Physicochemical Characterization of Bilayer Hybrid Nanocellulose-Collagen as a Potential Wound Dressing
Abstract
1. Introduction
2. Materials and Methods
2.1. Collagen Extraction and Purification
2.2. Nanocellulose Preparation
2.3. Fabrication of Bilayer Hybrid Scaffold
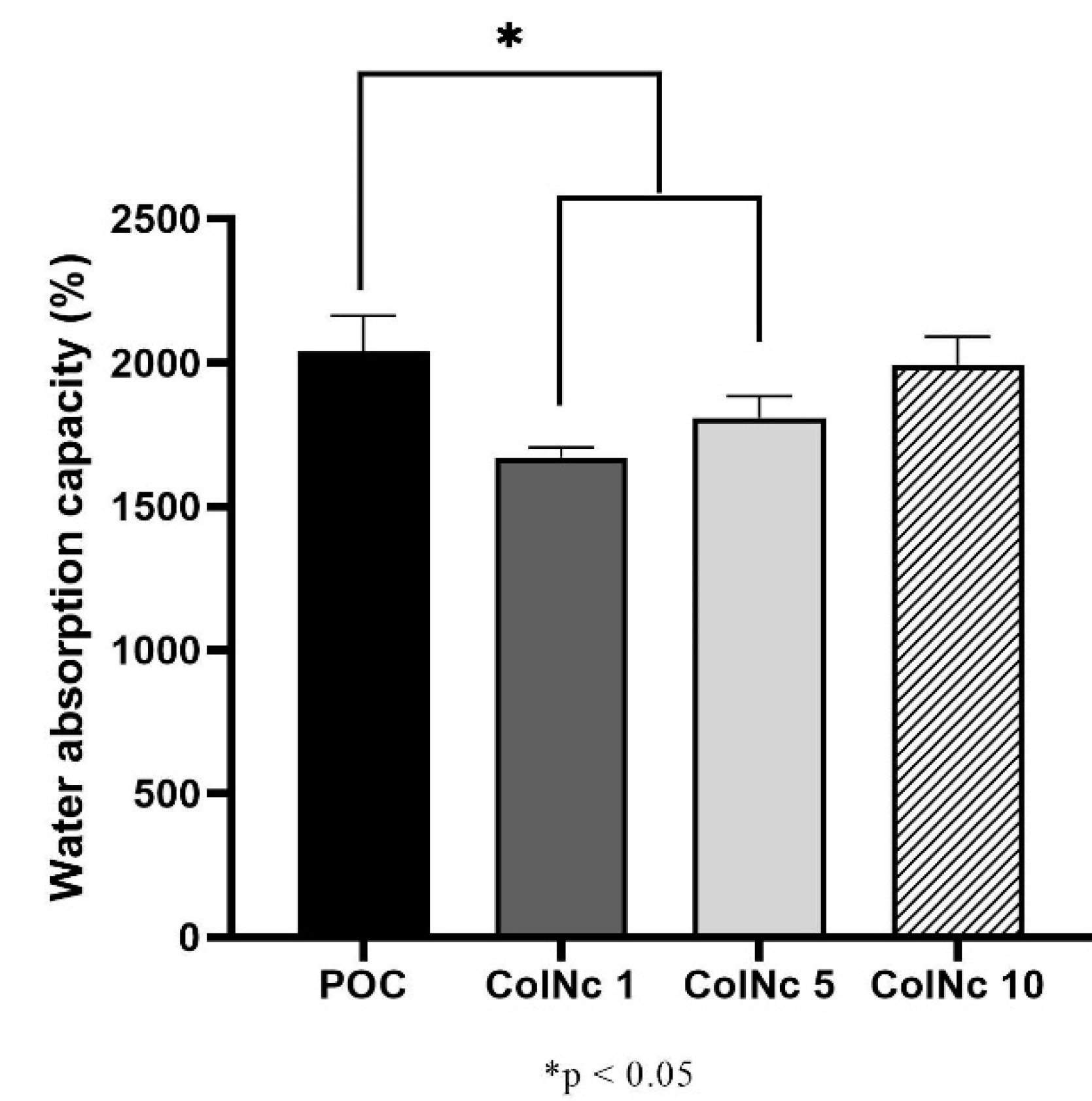
2.4. Water Absorption Capacity
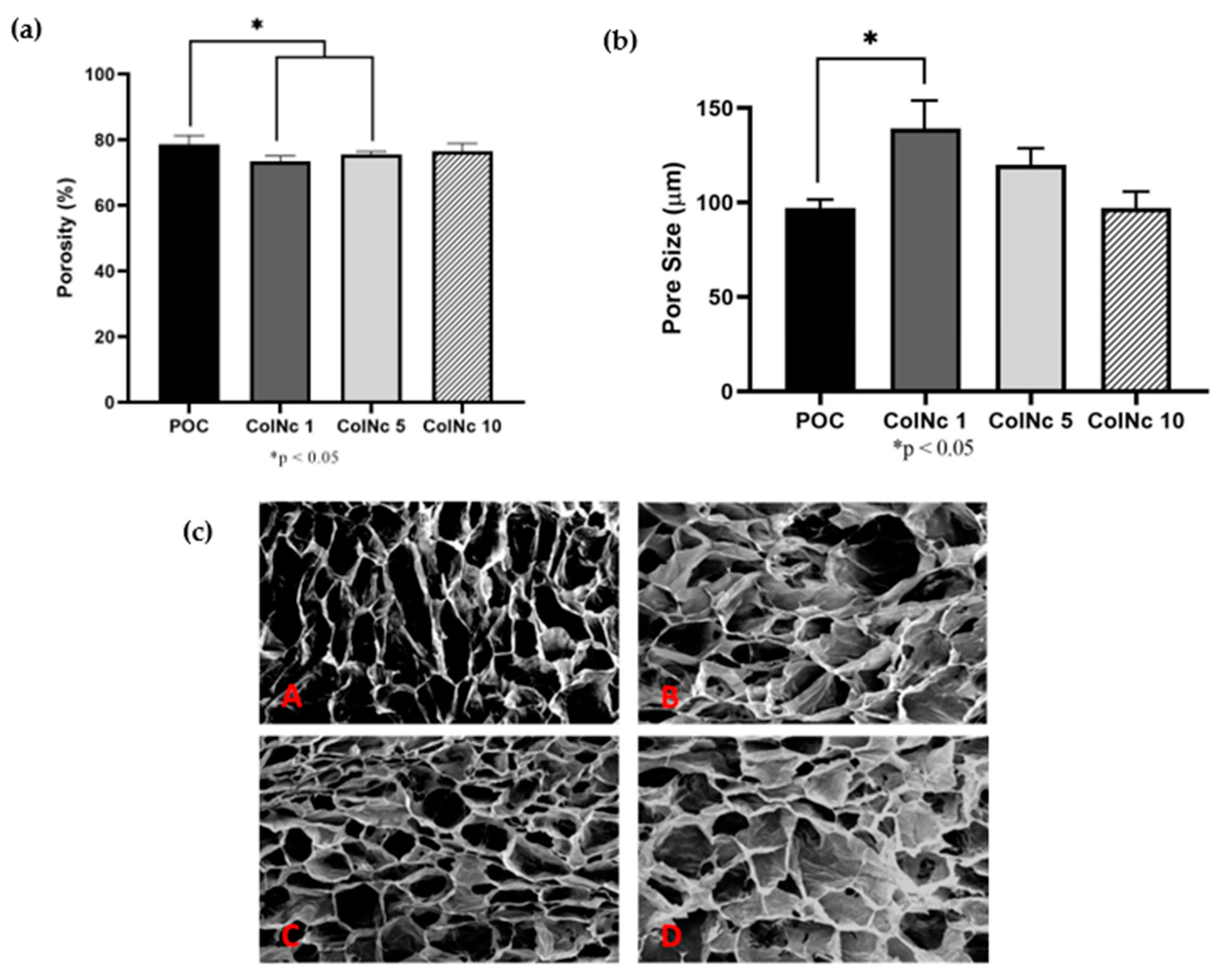
2.5. Porosity
2.6. 3D-Microstructure
2.7. Biodegradation
2.8. Chemical Characterisation of Bilayered Hybrid Bioscaffold
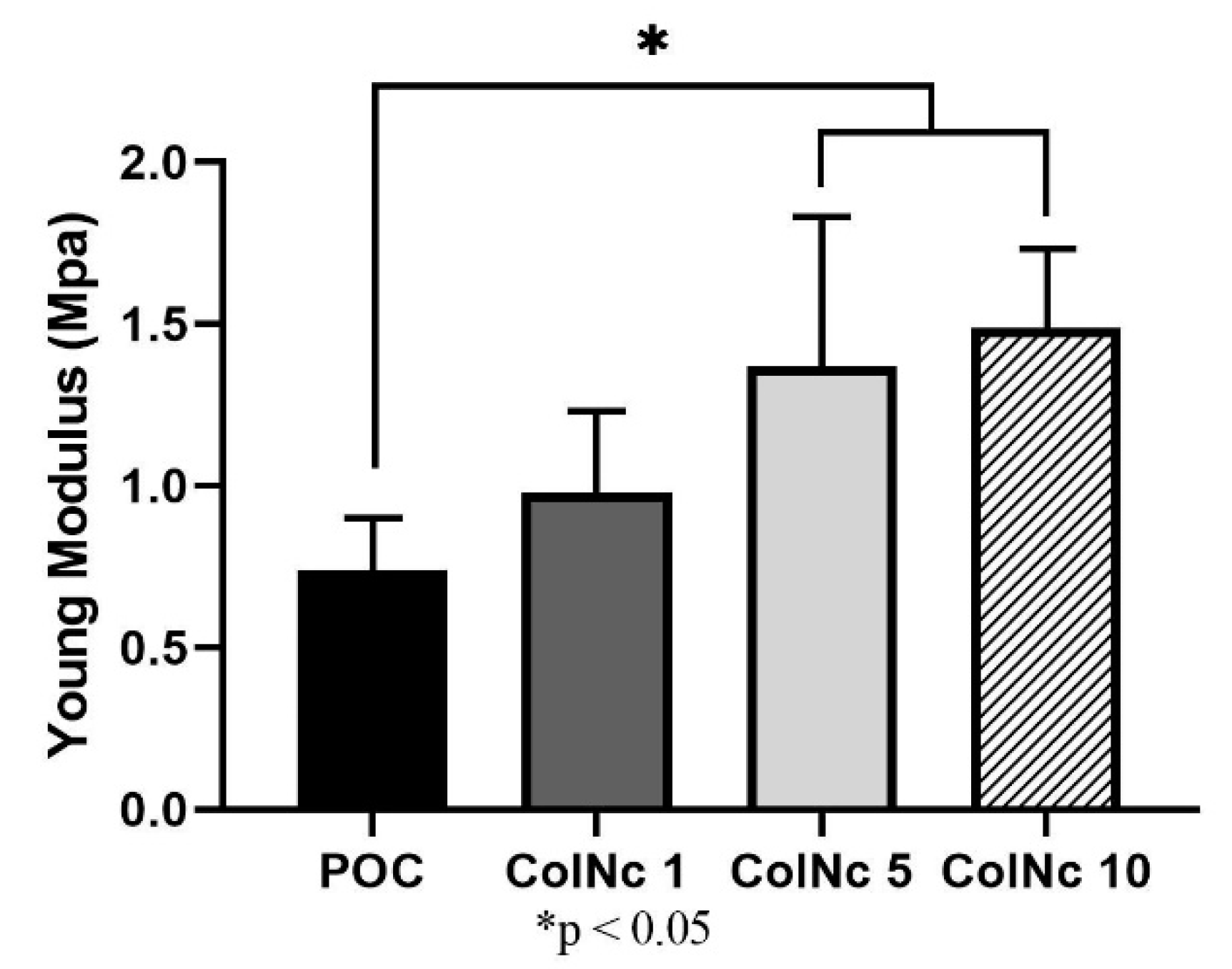
2.9. Mechanical Strength
2.10. Statistical Analysis
3. Results
3.1. Physical Characterisations of Hybrid Bioscaffold
3.1.1. Water Absorption Capacity
3.1.2. Porous Microstructure
3.1.3. Biodegradation
3.2. Chemical Characterisations of Hybrid Bioscaffold
3.2.1. Energy Dispersive X-ray Spectrometry (EDX)
3.2.2. Fourier Transform InfraRed (FTIR)
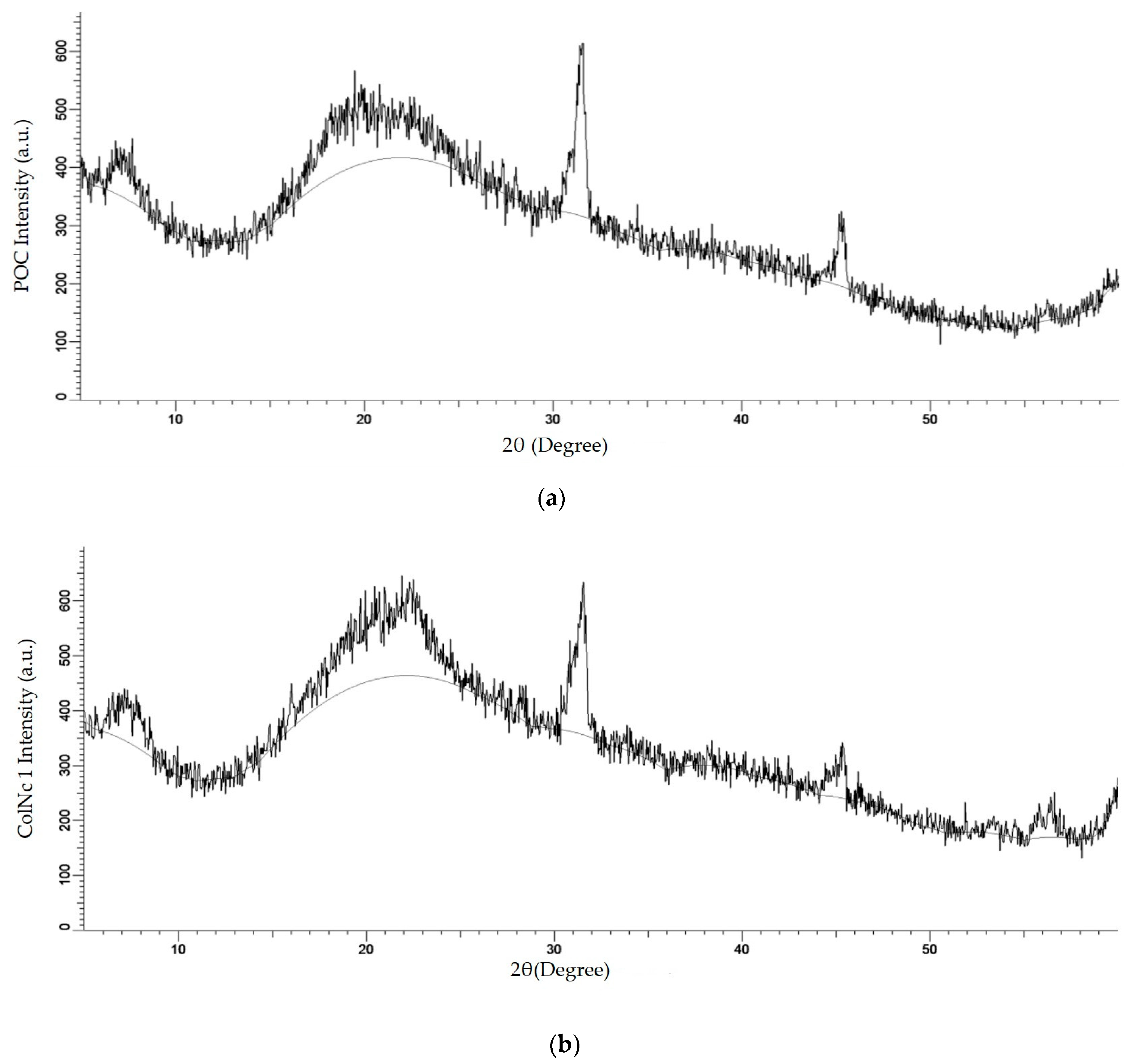
3.2.3. X-ray Diffraction (XRD)
3.2.4. Tensile Strength
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xie, H.; Chen, X.; Shen, X.; He, Y.; Chen, W.; Luo, Q.; Ge, W.; Yuan, W.; Tang, X.; Hou, D.; et al. Preparation of chitosan-collagen-alginate composite dressing and its promoting effects on wound healing. Int. J. Biol. Macromol. 2018, 107, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Gaspar-Pintiliescu, A.; Stanciuc, A.-M.; Craciunescu, O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 24–28. [Google Scholar] [CrossRef]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef]
- Chandan, K.S. Human wounds and its burden: An updated compendium of estimates. Adv. Wound Care. 2019, 8, 39–48. [Google Scholar] [CrossRef]
