The Effects of Grain Size and Twins Density on High Temperature Oxidation Behavior of Nickel-Based Superalloy GH738
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
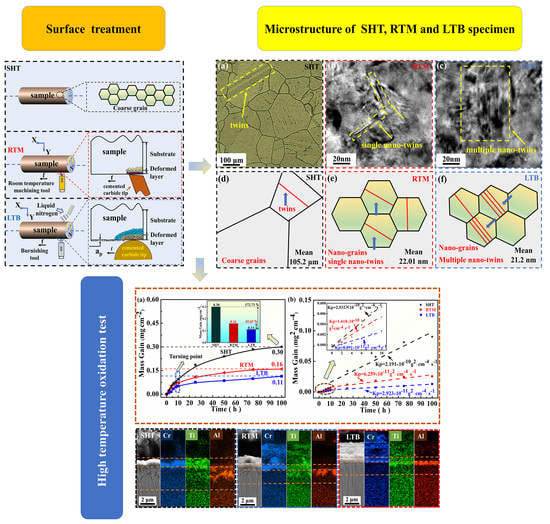
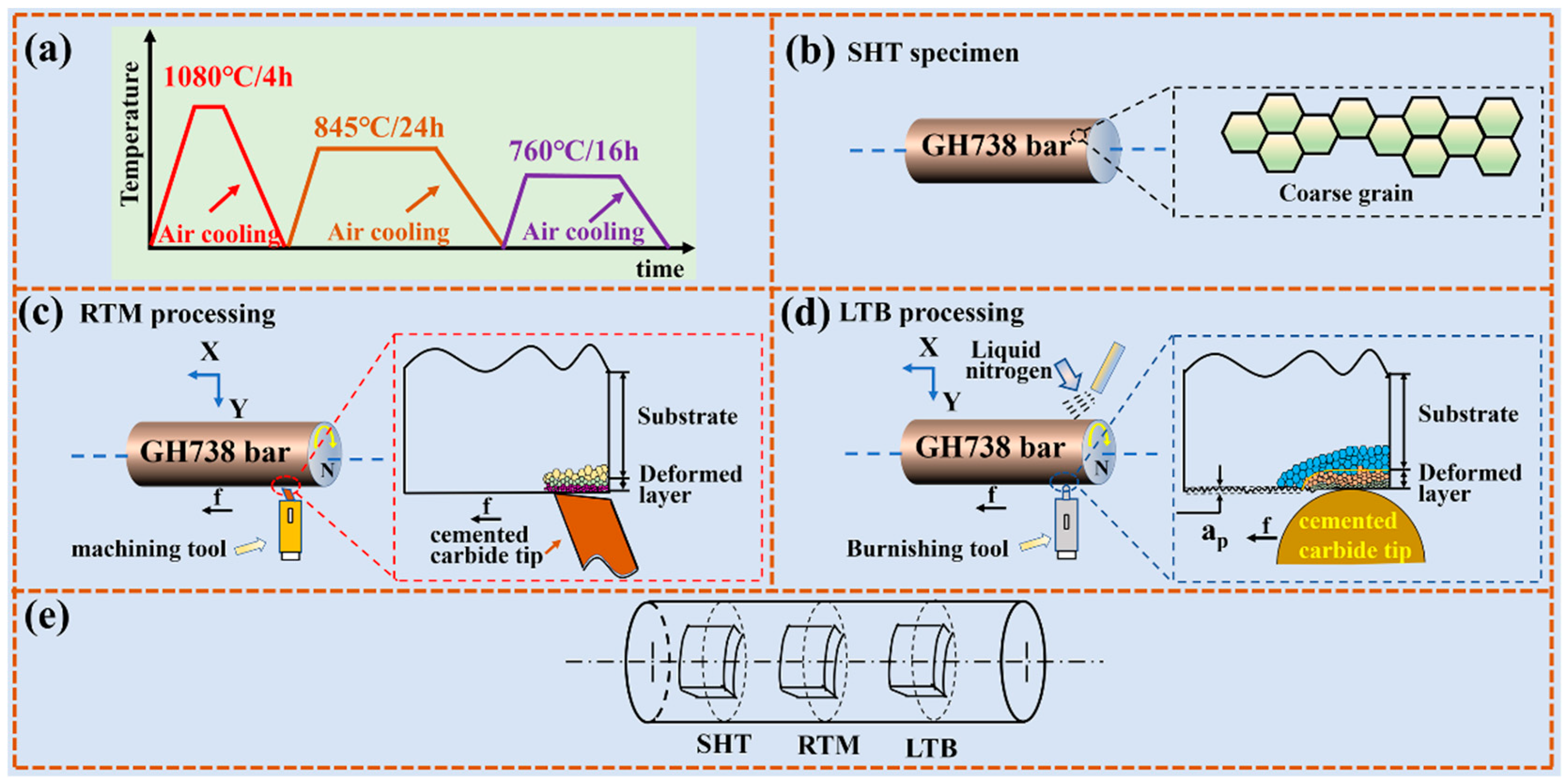
2.2. Surface Treatment Process
2.3. Oxidation Test
2.4. Microstructure Characterization
3. Results and Discussion
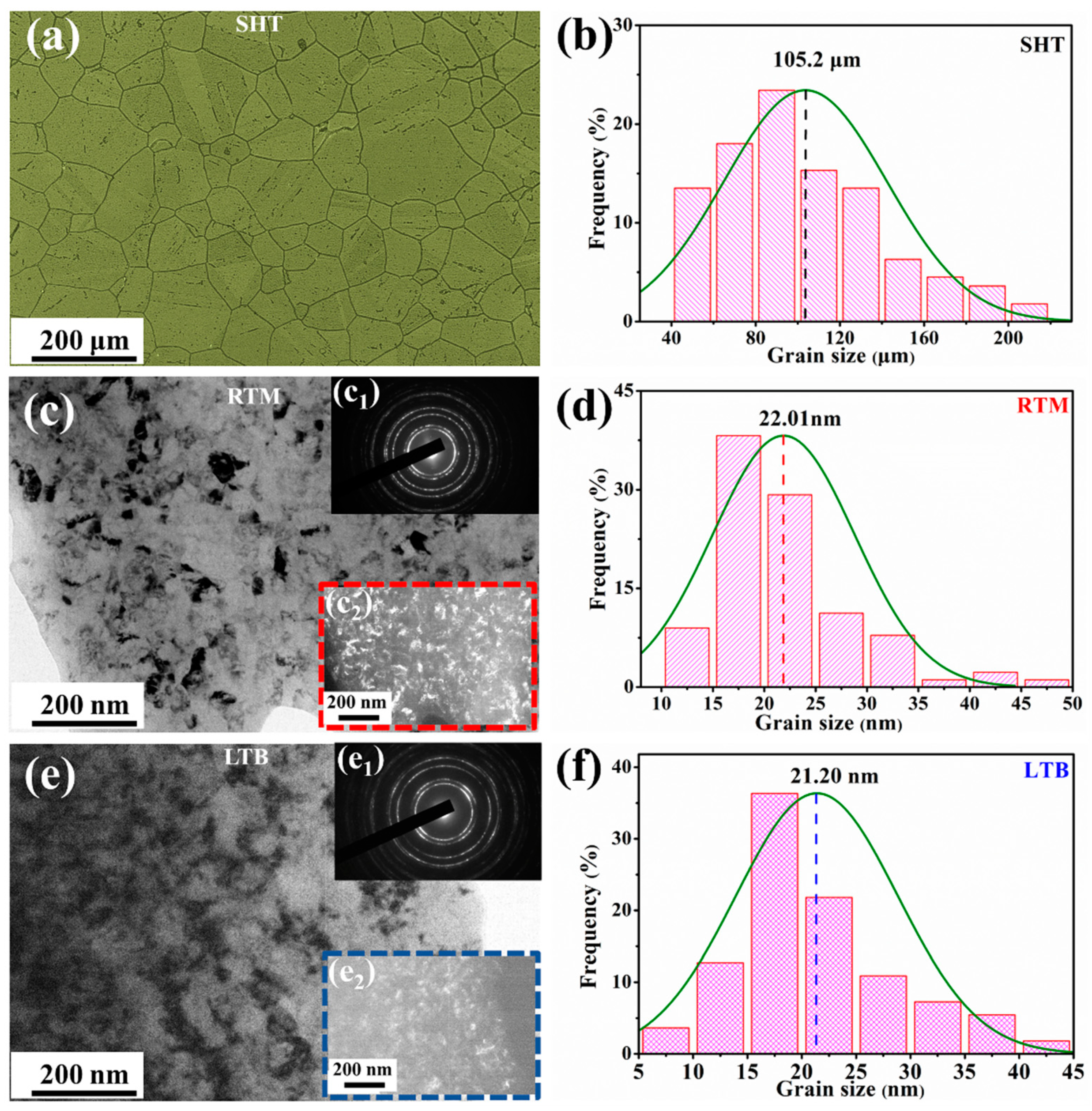
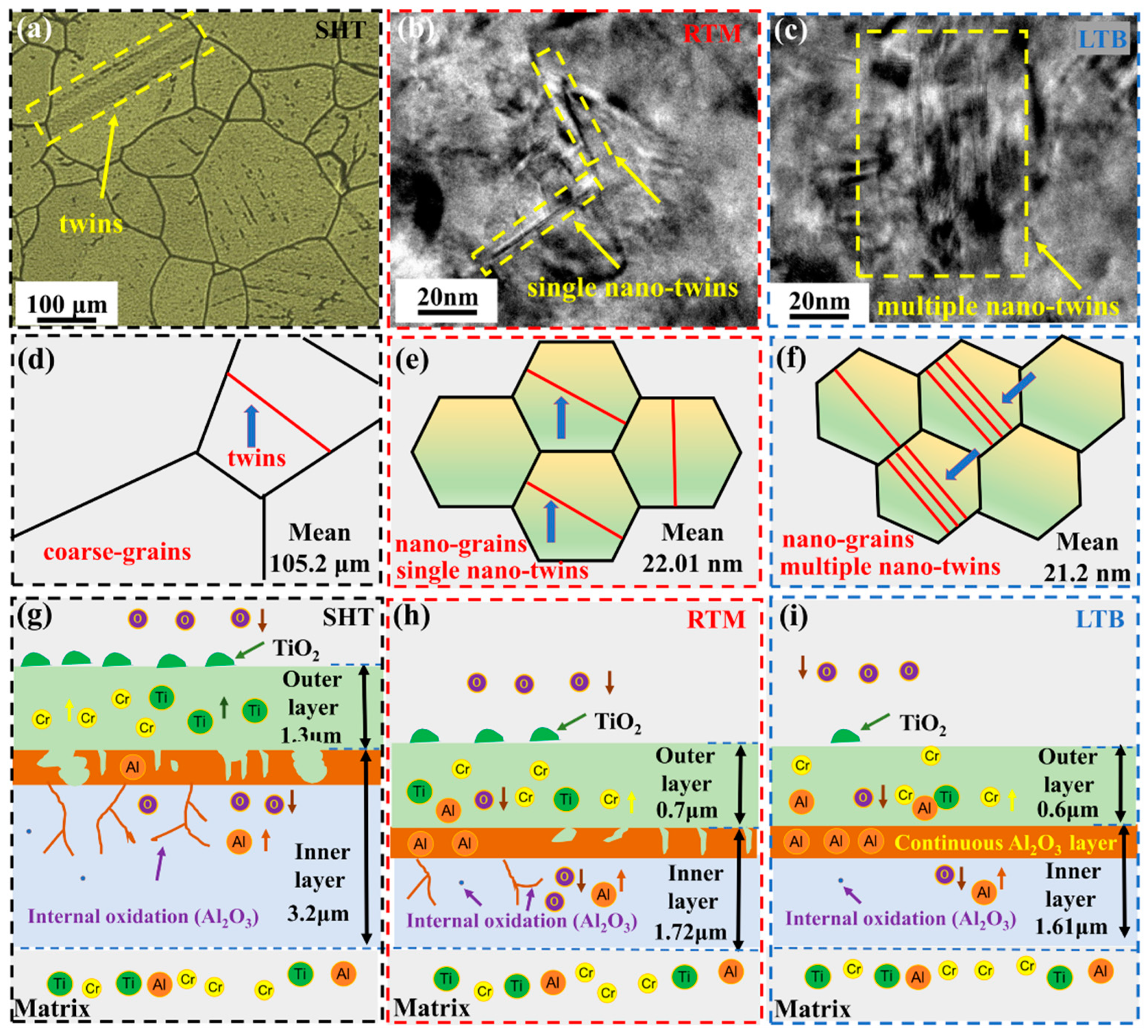
3.1. Surface Layer Characterization
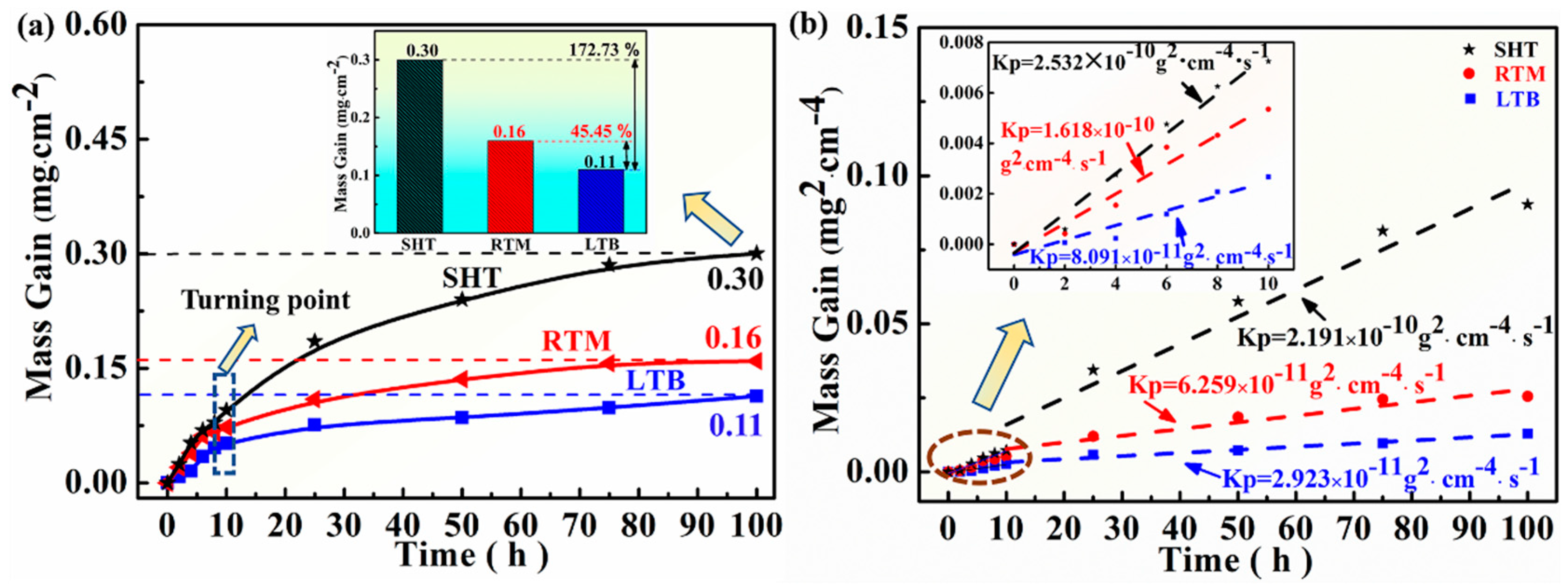
3.2. Oxidation Kinetics Analysis
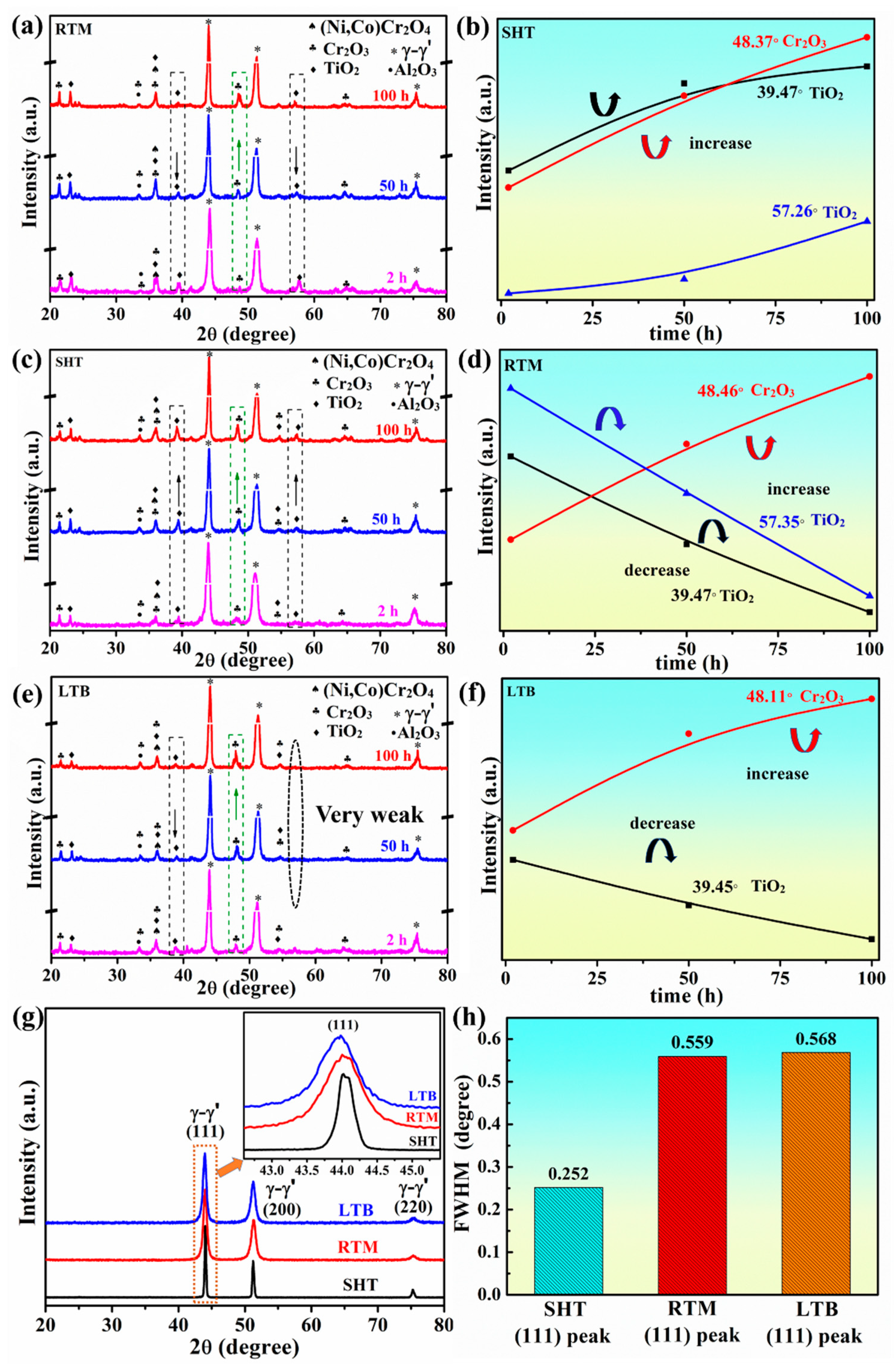
3.3. Phase Constitution of Surface Oxidation Product
3.4. Surface Morphology and Composition
3.5. Cross-Section Microstructure and Composition Distribution of Oxide Scales
3.6. High Temperature Oxidation Mechanism of GH738 Superalloy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, R.F.; Zhou, Y.X.; Li, X.; Yang, S.L.; Han, K.N.; Wang, S.J. The effect of milling cooling conditions on the surface integrity and fatigue behavior of the GH4169 superalloy. Metals 2019, 9, 1179. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Xu, D.K.; Lin, Y.C.; Chen, X. Low cycle fatigue and creep-fatigue interaction behavior of nickel-base superalloy GH4169 at elevated temperature of 650 °C. Mater. Sci. Eng. A 2016, 655, 175–182. [Google Scholar] [CrossRef]
- Wu, D.L.; Jiang, S.M.; Fan, Q.X.; Gong, J.; Sun, C. Hot corrosion behavior of a Cr-modified aluminide coating on a Ni-based superalloy. Acta Metall. Sin. (Engl. Lett.) 2014, 27, 627–634. [Google Scholar] [CrossRef]
- El-Awadi, G.A.; Abdel-Samad, S.; Elshazly, E.S. Hot corrosion behavior of Ni based Inconel 617 and Inconel 738 superalloys. Appl. Surf. Sci. 2016, 378, 224–230. [Google Scholar] [CrossRef]
- Mahobia, G.S.; Paulose, N.; Singh, V. Hot corrosion behavior of superalloy IN718 at 550 and 650 °C. J. Mater. Eng. Perform. 2013, 22, 2418–2435. [Google Scholar] [CrossRef]
