Relationship between Implant Geometry and Primary Stability in Different Bony Defects and Variant Bone Densities: An In Vitro Study
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Protocol
2.2. Statistical Analysis
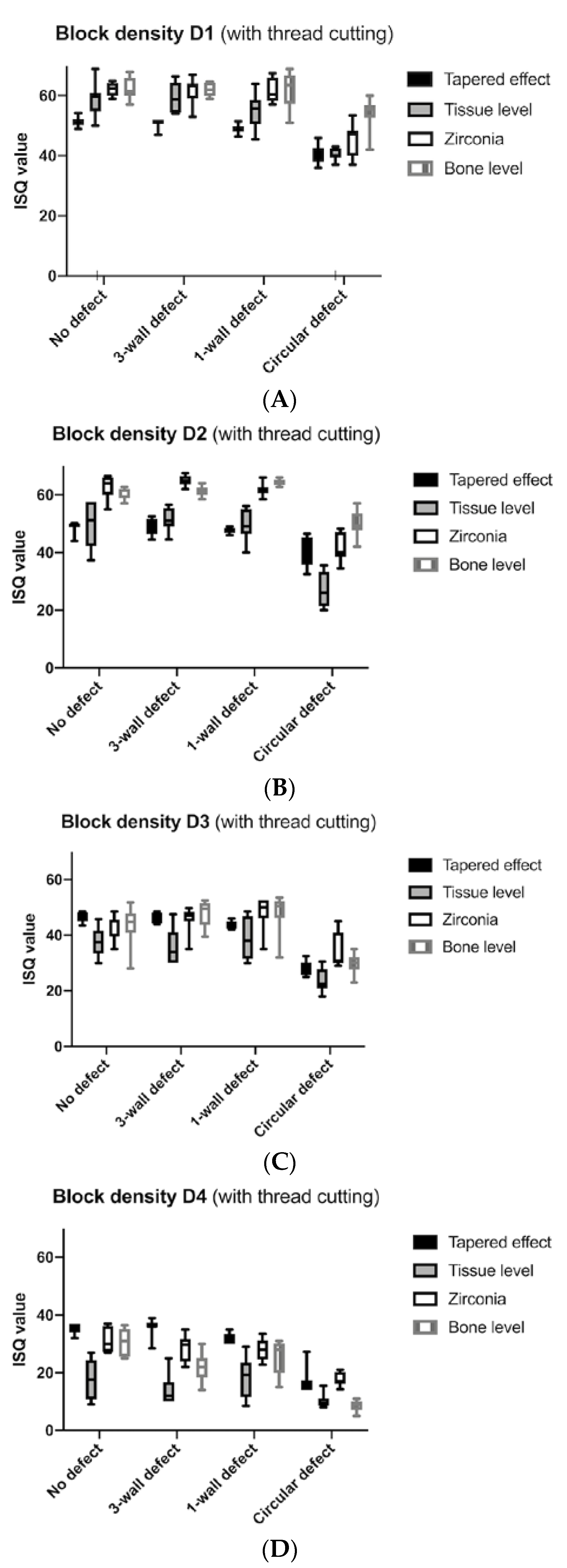
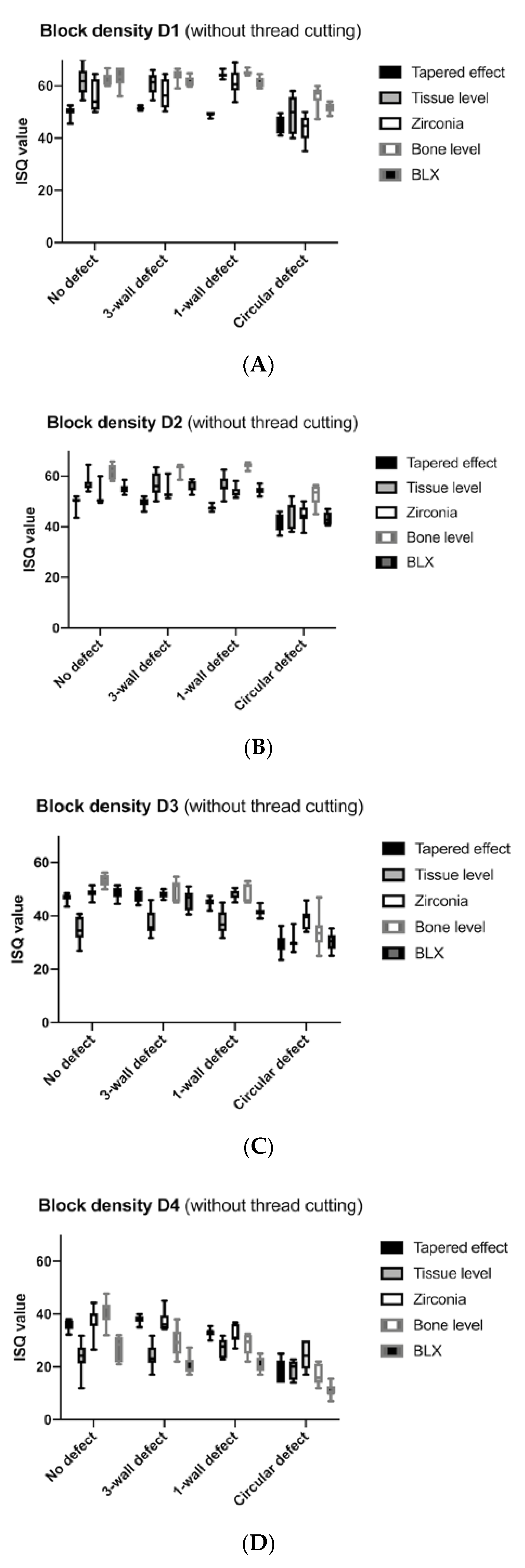
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Ellis, R.; Chen, S.; Davies, H.; Fitzgerald, W.; Xu, J.; Darby, I.B. Primary stability and healing outcomes of apically tapered and straight implants placed into fresh extraction sockets. A pre-clinical in vivo study. Clin. Oral Implant. Res. 2020, 31, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Davies, J. Mechanisms of endosseous integration. Int. J. Prosthodont. 1999, 11, 391–401. [Google Scholar]
- Ballini, A.; Cantore, S.; Scacco, S.; Coletti, D.; Tatullo, M. Mesenchymal Stem Cells as Promoters, Enhancers, and Playmakers of the Translational Regenerative Medicine 2018. Stem Cells Int. 2018, 2018, 6927401. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Scacco, S.; Coletti, D.; Pluchino, S.; Tatullo, M. Mesenchymal Stem Cells as Promoters, Enhancers, and Playmakers of the Translational Regenerative Medicine. Stem Cells Int. 2017, 2017, 3292810. [Google Scholar] [CrossRef]
- Staedt, H.; Kämmerer, P.W.; Goetze, E.; Thiem, D.G.E.; Al-Nawas, B.; Heimes, D. Implant primary stability depending on protocol and insertion mode—An ex vivo study. Int. J. Implant. Dent. 2020, 6, 49. [Google Scholar] [CrossRef]
- Friberg, B.; Ekestubbe, A.; Sennerby, L. Clinical outcome of Brånemark System implants of various diameters: A retrospective study. Int. J. Oral Maxillofac. Implant. 2002, 17, 671–677. [Google Scholar]
- Su, Y.-H.; Peng, B.-Y.; Wang, P.-D.; Feng, S.-W. Evaluation of the implant stability and the marginal bone level changes during the first three months of dental implant healing process: A prospective clinical study. J. Mech. Behav. Biomed. Mater. 2020, 110, 103899. [Google Scholar] [CrossRef]
- Chong, L.; Khocht, A.; Suzuki, J.B.; Gaughan, J. Effect of Implant Design on Initial Stability of Tapered Implants. J. Oral Implant. 2009, 35, 130–135. [Google Scholar] [CrossRef]
- Arosio, P.; Arosio, F.; Di Stefano, D.A. Implant Diameter, Length, and the Insertion Torque/Depth Integral: A Study Using Polyurethane Foam Blocks. Dent. J. 2020, 8, 56. [Google Scholar] [CrossRef]
- Cionca, N.; Hashim, D.; Mombelli, A. Zirconia dental implants: Where are we now, and where are we heading? Periodontology 2000 2016, 73, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Kniha, K.; Bock, A.; Peters, F.; Heitzer, M.; Modabber, A.; Kniha, H.; Hölzle, F.; Möhlhenrich, S. Aesthetic aspects of adjacent maxillary single-crown implants-influence of zirconia and titanium as implant materials. Int. J. Oral Maxillofac. Surg. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Kniha, K.; Schlegel, K.A.; Kniha, H.; Modabber, A.; Neukam, F.; Kniha, K. Papilla-Crown Height Dimensions around Zirconium Dioxide Implants in the Esthetic Area: A 3-Year Follow-Up Study. J. Prosthodont. 2018, 28, e694–e698. [Google Scholar] [CrossRef] [PubMed]
- Kniha, K.; Kniha, H.; Grunert, I.; Edelhoff, D.; Hölzle, F.; Modabber, A. Esthetic Evaluation of Maxillary Single-Tooth Zirconia Implants in the Esthetic Zone. Int. J. Periodontics Restor. Dent. 2019, 39, e195–e201. [Google Scholar] [CrossRef] [PubMed]
- Meredith, N. Assessment of implant stability as a prognostic determinant. Int. J. Prosthodont. 1999, 11, 491–501. [Google Scholar]
- Goldman, H.M.; Cohen, D.W. The Infrabony Pocket: Classification and Treatment. J. Periodontol. 1958, 29, 272–291. [Google Scholar] [CrossRef]
- Misch, C.E. Bone classification, training keys to implant success. Dent. Today 1989, 8, 39–44. [Google Scholar]