- Chanjuan, D.; Yonggang, L. Application of collagen scaffold in tissue engineering: Recent advances and new perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef]
- Ng, M.R.; Besser, A.; Danuser, G.; Brugge, J.S. Substrate stiffness regulates cadherin-dependent collective migration through myosin-II contractility. J. Cell Biol. 2012, 199, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, A.; Burcharth, J.; Rosenberg, J. Animal derived products may conflict with religious patients’ beliefs. BMC Med. Ethics 2013, 14. [Google Scholar] [CrossRef]
- Fauzi, M.B.; Rajab, N.F.; Tabata, Y.; Saim, A.B.; Ruszymah, B.H.I.; Chowdhury, S.R. Rapid treatment of full-thickness skin loss using ovine tendon collagen type I scaffold with skin cells. J. Tissue Eng. Regen. Med. 2019, 13, 874–891. [Google Scholar] [CrossRef]
- Zuber, M.; Zia, F.; Zia, K.M.; Tabasum, S.; Salman, M.; Sultan, N. Collagen based polyurethanes—A review of recent advances and perspective. Int. J. Biol. Macromol. 2015, 80, 366–374. [Google Scholar] [CrossRef]
- Yunus, M.H.M.; Shuid, A.N.; Busra, M.F.; Hui, C.K.; Ghafar, N.A.; Rani, R.A. The effect of stichopus chloronotus aqueous extract on human osteoarthitis chondrocytes cartilage in three-dimensional collagen type I hydrogel in vitro. Sains Malaysiana 2019, 48, 1671–1683. [Google Scholar] [CrossRef]
- Bohn, G.; Liden, B.; Schultz, G.; Yang, Q.; Gibson, J.D. Ovine-based collagen matrix dressing: Next-generation collagen dressing for wound care. Adv. Wound Care 2016, 5, 1–10. [Google Scholar] [CrossRef]
- Rýglová, Š.; Braun, M.; Suchý, T. Collagen and its modifications-crucial aspects with concern to its processing and analysis. Macromol. Mater. Eng. 2017, 302. [Google Scholar] [CrossRef]