- Cho, S.H.; Kwon, S.C.; Kim, D.Y.; Choi, W.S.; Kim, Y.S.; Lee, J.H. Hot corrosion behaviour of nickel-cobalt-based alloys in a lithium molten salt. Corros. Sci. 2019, 151, 20–26. [Google Scholar] [CrossRef]
- Pradhan, D.; Mahobia, G.S.; Chattopadhyay, K.; Singh, V. Salt induced corrosion behaviour of superalloy IN718. Mater. Today Proc. 2018, 5, 7047–7054. [Google Scholar] [CrossRef]
- Zhao, L.H.; Tan, Y.; Shi, S.; Zhuang, X.P.; Niu, S.Q.; You, Q.F.; Wang, Y.N. High temperature oxidation behavior of electron beam smelted K417 superalloy. Vacuum 2019, 170, 108979. [Google Scholar] [CrossRef]
- Cao, J.D.; Zhang, J.S.; Chen, R.F.; Ye, Y.X.; Hua, Y.Q. High temperature oxidation behavior of Ni-based superalloy GH202. Mater. Charact. 2016, 118, 122–128. [Google Scholar] [CrossRef]
- Cao, J.D.; Zhang, J.S.; Hua, Y.Q.; Rong, Z.; Chen, F.R.; Ye, Y.X. High temperature oxidation behavior of Ni-based superalloy GH586 in air. Rare Met. 2017, 36, 878–885. [Google Scholar] [CrossRef]
- Park, S.J.; Seo, S.M.; Yoo, Y.S.; Jeong, H.W.; Jang, H.J. Effects of Cr, W, and Mo on the high temperature oxidation of Ni-based superalloys. Materials 2019, 12, 2934. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.F.; Ren, X.D.; Yang, Y.; Tong, Z.P.; Chen, L. Tensile behavior of nickel with gradient microstructure produced by laser shock peening. Mater. Sci. Eng. A 2020, 771, 138603. [Google Scholar] [CrossRef]
- Li, X.G.; Lu, L.; Li, J.G.; Zhang, X.; Gao, H.J. Mechanical properties and deformation mechanisms of gradient nanostructured metals and alloys. Nat. Rev. Mater. 2020, 5, 706–723. [Google Scholar] [CrossRef]
- Tong, Z.P.; Ren, X.D.; Ren, Y.P.; Dai, F.Z.; Ye, Y.X.; Zhou, W.F. Effect of laser shock peening on microstructure and hot corrosion of TC11 alloy. Surf. Coat. Technol. 2018, 335, 32–40. [Google Scholar] [CrossRef]
- Lu, J.Z.; Luo, K.Y.; Yang, D.K.; Cheng, X.N.; Hu, J.L.; Dai, F.Z. Effects of laser peening on stress corrosion cracking (SCC) of ANSI 304 austenitic stainless steel. Corros. Sci. 2012, 60, 145–152. [Google Scholar] [CrossRef]