- Shin, S.-Y.; Kye, S.-B.; Hong, J.; Paeng, J.-Y.; Chang, S.-W.; Yang, S.-M.; Shin, S. The Effects of Defect Type and Depth, and Measurement Direction on the Implant Stability Quotient Value. J. Oral Implant. 2015, 41, 652–656. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Tözüm, T.F.; Turkyilmaz, I.; McGlumphy, E.A. Relationship between dental implant stability determined by resonance frequency analysis measurements and peri-implant vertical defects: Anin vitrostudy. J. Oral Rehabil. 2008, 35, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Prion, S.; Haerling, K.A. Making Sense of Methods and Measurement: Spearman-Rho Ranked-Order Correlation Coefficient. Clin. Simul. Nurs. 2014, 10, 535–536. [Google Scholar] [CrossRef]
- Alsaadi, G.; Quirynen, M.; Michiels, K.; Jacobs, R.; Van Steenberghe, D. A biomechanical assessment of the relation between the oral implant stability at insertion and subjective bone quality assessment. J. Clin. Periodontol. 2007, 34, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.-J.; Lim, H.-C.; Hong, J.-Y.; Shin, S.; Chung, J.-H.; Herr, Y.; Shin, S.-Y. Primary stability of implants with peri-implant bone defects of various widths: An in vitro investigation. J. Periodontal Implant. Sci. 2019, 49, 39–46. [Google Scholar] [CrossRef]
- Merheb, J.; Coucke, W.; Jacobs, R.; Naert, I.E.; Quirynen, M. Influence of bony defects on implant stability. Clin. Oral Implant. Res. 2010, 21, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Udomsawat, C.; Rungsiyakull, P.; Rungsiyakull, C.; Khongkhunthian, P. Comparative study of stress characteristics in surrounding bone during insertion of dental implants of three different thread designs: A three-dimensional dynamic finite element study. Clin. Exp. Dent. Res. 2018, 5, 26–37. [Google Scholar] [CrossRef]
- Misch, C.E. Contemporary Implant, Dentistry; Mosby Elsevier: St-Louis, MO, USA, 2008. [Google Scholar]
- Kim, D.-R.; Lim, Y.-J.; Kim, M.-J.; Kwon, H.-B.; Kim, S.-H. Self-cutting blades and their influence on primary stability of tapered dental implants in a simulated low-density bone model: A laboratory study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 112, 573–580. [Google Scholar] [CrossRef]
- Menicucci, G.; Pachie, E.; Lorenzetti, M.; Migliaretti, G.; Carossa, S. Comparison of primary stability of straight-walled and tapered implants using an insertion torque device. Int. J. Prosthodont. 2012, 25, 465–471. [Google Scholar]
- Rokn, A.; Rasouli-Ghahroudi, A.A.; Mesgarzadeh, A.; Miremadi, A.; Yaghoobi, S. Evaluation of Stability Changes in Tapered and Parallel Wall Implants: A Human Clinical Trial. J. Dent. (Tehran Iran.) 2011, 8, 186–200. [Google Scholar]
- Östman, P.-O.; Hellman, M.; Wendelhag, I.; Sennerby, L. Resonance frequency analysis measurements of implants at placement surgery. Int. J. Prosthodont. 2006, 19, 77. [Google Scholar]
- Cehreli, M.; Şahin, S.; Akça, K. Role of mechanical environment and implant design on bone tissue differentiation: Current knowledge and future contexts. J. Dent. 2004, 32, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Möhlhenrich, S.; Kniha, K.; Heussen, N.; Hölzle, F.; Modabber, A. Effects on primary stability of three different techniques for implant site preparation in synthetic bone models of different densities. Br. J. Oral Maxillofac. Surg. 2016, 54, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Ting, C.-C.; Hsu, K.-J.; Hsiao, S.-Y.; Chen, C.-M. The correlation among gripping volume, insertion torque, and pullout strength of micro-implant. J. Dent. Sci. 2020, in press. [Google Scholar] [CrossRef]
- Romanos, G.E.; Basha-Hijazi, A.; Gupta, B.; Ren, Y.F.; Malmstrom, H. Role of clinician’s experience and implant design on implant stability. An ex vivo study in artificial soft bones. Clin. Implant. Dent. Relat. Res. 2014, 16, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G.E.; Ciornei, G.; Jucan, A.; Malmstrom, H.; Gupta, B. In Vitro Assessment of Primary Stability of Straumann® Implant Designs. Clin. Implant. Dent. Relat. Res. 2012, 16, 89–95. [Google Scholar] [CrossRef] [PubMed]
