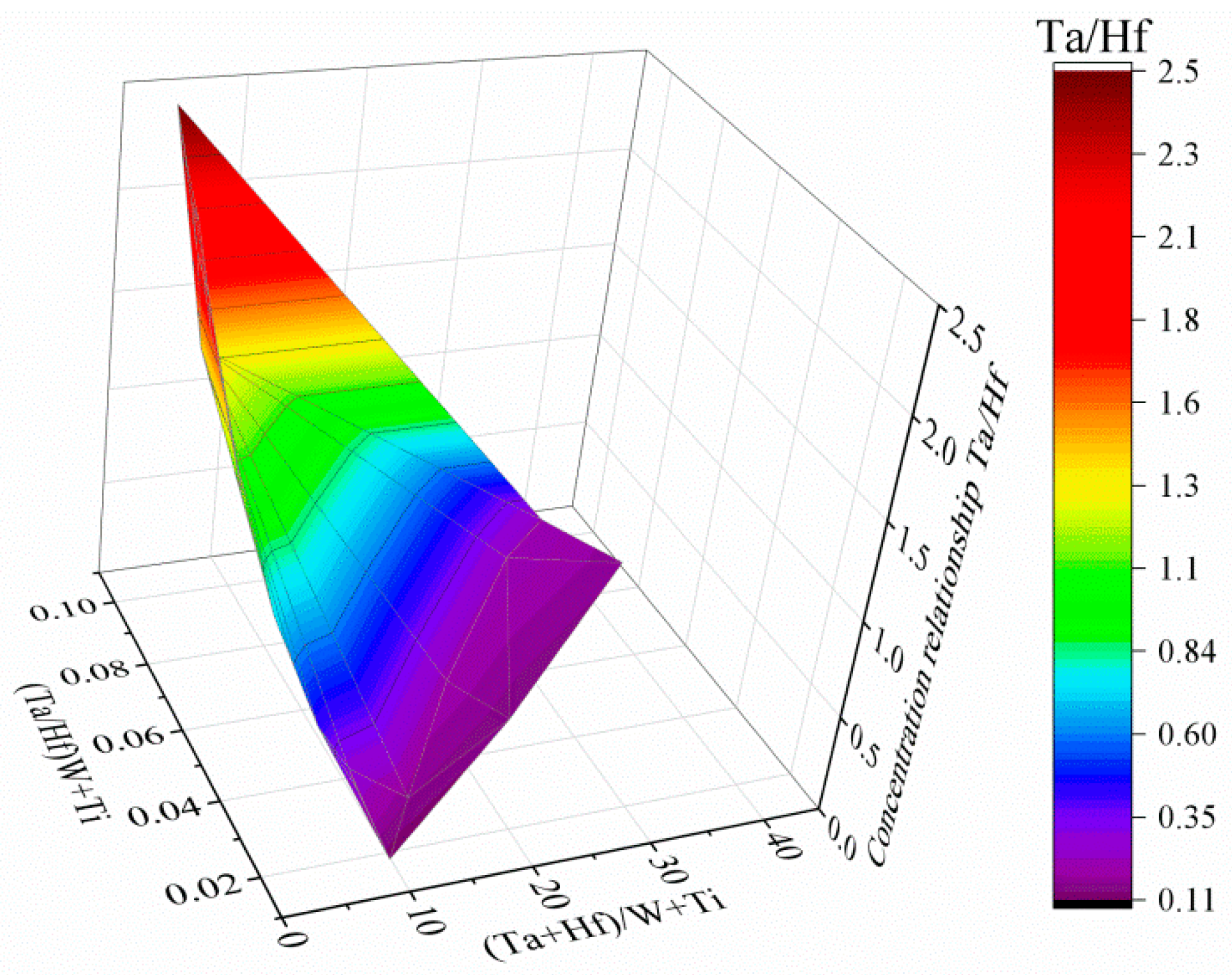
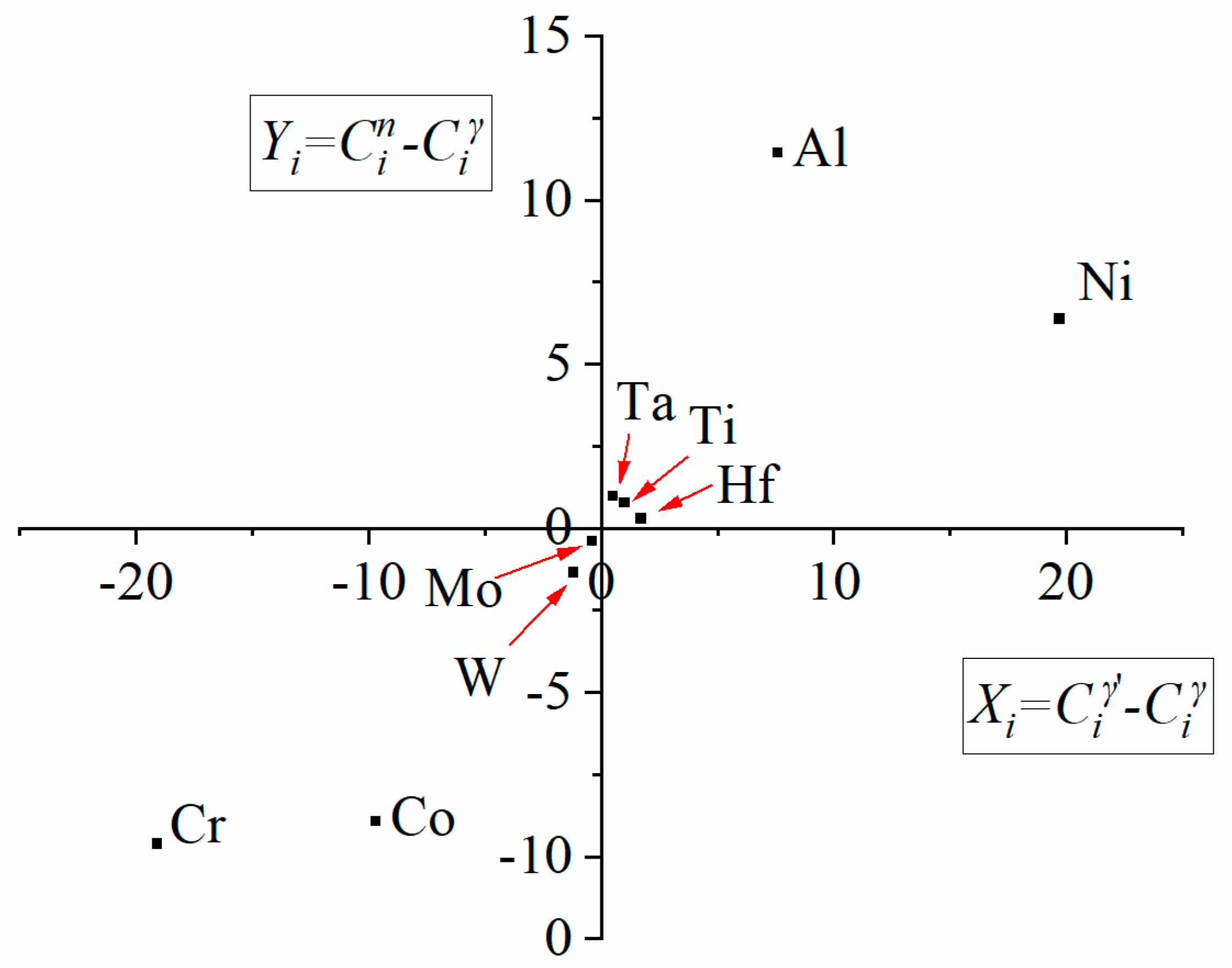
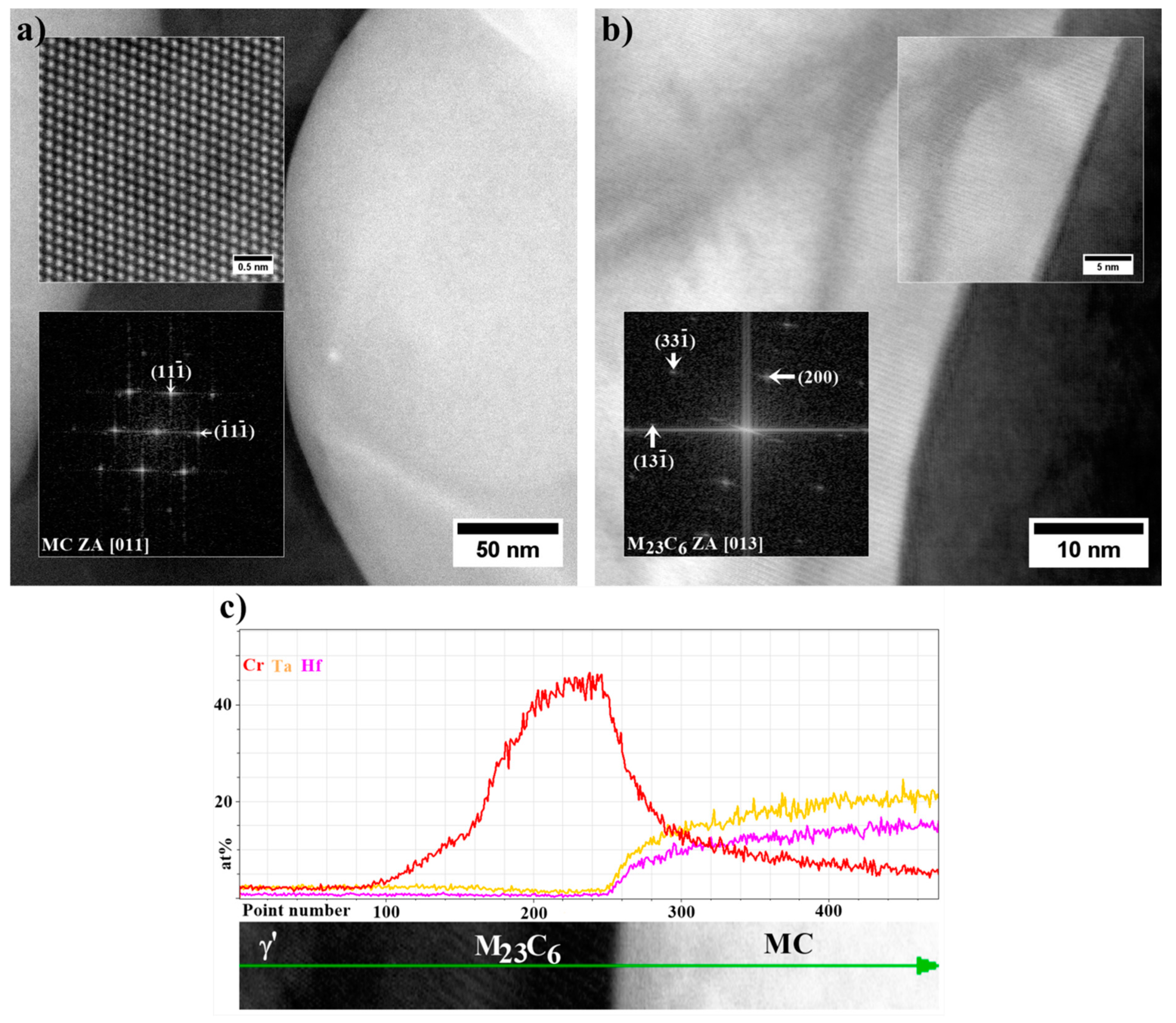
- The MC carbides are preferentially precipitated in the interdendritic areas. The “M” position is occupied mainly by Ta, Hf, Ti, and W, while the mutual concentration relationships depend on the morphology.
