Synthesis, Characterization and Use of Mesoporous Silicas of the Following Types SBA-1, SBA-2, HMM-1 and HMM-2
Abstract
1. Introduction
2. SBA-1 Silicas
2.1. General Characterization of SBA-1
2.2. Selected Procedures for the Synthesis of SBA-1 Silicas
2.2.1. The Use Surfactants Other Than C16TEAB in SBA-1 Synthesis
2.2.2. Precursors of Silicon Applied in the Synthesis of SBA-1
2.2.3. The Reaction Environment and Stability of SBA-1 Materials
2.2.4. The Auxiliary Compounds Stabilizing the Structure of SBA-1
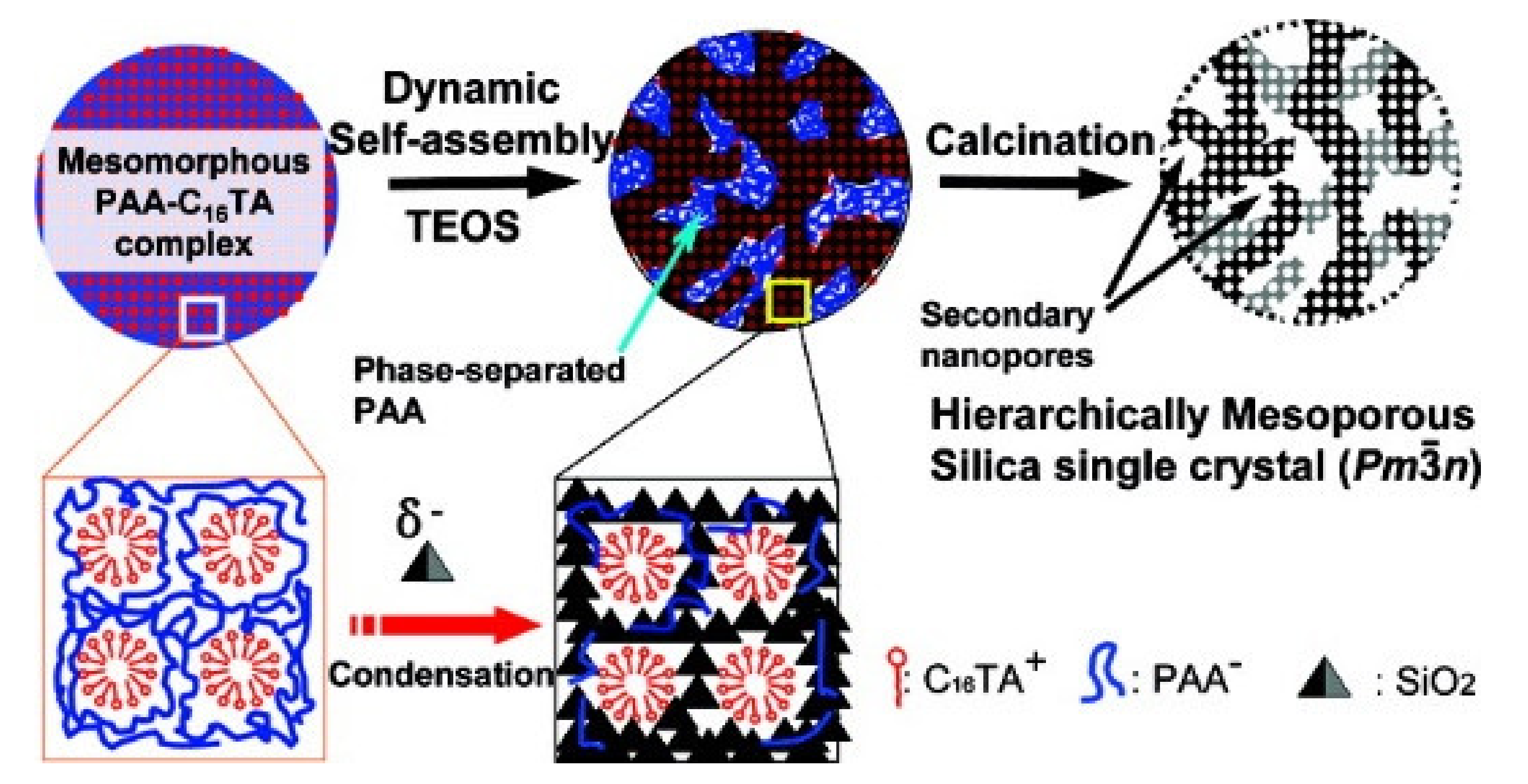
2.2.5. Complex of Polyelectrolyte-Surfactant as a Template in Acidic Conditions
2.2.6. Modification of SBA-1 With Metal Ions in Acidic Conditions and the Use of Modified Silicas in Catalytic Reactions
2.2.7. Modification of SBA-1 with Organic Functional Groups in Acidic Conditions
2.2.8. Application of Unmodified SBA-1 Obtained in Acidic Conditions
2.2.9. Synthesis of SBA-1 in Basic Conditions
2.2.10. Applications of SBA-1 Silicas Synthesized in Basic Conditions
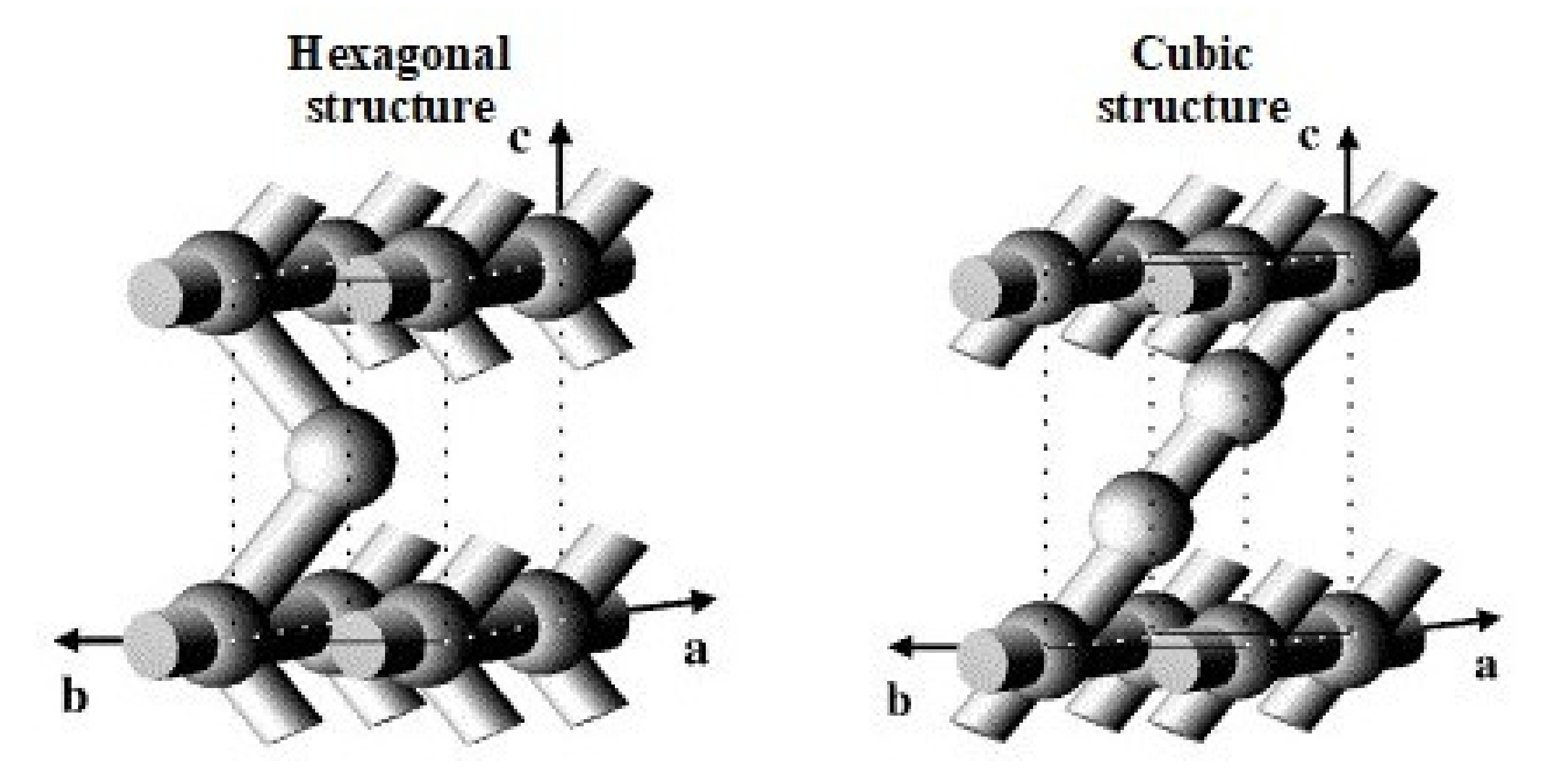
3. SBA-2 Silicas
3.1. General Characterization of SBA-2 Silicas
3.2. Syntheses and Structural Characterization of SBA-2 Silicas
3.3. Application of SBA-2 Materials
4. HMM Type Materials
4.1. General Characterization of HMM Type Materials
4.2. Synthesis of HMM Materials
4.3. HMM Modifications and Applications
5. Comparison of SBA and HMM Materials
6. Application of Mesoporous Silica Materials—Summary
7. Summary
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| APTMS | (3-Aminopropyl)trimethoxysilane |
| BJH | Barrett- Joyner-Halenda method |
| BTME | 1, 2-Bis(trimethoxysilyl)ethane |
| BTMB | 1, 4-Bis(triethoxysilyl)benzene |
| C16TAB | hexadecyltrimethylammonium bromide |
| C16TEAB | hexadecyltriethylammonium bromide |
| C16TPAB | hexadecyltripropylammonium bromide |
| C18TMACl = ODTMACl | octadecyltrimethylammonium chloride |
| CES | carboxyethyl silanetriol sodium salt |
| CPC | hexadecylpyridinium chloride |
| FDU | group of mesoporous materials named after Fudan University |
| FSM | group of mesoporous materials named after Folded Sheet Materials |
| HAADF- STEM | high-angle annular dark-field imaging scanning transmission microscopy |
| HMM | group of mesoporous materials named after Hybrid Mesoporous Materials |
| HRTEM | high-resolution transmission electron microscopy |
| IEP | isoelectric point |
| IUPAC | International Union of Pure and Applied Chemistry |
| KIT | group of mesoporous materials named after Korean Advanced Institute of Science and Technology |
| MCM | group of mesoporous materials named after Mobile Composition of Matter |
| MPTMS | (3-Mercaptopropyl)trimethoxysilane |
| MSU | group of mesoporous materials named after Michigan State University |
| PAA | poly(acrylic acid) |
| PMOs | Periodic Mesoporous Silicas |
| SBA | group of mesoporous materials named after Santa Barbara University |
| SEM | scanning electron microscopy |
| scCO2 | supercritical carbon(IV) oxide |
| TBHP | tert-Butyl hydroperoxide |
| TEM | transmission electron microscopy |
| TEOS | tetraethyl orthosilicate (tetraethoxysilane) |
| TGA | thermogravimetric analysis |
| THF | tetrahydrofuran |
| TMB | 1,3,5-trimethylbenzene |
| TMOS | Tetramethyl orthosilicate (tetramethoxysilane) |
| TOF | turnover frequency |
| TPOS | tetrapropyl orthosilicate (tetrapropoxysilane) |
| UV-VIS | ultraviolet-visible spectroscopy |
| XAFS | X-ray absorption fine structure spectroscopy |
| XRD | X-ray diffraction |
References
- Sharma, N.; Ojha, H.; Bharadwaj, A.; Pathak, D.P.; Sharma, R.K. Preparation and catalytic applications of nanomaterials: A review. RSC Adv. 2015, 5, 53381–53403. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Welborn, S.S.; Detsi, E. Small-angle X-ray scattering of nanoporous materials. Nanoscale Horiz. 2020, 5, 12–24. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Feliczak-Guzik, A. Hierarchical zeolites: Synthesis and catalytic properties. Microporous Mesoporous Mater. 2018, 259, 33–45. [Google Scholar] [CrossRef]