- González-Serrano, J.; Ortega-Aranegui, R.; López-Quiles, J. In vitro comparison of primary stability of two implant designs in D3 bone. Med. Oral. Patol. Oral. Cir. Bucal. 2017, 22, e473–e477. [Google Scholar]
- Lachmann, S.; Jäger, B.; Axmann, D.; Gomez-Roman, G.; Groten, M.; Weber, H. Resonance frequency analysis and damping capacity assessment. Part I: An in vitro study on measurement reliability and a method of comparison in the determination of primary dental implant stability. Clin. Oral Implant. Res. 2006, 17, 75–79. [Google Scholar] [CrossRef]
- Lachmann, S.; Laval, J.Y.; Jäger, B.; Axmann, D.; Gomez-Roman, G.; Groten, M.; Weber, H. Resonance frequency analysis and damping capacity assessment. Part 2: Peri-implant bone loss follow-up. An in vitro study with the Periotest and Osstell instruments. Clin. Oral Implant. Res. 2006, 17, 80–84. [Google Scholar] [CrossRef]
- Marrelli, M.; Tatullo, M. Influence of PRF in the healing of bone and gingival tissues. Clinical and histological evaluations. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1958–1962. [Google Scholar]
- Bardyn, T.; Gédet, P.; Hallermann, W.; Büchler, P. Quantifying the influence of bone density and thickness on resonance frequency analysis: An in vitro study of biomechanical test materials. Int. J. Oral Maxillofac. Implant. 2010, 24, 1006–1014. [Google Scholar]

| Type of Implant | Defect Size | With Thread Cutting | Without Thread Cutting | |||||
|---|---|---|---|---|---|---|---|---|
| Block Density | ISQ Mean (SD) | Max Torque in Value (SD) | Max Torque out Value (SD) | ISQ Mean (SD) | Max Torque in Value (SD) | Max Torque out Value (SD) | ||
| D1 | Tapered effect | no defect | 51.3 (1.5) | 49.9 (0.3) | 38.6 (6.3) | 50.0 (0.0) | 50.0 (0.0) | 37.5 (3.7) |
| 3-wall defect | 51.0 (1.5) | 43.9 (7.4) | 30.9 (4.9) | 51.4 (0.6) | 47.5 (3.3) | 33.2 (1.9) | ||
| 1-wall defect | 49.0 (1.5) | 47.2 (3.3) | 33.9 (2.0) | 49.2 (0.8) | 50.0 (0.3) | 35.2 (1.9) | ||
| Circular defect | 40.6 (3.0) | 41.4 (4.9) | 26.7 (2.3) | 45.4 (3.1) | 47.3 (2.5) | 31.9 (2.6) | ||
| Tissue level | no defect | 58.9 (5.1) | 15.3 (5.3) | 12.5 (3.4) | 61.9 (5.0) | 35.4 (9.0) | 27.1 (5.2) | |
| 3-wall defect | 59.2 (5.0) | 24.5 (9.2) | 13.1 (5.1) | 60.9 (3.9) | 35.5 (6.9) | 21.6 (2.3) | ||
| 1-wall defect | 55.4 (5.5) | 21.5 (5.4) | 10.3 (2.8) | 64.2 (1.1) | 37.2 (5.7) | 22.8 (4.7) | ||
| Circular defect | 40.6 (1.9) | 16.9 (3.0) | 6.2 (1.8) | 49.5 (6.9) | 25.4 (6.0) | 12.4 (2.8) | ||
| Zirconia | no defect | 62.3 (2.2) | 26.9 (4.6) | 24.7 (4.3) | 56.0 (5.8) | 50.0 (0.0) | 38.5 (3.4) | |
| 3-wall defect | 61.6 (4.1) | 40.0 (4.7) | 29.2 (1.6) | 56.8 (5.5) | 48.7 (2.4) | 32.6 (3.4) | ||
| 1-wall defect | 61.6 (3.8) | 47.6 (3.4) | 28.0 (4.2) | 61.2 (4.6) | 49.7 (0.9) | 31.4 (2.6) | ||
| Circular defect | 45.6 (5.1) | 29.3 (5.8) | 14.8 (2.8) | 44.1 (4.7) | 32.8 (5.7) | 17.7 (3.3) | ||
| Bone level | no defect | 62.3 (3.4) | 41.6 (7.2) | 26.3 (8,5) | 62.0 (2.3) | 50.0 (0.0) | 37.1 (2.2) | |
| 3-wall defect | 62.0 (2.1) | 24.5 (5.0) | 21.0 (5.2) | 63.9 (2.2) | 38.9 (3.1) | 31.2 (2.6) | ||
| 1-wall defect | 62.1 (6.0) | 38.1 (10.3) | 27.1 (9.8) | 65.1 (0.8) | 47.5 (3.6) | 37.1 (3.1) | ||
| Circular defect | 54.1 (5.0) | 28.6 (4.4) | 20.8 (3.3) | 56.1 (3.9) | 33.3 (7.6) | 22.7 (2.7) | ||
| BLX | no defect | 63.2 (3.8) | 40.5 (3.9) | 26.0 (2.4) | ||||
| 3-wall defect | 61.7 (1.9) | 33.4 (3.9) | 19.9 (2.8) | |||||
| 1-wall defect | 61.3 (1.8) | 39.2 (5.2) | 25.5 (2.1) | |||||
| Circular defect | 51.4 (1.8) | 28.3 (5.8) | 17.8 (8.6) | |||||
| D2 | Tapered effect | no defect | 49.0 (1.9) | 29.2 (4.1) | 27.4 (3.7) | 49.9 (2.3) | 39.8 (5.7) | 30.5 (5.9) |
| 3-wall defect | 49.5 (3.0) | 41.5 (5.6) | 26.2 (3.6) | 49.7 (1.9) | 46.5 (3.7) | 29.4 (2.1) | ||
| 1-wall defect | 47.7 (1.1) | 39.0 (3.7) | 27.4 (2.8) | 47.4 (1.0) | 49.7 (0.7) | 31.5 (2.3) | ||