- Tao, N.R.; Wang, Z.B.; Tong, W.P.; Sui, M.L.; Lu, J.; Lu, K. An investigation of surface nanocrystallization mechanism in Fe induced by surface mechanical attrition treatment. Acta Mater. 2002, 50, 4603–4616. [Google Scholar] [CrossRef]
- Wen, L.; Wang, Y.M.; Zhou, Y.; Guo, L.X.; Ouyang, J.H. Microstructure and corrosion resistance of modified 2024 Al alloy using surface mechanical attrition treatment combined with microarc oxidation process. Corros. Sci. 2011, 53, 473–480. [Google Scholar] [CrossRef]
- Chan, H.L.; Ruan, H.H.; Chen, A.Y.; Lu, J. Optimization of the strain rate to achieve exceptional mechanical properties of 304 stainless steel using high speed ultrasonic surface mechanical attrition treatment. Acta Mater. 2010, 58, 5086–5096. [Google Scholar] [CrossRef]
- Huang, H.W.; Wang, Z.B.; Lu, J.; Lu, K. Fatigue behaviors of AISI 316L stainless steel with a gradient nanostructured surface layer. Acta Mater. 2015, 87, 150–160. [Google Scholar] [CrossRef]
- Li, W.L.; Tao, N.R.; Lu, K. Fabrication of a gradient nano-micro-structured surface layer on bulk copper by means of a surface mechanical grinding treatment. Scr. Mater. 2008, 59, 546–549. [Google Scholar] [CrossRef]
- Fang, T.H.; Li, W.L.; Tao, N.R.; Lu, K. Revealing extraordinary intrinsic tensile plasticity in gradient nano-grained copper. Science 2011, 331, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Song, G.D.; Wang, Y.M.; Zheng, M.; Xiao, G.Z.; Yang, J. Effect of surface nanocrystallization on fatigue crack initiation and propagation behavior in pure Zr. Mater. Sci. Eng. A 2020, 794, 139831. [Google Scholar] [CrossRef]
- Swaminathan, S.; Ravi, S.M.; Rao, B.C.; Compton, W.D.; Chandrasekar, S.; King, A.H.; Trumble, K.P. Severe plastic deformation (SPD) and nanostructured materials by machining. J. Mater. Sci. 2007, 42, 1529–1541. [Google Scholar] [CrossRef]
- Tang, J.; Luo, H.Y.; Guo, J.J.; Lv, J.L.; Zhang, Z.; Ma, Y. Effects of nano-grains and deformation nano-twins on electrochemical corrosion behavior of DZ125 nickel-based superalloy. Adv. Eng. Mater. 2018, 20, 1800279. [Google Scholar] [CrossRef]