- Malgras, V.; Tang, J.; Wang, J.; Kim, J.; Torad, N.L.; Dutta, S.; Ariga, K.; Hossain, M.S.A.; Yamauchi, Y.; Wu, K.C.W. Fabrication of nanoporous carbon materials with hard- and soft-templating approaches: A review. J. Nanosci. Nanotechnol. 2019, 19, 3673–3685. [Google Scholar] [CrossRef] [PubMed]
- Naik, B.; Ghosh, N. A review on chemical methodologies for preparation of mesoporous silica and alumina based materials. Recent Pat. Nanotechnol. 2009, 3, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Malik, M.M.; Purohit, R. Synthesis methods of mesoporous silica materials. Mater. Today Proc. 2017, 4, 350–357. [Google Scholar] [CrossRef]
- Alothman, Z.A. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Pal, N.; Bhaumik, A. Soft templating strategies for the synthesis of mesoporous materials: Inorganic, organic-inorganic hybrid and purely organic solids. Adv. Colloid Interface Sci. 2013, 189–190, 21–41. [Google Scholar] [CrossRef]
- Perego, C.; Millini, R. Porous materials in catalysis: Challenges for mesoporous materials. Chem. Soc. Rev. 2013, 42, 3956–3976. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, D. An overview of the synthesis of ordered mesoporous materials. Chem. Commun. 2013, 49, 943–946. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Chen, H.; Yang, H.; Xi, Y. Highly ordered and hexagonal mesoporous silica materials with large specific surface from natural rectorite mineral. Microporous Mesoporous Mater. 2019, 279, 53–60. [Google Scholar] [CrossRef]
- Asefa, T.; Tao, Z. Mesoporous silica and organosilica materials—Review of their synthesis and organic functionalization. Can. J. Chem. 2012, 90, 1015–1031. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, R.; Setiabudi, H.D.; Nanda, S.; Vo, D.V.N. Advanced synthesis strategies of mesoporous SBA-15 supported catalysts for catalytic reforming applications: A state-of-the-art review. Appl. Catal. A Gen. 2018, 559, 57–74. [Google Scholar] [CrossRef]
- Meynen, V.; Cool, P.; Vansant, E.F. Verified syntheses of mesoporous materials. Microporous Mesoporous Mater. 2009, 125, 170–223. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-based mesoporous organic-inorganic hybrid materials. Angew. Chemie Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric sufactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Huo, Q.; Margolese, D.I.; Ciesla, U.; Feng, P.; Gier, T.E.; Sieger, P.; Leon, R.; Petroff, P.M.; Schüth, F.; Stucky, G.D. Generalized synthesis of periodic surfactant/inorganic composite materials. Nature 1994, 368, 317–321. [Google Scholar] [CrossRef]
- Liu, M.C.; Sheu, H.S.; Cheng, S. Drying induced phase transformation of mesoporous silica. Chem. Commun. 2002, 23, 2854–2855. [Google Scholar] [CrossRef] [PubMed]
- Che, S.; Sakamoto, Y.; Yoshitake, H.; Terasaki, O.; Tatsumi, T. Synthesis and characterization of Mo–SBA-1 cubic mesoporous molecular sieves. J. Phys. Chem. B 2001, 105, 10565–10572. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Kaneda, M.; Terasaki, O.; Zhao, D.Y.; Kim, J.M.; Stucky, G.; Shin, H.J.; Ryoo, R. Direct imaging of the pores and cages of three-dimensional mesoporous materials. Nature 2000, 408, 449–453. [Google Scholar] [CrossRef]
- Srinivasu, P.; Vinu, A. Three-dimensional mesoporous gallosilicate with Pm3n symmetry and its unusual catalytic performances. Chem. Eur. J. 2008, 14, 3553–3561. [Google Scholar] [CrossRef]
- Michorczyk, P.; Ogonowski, J.; Niemczyk, M. Investigation of catalytic activity of CrSBA-1 materials obtained by direct method in the dehydrogenation of propane with CO2. Appl. Catal. A Gen. 2010, 374, 142–149. [Google Scholar] [CrossRef]
- Kao, H.M.; Cheng, C.C.; Ting, C.C.; Hwang, L.Y. Phase control of cubic SBA-1 mesostructures via alcohol-assisted synthesis. J. Mater. Chem. 2005, 15, 2989–2992. [Google Scholar] [CrossRef]
- Huo, Q.; Margolese, D.I.; Ciesla, U.; Demuth, D.G.; Feng, P.; Gier, T.E.; Sieger, P.; Firouzi, A.; Chmelka, B.F.; Schüth, F.; et al. Organization of organic molecules with inorganic molecular species into nanocomposite biphase arrays. Chem. Mater. 1994, 6, 1176–1191. [Google Scholar] [CrossRef]