- Purcel, G.; Meliţă, D.; Andronescu, E.; Grumezescu, A.M. Collagen-based nanobiomaterials. In Nanobiomaterials in Soft Tissue Engineering; Elsevier: Oxford, UK, 2016; pp. 173–200. [Google Scholar] [CrossRef]
- Aspler, J.O.E.; Bouchard, J.; Hamad, W.; Berry, R.; Beck, S.; Drolet, F.; Zou, X.A. Review of nanocellulosic products and their application. Biopolym. Nanocompos. 2013, 461–508. [Google Scholar] [CrossRef]
- Xue, Y.M.; Xiao, H. Nanocellulose as a sustainable biomass material: Structure, properties, present status and future prospects in biomedical applications. Nanoscale 2017, 9, 14758–14781. [Google Scholar] [CrossRef]
- Tayeb, A.; Amini, E.; Ghasemi, S.; Tajvidi, M. Cellulose nanomaterials—Binding properties and applications: A review. Molecules 2018, 23, 2684. [Google Scholar] [CrossRef]
- Li, W.; Guo, R.; Lan, Y.; Zhang, Y.; Xue, W.; Zhang, Y. Preparation and properties of cellulose nanocrystals reinforced collagen composite films. J. Biomed. Mater. Res. Part A 2013, 102, 1131–1139. [Google Scholar] [CrossRef]
- Gupta, P.K.; Raghunath, S.S.; Prasanna, D.V.; Venkat, P.; Shree, V.; Chithananthan, C.; Choudhari, S.; Surender, K.; Geetha, K. An update on overview of cellulose, its structure and applications. In Cellulose—Biomass Conversion; IntechOpen: Rijeka, Croatia, 2019; Volume 216, pp. 1066–1076. [Google Scholar] [CrossRef]
- Costa, A.F.S.; Almeida, F.C.G.; Vinhas, G.M.; Sarubbo, L.A. Production of bacterial cellulose by gluconacetobacter hansenii using corn steep liquor as nutrient sources. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.C.; Fontão, A.I.; Coelho, A.; Leal, M.; Da Silva, F.A.S.; Wan, Y.; Gama, M.; Dourado, F. Response surface statistical optimization of bacterial nanocellulose fermentation in static culture using a low-cost medium. New Biotechnol. 2019, 49, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Farah, N.M.P.; Lee, S.H.; Zuriyati, M.A.A.; Lee, C.H.; Luqman, C.A. Potential of oil palm empty fruit bunch resources in nanocellulose hydrogel production for versatile applications: A review. Material 2020, 13, 1245. [Google Scholar] [CrossRef]
- Fauzi, M.B.; Lokanathan, Y.; Aminuddin, B.S.; Ruszymah, B.H.I.; Chowdhury, S.R. Ovine tendon collagen: Extraction, characterisation and fabrication of thin films for tissue engineering applications. Mater. Sci. Eng. C 2016, 68, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Mohd, N.H.; Ismail, N.F.H.; Zahari, J.I.; Farahhanim, W.; Fathilah, W.; Kargarzadeh, H.; Ramli, S.; Ahmad, I.; Ambar, M.; Yarmo Rizafizah, Y.; et al. Effect of aminosilane modification on nanocrystalline cellulose properties. J. Nanomater. 2016, 1–8. [Google Scholar] [CrossRef]
- Zahari, M.J.I.; Mohd Jahi, N.; Mohd, N.H.; Ahmad, I.; Baharum, A.; Lazim, A.M.; Ramli, S.; Othaman, R. Enhanced performance of cellulose from palm oil empty fruit bunch (Efb) Via acetylation and silylation. Preprints 2018, 2018070314. [Google Scholar] [CrossRef]
- Halib, N.; Perrone, F.; Cemazar, M.; Dapas, B.; Farra, R.; Abrami, M.; Chiarappa, G.; Forte, G.; Zanconati, F.; Pozzato, G.; et al. Potential applications of nanocellulose-containing materials in the biomedical field. Materials 2017, 10, 977. [Google Scholar] [CrossRef]
- Shah, R.; Stodulka, P.; Skopalova, K.; Saha, P. Dual crosslinked collagen/chitosan film for potential biomedical applications. Polymers 2019, 11, 2094. [Google Scholar] [CrossRef]