- Tang, J.; Luo, H.Y.; Qi, Y.M.; Xu, P.W.; Lv, J.L.; Ma, Y.; Zhang, Z. Effect of nano-scale martensite and β phase on the passive film formation and electrochemical behaviour of Ti-10V-2Fe-3Al alloy in 3.5% NaCl solution. Electrochim. Acta 2018, 283, 1300–1312. [Google Scholar] [CrossRef]
- Maamoun, A.H.; Elbestawi, M.A.; Veldhuis, S.C. Influence of shot peening on AlSi10Mg parts fabricated by additive manufacturing. J. Manuf. Mater. Process. 2018, 2, 40. [Google Scholar] [CrossRef]
- Estrin, Y.; Beygelzimer, Y.; Kulagin, R. Design of architectured materials based on mechanically driven structural and compositional patterning. Adv. Eng. Mater. 2019, 21, 1900487. [Google Scholar] [CrossRef]
- Zhou, L.C.; Long, C.B.; He, W.F.; Tian, L.; Jia, W.T. Improvement of high-temperature fatigue performance in the nickel-based alloy by LSP-induced surface nanocrystallization. J. Alloys Compd. 2018, 744, 156–164. [Google Scholar] [CrossRef]
- Cao, J.D.; Zhang, J.S.; Hua, Y.Q.; Chen, R.F.; Ye, Y.X. Improving the high temperature oxidation resistance of Ni-based superalloy GH202 induced by laser shock processing. J. Mater. Process. Technol. 2017, 243, 31–39. [Google Scholar] [CrossRef]
- Xia, Z.X.; Zhang, C.; Huang, X.F.; Liu, W.B.; Yang, Z.G. Improve oxidation resistance at high temperature by nanocrystalline surface layer. Sci. Rep. 2015, 5, 13027. [Google Scholar] [CrossRef]
- Jelliti, S.; Richard, C.; Retraint, D.; Roland, T.; Chemkhi, M.; Demangel, C. Effect of surface nanocrystallization on the corrosion behavior of Ti–6Al–4V titanium alloy. Surf. Coat. Technol. 2013, 224, 82–87. [Google Scholar] [CrossRef]
- Ren, N.F.; Yang, H.M.; Yuan, S.Q.; Wang, Y.; Tang, S.X.; Zheng, L.M.; Ren, X.D.; Dai, F.Z. High temperature mechanical properties and surface fatigue behavior improving of steel alloy via laser shock peening. Mater. Des. 2014, 53, 452–456. [Google Scholar] [CrossRef]
- Altenberger, I.; Nalla, R.K.; Sano, Y.J.; Wagner, L.; Ritchie, R.O. On the effect of deep-rolling and laser-peening on the stress-controlled low- and high-cycle fatigue behavior of Ti–6Al–4V at elevated temperatures up to 550 °C. Int. J. Fatigue 2012, 44, 292–302. [Google Scholar] [CrossRef]