- Huo, Q.; Leon, R.; Petroff, P.M.; Stucky, G.D. Mesostructure design with gemini surfactants: Supercage formation in a three-dimensional hexagonal array. Science 1995, 268, 1324–1327. [Google Scholar] [CrossRef]
- Kim, M.J.; Ryoo, R. Synthesis and pore size control of cubic mesoporous silica SBA-1. Chem. Mater. 1999, 11, 487–491. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Ryoo, R.; Kim, J.M. Characterization of high-quality MCM-48 and SBA-1 mesoporous silicas. Chem. Mater. 1999, 11, 2568–2572. [Google Scholar] [CrossRef]
- Che, S.; Sakamoto, Y.; Terasaki, O.; Tatsumi, T. Control of crystal morphology of SBA-1 mesoporous silica. Chem. Mater. 2001, 13, 2237–2239. [Google Scholar] [CrossRef]
- Kao, H.M.; Liao, Y.W.; Ting, C.C. Synthesis of cubic mesoporous silica SBA-1 with bulky headgroup surfactant cetyltripropylammonium bromide. Microporous Mesoporous Mater. 2007, 98, 80–88. [Google Scholar] [CrossRef]
- Che, S.; Sakamoto, Y.; Terasaki, O.; Tatsumi, T. Synthesis and morphology control of SBA-1 mesoporous silica with surfactant of cetyltrimethylammonium bromide (CTMABr). Chem. Lett. 2002, 31, 214–215. [Google Scholar] [CrossRef]
- Chao, M.C.; Wang, D.S.; Lin, H.P.; Mou, C.Y. Control of single crystal morphology of SBA-1 mesoporous silica. J. Mater. Chem. 2003, 13, 2853–2854. [Google Scholar] [CrossRef]
- Chao, M.C.; Lin, H.P.; Wang, D.S.; Tang, C.Y. Controlling the crystal morphology of mesoporous silica SBA-1. Microporous Mesoporous Mater. 2005, 83, 269–276. [Google Scholar] [CrossRef]
- Kao, H.M.; Cheng, C.C. Tetramethoxysilane as a convenient silicon source for phase control of cubic SBA-1 mesostructures. Mater. Lett. 2006, 60, 2594–2599. [Google Scholar] [CrossRef]
- Tanglumlert, W.; Imae, T.; White, T.J.; Wongkasemjit, S. Structural aspects of SBA-1 cubic mesoporous silica synthesized via a sol-gel process using a silatrane precursor. J. Am. Ceram. Soc. 2007, 90, 3992–3997. [Google Scholar] [CrossRef]
- Voronkov, M.G.; Belyaeva, V.V.; Abzaeva, K.A. Basicity of silatranes (review). Chem. Heterocycl. Compd. 2012, 47, 1330–1338. [Google Scholar] [CrossRef]
- Phiriyawirut, P.; Jamieson, A.M.; Wongkasemjit, S. VS-1 zeolite synthesized directly from silatrane. Microporous Mesoporous Mater. 2005, 77, 203–213. [Google Scholar] [CrossRef]
- Thanabodeekij, N.; Tanglumlert, W.; Gulari, E.; Wongkasemjit, S. Synthesis of Ti-MCM-41 directly from silatrane and titanium glycolate and its catalytic activity. Appl. Organomet. Chem. 2005, 19, 1047–1054. [Google Scholar] [CrossRef]
- Tanglumlert, W.; Imae, T.; White, T.J.; Wongkasemjit, S. Preparation of highly ordered Fe-SBA-1 and Ti-SBA-1 cubic mesoporous silica via sol-gel processing of silatrane. Mater. Lett. 2008, 62, 4545–4548. [Google Scholar] [CrossRef]
- Tanglumlert, W.; Imae, T.; White, T.J.; Wongkasemjit, S. Styrene oxidation with H2O2 over Fe- and Ti-SBA-1 mesoporous silica. Catal. Commun. 2009, 10, 1070–1073. [Google Scholar] [CrossRef]
- Vinu, A.; Murugesan, V.; Hartmann, M. Pore size engineering and mechanical stability of the cubic mesoporous molecular sieve SBA-1. Chem. Mater. 2003, 15, 1385–1393. [Google Scholar] [CrossRef]
- Kao, H.M.; Ting, C.C.; Chiang, A.S.T.; Teng, C.C.; Chen, C.H. Facile synthesis of stable cubic mesoporous silica SBA-1 over a broad temperature range with the aid of D-fructose. Chem. Commun. 2005, 8, 1058–1060. [Google Scholar] [CrossRef]
- Pantazis, C.C.; Pomonis, P.J. Mesostructure design via poly(acrylic acid)-CnTAB complexes: A new route for SBA-1 mesoporous silica. Chem. Mater. 2003, 15, 2299–2300. [Google Scholar] [CrossRef]
- Morey, M.S.; Davidson, A.; Stucky, G.D. Silica-based, cubic mesostructures: Synthesis, characterization and relevance for catalysis. J. Porous Mater. 1998, 5, 195–204. [Google Scholar] [CrossRef]
- Huo, Q.; Margolese, D.I.; Stucky, G.D. Surfactant control of phases in the synthesis of mesoporous silica-based materials. Chem. Mater. 1996, 8, 1147–1160. [Google Scholar] [CrossRef]
- Dai, L.-X.; Teng, Y.-H.; Tabata, K.; Suzuki, E.; Tatsumi, T. Mo-containing SBA-1 mesoporous molecular sieves as catalysts. Chem. Lett. 2000, 29, 794–795. [Google Scholar] [CrossRef]