- Výborný, K.; Vallová, J.; Kočí, Z.; Kekulová, K.; Jiráková, K.; Jendelová, P.; Jedaleová, P.; Kubinová, Š. Genipin and EDC crosslinking of extracellular matrix hydrogel derived from human umbilical cord for neural tissue repair. Sci. Rep. 2019, 9, 10674. [Google Scholar] [CrossRef]
- Suesca, E.; Dias, A.M.A.; Braga, M.E.M.; de Sousa, H.C.; Fontanilla, M.R. Multifactor analysis on the effect of collagen concentration, cross-linking and fiber/pore orientation on chemical, microstructural, mechanical and biological properties of collagen type I scaffolds. Mater. Sci. Eng. C 2017, 77, 333–341. [Google Scholar] [CrossRef]
- Sahana, T.G.; Rekha, P.D. Bioplymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef]
- Păunica-Panea, G.; Ficai, A.; Marin, M.M.; Marin, Ș.; Albu, M.G.; Constantin, V.D.; Corobea, M.C.; Ghica, M.V. New collagen-dextran-zinc oxide composites for wound dressing. J. Nanomater. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Wang, H.M.; Chou, Y.T.; Wen, Z.H.; Wang, Z.R.; Chen, C.H.; Ho, M.L. Novel biodegradable porous scaffold applied to skin regeneration. PLoS ONE 2013, 8, 56330. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.M.A.; Mohd Yunus, M.H.; Mh Busra, M.F. Genipin-Crosslinked Gelatin Scaffold in Tissue Engineering: A Systematic Review. Med. Health 2019, 14, 1–16. [Google Scholar]
- Han, F.; Dong, Y.; Su, Z.; Yin, R.; Song, A.; Li, S. Preparation, characteristics and assessment of a novel gelatin-chitosan sponge scaffold as skin tissue engineering material. Int. J. Pharm. 2014, 476, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Tronci, G. The application of collagen in advanced wound dressings. Adv. Textiles Wound Care 2019, 363–389. [Google Scholar] [CrossRef]