| Circular defect | 41.1 (4.9) | 30.8 (2.3) | 18.0 (1.9) | 41.3 (3.4) | 36.7 (2.3) | 24.1 (2.8) | ||
| Tissue level | no defect | 49.5 (8.3) | 8.3 (4.1) | 4.8 (3.3) | 56.8 (3.0) | 21.7 (5.5) | 14.8 (2.4) | |
| 3-wall defect | 51.7 (3.9) | 16.4 (4.3) | 7.8 (2.2) | 56.7 (4.3) | 27.8 (6.4) | 14.2 (1.9) | ||
| 1-wall defect | 49.4 (5.0) | 31.3 (2.1) | 10.9 (2.1) | 56.2 (3.4) | 40.7 (4.4) | 17.7 (2.8) | ||
| Circular defect | 27.2 (5.7) | 12.3 (1.6) | 2.3 (0.9) | 45.8 (5.2) | 26.1 (3.3) | 8.8 (1.6) | ||
| Zirconia | no defect | 62.9 (3.9) | 27.8 (3.0) | 21.7 (3.6) | 51.2 (3.1) | 39.8 (4.8) | 30.7 (4.3) | |
| 3-wall defect | 64.9 (1.8) | 30.7 (3.5) | 21.0 (2.1) | 53.3 (2.8) | 38.8 (2.1) | 28.5 (2.0) | ||
| 1-wall defect | 61.6 (2.0) | 35.1 (4.4) | 23.9 (4.2) | 53.7 (1.9) | 45.5 (4.4) | 29.7 (2.5) | ||
| Circular defect | 41.4 (4.6) | 21.7 (6.2) | 10.7 (2.9) | 44.8 (3.6) | 28.5 (5.8) | 17.1 (2.3) | ||
| Bone level | no defect | 60.7 (1.9) | 24.9 (6.5) | 20.8 (6.8) | 61.6 (3.0) | 49.2 (2.5) | 39.7 (6.4) | |
| 3-wall defect | 61.3 (1.6) | 23.1 (3.0) | 17.4 (3.0) | 63.2 (1.7) | 32.6 (1.7) | 25.6 (1.9) | ||
| 1-wall defect | 64.3 (1.1) | 32.5 (4.0) | 26.2 (2.9) | 64.2 (1.2) | 43.4 (3.8) | 31.0 (2.3) | ||
| Circular defect | 50.0 (4.2) | 27.5 (6.4) | 17.0 (4.8) | 52.5 (4.3) | 28.4 (4.6) | 18.3 (3.4) | ||
| BLX | no defect | 55.2 (2.0) | 24.1 (3.1) | 15.1 (1.7) | ||||
| 3-wall defect | 56.5 (2.3) | 25.2 (3.2) | 15.8 (2.0) | |||||
| 1-wall defect | 54.3 (1.6) | 19.4 (2.5) | 13.6 (3.0) | |||||
| Circular defect | 43.4 (2.4) | 15.2 (2.4) | 8.6 (0.7) | |||||
| D3 | Tapered effect | no defect | 46.3 (1.7) | 12.7 (1.1) | 8.8 (0.6) | 46.9 (1.6) | 22.7 (1.5) | 16.0 (1.3) |
| 3-wall defect | 45.9 (1.8) | 21.3 (3.5) | 16.0 (2.0) | 47.6 (2.2) | 24.3 (2.6) | 18.4 (2.8) | ||
| 1-wall defect | 43.8 (1.4) | 20.4 (1.4) | 13.1 (1.5) | 45.1 (1.6) | 25.2 (2.8) | 17.8 (1.8) | ||
| Circular defect | 28.5 (2.5) | 8.1 (0.9) | 4.8 (1.0) | 29.4 (3.8) | 15.7 (2.0) | 9.5 (1.7) | ||
| Tissue level | no defect | 37.4 (5.3) | 9.7 (3.7) | 4.0 (2.6) | 35.0 (4.5) | 7.1 (1.0) | 4.2 (1.6) | |
| 3-wall defect | 35.6 (6.2) | 8.8 (4.6) | 2.1 (2.5) | 37.4 (4.6) | 7.7 (0.7) | 5.2 (0.8) | ||
| 1-wall defect | 38.9 (7.0) | 10.2 (1.0) | 1.3 (0.9) | 37.6 (4.6) | 16.3 (3.0) | 5.6 (0.7) | ||
| Circular defect | 24.0 (4.3) | 7.1 (1.0) | 0.9 (0.7) | 30.2 (2.7) | 8.6 (0.8) | 3.0 (1.6) | ||
| Zirconia | no defect | 41.5 (4.2) | 14.3 (2.2) | 10.2 (1.8) | 48.5 (2.1) | 22.4 (1.6) | 17.2 (0.9) | |
| 3-wall defect | 46.0 (4.1) | 15.2 (3.6) | 10.9 (2.7) | 47.9 (1.2) | 23.7 (2.3) | 16.5 (1.6) | ||
| 1-wall defect | 48.3 (5.4) | 14.1 (1.4) | 10.4 (1.3) | 47.7 (1.7) | 18.7 (1.3) | 15.9 (1.4) | ||
| Circular defect | 33.8 (6.0) | 9.1 (1.9) | 5.8 (0.6) | 38.9 (3.9) | 12.9 (1.5) | 8.0 (1.2) | ||
| Bone level | no defect | 43.7 (6.9) | 9.3 (2.5) | 4.3 (2.9) | 53.3 (2.0) | 15.7 (2.0) | 11.5 (1.0) | |
| 3-wall defect | 47.6 (4.8) | 8.4 (2.6) | 5.1 (2.7) | 49.9 (3.8) | 13.5 (1.8) | 9.4 (1.4) | ||
| 1-wall defect | 48.1 (6.9) | 13.1 (2.0) | 8.6 (2.0) | 49.5 (3.2) | 16.8 (1.8) | 11.5 (1.4) | ||
| Circular defect | 29.7 (3.4) | 7.8 (1.0) | 4.7 (1.3) | 34.0 (5.9) | 9.1 (1.7) | 5.5 (1.4) | ||
| BLX | no defect | 48.5 (2.1) | 10.6 (1.2) | 7.0 (0.7) | ||||
| 3-wall defect | 46.0 (3.5) | 8.7 (1.4) | 5.6 (1.0) | |||||
| 1-wall defect | 41.4 (1.7) | 7.5 (0.5) | 6.7 (0.9) | |||||
| Circular defect | 30.3 (3.4) | 6.5 (0.7) | 4.1 (1.0) | |||||
| D4 | Tapered effect | no defect | 35.5 (1.7) | 7.0 (0.7) | 5.5 (0.7) | 36.2 (2.0) | 11.2 (1.4) | 7.7 (1.1) |
| 3-wall defect | 35.8 (2.8) | 6.7 (0.5) | 3.4 (1.2) | 38.1 (1.5) | 8.8 (0.9) | 5.3 (0.9) | ||
| 1-wall defect | 31.8 (1.7) | 7.9 (1.0) | 5.2 (0.9) | 32.9 (1.5) | 8.4 (1.1) | 6.3 (0.8) | ||
| Circular defect | 16.8 (4.0) | 6.4 (0.8) | 4.3 (1.2) | 18.7 (4.2) | 7.5 (1.3) | 4.9 (1.1) | ||
| Tissue level | no defect | 17.8 (6.8) | 1.0 (2.1) | 0.1 (0.3) | 23.7 (5.7) | 0.5 (1.0) | 0.6 (1.0) | |