- Dai, L.-X.; Tabata, K.; Suzuki, E.; Tatsumi, T. Synthesis and characterization of V-SBA-1 cubic mesoporous molecular sieves. Chem. Mater. 2001, 13, 208–212. [Google Scholar] [CrossRef]
- Hartmann, M.; Vinu, A.; Elangovan, S.P.; Murugesan, V.; Böhlmann, W. Direct synthesis and catalytic evaluation of AlSBA-1. Chem. Commun. 2002, 11, 1238–1239. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.M.; Hemalatha, P.; Ganesh, M.; Palanichamy, M.; Jang, H.T. Solvent free synthesis of coumarin derivative by the use of AlSBA-1 molecular sieves. J. Ind. Eng. Chem. 2014, 20, 953–960. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Palanichamy, M.; Murugesan, V. Acetalization of heptanal over Al-SBA-1 molecular sieve. Catal. Commun. 2010, 12, 299–303. [Google Scholar] [CrossRef]
- Ji, D.; Ren, T.; Yan, L.; Suo, J. Synthesis of Ti-incorporated SBA-1 cubic mesoporous molecular sieves. Mater. Lett. 2003, 57, 4474–4477. [Google Scholar] [CrossRef]
- Ji, D.; Zhao, R.; Lv, G.; Qian, G.; Yan, L.; Suo, J. Direct synthesis, characterization and catalytic performance of novel Ti-SBA-1 cubic mesoporous molecular sieves. Appl. Catal. A Gen. 2005, 281, 39–45. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X. Synthesis, characterization and catalytic application of Cr-SBA-1 mesoporous molecular sieves. J. Mol. Catal. A Chem. 2007, 261, 225–231. [Google Scholar] [CrossRef]
- Michorczyk, P.; Pietrzyk, P.; Ogonowski, J. Preparation and characterization of SBA-1-supported chromium oxide catalysts for CO2 assisted dehydrogenation of propane. Microporous Mesoporous Mater. 2012, 161, 56–66. [Google Scholar] [CrossRef]
- Brzozowski, R.; Vinu, A. Alkylation of naphthalene over mesoporous Ga-SBA-1 catalysts. Top. Catal. 2009, 52, 1001–1004. [Google Scholar] [CrossRef]
- Imran, G.; Maheswari, R. Mn-incorporated SBA-1 cubic mesoporous silicates: Synthesis and characterization. Mater. Chem. Phys. 2015, 161, 237–242. [Google Scholar] [CrossRef]
- Gracia, M.D.; Jurado, M.J.; Luque, R.; Campelo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A. Modified SBA-1 materials for the Knoevenagel condensation under microwave irradiation. Microporous Mesoporous Mater. 2009, 118, 87–92. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S. Optimization of alkaline earth metal oxide and hydroxide catalysts for base-catalyzed reactions. Adv. Catal. 2006, 49, 239–302. [Google Scholar] [CrossRef]
- Yoshitake, H.; Yokoi, T.; Tatsumi, T. Adsorption of chromate and arsenate by amino-functionalized MCM-41 and SBA-1. Chem. Mater. 2002, 14, 4603–4610. [Google Scholar] [CrossRef]
- Kao, H.-M.; Liao, C.-H.; Palani, A.; Liao, Y.-C. One-pot synthesis of ordered and stable cubic mesoporous silica SBA-1 functionalized with amino functional groups. Microporous Mesoporous Mater. 2008, 113, 212–223. [Google Scholar] [CrossRef]
- Kao, H.-M.; Wu, J.-D.; Cheng, C.-C.; Chiang, A.S.T. Direct synthesis of vinyl-functionalized cubic mesoporous silica SBA-1. Microporous Mesoporous Mater. 2006, 88, 319–328. [Google Scholar] [CrossRef]
- Pan, Y.-C.; Wu, H.-Y.; Lee, L.-P.; Jheng, G.-L.; Fey, G.T.K.; Kao, H.-M. Cyanide- and carboxylate-functionalized cubic mesoporous silicas SBA-1: Synthesis, characterization and reactivity of organic functional groups. Microporous Mesoporous Mater. 2009, 123, 78–90. [Google Scholar] [CrossRef]
- Tsai, H.-H.G.; Chiu, P.-J.; Jheng, G.-L.; Ting, C.-C.; Pan, Y.-C.; Kao, H.-M. Synthesis and solid-state NMR characterization of cubic mesoporous silica SBA-1 functionalized with sulfonic acid groups. J. Colloid Interface Sci. 2001, 359, 86–94. [Google Scholar] [CrossRef]
- Yang, H.; Ma, Z.; Wang, Y.; Wang, Y.; Fang, L. Hoveyda-Grubbs catalyst confined in the nanocages of SBA-1: Enhanced recyclability for olefin metathesis. Chem. Commun. 2010, 46, 8659–8661. [Google Scholar] [CrossRef]
- Li, H.; Sakamoto, Y.; Li, Y.; Terasaki, O.; Thommes, M.; Che, S. Synthesis of carbon replicas of SBA-1 and SBA-7 mesoporous silicas. Microporous Mesoporous Mater. 2006, 95, 193–199. [Google Scholar] [CrossRef]