- Hua, Y.Q.; Rong, Z.; Ye, Y.X.; Chen, K.M.; Chen, R.F.; Xue, Q. Laser shock processing effects on isothermal oxidation resistance of GH586 superalloy. Appl. Surf. Sci. 2015, 330, 439–444. [Google Scholar] [CrossRef]
- Tan, L.; Ren, X.; Sridharan, K.; Allen, T.R. Effect of shot-peening on the oxidation of alloy 800H exposed to supercritical water and cyclic oxidation. Corros. Sci. 2008, 50, 2040–2046. [Google Scholar] [CrossRef]
- Wu, M.Y.; Chen, M.H.; Zhu, S.L.; Wang, F.H. Effect of sand blasting on oxidation behavior of K38G superalloy at 1000 °C. Corros. Sci. 2015, 92, 256–262. [Google Scholar] [CrossRef]
- Caudill, J.; Huang, B.; Arvin, C.; Schoop, J.; Meyer, K.; Jawahir, I.S. Enhancing the surface integrity of Ti-6Al-4V alloy through cryogenic burnishing. Procedia CIRP 2014, 13, 243–248. [Google Scholar] [CrossRef]
- Tang, J.; Luo, H.Y.; Zhang, Y.B. Enhancing the surface integrity and corrosion resistance of Ti-6Al-4V titanium alloy through cryogenic burnishing. Int. J. Adv. Manuf. Technol. 2016, 88, 2785–2793. [Google Scholar] [CrossRef]
- Pu, Z.; Yang, S.; Song, G.L.; Dillon, O.W.; Puleo, D.A.; Jawahir, I.S. Ultrafine-grained surface layer on Mg-Al-Zn alloy produced by cryogenic burnishing for enhanced corrosion resistance. Scr. Mater. 2011, 65, 520–523. [Google Scholar] [CrossRef]
- Altenberger, I.; Stach, E.A.; Liu, G.; Nalla, R.K.; Ritchie, R.O. An in situ transmission electron microscope study of the thermal stability of near-surface microstructures induced by deep rolling and laser-shock peening. Scr. Mater. 2003, 48, 1593–1598. [Google Scholar] [CrossRef]
- Lv, J.L.; Luo, H.Y. Effect of surface burnishing on texture and corrosion behavior of 2024 aluminum alloy. Surf. Coat. Technol. 2013, 235, 513–520. [Google Scholar]
- Luo, H.Y.; Liu, J.Y.; Wang, L.J.; Zhong, Q.P. Investigation of the burnishing process with PCD tool on non-ferrous metals. Int. J. Adv. Manuf. Technol. 2004, 25, 454–459. [Google Scholar] [CrossRef]