- The MC carbide degradation occurred during ageing according to the phase transformation reaction: MC + γ → M23C6 + γ′. The M23C6 carbides are revealed at the MC edges as nano-layers with width 5-15 nm.
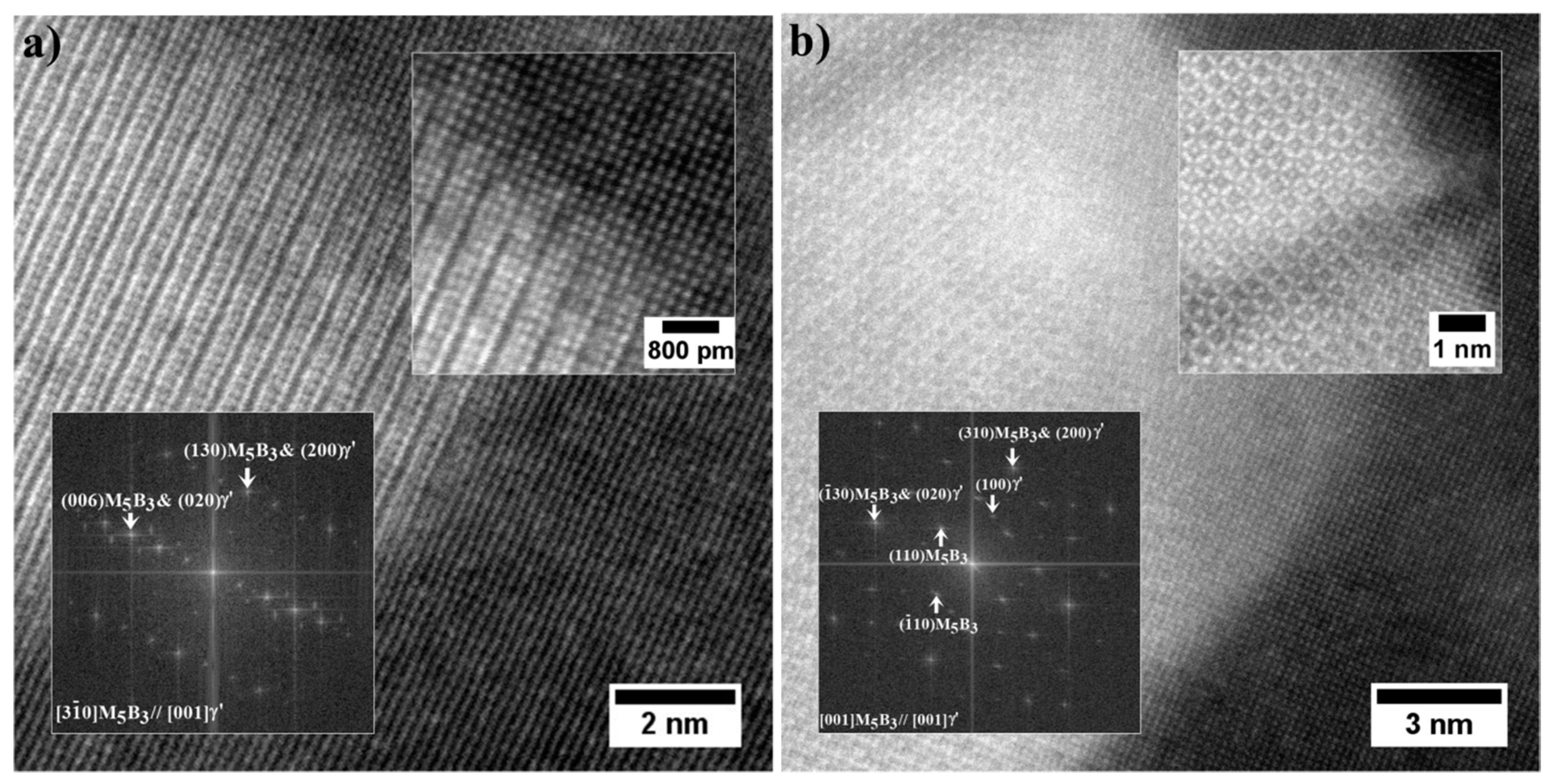
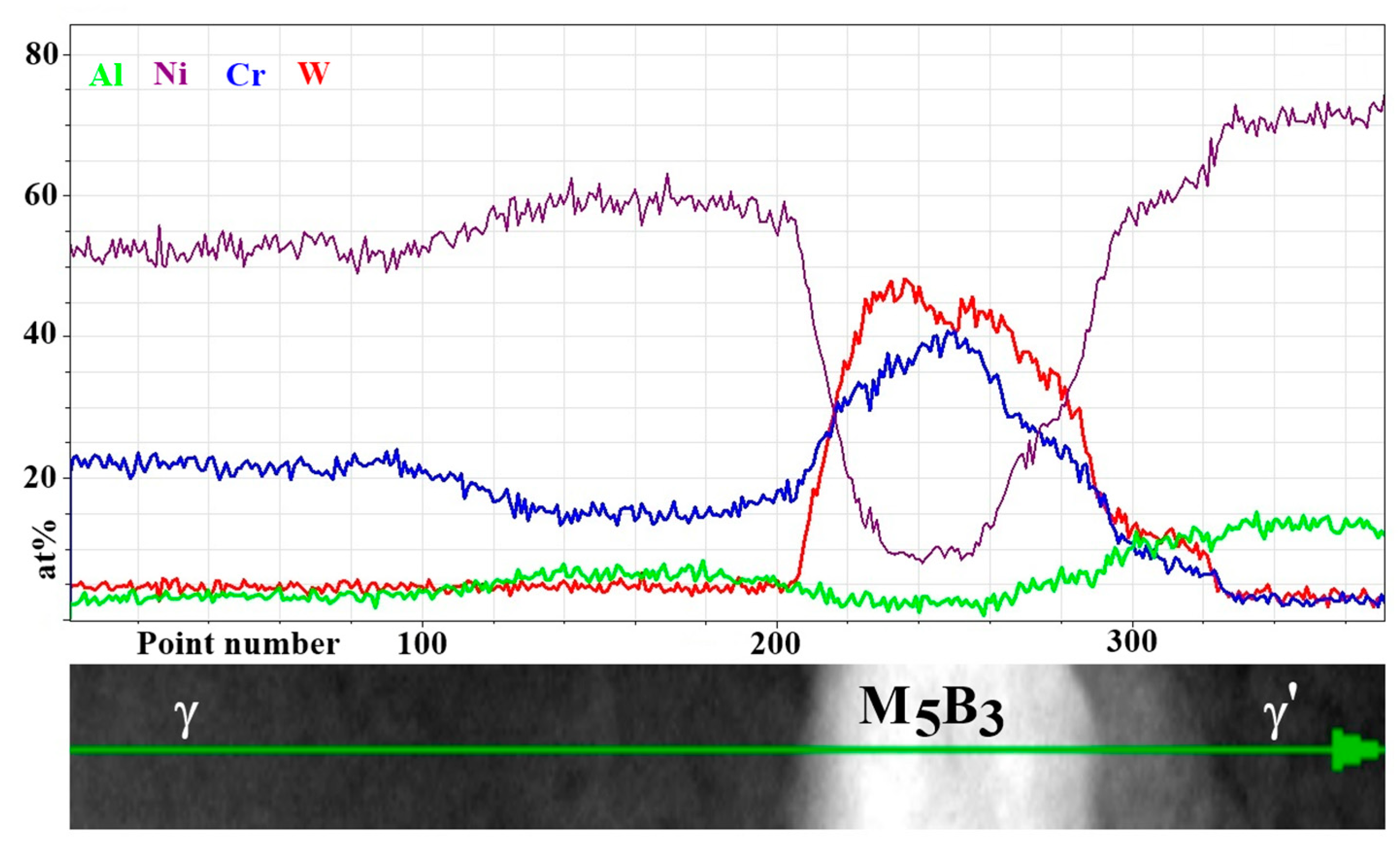
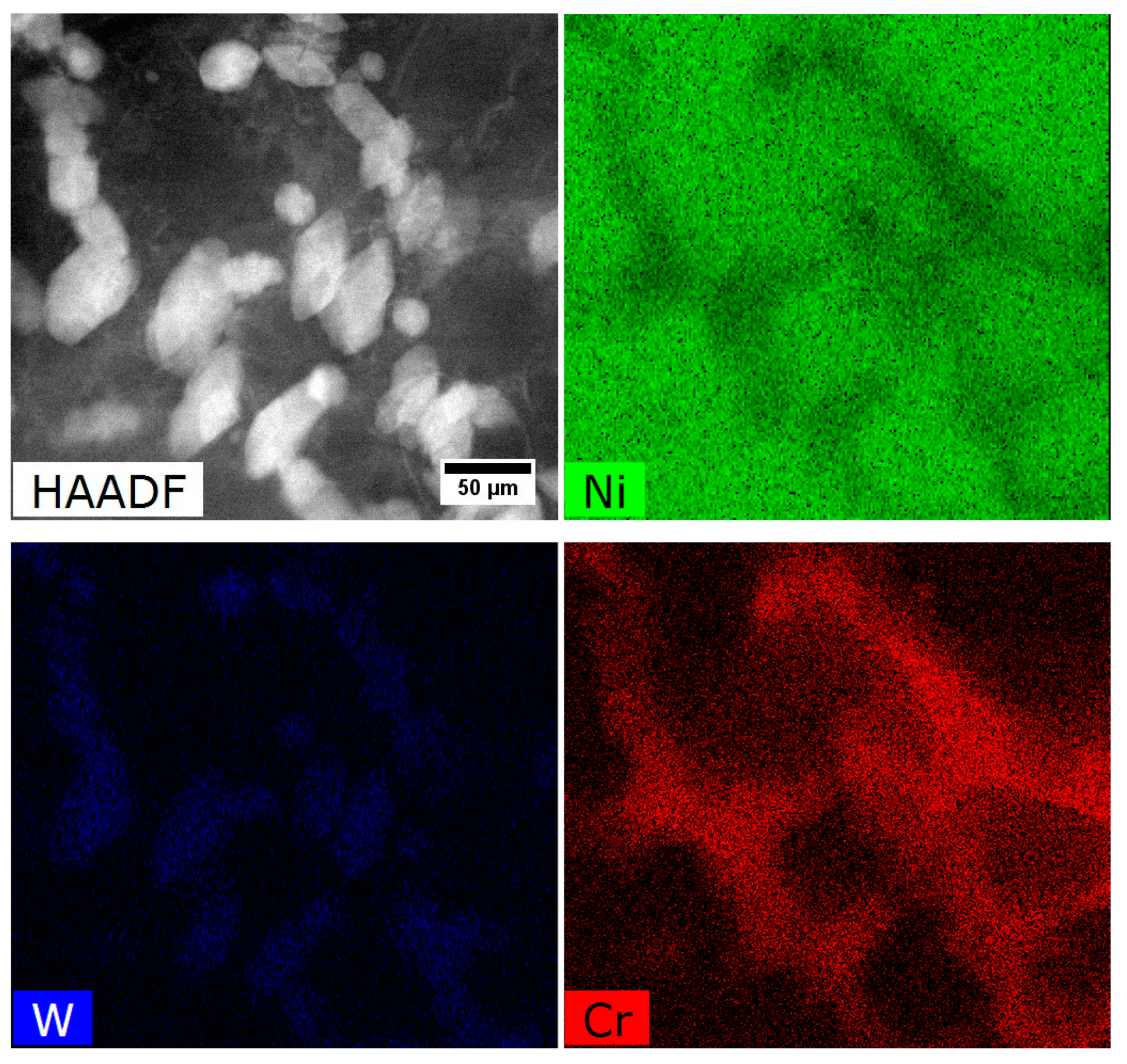
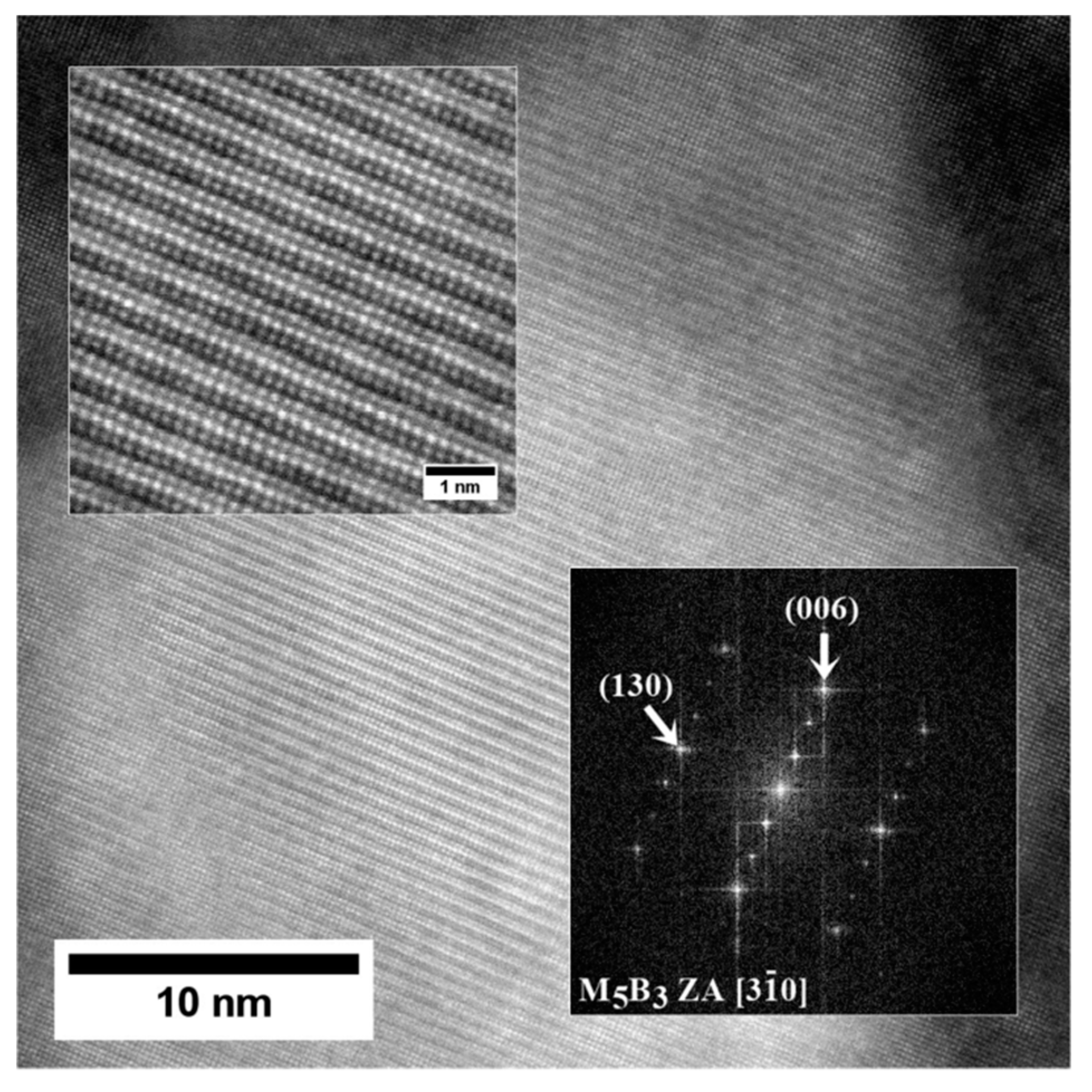
- The M5B3 borides characterised by body-centered tetragonal I4/mcm crystal structure have polygon and rhombus forms in thin foils. They are preferentially formed at the interfaces of secondary γ′ with matrix as nano-precipitates, both in the dendritic and interdendritic regions, and also on the grain boundaries. The STEM-EDX analysis revealed that they are enriched mainly in W, Cr, and Mo.