| 3-wall defect | 14.0 (5.4) | 2.0 (2.3) | 0.1 (0.3) | 24.2 (4.3) | 4.7 (2.1) | 0.4 (0.5) | ||
| 1-wall defect | 18.2 (6.8) | 1.8 (2.4) | 0.3 (0.9) | 27.3 (3.4) | 5.2 (1.0) | 0.4 (0.5) | ||
| Circular defect | 10.3 (2.6) | 3.2 (1.5) | 0.4 (0.5) | 19.1 (3.4) | 4.9 (2.0) | 0.4 (0.5) | ||
| Zirconia | no defect | 31.7 (4.3) | 6.3 (0.9) | 5.0 (1.3) | 36.6 (4.8) | 8.8 (1.0) | 6.9 (0.3) | |
| 3-wall defect | 28.6 (4.5) | 5.4 (0.8) | 2.5 (1.8) | 37.4 (3.7) | 8.1 (1.0) | 6.0 (0.7) | ||
| 1-wall defect | 28.1 (3.6) | 3.6 (2.0) | 1.4 (1.1) | 32.3 (3.3) | 6.4 (0.8) | 7.1 (0.9) | ||
| Circular defect | 17.9 (2.5) | 4.4 (1.2) | 0.6 (0.7) | 24.2 (5.8) | 5.8 (0.8) | 1.5 (0.8) | ||
| Bone level | no defect | 30.7 (4.5) | 2.7 (1.8) | 1.7 (1.5) | 41.4 (4.5) | 7.0 (0.8) | 5.4 (0.7) | |
| 3-wall defect | 21.8 (5.3) | 2.0 (0.0) | 1.6 (1.8) | 29.4 (5.1) | 5.0 (1.2) | 1.6 (1.0) | ||
| 1-wall defect | 25.3 (5.7) | 5.0 (0.7) | 1.7 (1.7) | 28.6 (3.7) | 6.2 (0.8) | 3.3 (1.5) | ||
| Circular defect | 8.6 (1.8) | 1.0 (1.1) | 0.3 (0.7) | 16.9 (3.6) | 3.6 (1.8) | 1.1 (0.9) | ||
| BLX | no defect | 27.3 (4.4) | 3.9 (1.5) | 0.9 (1.0) | ||||
| 3-wall defect | 20.3 (3.7) | 0.5 (1.6) | 0.6 (0.5) | |||||
| 1-wall defect | 20.8 (2.9) | 1.5 (2.4) | 0.5 (0.7) | |||||
| Circular defect | 11.1 (2.5) | 0.8 (1.6) | 0.0 (0.0) | |||||
| p-Values (with Thread Cutting) | Block Density | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | |||||||||||||
| Statistical Analysis of ISQ Values | Tapered Effect | Tissue Level | Zirconia | Bone Level | Tapered Effect | Tissue Level | Zirconia | Bone Level | Tapered Effect | Tissue Level | Zirconia | Bone Level | Tapered Effect | Tissue Level | Zirconia | Bone Level |
| No defect vs. 3-wall defect | 0.9487 | 0.9992 | 0.9598 | 0.9935 | 0.9465 | 0.9269 | 0.4023 | 0.7791 | 0.8801 | 0.8869 | 0.1027 | 0.4707 | 0.9782 | 0.4390 | 0.3070 | 0.0091 |
| No defect vs. 1-wall defect | 0.0593 | 0.4803 | 0.9389 | 0.9988 | 0.2165 | >0.9999 | 0.5393 | 0.0032 | 0.0950 | 0.8930 | 0.0785 | 0.4634 | 0.0008 | 0.9983 | 0.2675 | 0.2013 |
| No defect vs. circular defect | <0.0001 | <0.0001 | <0.0001 | 0.0084 | 0.0091 | 0.0004 | <0.0001 | 0.0006 | <0.0001 | 0.0020 | 0.0191 | 0.0025 | <0.0001 | 0.0087 | 0.0002 | <0.0001 |
| 3-wall defect vs. 1-wall defect | 0.0320 | 0.3744 | >0.9999 | >0.9999 | 0.2500 | 0.6505 | 0.0115 | 0.0034 | 0.1219 | 0.4591 | 0.7565 | 0.9870 | 0.0096 | 0.5673 | 0.9836 | 0.1446 |
| 3-wall defect vs. circular defect | <0.0001 | <0.0001 | 0.0005 | 0.0019 | 0.0047 | <0.0001 | <0.0001 | 0.0003 | <0.0001 | 0.0024 | 0.0026 | <0.0001 | <0.0001 | 0.1916 | 0.0001 | 0.0002 |
| 1-wall defect vs. circular defect | 0.0003 | 0.0001 | 0.0001 | 0.0548 | 0.0184 | 0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0009 | 0.0008 | 0.0002 | <0.0001 | 0.0396 | <0.0001 | 0.0001 |
| No defect | 3-wall defect | 1-wall defect | circular defect | No defect | 3-wall defect | 1-wall defect | circular defect | No defect | 3-wall defect | 1-wall defect | circular defect | No defect | 3-wall defect | 1-wall defect | circular defect | |
| Tapered effect vs. Tissue level | 0.0001 | <0.0001 | 0.0018 | >0.9999 | 0.9984 | 0.5023 | 0.7124 | 0.0061 | 0.0009 | 0.0038 | 0.2347 | 0.0430 | 0.0001 | <0.0001 | 0.0004 | 0.0006 |
| Tapered effect vs. Zirconia | <0.0001 | <0.0001 | <0.0001 | 0.0221 | <0.0001 | <0.0001 | <0.0001 | 0.9982 | 0.0100 | 0.9998 | 0.1205 | 0.0342 | 0.0410 | 0.0197 | 0.0671 | 0.9240 |
| Tapered effect vs. Bone level | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0149 | 0.6010 | 0.6515 | 0.2585 | 0.7754 | 0.0609 | 0.0004 | 0.0287 | 0.0003 |
| Tissue level vs. Zirconia | 0.2012 | 0.4833 | 0.0026 | 0.0221 | 0.0030 | <0.0001 | 0.0004 | 0.0018 | 0.0472 | 0.0125 | 0.0350 | 0.0003 | 0.0021 | 0.0016 | 0.0035 | 0.0032 |
| Tissue level vs. Bone level | 0.2012 | 0.3644 | 0.0009 | <0.0001 | 0.0076 | <0.0001 | <0.0001 | <0.0001 | 0.0137 | 0.0033 | 0.0275 | 0.0597 | 0.0081 | 0.0842 | 0.0934 | 0.2921 |