- Hoque, M.E.; Nuge, T.; Yeow, T.K.; Nordin, N.P. Gelatin based scaffolds for tissue engineering—A review. Polym. Res. J. 2015, 9, 15–32. [Google Scholar]
- Paolo, A.N. Biomedical Foams for Tissue Engineering Applications; Elsevier: Cambridge, UK, 2014; ISBN 9780857097033. [Google Scholar]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Lim, M.M.; Sun, T.; Sultana, N. In vitro biological evaluation of electrospun polycaprolactone/gelatine nanofibrous scaffold for tissue engineering. J. Nanomater. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Moraes, P.R.F.S.; Saska, S.; Lima, L.R.D.; Martins, V.D.C.A.; Plepis, A.M.D.G.; Riberio, S.J.L.; Gaspar, A.M.M. Bacterial cellulose/collagen hydrogel for wound healing. Mater. Res. 2016, 19, 106–116. [Google Scholar] [CrossRef]
- Junka, R.A.; Daly, L.E.; Yu, X. Characterization of Biomaterials: 5. Bioreactors for Evaluating Cell Infiltration and Tissue Formation in Biomaterials; Elsevier: Cambridge, UK, 2012; ISBN 9780857093684. [Google Scholar]
- Yang, Y.; Zhu, X.; Cui, W.; Li, X.J. Electrospun composite mats of poly[(D,llactide)-co-glycolide] and collagen with high porosity as potential scaffolds for skin tissue engineering. Macromol. Mater. Eng. 2009, 294, 611–619. [Google Scholar] [CrossRef]
- Tan, H.B.; Wang, F.Y.; Wei, D.; Zhang, Y.; Jing, D.I.N.G.; Di Xin, C.A.I.; XU, Y.Q. Fabrication and evaluation of porous keratin/Chitosan (Kcs) Scaffolds for effectively accelerating wound healing. Biomed. Environ. Sci. BES 2015, 28, 178–189. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.R.; Mh Busra, M.F.; Lokanathan, Y.; Ng, M.H.; Law, J.X.; Cletus, U.C.; Binti Haji Idrus, R. Collagen type I: A versatile biomaterial. Adv. Exp. Med. Biol. 2018, 1077, 389–414. [Google Scholar] [PubMed]