Author Contributions
Funding
Conflicts of Interest
References
- Reed, R.C. The Superalloys: Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Grudzień-Rakoczy, M.; Rakoczy, Ł.; Cygan, R.; Kromka, F.; Pirowski, Z.; Milkovič, O. Fabrication and characterization of the newly developed superalloys based on Inconel 740. Materials 2020, 13, 2362. [Google Scholar] [CrossRef] [PubMed]
- Rogalski, G.; Świerczyńska, A.; Landowski, M.; Fydrych, D. Mechanical and microstructural characterization of TIG welded dissimilar joints between 304L austenitic stainless steel and Incoloy 800HT nickel alloy. Metals 2020, 10, 559. [Google Scholar] [CrossRef]
- Rakoczy, Ł.; Cempura, G.; Kruk, A.; Czyrska-Filemonowicz, A.; Zielińska-Lipiec, A. Evolution of γ′ morphology and γ/γ′ lattice parameter misfit in a nickel-based superalloy during non-equilibrium cooling. Int. J. Mater. Res. 2019, 110, 66–69. [Google Scholar] [CrossRef]
- Sims, C.T. A History of superalloy metallurgy for superalloy metallurgists. In Proceedings of the Fifth International Symposium on Superalloys, Pittsburgh, PA, USA, 7–11 October 1984; pp. 399–419. [Google Scholar] [CrossRef]
- Reed, R.C.; Rae, C.M.F. 22—Physical metallurgy of the nickel-based superalloys. In Physical Metallurgy, 5th ed.; Laughlin, D.E., Hono, K., Eds.; Elsevier: Oxford, UK, 2014; pp. 2215–2290. ISBN 978-0-444-53770-6. [Google Scholar]
- Satyanarayana, D.; Prasad, N.E. Nickel-based superalloys technologies. In Aerospace Materials and Material Technologies; Eswara Prasad, N., Wanhill, R.J.H., Eds.; Springer: Singapore, 2017; pp. 199–228. [Google Scholar] [CrossRef]
- Kishawy, H.A.; Hosseini, A. Superalloys. In Machining Difficult-to-Cut Materials. Basic Principles and Challenges; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Sabol, G.P.; Stickler, R. Microstructure of nickel-based superalloys. Phys. Stat. Sol. 1969, 35, 11–52. [Google Scholar] [CrossRef]
- Geddes, B.; Leon, H.; Huang, X. Superalloys: Alloying and Performance; ASM International: Materials Park, OH, USA, 2010; ISBN 978-1-61503-040-8. [Google Scholar]
- Bradley, A.J.; Taylor, A. An X-ray analysis of the nickel–aluminium system. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1937, 159, 56–72. [Google Scholar]
- Calmon, J. From Sir Frank Whittle to the year 2000—what is new in propulsion? Aeronaut. Astronaut. 1988, 6, 32–46. [Google Scholar] [CrossRef]
- Ennis, P. 6- Nickel-base alloys for advanced power plant components. In Coal Power Plant Materials and Life Assessment; Shimbli, A., Ed.; Woodhead Publishing: Shaston/Cambridge, UK, 2014; pp. 147–167. [Google Scholar] [CrossRef]
- White, C.H. Nickel base alloys. In The Development of Gas Turbine Materials, 1st ed.; Meetham, G.W., Ed.; Springer: Dordrecht, The Netherlands, 1981; pp. 89–119. [Google Scholar] [CrossRef]
- Rakoczy, Ł.; Cygan, R. Analysis of temperature distribution in shell mould during thin-wall superalloy casting and its effect on the resultant microstructure. Arch. Civ. Mech. Eng. 2018, 18, 144–1450. [Google Scholar] [CrossRef]
- Rakoczy, Ł.; Grudzień, M.; Cygan, R.; Zielińska-Lipiec, A. Effect of cobalt aluminate content and pouring temperature on macrostructure, tensile strength and creep rupture of Inconel 713 castings. Arch. Metall. Mater. 2019, 63, 1537–1545. [Google Scholar] [CrossRef]