- Nouduru, S.K.; Kumar, M.K.; Kain, V.; Khanna, A.S.; Saibaba, N.; Dey, G.K. High temperature and high pressure oxidation behavior of Zr-2.5Nb pressure tube material—Effect of β phase composition and surface machining. J. Nucl. Mater. 2016, 470, 197–207. [Google Scholar] [CrossRef]
- Tang, J.; Luo, H.Y.; Qi, Y.M.; Xu, P.W.; Ma, S.; Zhang, Z. The effect of cryogenic burnishing on the formation mechanism of corrosion product film of Ti-6Al-4V titanium alloy in 0.9% NaCl solution. Surf. Coat. Technol. 2018, 345, 123–131. [Google Scholar] [CrossRef]
- Xu, P.W.; Luo, H.Y.; Han, Z.Y.; Zou, J. Tailoring a gradient nanostructured age-hardened aluminum alloy using high-gradient strain and strain rate. Mater. Des. 2015, 85, 240–247. [Google Scholar] [CrossRef]
- Lou, S.; Li, Y.; Zhou, L.; Nie, X.; He, G.; Li, Y. Surface nanocrystallization of metallic alloys with different stacking fault energy induced by laser shock processing. Mater. Des. 2016, 104, 320–326. [Google Scholar] [CrossRef]
- Meng, G.Z.; Li, Y.; Shao, Y.W.; Zhang, T.; Wang, Y.Q.; Wang, F.H.; Cheng, X.Q.; Dong, C.F.; Li, X.G. Effect of microstructures on corrosion behavior of nickel coatings: (II) Competitive effect of grain size and twins density on corrosion behavior. J. Mater. Sci. Technol. 2016, 32, 465–469. [Google Scholar] [CrossRef]
- Wang, K.; Tao, N.R.; Liu, G.; Lu, J.; Lu, K. Plastic strain-induced grain refinement at the nanometer scale in copper. Acta Mater. 2006, 54, 5281–5291. [Google Scholar] [CrossRef]
- Ren, X.D.; Zhou, W.F.; Ren, Y.P.; Xu, S.D.; Liu, F.F.; Yuan, S.Q. Dislocation evolution and properties enhancement of GH2036 by laser shock processing: Dislocation dynamics simulation and experiment. Mater. Sci. Eng. A 2016, 654, 184–192. [Google Scholar] [CrossRef]
- Fan, Q.X.; Peng, X.; Yu, H.J.; Jiang, S.M.; Gong, J.; Sun, C. The isothermal and cyclic oxidation behaviour of two Co modified aluminide coatings at high temperature. Corros. Sci. 2014, 84, 42–53. [Google Scholar] [CrossRef]