- Cai, Z.; Guang, Y. Bacterial cellulose/Collagen composite: Characterization and first evaluation of cytocompatibility. J. Appl. Polym. Sci. 2011, 120, 2938–2944. [Google Scholar] [CrossRef]
- Ge, L.; Xu, Y.; Li, X.; Yuan, L.; Tan, H.; Li, D.; Mu, C. Fabrication of antibacterial collagen-based composite wound dressing. ACS Sustain. Chem. Eng. 2018, 6, 9153–9166. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Pierron, D.; Harmand, M.F. Biocompatible fibrous networks of cellulose nanofibres and collagen crosslinked using genipin: Potential as artificial ligament/Tendons. Macromol. Biosci. 2013, 12, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, D.M.; Nunes, Y.L.; de Azeredo, H.M.; Aouada, F.A.; Rosa, M.F.; Feitosa, J.P.; Dufresne, A. Nanocell51ulose nanocomposite hydrogels: Technological and environmental issues. Green Chem. 2018, 20, 2428–2448. [Google Scholar] [CrossRef]



| (a) | |||||
| Type of BHS Element | POC | ColNc 1 | ColNc 5 | ColNc 10 | Nanocellulose |
| Weight (%) | |||||
| Carbon | 44.34 | 47.77 | 45.60 | 46.26 | 53.3 |
| Nitrogen | 19.31 | 15.79 | 18.55 | 13.45 | – |
| Oxygen | 36.34 | 36.44 | 35.85 | 40.29 | 46.4 |
| Sulphur | – | – | – | – | 0.4 |
| (b) | |||||
| Type of BHS Element | POC | ColNc 1 | ColNc 5 | ColNc 10 | Nanocellulose |
| Atomic (%) | |||||
| Carbon | 50.28 | 53.88 | 51.57 | 52.55 | 60.37 |
| Nitrogen | 18.78 | 15.27 | 17.99 | 13.10 | – |
| Oxygen | 30.94 | 30.86 | 30.44 | 34.36 | 39.46 |