- Eiselstein, H.L. Metallurgy of a columbium-hardened nickel-chromium-iron alloy. In Advances in the Technology of Stainless Steels and Related Alloys; ASTM STP 369: West Conshohocken, PA, USA, 1965; pp. 62–77. [Google Scholar] [CrossRef]
- Smith, S.R.; Carter, W.J.; Mateescu, G.D.; Kohl, F.J.; Fryburg, G.C.; Stearns, C.A. ESCA study of oxidation and hot corrosion of nickel-base superalloys. Oxid Met. 1980, 14, 415–435. [Google Scholar] [CrossRef]
- Wilson, A.S. Formation and effect of topologically close-packed phases in nickel-base superalloys. Mat. Sci. Tech. 2017, 33, 1108–1118. [Google Scholar] [CrossRef]
- Morinaga, M.; Yukawa, N.; Adachi, H.; Ezaki, H. New phacomp and its applications to alloy design. In Proceedings of the Fifth International Symposium on Superalloys, Pittsburgh, PA, USA, 7–11 October 1984; pp. 523–532. [Google Scholar]
- Muktinutalapati, R.N. Materials for gas turbines—An overview. In Advances in Gas Turbine Technology; Benini, E., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 293–314. [Google Scholar] [CrossRef] [Green Version]
- Rakoczy, Ł.; Milkovič, O.; Rutkowski, B.; Cygan, R.; Grudzień-Rakoczy, M.; Kromka, F.; Zielińska-Lipiec, A. Characterization of γ′ precipitates in cast Ni-based superalloy and their behaviour at high-homologous temperatures studied by TEM and in Situ XRD. Materials 2020, 13, 2397. [Google Scholar] [CrossRef] [PubMed]
- Donachie, M.J.; Donachie, S.J. Superalloys: A Technical Guide, 2nd ed.; ASM International: Materials Park, OH, USA, 2002. [Google Scholar]
- Burt, H.; Dennison, J.P.; Elliot, I.C.; Wilshire, B. The effect of hot isostatic pressing on the creep and fracture behaviour of the cast superalloy Mar 002. Mat. Sci. Eng. 1982, 53, 245–250. [Google Scholar] [CrossRef]
- Maslenkov, S.B.; Burova, N.N.; Khangulov, V.V. Effect of hafnium on the structure and properties of nickel alloys. Met. Sci. Heat Treat. 1980, 22, 283–285. [Google Scholar] [CrossRef]
- Dahl, J.M.; Danesi, W.F.; Dunn, R.G. The partitioning of refractory metal elements in hafnium-modified cast nickel-base superalloys. Metall. Mater. Trans. B 1973, 4, 1641. [Google Scholar] [CrossRef]
- da Silva Costa, A.M.; Nunes, C.A.; Baldan, R.; Coelho, G.C. Thermodynamic evaluation of the phase stability and microstructural characterization of a cast B1914 superalloy. J. Mat. Eng. Perform. 2014, 23, 819–825. [Google Scholar] [CrossRef]
- Maxwell, D.H.; Baldwin, J.F.; Radavich, J.F. New concept in superalloy ductility. Metall. Metal. Form. 1975, 42, 332. [Google Scholar]
- Ouichou, L.; Lavaud, F.; Lesoult, G. Influence of the chemical composition of nickel-base superalloys on their solidification behavior and foundry performance. In Proceedings of the Superalloys 1980 (Fourth International Symposium), The Seven Springs International Symposium Committee, Champion, PA, USA, 21–25 September 1980; pp. 235–244. [Google Scholar]
- Rakoczy, Ł.; Grudzień-Rakoczy, M.; Cygan, R. The influence of shell mold composition on the as-cast macro- and micro-structure of thin-walled IN713C superalloy casting. J. Mat. Eng. Perform. 2019, 28, 3974–3985. [Google Scholar] [CrossRef] [Green Version]
- Kontis, P.; Mohd Yusof, H.A.; Pedrazzini, S.; Danaie, M.; Moore, K.L.; Bagot, P.A.J.; Moody, M.P.; Grovenor, C.R.M.; Reed, R.C. On the effect of boron on grain boundary character in a new polycrystalline superalloy. Act. Mat. 2016, 103, 688–699. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, Y.; Sheng, N.; Ma, X.L. The Wyckoff positional order and polyhedral intergrowth in the M3B2- and M5B3-type boride precipitated in the Ni-based superalloys. Sci. Rep. 2014, 4, 7367. [Google Scholar] [CrossRef] [Green Version]