| Zirconia vs. Bone level | >0.9999 | 0.9971 | 0.9916 | <0.000 | 0.4831 | 0.0092 | 0.0150 | 0.0037 | 0.6609 | 0.8336 | 0.9998 | 0.1892 | 0.9162 | 0.0438 | 0.5015 | <0.0001 |
| Statistical analysis of torque in values | Tapered effect | Tissue level | Zirconia | Bone level | Tapered effect | Tissue level | Zirconia | Bone level | Tapered effect | Tissue level | Zirconia | Bone level | Tapered effect | Tissue level | Zirconia | Bone level |
| No defect vs. 3-wall defect | 0.1131 | 0.0946 | <0.0001 | 0.0001 | 0.0036 | 0.0012 | 0.3156 | 0.8213 | <0.0001 | 0.9556 | 0.8964 | 0.8686 | 0.6689 | 0.7050 | 0.1513 | 0.6360 |
| No defect vs. 1-wall defect | 0.1290 | 0.1378 | <0.0001 | 0.7941 | 0.0006 | <0.0001 | 0.0102 | 0.0538 | <0.0001 | 0.9783 | 0.9968 | 0.0047 | 0.0739 | 0.9070 | 0.0167 | 0.0233 |
| No defect vs. circular defect | 0.0020 | 0.7579 | 0.3295 | 0.0003 | 0.7281 | 0.0723 | 0.0117 | 0.8542 | <0.0001 | 0.2109 | 0.0022 | 0.3210 | 0.3482 | 0.0471 | 0.0033 | 0.1293 |
| 3-wall defect vs. 1-wall defect | 0.7073 | 0.7766 | 0.0119 | 0.0432 | 0.7670 | <0.0001 | 0.0421 | 0.0067 | 0.7207 | 0.7996 | 0.8186 | 0.0155 | 0.0219 | 0.9975 | 0.0412 | <0.0001 |
| 3-wall defect vs. circular defect | 0.7404 | 0.1172 | 0.0001 | 0.2238 | 0.0015 | 0.1331 | 0.0270 | 0.3107 | <0.0001 | 0.5809 | 0.0054 | 0.9256 | 0.7533 | 0.6479 | 0.0889 | 0.0601 |
| 1-wall defect vs. circular defect | 0.0808 | 0.1458 | <0.0001 | 0.0236 | 0.0012 | <0.0001 | 0.0053 | 0.2289 | <0.0001 | 0.0006 | 0.0006 | 0.0001 | 0.0378 | 0.4954 | 0.6850 | <0.0001 |
| No defect | 3-wall defect | 1-wall defect | circular defect | No defect | 3-wall defect | 1-wall defect | circular defect | No defect | 3-wall defect | 1-wall defect | circular defect | No defect | 3-wall defect | 1-wall defect | circular defect | |
| Tapered effect vs. Tissue level | <0.0001 | 0.0002 | 0.0003 | <0.0001 | <0.0001 | <0.0001 | 0.0014 | <0.0001 | 0.1417 | 0.0014 | <0.0001 | 0.0601 | 0.0001 | 0.0002 | 0.0010 | 0.0030 |
| Tapered effect vs. Zirconia | <0.0001 | 0.7026 | 0.9931 | 0.0025 | 0.5478 | 0.0035 | 0.2119 | 0.0125 | 0.3608 | 0.0082 | <0.0001 | 0.4118 | 0.0394 | 0.0008 | 0.0019 | 0.0069 |
| Tapered effect vs. Bone level | 0.0222 | 0.0003 | 0.1089 | 0.0013 | 0.2796 | <0.0001 | 0.0037 | 0.5122 | 0.0030 | 0.0001 | <0.0001 | 0.8711 | 0.0001 | <0.0001 | 0.0001 | <0.0001 |
| Tissue level vs. Zirconia | 0.0075 | 0.0002 | 0.0003 | 0.0010 | <0.0001 | <0.0001 | 0.1871 | 0.0137 | 0.0260 | 0.0446 | <0.0001 | 0.0163 | 0.0007 | 0.0089 | 0.2505 | 0.3664 |
| Tissue level vs. Bone level | <0.0001 | 0.7438 | 0.0984 | <0.0001 | 0.0003 | 0.0111 | 0.7404 | 0.0002 | 0.9888 | 0.9947 | 0.0374 | 0.5853 | 0.3033 | >0.9999 | 0.0196 | 0.0373 |
| Zirconia vs. Bone level | 0.0058 | 0.0001 | 0.0822 | 0.9942 | 0.5532 | 0.0015 | 0.4845 | 0.2055 | 0.0057 | 0.0006 | 0.6793 | 0.3098 | 0.0009 | <0.0001 | 0.1779 | 0.0005 |
| p Values(without Thread Cutting) | Block Density | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | |||||||||||||||||
| Statistical Analysis of ISQ Values | Tapered Effect | Tissue Level | Zirconia | Bone Level | BLX | Tapered Effect | Tissue Level | Zirconia | Bone Level | BLX | Tapered Effect | Tissue Level | Zirconia | Bone Level | BLX | Tapered Effect | Tissue Level | Zirconia | Bone Level | BLX |
| No defect vs. 3-wall defect | 0.3387 | 0.9775 | 0.8464 | 0.4417 | 0.7500 | 0.9987 | 0.9997 | 0.4654 | 0.5492 | 0.6482 | 0.8811 | 0.2633 | 0.8812 | 0.1904 | 0.2622 | 0.2558 | 0.9974 | 0.9818 | 0.0003 | 0.0242 |
| No defect vs. 1-wall defect | 0.3351 | 0.5516 | 0.3889 | 0.0073 | 0.3386 | 0.0766 | 0.9770 | 0.0153 | 0.1285 | 0.6545 | 0.0199 | 0.3828 | 0.7801 | 0.0602 | <0.0001 | 0.0332 | 0.3379 | 0.1511 | 0.0001 | 0.0328 |
| No defect vs. circular defect | 0.0047 | 0.0191 | 0.0034 | 0.0099 | 0.0001 | <0.0001 | 0.0005 | 0.0077 | 0.0003 | <0.0001 | <0.0001 | 0.0283 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.2540 | 0.0035 | <0.0001 | <0.0001 |