- Athreya, C.N.; Deepak, K.; Kim, D.I.; Boer, D.B.; Mandal, S.; Sarma, S.V. Role of grain boundary engineered microstructure on high temperature steam oxidation behaviour of Ni based superalloy alloy 617. J. Alloys Compd. 2019, 778, 224–233. [Google Scholar] [CrossRef]
- Park, S.J.; Seo, S.M.; Yoo, Y.S.; Jeong, H.W.; Jang, H. Effects of Al and Ta on the high temperature oxidation of Ni-based superalloys. Corros. Sci. 2015, 90, 305–312. [Google Scholar] [CrossRef]
- Chen, M.H.; Shen, M.L.; Zhu, S.L.; Wang, F.H.; Wang, X.L. Effect of sand blasting and glass matrix composite coating on oxidation resistance of a nickel-based superalloy at 1000 °C. Corros. Sci. 2013, 73, 331–341. [Google Scholar] [CrossRef]
- Liu, P.S.; Liang, K.M.; Gu, S.R. High-temperature oxidation behavior of aluminide coatings on a new cobalt-base superalloy in air. Corros. Sci. 2001, 43, 1217–1226. [Google Scholar] [CrossRef]
- Maamoun, A.H.; Elbestawi, M.; Dosbaeva, G.K.; Veldhuis, S.C. Thermal post-processing of AlSi10Mg parts produced by selective laser melting using recycled powder. Addit. Manuf. 2018, 21, 234–247. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years A survey and some new results in the determination. J. Appl. Cryst. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Kang, Y.J.; Yang, S.S.; Kima, Y.K.; AlMangour, B.; Lee, K.A. Effect of post-treatment on the microstructure and high-temperature oxidation behaviour of additively manufactured inconel 718 alloy. Corros. Sci. 2019, 158, 108082. [Google Scholar] [CrossRef]
- Niu, J.M.; Wang, W.; Zhu, S.L.; Wang, F.H. The scaling behavior of sputtered Ni3Al coatings with and without Pt modification. Corros. Sci. 2012, 58, 115–120. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, M.C.; Dong, J.X. Oxidation behavior and mechanism of powder metallurgy Rene95 nickel based superalloy between 800 and 1000 °C. Appl. Surf. Sci. 2010, 256, 7510–7515. [Google Scholar] [CrossRef]