- Strunz, P.; Petrenec, M.; Polák, J.; Gasser, U.; Farkas, G. Formation and dissolution of γ′ precipitates in IN792 superalloy at elevated temperatures. Metals 2016, 6, 37. [Google Scholar] [CrossRef]
- Jena, A.K.; Chaturvedi, M.C. The role of alloying elements in the design of nickel-base superalloys. J. Mat. Sci. 1984, 19, 3121–3139. [Google Scholar] [CrossRef]
- Rakoczy, Ł.; Grudzień, M.; Zielińska-Lipiec, A. Contribution of microstructural constituents on hot cracking of MAR-M247 nickel based supealloy. Arch. Met. Mat. 2018, 63, 181–189. [Google Scholar] [CrossRef]
- Clementi, E.; Raimondi, D.L.; Reinhardt, W.P. Atomic screening constants from SCF functions. II. Atoms with 37 to 86 electrons. J. Chem. Phys. 1967, 47, 1300–1307. [Google Scholar] [CrossRef]
- Davis, J.R. Heat-Resistant Materials; ASM International: Materials Park, OH, USA, 1997. [Google Scholar]
- Bertaut, F.; Blum, P. Etude des borures de chrome. Compt. Rend. Hebd. Seances Acad. Sci. 1953, 236, 1055–1056. [Google Scholar]
- Han, F.F.; Chang, J.X.; Li, H.; Lou, L.H.; Zhang, J. Influence of Ta content on hot corrosion behaviour of a directionally solidified nickel base superalloy. J. All. Comp. 2015, 619, 102–108. [Google Scholar] [CrossRef]
- Sheng, N.; Li, B.; Liu, J.; Jin, T.; Sun, X.; Hu, Z. Influence of the substrate orientation on the isothermal solidification during TLP bonding single crystal superalloys. J. Mater. Sci. Technol. 2014, 30, 213–216. [Google Scholar] [CrossRef]
- Zhang, H.R.; Ojo, O.A. TEM analysis of Cr-Mo-W-B phase in a DS nickel based superalloy. J. Mater. Sci. 2008, 43, 6024–6028. [Google Scholar] [CrossRef]
- Long, H.; Mao, S.; Liu, Y.; Zhang, Z.; Han, X. Microstructural and compositional design of Ni-based single crystalline superalloys. J. All. Comp. 2018, 743, 203–220. [Google Scholar] [CrossRef]
- Ducki, K. Microstructural Aspects of Deformation, Precipitation and Strengthening Processes in Austenitic Fe-Ni Superalloy; Silesian University of Technology: Gliwice, Poland, 2010. (In Polish) [Google Scholar]
- Grudzień, M.; Tuz, L.; Pańcikiewicz, K.; Zielińska-Lipiec, A. microstructure and properties of a repair weld in a nickel based superalloy gas turbine component. Adv. Mater. Sci. 2017, 17, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Hanning, F.; Khan, A.K.; Steffenburg-Nordenström, J.; Ojo, O.; Andersson, J. Investigation of the effect of short exposure in the temperature range of 750–950 °C on the ductility of Haynes® 282® by advanced microstructural characterization. Metals 2019, 9, 1357. [Google Scholar] [CrossRef] [Green Version]
- Rakoczy, Ł.; Grudzień, M.; Cygan, R. Influence of melt-pouring temperature and composition of primary coating of shell mold on tensile strength and creep resistance of Ni-based superalloy. J. Mat. Eng. Perform. 2019, 28, 3826–3834. [Google Scholar] [CrossRef]
- Grudzień, M.; Cygan, R.; Pirowski, Z.; Rakoczy, Ł. Microstructural characterization of Inconel 713C superalloy after creep testing. Trans. Found. Res. Inst. 2018, 58, 39–45. [Google Scholar] [CrossRef]
- Choi, B.G.; Kin, I.S.; Kim, D.H.; Seo, S.M.; Jo, C.Y. Eta phase formation during thermal exposure and its effect on mechanical properties in Ni-base superalloy GTD 111. In Proceedings of the 10th International Symposium Superalloys, Pittsburgh, PA, USA, 19–23 September 1996; pp. 163–172. [Google Scholar]