| 3-wall defect vs. 1-wall defect | 0.0003 | 0.0781 | 0.4268 | 0.5591 | 0.9755 | 0.0308 | 0.9788 | 0.9778 | 0.5764 | 0.0465 | 0.0803 | 0.9785 | 0.8520 | 0.9885 | 0.0144 | 0.0004 | 0.5076 | 0.0457 | 0.9833 | 0.9798 |
| 3-wall defect vs. circular defect | 0.0017 | 0.0016 | 0.0018 | 0.0006 | <0.0001 | 0.0003 | 0.0012 | 0.0016 | 0.0002 | <0.0001 | <0.0001 | 0.0079 | 0.0004 | 0.0014 | <0.0001 | <0.0001 | 0.0246 | 0.0007 | 0.0005 | 0.0007 |
| 1-wall defect vs. circular defect | 0.0168 | 0.0002 | <0.0001 | 0.0003 | <0.0001 | 0.0014 | 0.0020 | 0.0002 | 0.0002 | <0.0001 | <0.0001 | 0.0062 | 0.0004 | 0.0003 | <0.0001 | <0.0001 | 0.0006 | 0.0338 | <0.0001 | 0.0002 |
| No defect | 3-wall defect | 1-wall defect | circular defect | No defect | 3-wall defect | 1-wall defect | circular defect | No defect | 3-wall defect | 1-wall defect | circular defect | No defect | 3-wall defect | 1-wall defect | circular defect | |||||
| Tapered effect vs. Tissue level | 0.0004 | 0.0001 | <0.0001 | 0.5537 | 0.0032 | 0.0052 | 0.0004 | 0.3377 | 0.0003 | 0.0004 | 0.0086 | 0.9802 | 0.0002 | <0.0001 | 0.0003 | 0.9988 | ||||
| Tapered effect vs. Zirconia | 0.0790 | 0.0976 | 0.0003 | 0.9339 | 0.7823 | 0.0286 | <0.0001 | 0.3031 | 0.2588 | 0.9955 | 0.0182 | 0.0021 | 0.9994 | 0.9866 | 0.9904 | 0.2197 | ||||
| Tapered effect vs. Bone level | <0.0001 | <0.0001 | <0.0001 | 0.0017 | <0.0001 | <0.0001 | <0.0001 | 0.0014 | <0.0001 | 0.6752 | 0.0578 | 0.5208 | 0.0653 | 0.0046 | 0.0404 | 0.8670 | ||||
| Tapered effect vs. BLX | <0.0001 | <0.0001 | <0.0001 | 0.0102 | 0.0004 | 0.0014 | <0.0001 | 0.4273 | 0.2189 | 0.4479 | 0.0012 | 0.9879 | 0.0007 | <0.0001 | <0.0001 | 0.0069 | ||||
| Tissue level vs. Zirconia | 0.0544 | 0.5475 | 0.3229 | 0.3580 | <0.0001 | 0.1391 | 0.3373 | 0.9851 | 0.0002 | 0.0007 | 0.0022 | 0.0001 | 0.0020 | 0.0010 | 0.1347 | 0.1427 | ||||
| Tissue level vs. Bone level | >0.9999 | 0.3265 | 0.3502 | 0.2188 | 0.0196 | 0.0049 | <0.0001 | 0.0669 | <0.0001 | 0.0005 | 0.0005 | 0.4837 | 0.0008 | 0.2091 | 0.9520 | 0.5139 | ||||
| Tissue level vs. BLX | 0.9539 | 0.8573 | 0.0250 | 0.8626 | 0.4586 | >0.9999 | 0.2011 | 0.6643 | 0.0002 | 0.0097 | 0.1065 | >0.9999 | 0.6327 | 0.1159 | 0.0092 | 0.0012 | ||||
| Zirconia vs. Bone level | 0.0814 | 0.0437 | 0.0812 | 0.0033 | <0.0001 | <0.0001 | <0.0001 | 0.0010 | 0.0004 | 0.6233 | 0.3851 | 0.2777 | 0.2822 | 0.0080 | 0.1773 | 0.0092 | ||||
| Zirconia vs. BLX | 0.0118 | 0.2155 | >0.9999 | 0.0221 | 0.0064 | 0.2658 | 0.8947 | 0.8753 | >0.9999 | 0.3670 | <0.0001 | 0.0001 | 0.0003 | <0.0001 | 0.0003 | 0.0023 | ||||
| Bone level vs. BLX | 0.9388 | 0.3057 | 0.0025 | 0.0087 | <0.0001 | 0.0002 | <0.0001 | 0.0026 | 0.0002 | 0.4057 | 0.0008 | 0.4588 | 0.0002 | 0.0095 | 0.0010 | 0.0535 | ||||
| Statistical analysis of torque in values | Tapered effect | Tissue level | Zirconia | Bone level | BLX | Tapered effect | Tissue level | Zirconia | Bone level | BLX | Tapered effect | Tissue level | Zirconia | Bone level | BLX | Tapered effect | Tissue level | Zirconia | Bone level | BLX |
| No defect vs. 3-wall defect | 0.1539 | 0.9885 | 0.3582 | <0.0001 | 0.0167 | 0.0433 | 0.2306 | 0.8411 | <0.0001 | 0.9244 | 0.2614 | 0.1816 | 0.3881 | 0.1733 | 0.0631 | 0.0032 | 0.0003 | 0.5227 | 0.0127 | 0.0036 |
| No defect vs. 1-wall defect | >0.9999 | 0.8026 | 0.7533 | 0.1956 | 0.5601 | 0.0022 | <0.0001 | 0.0762 | 0.0437 | 0.0162 | 0.1591 | <0.0001 | 0.0017 | 0.6875 | <0.0001 | 0.0039 | <0.0001 | 0.0043 | 0.0440 | 0.0668 |
| No defect vs. circular defect | 0.0291 | 0.0889 | <0.0001 | 0.0003 | 0.0022 | 0.4924 | 0.1868 | 0.0050 | <0.0001 | 0.0003 | 0.0004 | 0.0199 | <0.0001 | 0.0001 | <0.0001 | <0.0001 | 0.0009 | 0.0003 | 0.0036 | 0.0127 |
| 3-wall defect vs. 1-wall defect | 0.1539 | 0.9029 | 0.6602 | 0.0005 | 0.0835 | 0.0870 | 0.0008 | 0.0021 | <0.0001 | 0.0026 | 0.9062 | <0.0001 | 0.0004 | 0.0248 | 0.1363 | 0.7109 | 0.9194 | 0.0030 | 0.0717 | 0.7533 |