- Liu, M.-C.; Chang, C.-S.; Chan, J.C.C.; Sheu, H.-S.; Cheng, S. An alkaline route to prepare hydrothermally stable cubic Pm3n mesoporous silica using CTEA template. Microporous Mesoporous Mater. 2009, 121, 41–51. [Google Scholar] [CrossRef]
- Lin, T.-H.; Chen, C.-H.; Chang, C.-S.; Liu, M.-C.; Huang, S.-J.; Cheng, S. Cubic Pm3n mesoporous aluminosilicate assembled from zeolite seeds as strong acidic catalysts. Catal. Sci. Technol. 2015, 5, 3182–3193. [Google Scholar] [CrossRef]
- Wang, J.-G.; Zhou, H.-J.; Sun, P.-C.; Ding, D.-T.; Chen, T.-H. Hollow carved single-crystal mesoporous silica templated by mesomorphous polyelectrolyte-surfactant complexes. Chem. Mater. 2010, 22, 3829–3831. [Google Scholar] [CrossRef]
- Li, N.; Wang, J.-G.; Xu, J.-X.; Liu, J.-Y.; Zhou, H.-J.; Sun, P.-C.; Chen, T.-H. Synthesis of hydrothermally stable, hierarchically mesoporous aluminosilicate Al-SBA-1 and their catalytic properties. Nanoscale 2012, 4, 2150–2156. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-H.; Chen, C.-C.; Jang, L.-Y.; Lee, J.-F.; Cheng, S. Preparation and catalytic properties of mesoporous titanosilicate of cubic Pm3n structure. Microporous Mesoporous Mater. 2014, 198, 194–202. [Google Scholar] [CrossRef]
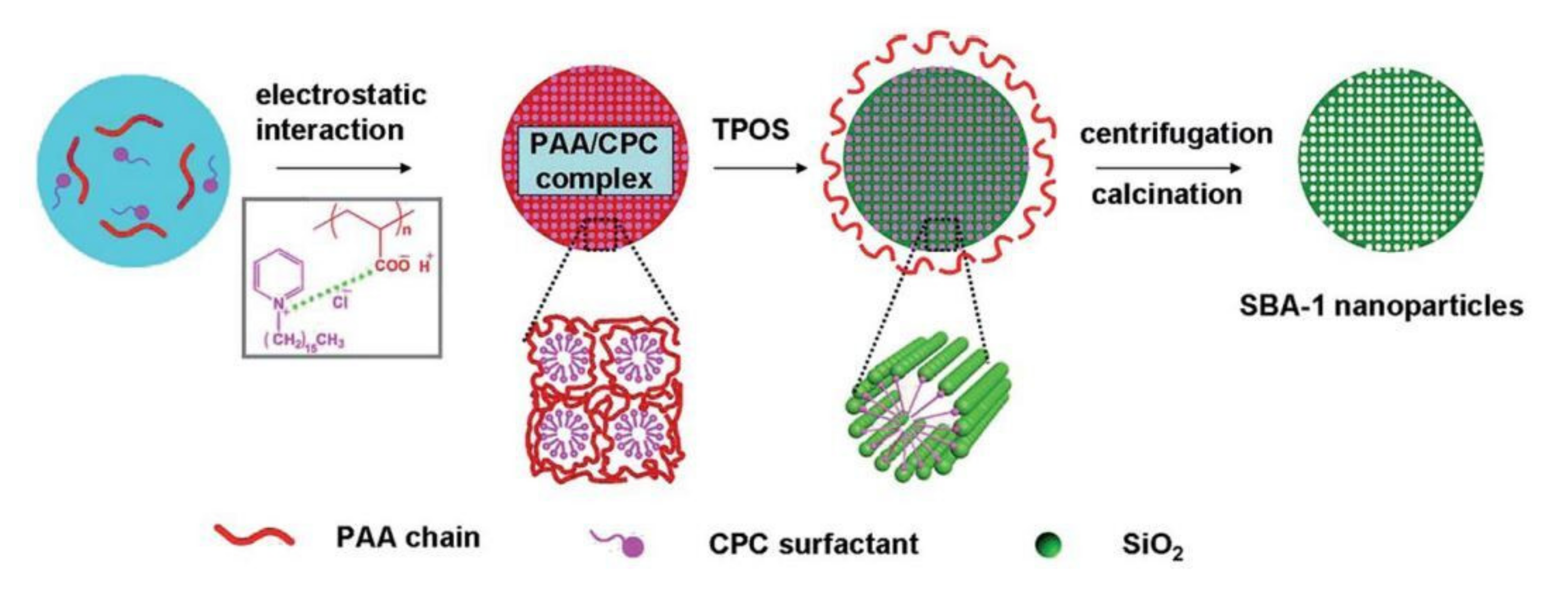
- Li, N.; Wang, J.-G.; Zhou, H.-J.; Sun, P.-C.; Chen, T.-H. Facile fabrication of hierarchically nanoporous SBA-1 nanoparticles. RSC Adv. 2012, 2, 2229–2231. [Google Scholar] [CrossRef]
- Xu, J.; Liu, W.; Yu, Y.; Du, J.; Li, N.; Xu, L. Synthesis of mono-dispersed mesoporous SBA-1 nanoparticles with tunable pore size and their application in lysozyme immobilization. RSC Adv. 2014, 4, 37470–37478. [Google Scholar] [CrossRef]
- Saikia, D.; Deka, J.R.; Wu, C.-E.; Yang, Y.-C.; Kao, H.-M. pH responsive selective protein adsorption by carboxylic acid functionalized large pore mesoporous silica nanoparticles SBA-1. Mater. Sci. Eng. C 2019, 94, 344–356. [Google Scholar] [CrossRef]
- Lin, C.-H.; Deka, J.R.; Wu, C.-E.; Tsai, C.-H.; Saikia, D.; Yang, Y.-C.; Kao, H.-M. Bifunctional cage-type cubic mesoporous silica SBA-1 nanoparticles for selective adsorption of dyes. Chem. An. Asian J. 2017, 12, 1314–1325. [Google Scholar] [CrossRef]
- Pérez-Mendoza, M.; Gonzalez, J.; Wright, P.A.; Seaton, N.A. Structure of the mesoporous silica SBA-2, determined by a percolation analysis of adsorption. Langmuir 2004, 20, 9856–9860. [Google Scholar] [CrossRef]
- Shi, C.; Wang, W.; Liu, N.; Xu, X.; Wang, D.; Zhang, M.; Sun, P.; Chen, T. Low temperature oxidative desulfurization with hierarchically mesoporous titaniumsilicate Ti-SBA-2 single crystals. Chem. Commun. 2015, 51, 11500–11503. [Google Scholar] [CrossRef]
- Zhao, D.; Wan, Y.; Zhou, W. Ordered Mesoporous Materials; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 171–181. [Google Scholar]
- Sakamoto, Y.; Díaz, I.; Terasaki, O.; Zhao, D.; Pérez-Pariente, J.; Kim, J.M.; Stucky, G.D. Three-dimensional cubic mesoporous structures of SBA-12 and related materials by electron crystallography. J. Phys. Chem. B 2002, 106, 3118–3123. [Google Scholar] [CrossRef]
- Garcia-Bennett, A.E.; Williamson, S.; Wright, P.A.; Shannon, I.J. Control of structure, pore size and morphology of three-dimensionally ordered mesoporous silicas prepared using the dicationic surfactant [CH3(CH2)15N(CH3)2(CH2)3N(CH3)3]Br2. J. Mater. Chem. 2002, 12, 3533–3540. [Google Scholar] [CrossRef]
- Zhou, W.; Hunter, H.M.A.; Wright, P.A.; Ge, Q.; Thomas, J.M. Imaging the pore structure and polytypic intergrowths in mesoporous silica. J. Phys. Chem. B 1998, 102, 2–5. [Google Scholar] [CrossRef]
- Pérez-Pariente, J.; Díaz, I.; Mohino, F.; Sastre, E. Selective synthesis of fatty monoglycerides by using functionalised mesoporous catalysts. Appl. Catal. A Gen. 2003, 254, 173–188. [Google Scholar] [CrossRef]
- Hunter, H.M.A.; Wright, P.A. Synthesis and characterisation of the mesoporous silicate SBA-2 and its performance as an acid catalyst. Microporous Mesoporous Mater. 2001, 43, 361–373. [Google Scholar] [CrossRef]