- Nunomura, Y.; Kaneno, Y.; Tsuda, H.; Takasugi, T. Phase relation and microstructure in multi-phase intermetallic alloys based on Ni3Al-Ni3Ti-Ni3V pseudo-ternary alloy system. Intermetallics 2004, 12, 389–399. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, C.; Xiao, X.; Cao, X.; Jia, G.; Shen, Z. Evolution of microstructure and mechanical properties of Ti modified superalloy Nimonic 80A. Mat. Sci. Eng. A 2001, 530, 315–326. [Google Scholar] [CrossRef]
- Xie, J.; Yu, J.; Sun, X.; Jin, T. Thermodynamics analysis and precipitation behavior of fine carbide in K416B Ni-based superalloy with high W content during creep. Trans. Nonferrous Met. Soc. China 2015, 25, 1478–1483. [Google Scholar] [CrossRef]
- Li, Q.; Lin, X.; Wang, X.; Yang, H.; Song, M.; Huang, W. Research on the grain boundary liquation mechanism in heat affected zones of laser forming repaired K465 nickel-based superalloy. Metals 2016, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.; Han, C.; Shin, Y.; Youn, J.; Kim, Y. Effects of boron and zirconium on grain boundary morphology and creep resistance in Nickel-based superalloy. J. Mat. Eng Perform. 2019, 28, 7025–7035. [Google Scholar] [CrossRef]
- Tytko, D.; Choi, P.P.; Klöwer, J.; Kostka, A.; Inden, G.; Raabe, D. Microstructural evolution of a Ni-based superalloy (617B) at 700 °C studied by electron microscopy and atom probe tomography. Acta Mater. 2012, 60, 1731–1740. [Google Scholar] [CrossRef]
- Xiao, L.; Chen, D.L.; Chaturvedi, M.C. Effect of boron on fatigue crack growth behavior in superalloy IN718 at RT and 650 °C. Mater. Sci. Eng. A 2006, 428, 1–11. [Google Scholar] [CrossRef]
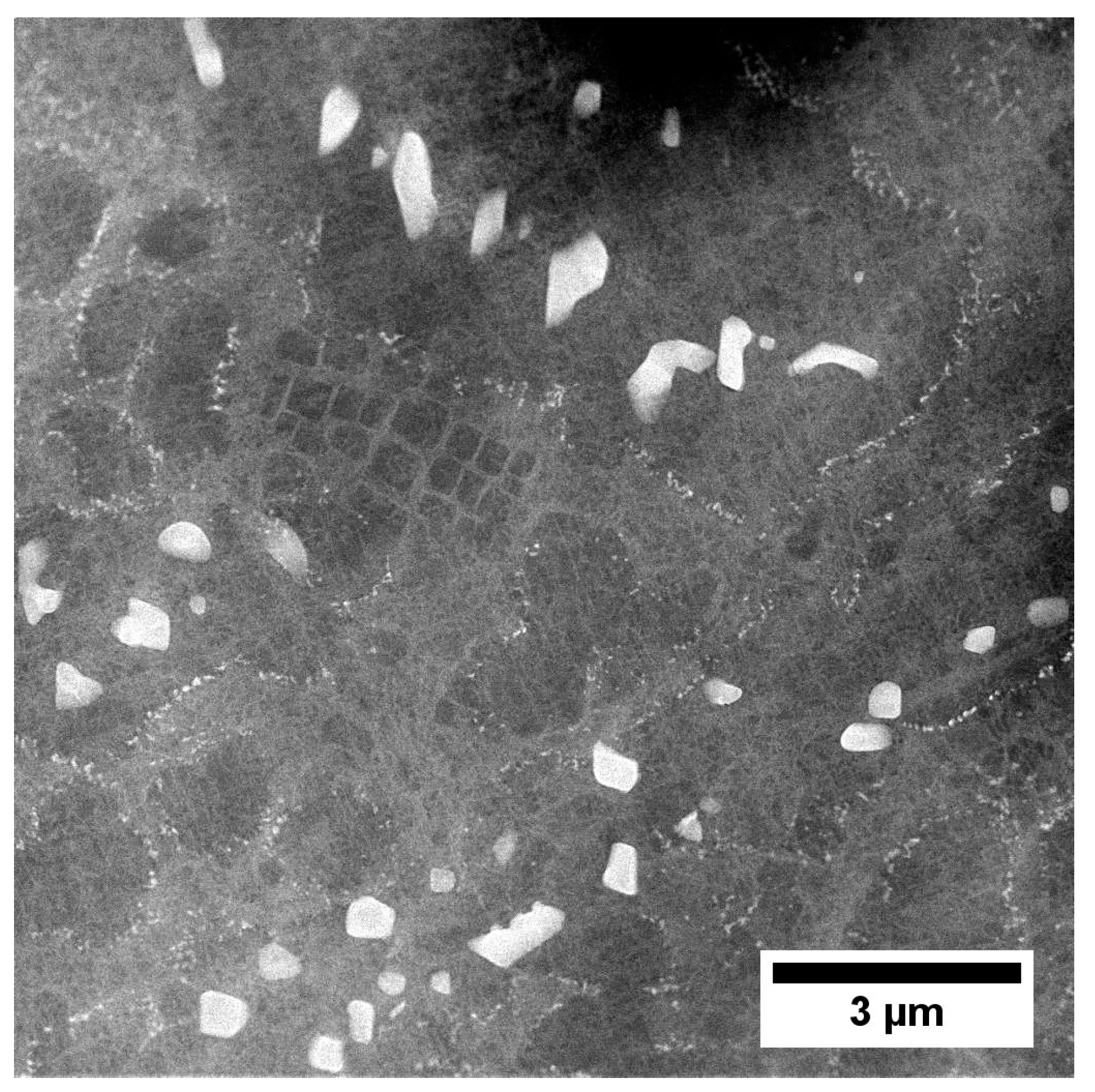
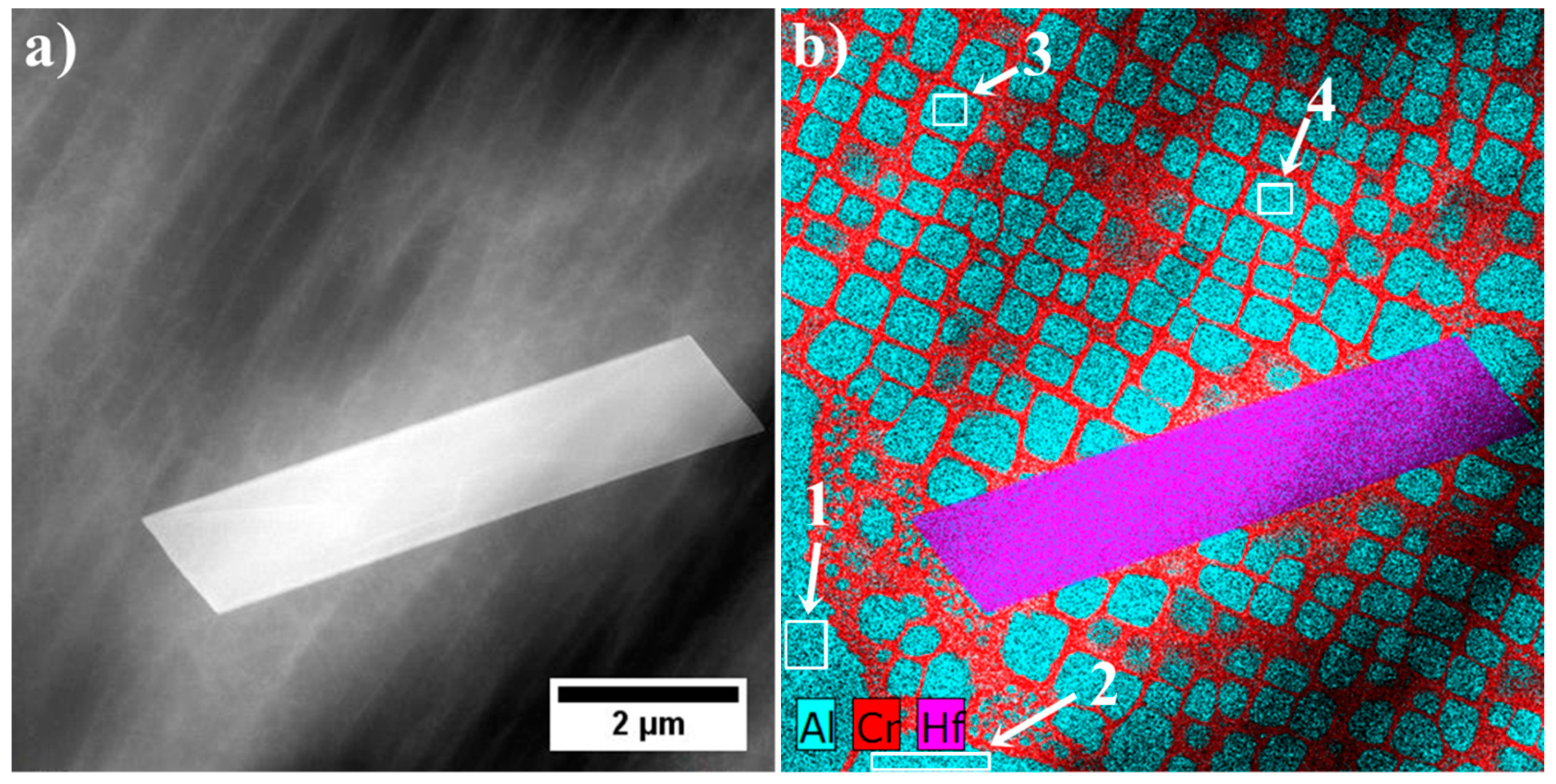
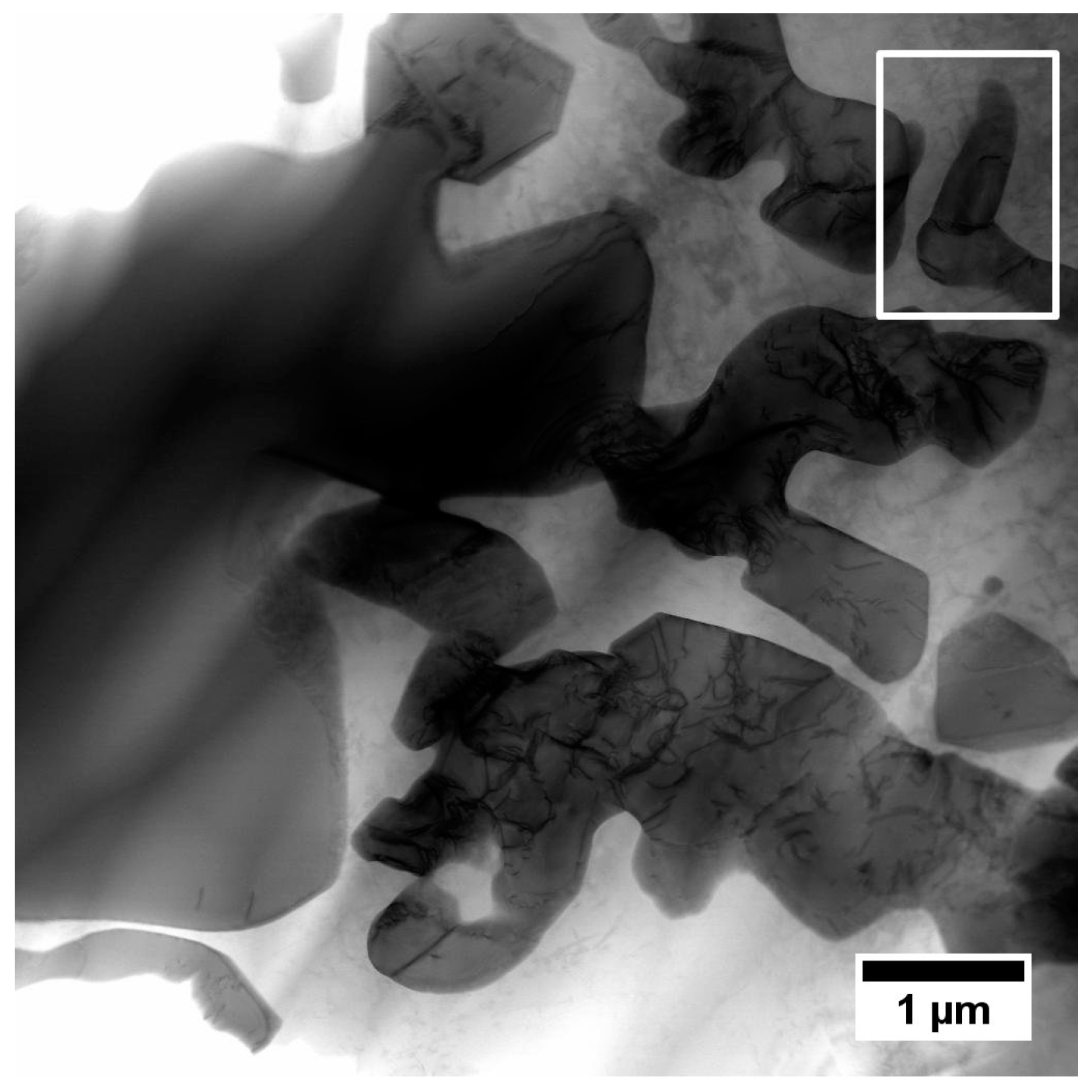
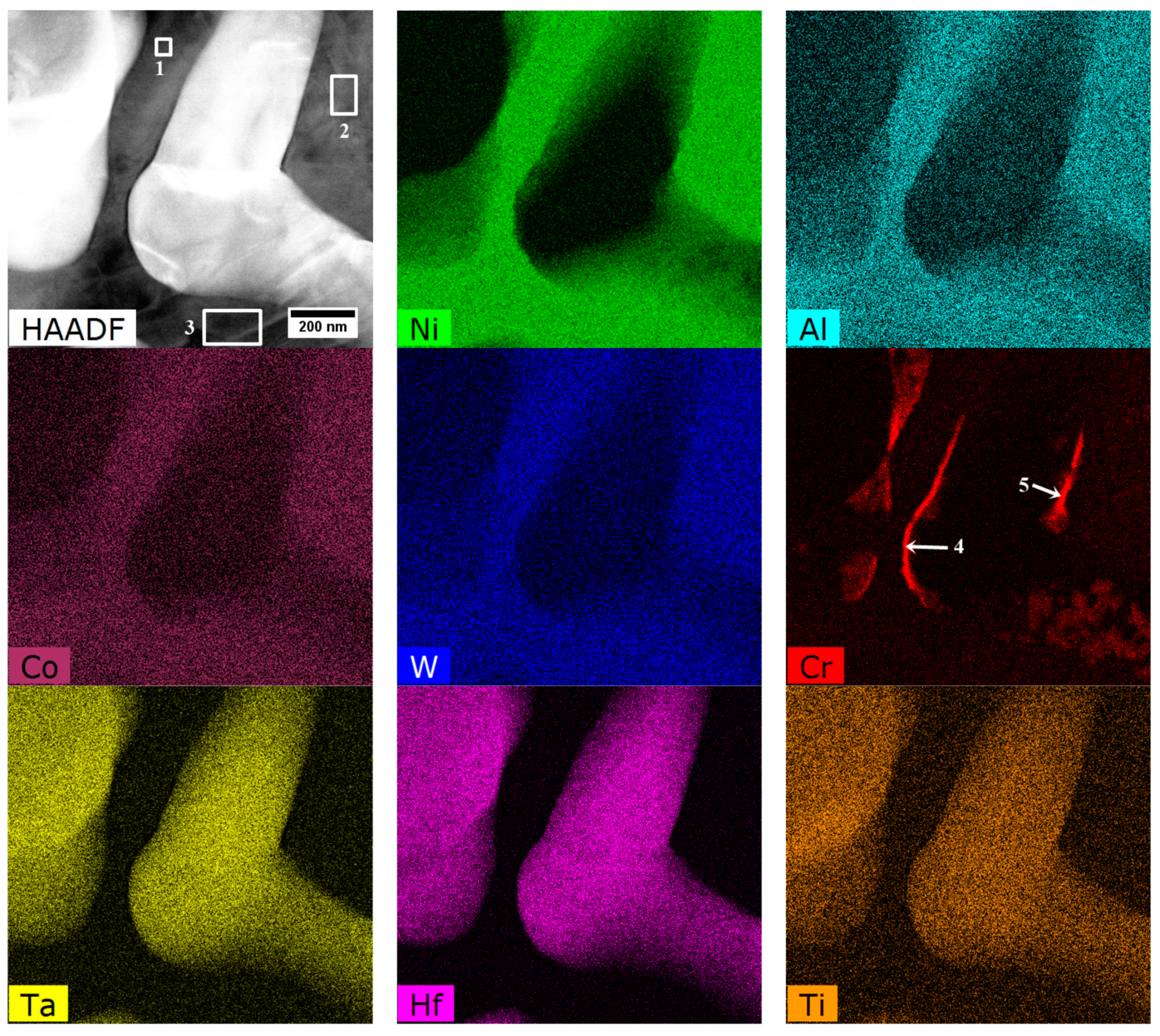
| Element | Cr | W | Co | Al | Ta | Hf | Ti | Mo | C | B | Zr | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | 11.4 | 8.9 | 8.2 | 6.4 | 3.6 | 1.5 | 0.8 | 0.5 | 0.08 | 0.02 | 0.0125 | Bal. |
| Element | Radius, Å | Solubility in Nickel, wt%/at% | |
|---|---|---|---|
| 1200 °C | 900 °C | ||
| Ni | 1.49 | ||
| Cr | 1.66 | 46.50/49.53 | 38.49/41.40 |
| Co | 1.52 | 100/100 | 100/100 |
| W | 1.93 | 39.01/16.96 | 35.31/14.84 |
| Al | 1.18 | 9.38/18.38 | 7.17/14.38 |
| Ta | 2.00 | 20.19/7.58 | 12.87/4.57 |
| Hf | 2.08 | 3.67/1.24 | 0.78/0.26 |
| Ti | 1.76 | 11.98/14.30 | 8.58/10.32 |
| Mo | 1.90 | 36.45/25.97 | 27.77/19.04 |
| B | 0.87 | 0.067/0.36 | 0.019/0.10 |
| C | 0.67 | 0.426/2.05 | 0.162/0.79 |
| Zr | 2.06 | 1.047/0.68 | 0.591/0.38 |
| (hkl) | dhkl, Å | a, Å |
|---|---|---|
| γ matrix FCC Fmm (225): PDF No. 47-1417 | ||
| (111) | 2.07 | 3.59 |
| (200) | 1.80 | 3.60 |
| (220) | 1.27 | 3.58 |
| (311) | 1.08 | 3.58 |
| (222) | 1.04 | 3.59 |
| γ′ FCC ordered L12: PDF No. 65-3245 | ||
| (111) | 2.09 | 3.62 |
| (200) | 1.81 | 3.63 |
| (210) | 1.60 | 3.58 |
| (220) | 1.27 | 3.59 |
| (221) | 1.20 | 3.60 |
| (311) | 1.08 | 3.60 |
| (222) | 1.04 | 3.60 |
| MC FCC Fmm (225): PDF No. 74-1223 | ||
| (111) | 2.61 | 4.52 |
| (200) | 2.26 | 4.52 |
| No. | Ta | Hf | Ti | Ni | W | Co | Cr | Mo | Ta/Hf | (Ta + Hf)/(W + Ti) | (Ta/Hf)/(W + Ti) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12.2 | 75.0 | 1.1 | 6.9 | 1.0 | 1.4 | 1.0 | 1.4 | 0.16 | 42.95 | 0.08 |
| 2 | 16.3 | 73.3 | 1.2 | 5.4 | 1.2 | 1.0 | 0.9 | 0.7 | 0.22 | 37.99 | 0.09 |