| 3-wall defect vs. circular defect | 0.9991 | 0.0494 | 0.0002 | 0.3016 | 0.1417 | 0.0001 | 0.8544 | 0.0014 | 0.0343 | 0.0002 | 0.0006 | 0.1513 | <0.0001 | 0.0046 | 0.0113 | 0.1435 | 0.9927 | 0.0007 | 0.2745 | 0.9600 |
| 1-wall defect vs. circular defect | 0.0291 | 0.0078 | <0.0001 | 0.0028 | 0.0090 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0408 | <0.0001 | 0.0002 | <0.0001 | <0.0001 | 0.0163 | 0.3057 | 0.9820 | 0.1816 | 0.0161 | 0.8947 |
| No defect | 3-wall defect | 1-wall defect | circular defect | No defect | 3-wall defect | 1-wall defect | circular defect | No defect | 3-wall defect | 1-wall defect | circular defect | No defect | 3-wall defect | 1-wall defect | circular defect | |||||
| Tapered effect vs. Tissue level | 0.0004 | 0.0105 | 0.0004 | <0.0001 | 0.0002 | 0.0001 | 0.0009 | <0.0001 | <0.0001 | <0.0001 | 0.0014 | <0.0001 | <0.0001 | 0.0046 | 0.0011 | 0.0204 | ||||
| Tapered effect vs. Zirconia | >0.9999 | 0.9030 | 0.8491 | 0.0003 | >0.9999 | 0.0024 | 0.1058 | 0.0335 | 0.9954 | 0.9888 | 0.0009 | 0.1075 | 0.0108 | 0.3772 | 0.0006 | 0.0082 | ||||
| Tapered effect vs. Bone level | >0.9999 | 0.0002 | 0.2624 | 0.0021 | 0.0067 | <0.0001 | 0.0029 | 0.0024 | 0.0003 | <0.0001 | 0.0006 | 0.0006 | 0.0008 | <0.0001 | 0.0012 | 0.0034 | ||||
| Tapered effect vs. BLX | 0.0002 | <0.0001 | 0.0007 | <0.0001 | 0.0004 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0002 | <0.0001 | ||||
| Tissue level vs. Zirconia | 0.0004 | 0.0008 | 0.0003 | 0.1723 | <0.0001 | 0.0026 | 0.1792 | 0.7451 | <0.0001 | <0.0001 | 0.2085 | <0.0001 | <0.0001 | 0.0160 | 0.1866 | 0.5668 | ||||
| Tissue level vs. Bone level | 0.0004 | 0.6647 | 0.0040 | 0.2661 | <0.0001 | 0.2904 | 0.6786 | 0.7311 | <0.0001 | <0.0001 | 0.9681 | 0.7686 | <0.0001 | 0.9917 | 0.2897 | 0.5368 | ||||
| Tissue level vs. BLX | 0.2483 | 0.8881 | 0.9380 | 0.8795 | 0.4958 | 0.6841 | <0.0001 | <0.0001 | 0.0014 | 0.3283 | <0.0001 | 0.0014 | 0.0006 | 0.0262 | 0.0139 | 0.0051 | ||||
| Zirconia vs. Bone level | >0.9999 | 0.0003 | 0.4451 | 0.9997 | 0.0040 | 0.0004 | 0.8596 | >0.9999 | <0.0001 | <0.0001 | 0.0596 | <0.0001 | 0.0180 | 0.0021 | 0.9837 | 0.0975 | ||||
| Zirconia vs. BLX | 0.0002 | <0.0001 | 0.0009 | 0.2931 | <0.0001 | <0.0001 | <0.0001 | 0.0025 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0009 | <0.0001 | 0.0015 | <0.0001 | ||||
| Bone level vs. BLX | 0.0002 | 0.0019 | 0.0438 | 0.4541 | <0.0001 | 0.0031 | <0.0001 | <0.0001 | <0.0001 | 0.0037 | <0.0001 | 0.0175 | 0.0006 | 0.0003 | 0.0040 | 0.1025 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, A.; Heitzer, M.; Bock, A.; Peters, F.; Möhlhenrich, S.C.; Hölzle, F.; Modabber, A.; Kniha, K. Relationship between Implant Geometry and Primary Stability in Different Bony Defects and Variant Bone Densities: An In Vitro Study. Materials 2020, 13, 4349. https://doi.org/10.3390/ma13194349
Ibrahim A, Heitzer M, Bock A, Peters F, Möhlhenrich SC, Hölzle F, Modabber A, Kniha K. Relationship between Implant Geometry and Primary Stability in Different Bony Defects and Variant Bone Densities: An In Vitro Study. Materials. 2020; 13(19):4349. https://doi.org/10.3390/ma13194349
Chicago/Turabian StyleIbrahim, Ahmad, Marius Heitzer, Anna Bock, Florian Peters, Stephan Christian Möhlhenrich, Frank Hölzle, Ali Modabber, and Kristian Kniha. 2020. "Relationship between Implant Geometry and Primary Stability in Different Bony Defects and Variant Bone Densities: An In Vitro Study" Materials 13, no. 19: 4349. https://doi.org/10.3390/ma13194349
APA StyleIbrahim, A., Heitzer, M., Bock, A., Peters, F., Möhlhenrich, S. C., Hölzle, F., Modabber, A., & Kniha, K. (2020). Relationship between Implant Geometry and Primary Stability in Different Bony Defects and Variant Bone Densities: An In Vitro Study. Materials, 13(19), 4349. https://doi.org/10.3390/ma13194349