- Hunter, H.M.A.; Garcia-Bennett, A.E.; Shannon, I.J.; Zhou, W.; Wright, P.A. Particle morphology and microstructure in the mesoporous silicate SBA-2. J. Mater. Chem. 2002, 12, 20–23. [Google Scholar] [CrossRef]
- Pérez-Mendoza, M.; Gonzalez, J.; Wright, P.A.; Seaton, N.A. Elucidation of the pore structure of SBA-2 using Monte Carlo simulation to interpret experimental data for the adsorption of light hydrocarbons. Langmuir 2004, 20, 7653–7658. [Google Scholar] [CrossRef]
- Pérez-Mendoza, M.; González, J.; Ferreiro-Rangel, C.A.; Lozinska, M.M.; Fairén-Jiménez, D.; Düren, T.; Wright, P.A.; Seaton, N.A. Pore-network connectivity and molecular sieving of normal and isoalkanes in the mesoporous silica SBA-2. J. Phys. Chem. C 2014, 118, 10183–10190. [Google Scholar] [CrossRef]
- Ferreiro-Rangel, C.A.; Lozinska, M.M.; Wright, P.A.; Seaton, N.A.; Düren, T. Kinetic Monte Carlo simulation of the synthesis of periodic mesoporous silicas SBA-2 and STAC-1: Generation of realistic atomistic models. J. Phys. Chem. C 2012, 116, 20966–20974. [Google Scholar] [CrossRef]
- Ferreiro-Rangel, C.A.; Seaton, N.A.; Düren, T. Grand-canonical Monte Carlo adsorption studies on SBA-2 periodic mesoporous silicas. J. Phys. Chem. C 2014, 118, 25441–25446. [Google Scholar] [CrossRef]
- Zapilko, C.; Anwander, R. Size-selective surface silylation of cagelike mesoporous silica SBA-2 with disilazane reagents. Chem. Mater. 2006, 18, 1479–1482. [Google Scholar] [CrossRef]
- Díaz, I.; Mohino, F.; Pérez-Pariente, J.; Sastre, E.; Wright, P.A.; Zhou, W. 07-P-17-A direct synthesis route to the mesoporous silicate SBA-2 bearing thiol groups. Stud. Surf. Sci. Catal. 2001, 135, 1253–1284. [Google Scholar] [CrossRef]
- Kosuge, K.; Kubo, S.; Kikukawa, N.; Takemori, M. Effect of pore structure in mesoporous silicas on VOC dynamic adsorption/desorption performance. Langmuir 2007, 23, 3095–3102. [Google Scholar] [CrossRef] [PubMed]
- Emparan-Legaspi, M.J.; Gonzalez, J.; Gonzalez-Carrillo, G.; Ceballos-Magaña, S.G.; Canales-Vazquez, J.; Aguayo-Villarreal, I.A.; Muñiz-Valencia, R. Dynamic adsorption separation of benzene/cyclohexane mixtures on micro-mesoporous silica SBA-2. Microporous Mesoporous Mater. 2020, 294, 109942. [Google Scholar] [CrossRef]
- Inagaki, S.; Guan, S.; Fukushima, Y.; Ohsuna, T.; Terasaki, O. Novel mesoporous materials with a uniform distribution of organic groups and inorganic oxide in their frameworks. J. Am. Chem. Soc. 1999, 121, 9611–9614. [Google Scholar] [CrossRef]
- Guan, S.; Inagaki, S.; Ohsuna, T.; Terasaki, O. Cubic hybrid organic-inorganic mesoporous crystal with a decaoctahedral shape. J. Am. Chem. Soc. 2000, 122, 5660–5661. [Google Scholar] [CrossRef]
- Guan, S.; Inagaki, S.; Ohsuna, T.; Terasaki, O. Hybrid ethane-siloxane mesoporous materials with cubic symmetry. Microporous Mesoporous Mater. 2001, 44–45, 165–172. [Google Scholar] [CrossRef]
- Dhepe, P.L.; Fukuoka, A.; Ichikawa, M. Novel fabrication and catalysis of nano-structured Rh and RhPt alloy particles occluded in ordered mesoporous silica templates using supercritical carbon dioxide. Phys. Chem. Chem. Phys. 2003, 5, 5565–5573. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Guan, S.; Inagaki, S. Adsorption and thermogravimetric characterization of mesoporous materials with uniform organic-inorganic frameworks. J. Phys. Chem. B 2001, 105, 681–689. [Google Scholar] [CrossRef]
- Fukuoka, A.; Araki, H.; Sakamoto, Y.; Inagaki, S.; Fukushima, Y.; Ichikawa, M. Palladium nanowires and nanoparticles in mesoporous silica templates. Inorg. Chim. Acta 2003, 350, 371–378. [Google Scholar] [CrossRef]
- Fukuoka, A.; Higuchi, T.; Ohtake, T.; Oshio, T.; Kimura, J.-I.; Sakamoto, Y.; Shimomura, N.; Inagaki, S.; Ichikawa, M. Nanonecklaces of platinum and gold with high aspect ratios synthesized in mesoporous organosilica templates by wet hydrogen reduction. Chem. Mater. 2006, 18, 337–343. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Neimark, A.V. Density functional theory of adsorption in spherical cavities and pore size characterization of templated nanoporous silicas with cubic and three-dimensional hexagonal structures. Langmuir 2002, 18, 1550–1560. [Google Scholar] [CrossRef]