| Sulphur | – | – | – | – | 0.17 |
| Sample | Modulus [MPa] | Tensile Strength at Break [MPa] | Tensile Strain (Extension) at Break [%] |
|---|---|---|---|
| POC | 0.74 ± 0.17 | 0.37 ± 0.07 | 67.15 ± 2.48 |
| ColNc 1 | 0.98 ± 0.30 | 0.28 ± 0.04 | 39.04 ± 8.17 |
| ColNc 5 | 1.37 ± 0.29 | 0.42 ± 0.11 | 47.04 ± 0.95 |
| ColNc 10 | 1.49 ± 0.21 | 0.57 ± 0.04 | 50.72 ± 10.89 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ooi, K.S.; Haszman, S.; Wong, Y.N.; Soidin, E.; Hesham, N.; Mior, M.A.A.; Tabata, Y.; Ahmad, I.; Fauzi, M.B.; Mohd Yunus, M.H. Physicochemical Characterization of Bilayer Hybrid Nanocellulose-Collagen as a Potential Wound Dressing. Materials 2020, 13, 4352. https://doi.org/10.3390/ma13194352
Ooi KS, Haszman S, Wong YN, Soidin E, Hesham N, Mior MAA, Tabata Y, Ahmad I, Fauzi MB, Mohd Yunus MH. Physicochemical Characterization of Bilayer Hybrid Nanocellulose-Collagen as a Potential Wound Dressing. Materials. 2020; 13(19):4352. https://doi.org/10.3390/ma13194352
Chicago/Turabian StyleOoi, Kai Shen, Shafieq Haszman, Yon Nie Wong, Emillia Soidin, Nadhirah Hesham, Muhammad Amirul Arif Mior, Yasuhiko Tabata, Ishak Ahmad, Mh Busra Fauzi, and Mohd Heikal Mohd Yunus. 2020. "Physicochemical Characterization of Bilayer Hybrid Nanocellulose-Collagen as a Potential Wound Dressing" Materials 13, no. 19: 4352. https://doi.org/10.3390/ma13194352
APA StyleOoi, K. S., Haszman, S., Wong, Y. N., Soidin, E., Hesham, N., Mior, M. A. A., Tabata, Y., Ahmad, I., Fauzi, M. B., & Mohd Yunus, M. H. (2020). Physicochemical Characterization of Bilayer Hybrid Nanocellulose-Collagen as a Potential Wound Dressing. Materials, 13(19), 4352. https://doi.org/10.3390/ma13194352