| 3 | 16.6 | 72.3 | 2.0 | 6.0 | 0.8 | 0.7 | 1.1 | 0.5 | 0.23 | 32.32 | 0.08 |
| 4 | 42.5 | 21.1 | 17.4 | 7.7 | 5.9 | 2.4 | 1.8 | 1.3 | 2.01 | 2.73 | 0.09 |
| 5 | 42.7 | 16.8 | 19.1 | 8.2 | 7.1 | 2.4 | 2.3 | 1.4 | 2.54 | 2.27 | 0.10 |
| 6 | 43.9 | 19.9 | 19.5 | 6.9 | 5.5 | 0.9 | 1.6 | 1.8 | 2.20 | 2.56 | 0.09 |
| 7 | 42.8 | 18.3 | 18.4 | 7.9 | 7.0 | 2.2 | 1.8 | 1.7 | 2.35 | 2.40 | 0.09 |
| 8 | 23.4 | 54.5 | 6.5 | 5.7 | 5.9 | 1.4 | 1.0 | 1.6 | 0.43 | 6.26 | 0.03 |
| 9 | 17.5 | 61.1 | 4.2 | 5.9 | 5.5 | 2.9 | 2.1 | 1.0 | 0.29 | 8.06 | 0.03 |
| 10 | 44.9 | 21.5 | 18.9 | 5.9 | 5.2 | 1.1 | 1.0 | 1.5 | 2.09 | 2.76 | 0.09 |
| 11 | 30.9 | 41.3 | 10.0 | 6.9 | 5.2 | 2.6 | 2.0 | 1.1 | 0.75 | 4.75 | 0.05 |
| 12 | 20.2 | 19.9 | 6.4 | 5.6 | 1.1 | 1.9 | 1.6 | 20.2 | 2.15 | 2.49 | 0.08 |
| Area | Phase | Ni | Cr | Co | W | Al | Ta | Hf | Ti | Mo |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Matrix γ | 52.1 | 21.5 | 16.9 | 4.7 | 3.4 | 0.3 | 0.2 | 0.2 | 0.7 |
| 2 | Secondary γ′ | 69.8 | 2.9 | 6.8 | 3.2 | 13.7 | 1.6 | 0.6 | 1.1 | 0.3 |
| 3 | Tertiary γ′ | 69.4 | 2.8 | 7.3 | 4.8 | 13.4 | 0.9 | 0.3 | 0.8 | 0.5 |
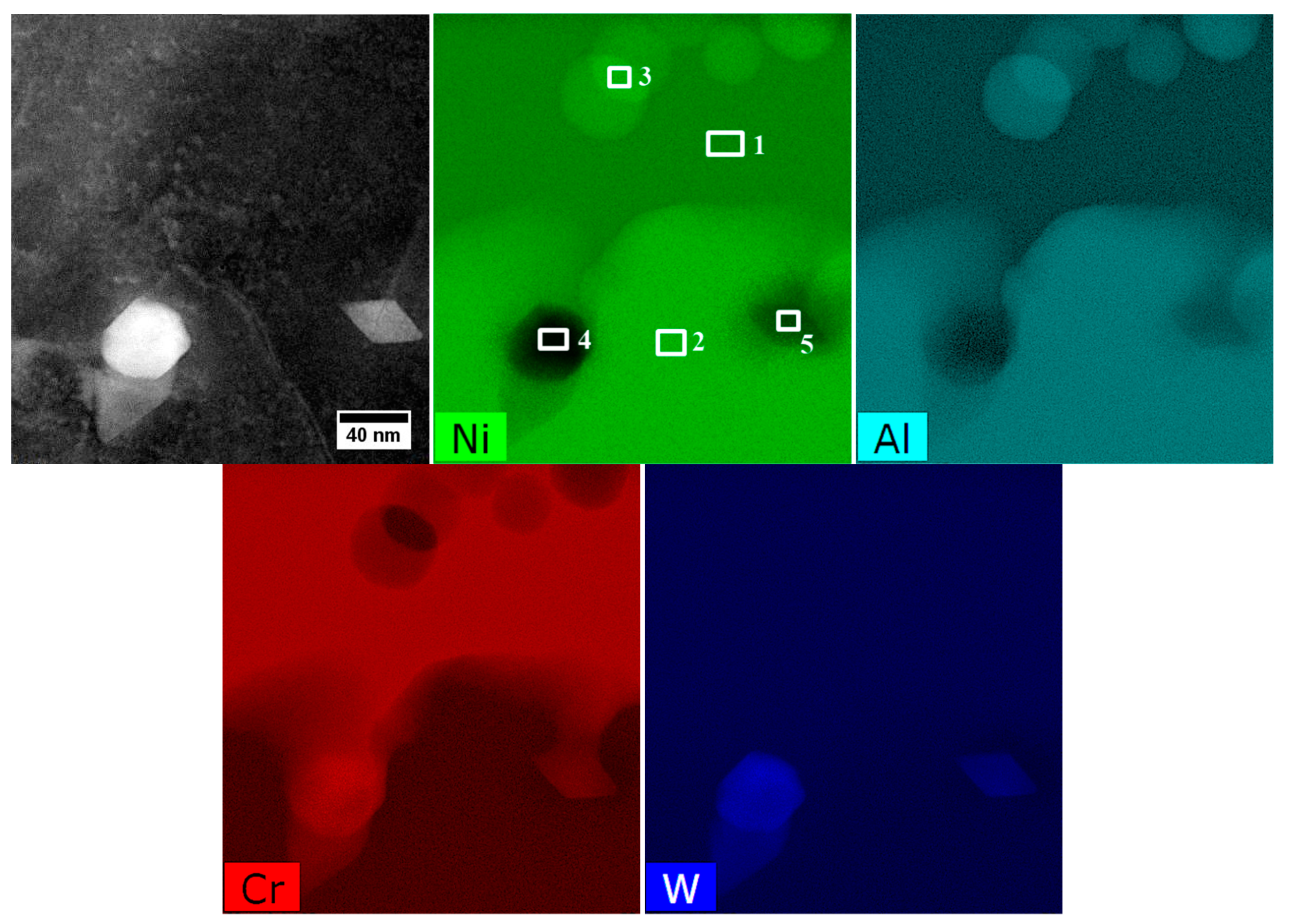
| 4 | Boride M5B3 | 10.0 | 35.5 | 3.2 | 42.0 | 2.3 | 1.2 | 0.3 | 0.4 | 5.2 |
| 5 | 50.9 | 12.4 | 5.9 | 17.1 | 9.4 | 1.3 | 0.4 | 0.7 | 1.9 |
| Area | Phase | Ni | Cr | Co | W | Al | Ta | Hf | Ti | Mo |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Primary γ′ | 71.0 | 3.5 | 7.1 | 2.6 | 11.6 | 1.4 | 1.1 | 1.4 | 0.3 |
| 2 | 70.2 | 3.6 | 7.0 | 2.8 | 12.3 | 1.5 | 1.0 | 1.3 | 0.3 | |
| 3 | Secondary γ′ | 71.8 | 2.7 | 6.5 | 2.7 | 11.2 | 1.3 | 1.0 | 1.4 | 0.6 |
| 4 | 74.1 | 2.7 | 6.5 | 3.1 | 10.0 | 1.3 | 0.9 | 1.1 | 0.5 |
| Area | Phase | Cr | Co | W | Al | Hf | Ta | Ti | Mo | Ni |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Coarse secondary γ′ | 3.0 | 7.5 | 3.3 | 11.8 | 1.1 | 2.7 | 1.7 | 0.5 | 68.4 |
| 2 | 3.5 | 7.3 | 2.7 | 10.9 | 1.0 | 2.6 | 1.8 | 0.8 | 69.6 | |
| 3 | 3.4 | 7.3 | 2.9 | 11.5 | 0.9 | 2.7 | 1.8 | 0.6 | 68.8 | |
| 4 | M23C6 | 43.7 | 4.5 | 2.0 | 6.2 | 3.3 | 6.5 | 2.7 | 1.9 | 29.4 |
| 5 | 53.3 | 6.3 | 4.0 | 2.5 | 2.8 | 5.3 | 1.6 | 2.2 | 22.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakoczy, Ł.; Rutkowski, B.; Grudzień-Rakoczy, M.; Cygan, R.; Ratuszek, W.; Zielińska-Lipiec, A. Analysis of γ′ Precipitates, Carbides and Nano-Borides in Heat-Treated Ni-Based Superalloy Using SEM, STEM-EDX, and HRSTEM. Materials 2020, 13, 4452. https://doi.org/10.3390/ma13194452
Rakoczy Ł, Rutkowski B, Grudzień-Rakoczy M, Cygan R, Ratuszek W, Zielińska-Lipiec A. Analysis of γ′ Precipitates, Carbides and Nano-Borides in Heat-Treated Ni-Based Superalloy Using SEM, STEM-EDX, and HRSTEM. Materials. 2020; 13(19):4452. https://doi.org/10.3390/ma13194452
Chicago/Turabian StyleRakoczy, Łukasz, Bogdan Rutkowski, Małgorzata Grudzień-Rakoczy, Rafał Cygan, Wiktoria Ratuszek, and Anna Zielińska-Lipiec. 2020. "Analysis of γ′ Precipitates, Carbides and Nano-Borides in Heat-Treated Ni-Based Superalloy Using SEM, STEM-EDX, and HRSTEM" Materials 13, no. 19: 4452. https://doi.org/10.3390/ma13194452
APA StyleRakoczy, Ł., Rutkowski, B., Grudzień-Rakoczy, M., Cygan, R., Ratuszek, W., & Zielińska-Lipiec, A. (2020). Analysis of γ′ Precipitates, Carbides and Nano-Borides in Heat-Treated Ni-Based Superalloy Using SEM, STEM-EDX, and HRSTEM. Materials, 13(19), 4452. https://doi.org/10.3390/ma13194452