- Dhepe, P.L.; Ohashi, M.; Inagaki, S.; Ichikawa, M.; Fukuoka, A. Hydrolysis of sugars catalyzed by water-tolerant sulfonated mesoporous silicas. Catal. Lett. 2005, 102, 163–169. [Google Scholar] [CrossRef]
- Fukuoka, A.; Sakamoto, Y.; Guan, S.; Inagaki, S.; Sugimoto, N.; Fukushima, Y.; Hirahara, K.; Iijima, S.; Ichikawa, M. Novel templating synthesis of necklace-shaped mono- and bimetallic nanowires in hybrid organic-inorganic mesoporous material. J. Am. Chem. Soc. 2001, 123, 3373–3374. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, A.; Sakamoto, Y.; Araki, H.; Sugimoto, N.; Inagaki, S.; Fukushima, Y.; Ichikawa, M. Template synthesis and catalysis of metal nanowires in mesoporous silicas. Stud. Surf. Sci. Catal. 2003, 146, 23–28. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Fukuoka, A.; Higuchi, T.; Shimomura, N.; Inagaki, S.; Ichikawa, M. Synthesis of platinum nanowires in organic-inorganic mesoporous silica templates by photoreduction: Formation mechanism and isolation. J. Phys. Chem. B 2004, 108, 853–858. [Google Scholar] [CrossRef]
- Fukuoka, A.; Sakamoto, Y.; Higuchi, T.; Shimomura, N.; Ichikawa, M. Synthesis and electronic property of platinum nanowire and nanoparticle in mesoporous silica template. J. Porous Mater. 2006, 13, 231–235. [Google Scholar] [CrossRef][Green Version]
- Fukuoka, A.; Kimura, J.-I.; Oshio, T.; Sakamoto, Y.; Ichikawa, M. Preferential oxidation of carbon monoxide catalyzed by platinum nanoparticles in mesoporous silica. J. Am. Chem. Soc. 2007, 129, 10120–10125. [Google Scholar] [CrossRef]
- Fukuoka, A.; Ichikawa, M. Nanoscale fabrication of metal particles and wires using mesoporous silica templates: Electronic properties and catalytic performances in PROX reaction. Top. Catal. 2006, 40, 103–109. [Google Scholar] [CrossRef]
- Fukuoka, A.; Ichikawa, M. Fabrication of metal nanowire and nanoparticle in mesoporous silica templates and their catalytic performances. Int. J. Nanosci. 2005, 4, 957–963. [Google Scholar] [CrossRef]
- Fukuoka, A.; Dhepe, P.L. Sustainable green catalysis by supported metal nanoparticles. Chem. Rec. 2009, 9, 224–235. [Google Scholar] [CrossRef]
- Fukuoka, A.; Araki, H.; Kimura, J.; Sakamoto, Y.; Higuchi, T.; Sugimoto, N.; Inagaki, S.; Ichikawa, M. Template synthesis of nanoparticle arrays of gold, platinum and palladium in mesoporous silica films and powders. J. Mater. Chem. 2004, 14, 752–756. [Google Scholar] [CrossRef]
- Bore, M.T.; Pham, H.N.; Switzer, E.E.; Ward, T.L.; Fukuoka, A.; Datye, A.K. The role of pore size and structure on the thermal stability of gold nanoparticles within mesoporous silica. J. Phys. Chem. B 2005, 109, 2873–2880. [Google Scholar] [CrossRef] [PubMed]
- Gabaldon, J.P.; Bore, M.; Datye, A.K. Mesoporous silica supports for improved thermal stability in supported Au catalysts. Top. Catal. 2007, 44, 253–262. [Google Scholar] [CrossRef]
- Yang, J.; Su, H.; Lian, C.; Shang, Y.; Liu, H.; Wu, J. Understanding surface charge regulation in silica nanopores. Phys. Chem. Chem. Phys. 2020, 22, 15373–15380. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.; Xian, K.; Liu, H.; Wu, J. Flow effects on silicate dissolution and ion transport at an aqueous interface. Phys. Chem. Chem. Phys. 2019, 21, 6970–6975. [Google Scholar] [CrossRef]
- Lis, D.; Backus, E.H.G.; Hunger, J.; Parekh, S.H.; Bonn, M. Liquid flow along a solid surface reversibly alters interfacial chemistry. Science 2014, 644, 1138–1142. [Google Scholar] [CrossRef] [PubMed]

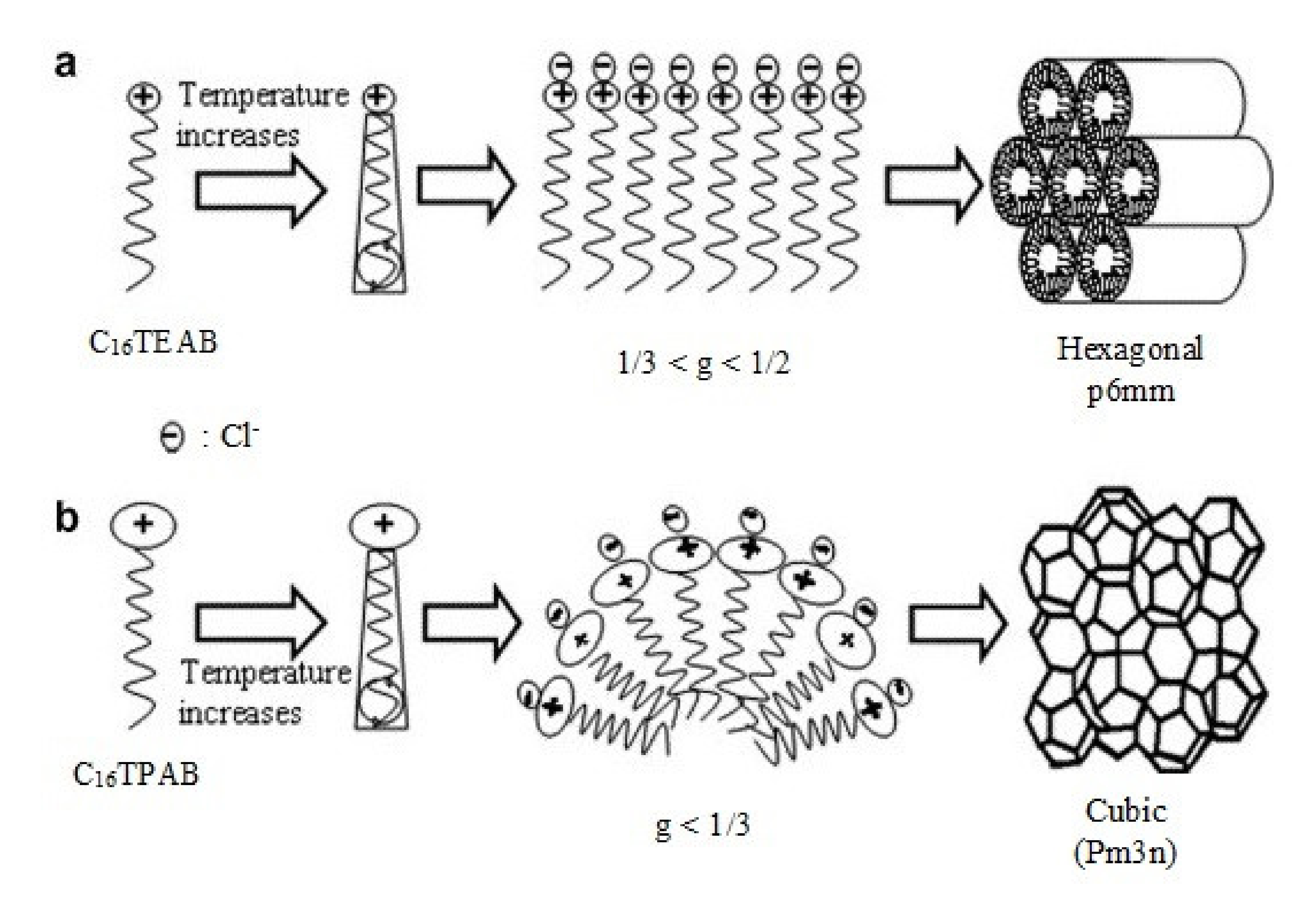
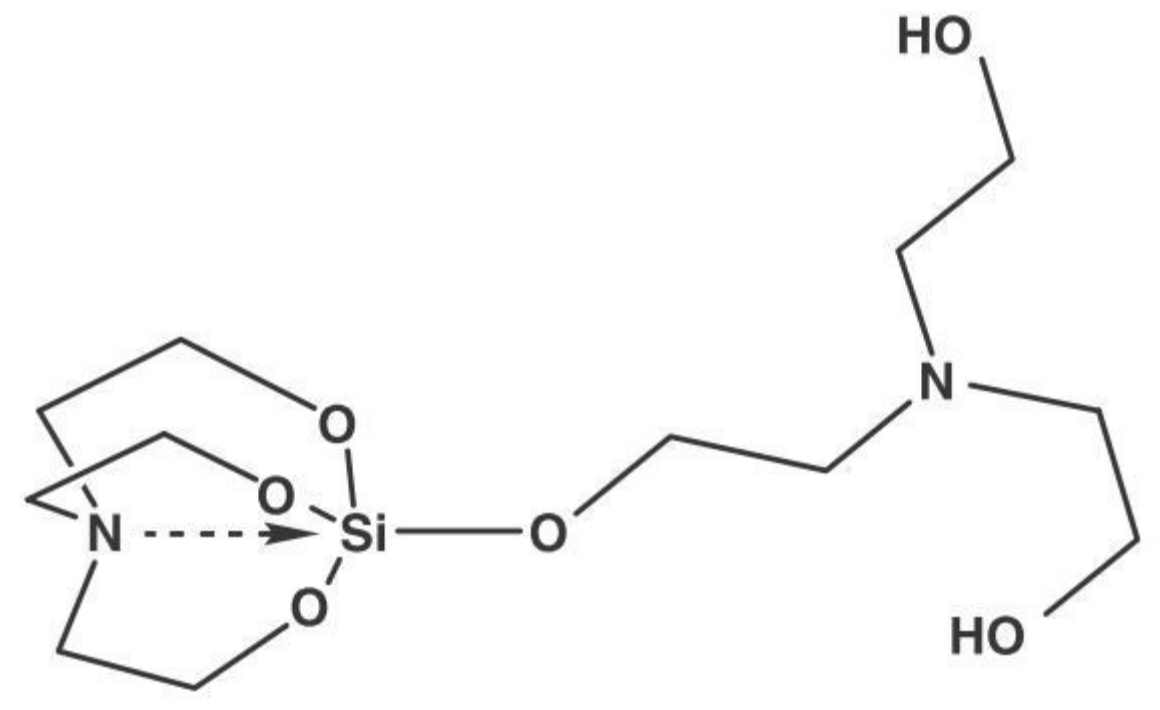
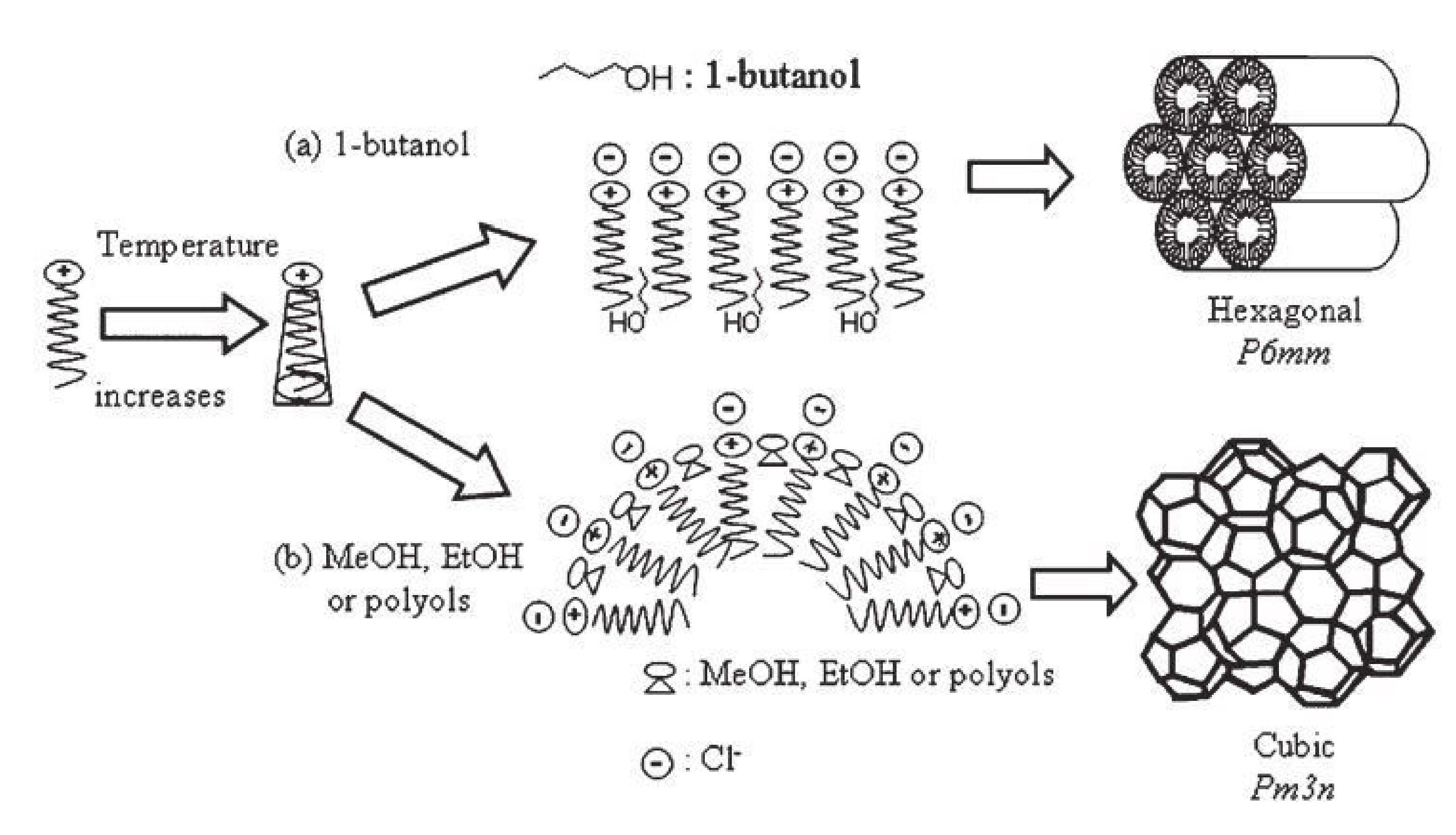

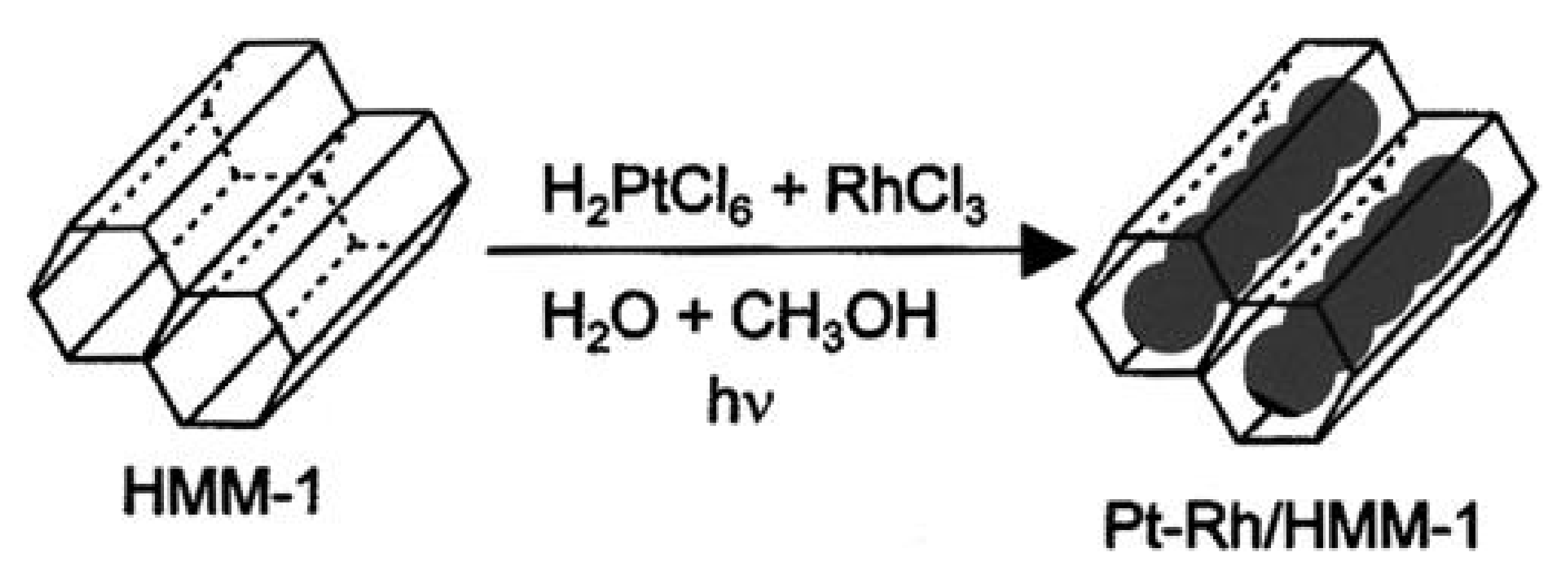
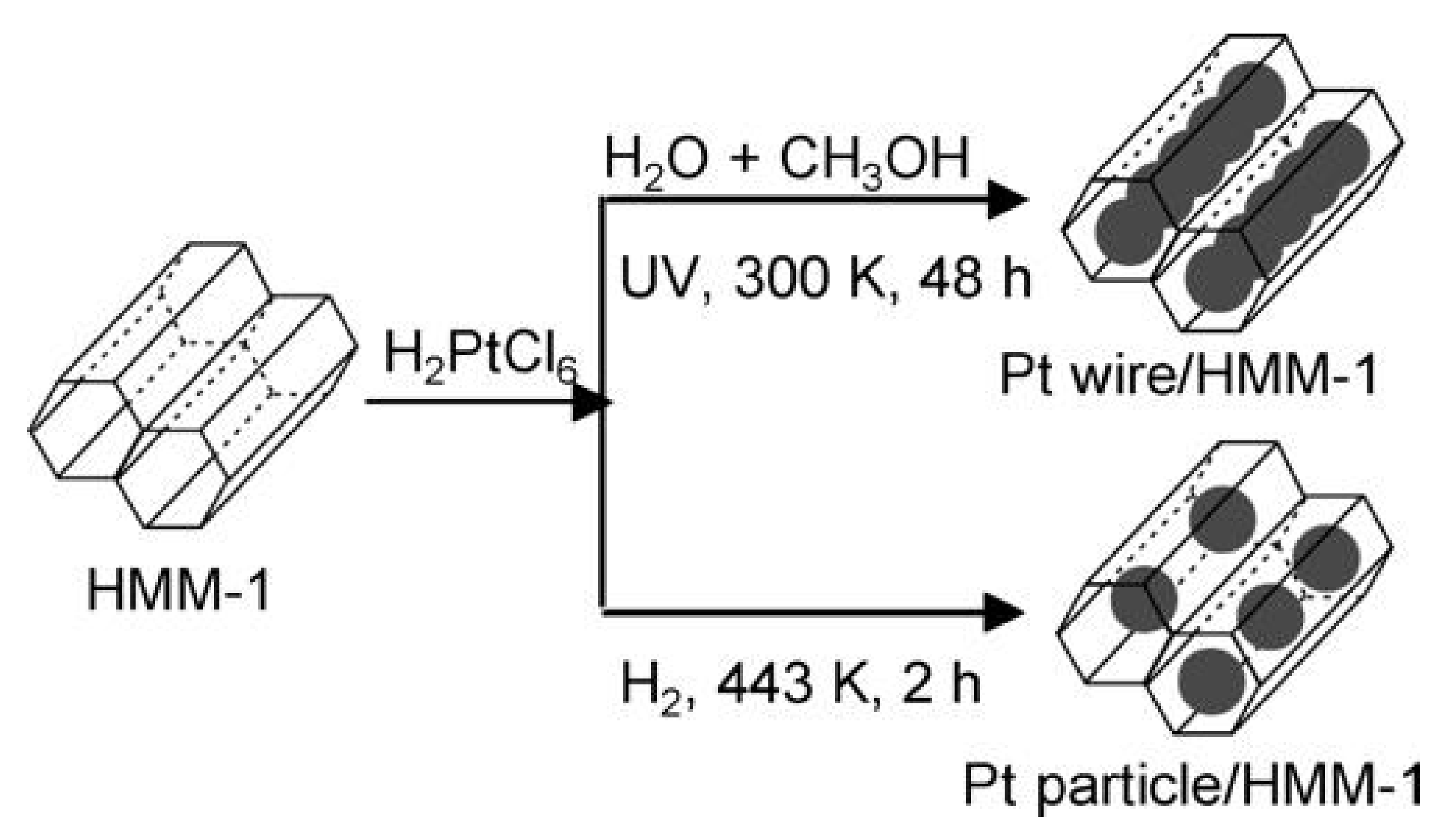
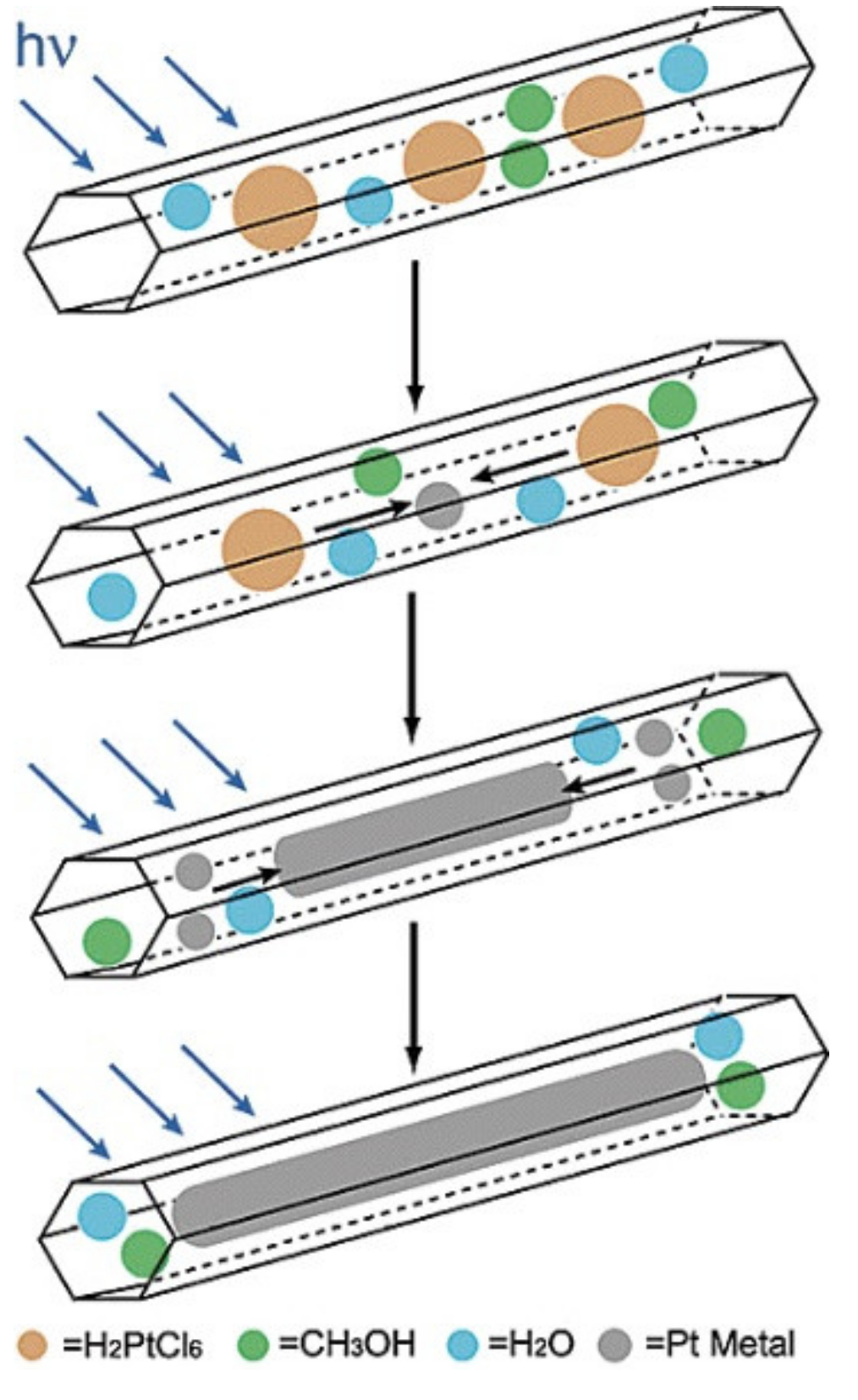
| SBA-1 | SBA-2 | HMM-1 | HMM-2 | |
|---|---|---|---|---|
| First synthesis | 1994 | 1995 | 1999 | 1999 |
| Type of material | Mesoporous Silica Materials | Periodic Mesoporous Organosilicas (PMOs) | ||
| Type of structure | 3-dimentional (3D) cubic | 3-dimentional (3D) mixed hexagonal and cubic | 2-dimentional (2D) hexagonal | 3-dimentional (3D) hexagonal |