- Nijdam, T.J.; Jeurgens, L.P.H.; Sloof, W.G. Promoting exclusive α-Al2O3 growth upon high-temperature oxidation of NiCrAl alloys: Experiment versus model predictions. Acta Mater. 2005, 53, 1643–1653. [Google Scholar] [CrossRef]
- Abe, F.; Araki, H.; Yoshida, H.; Okada, M. The role of Aluminiu and Titanium on the oxidation process of a nickel-based superalloy in steam at 800. Oxid. Met. 1987, 27, 21–36. [Google Scholar] [CrossRef]
- Wang, Z.B.; Tao, N.R.; Tong, W.P.; Lu, J.; Lu, K. Diffusion of chromium in nanocrystalline iron produced by means of surface mechanical attrition treatment. Acta Mater. 2003, 51, 4319–4329. [Google Scholar] [CrossRef]
- Jiang, L.; Fu, C.T.; Leng, B.; Jia, Y.Y.; Ye, X.X.; Zhang, W.Z. Influence of grain size on tellurium corrosion behaviors of GH3535 alloy. Corros. Sci. 2019, 148, 110–122. [Google Scholar] [CrossRef]
- Prescott, R.; Graham, M.J. The formation of aluminum oxide scales on high-temperature alloys. Oxid. Met. 1992, 38, 233–254. [Google Scholar] [CrossRef]



| C | Co | Al | Fe | Mo | B | Cr | Ti | Mg | Ni |
|---|---|---|---|---|---|---|---|---|---|
| 0.04 | 13.25 | 1.46 | 0.2 | 4.38 | 0.006 | 19.31 | 3.13 | 0.006 | Balance |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Luo, H.; Yang, X. The Effects of Grain Size and Twins Density on High Temperature Oxidation Behavior of Nickel-Based Superalloy GH738. Materials 2020, 13, 4166. https://doi.org/10.3390/ma13184166
Ma W, Luo H, Yang X. The Effects of Grain Size and Twins Density on High Temperature Oxidation Behavior of Nickel-Based Superalloy GH738. Materials. 2020; 13(18):4166. https://doi.org/10.3390/ma13184166
Chicago/Turabian StyleMa, Wenbin, Hongyun Luo, and Xiaoguang Yang. 2020. "The Effects of Grain Size and Twins Density on High Temperature Oxidation Behavior of Nickel-Based Superalloy GH738" Materials 13, no. 18: 4166. https://doi.org/10.3390/ma13184166
APA StyleMa, W., Luo, H., & Yang, X. (2020). The Effects of Grain Size and Twins Density on High Temperature Oxidation Behavior of Nickel-Based Superalloy GH738. Materials, 13(18), 4166. https://doi.org/10.3390/ma13184166