| Symmetry | Pmn | P63/mmc | p6mm | P63/mmc |
| Surface area (m2/g) | 1200–1450 | ~600 | ~1000 | ~1200 |
| Pore size (nm) | 2.1–2.6 | 3.5 | 3.1 | 2.7 |
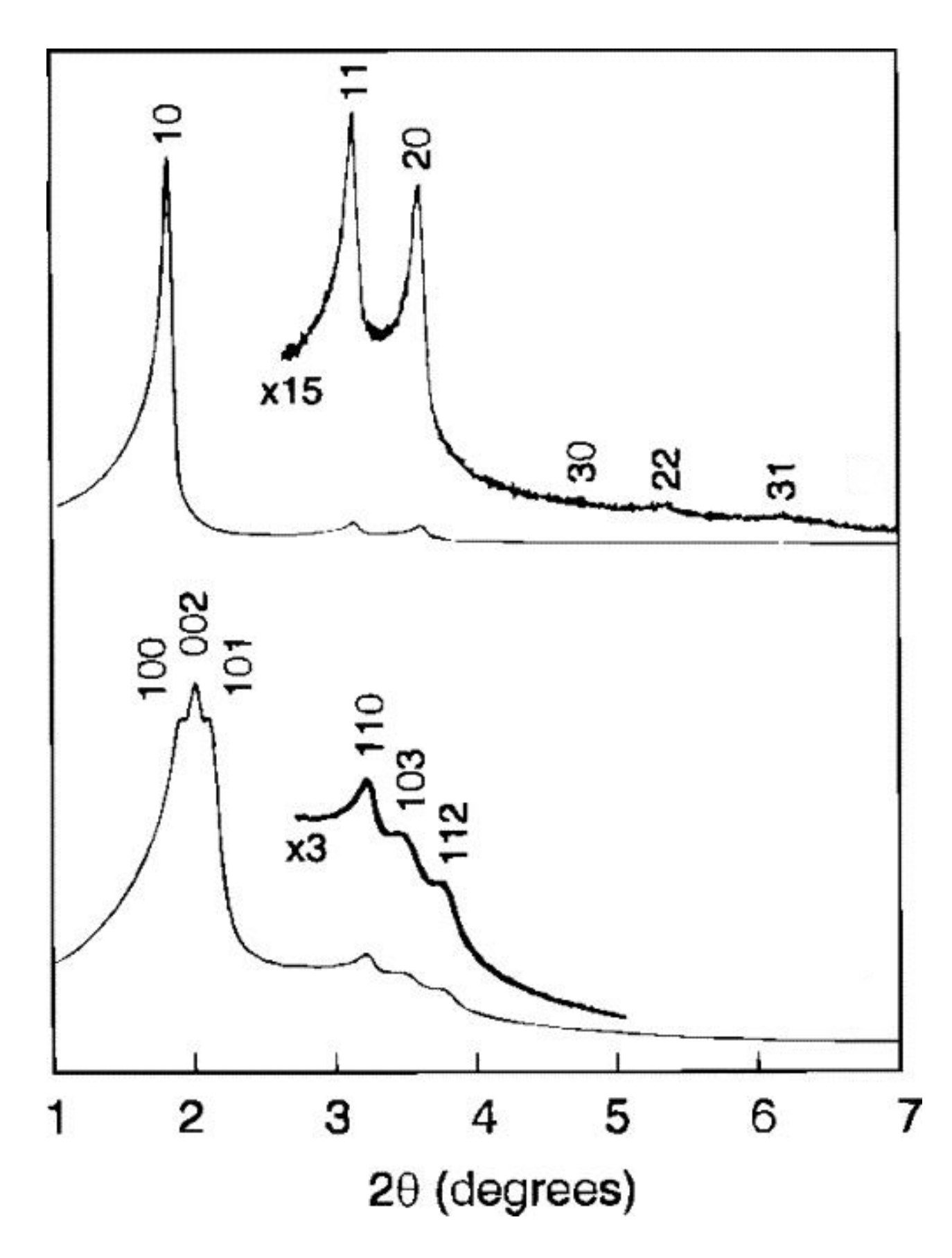
| Position of main X-ray reflections (2θ) | 2°–3° | 2°–7° | 1.8°–4° | 2°–4° |
| Crystallographic planes (hkl) | (200), (210), (211) | (100), (002), (101), (110), (103), (112), (211) | (100), (110), (200) | (100), (002), (101), (110), (103), (112) |
| Type of material | Metal/Organic Groups | Application | Ref. |
|---|---|---|---|
| Synthesis in Acidic Conditions | |||
| SBA-1 | Ti | Oxidation of styrene with hydrogen peroxide | [43] |
| SBA-1 | Fe | ||
| SBA-1 | Mo | Partial oxidation of methane with oxygen | [49] |
| SBA-1 | Al | Isomerization of n-decane | [51] |
| SBA-1 | Al | Synthesis of 7-methoxy-4-methylcoumarin | [52] |
| SBA-1 | Al Al and Mg | Acetalization of heptanal | [53] |
| SBA-1 | Ti | Epoxidation of styrene to styrene oxide | [54] |
| SBA-1 | Cr | Dehydrogenation of ethane with the use of CO2 | [56] |
| Dehydrogenation of propane with the use of CO2 | [57] | ||
| SBA-1 | Ga | tert-butylation of phenol | [25] |
| Alkylation of naphthalene with propylene | [58] | ||
| SBA-1 | Mn | Oxidation of ethylbenzene with the use of TBHP | [59] |
| SBA-1 | alkali metals Li, Na, K, Rb, Cs | Knoevenagel condensation between benzaldehyde or benzylacetone and ethyl cyanoacetate | [60] |
| SBA-1 | amino groups | Adsorption of oxyanions (chromates and arsenates) | [62] |
| SBA-1 | Hoveyd-Grubbs catalyst | Olefin metathesis catalyst | [67] |
| SBA-1 | unmodified | For obtaining highly-ordered carbon materials | [68] |
| Synthesis in Basic Conditions | |||
| SBA-1 | Al | Alkylation of 2, 4-Di-tert-butylphenol with cinnamyl alcohol | [69,70] |
| Alkylation of toluene with benzyl alcohol | [72] | ||
| SBA-1 | Ti | Oxidation of 2, 3, 6-trimethylphenol | [73] |
| SBA-1 | unmodified | Immobilization of lysozyme | [74] |
| SBA-1 | carboxyl groups | Immobilization of papain | [76] |
| SBA-1 | carboxyl and amino groups | Adsorption of toxic anionic or cationic dyes | [77] |
| SBA-2 | thiol groups | Esterification of glycerol with oleic acid (very low activity) | [92] |
| SBA-2 | sulfonic groups | Esterification of glycerol with oleic or lauric acid (very low activity) | [84] |
| SBA-2 | Ti | Oxidizing desulfurization of Diesel oil | [79] |
| SBA-2 | unmodified | Adsorbent of volatile organic compounds (adsorbent for separation of a mixture of benzene/cyclohexene) | [94] |
| HMM-1 | Rh or Pt and Rh | Hydrogenation of n-butane | [98] |
| HMM-1 | sulfonic groups | Hydrolysis of saccharose of starch | [103] |
| HMM-1 | unmodified | Matrices for syntheses of nanowires and metal nanoparticles (bimetallic Pt-Rh, Pt-Pd as well as monometallic Pt or Rh) | [104] |
| HMM-1 | Pd nanowires or nanoparticles | CO oxidation | [100] |
| HMM-2 | unmodified | Matrices for syntheses of nanowires and metal nanoparticles (Au, Pt) | [112] |
| HMM-2 | Au nanoparticles | CO oxidation | [113,114] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarmolińska, S.; Feliczak-Guzik, A.; Nowak, I. Synthesis, Characterization and Use of Mesoporous Silicas of the Following Types SBA-1, SBA-2, HMM-1 and HMM-2. Materials 2020, 13, 4385. https://doi.org/10.3390/ma13194385
Jarmolińska S, Feliczak-Guzik A, Nowak I. Synthesis, Characterization and Use of Mesoporous Silicas of the Following Types SBA-1, SBA-2, HMM-1 and HMM-2. Materials. 2020; 13(19):4385. https://doi.org/10.3390/ma13194385
Chicago/Turabian StyleJarmolińska, Sylwia, Agnieszka Feliczak-Guzik, and Izabela Nowak. 2020. "Synthesis, Characterization and Use of Mesoporous Silicas of the Following Types SBA-1, SBA-2, HMM-1 and HMM-2" Materials 13, no. 19: 4385. https://doi.org/10.3390/ma13194385
APA StyleJarmolińska, S., Feliczak-Guzik, A., & Nowak, I. (2020). Synthesis, Characterization and Use of Mesoporous Silicas of the Following Types SBA-1, SBA-2, HMM-1 and HMM-2. Materials, 13(19), 4385. https://doi.org/10.3390/ma13194385






