The Thermal Gelation Behavior and Performance Evaluation of High Molecular Weight Nonionic Polyacrylamide and Polyethyleneimine Mixtures for In-Depth Water Control in Mature Oilfields
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
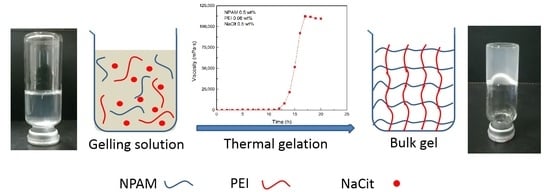
2.2.1. Preparation of Gelling Solutions
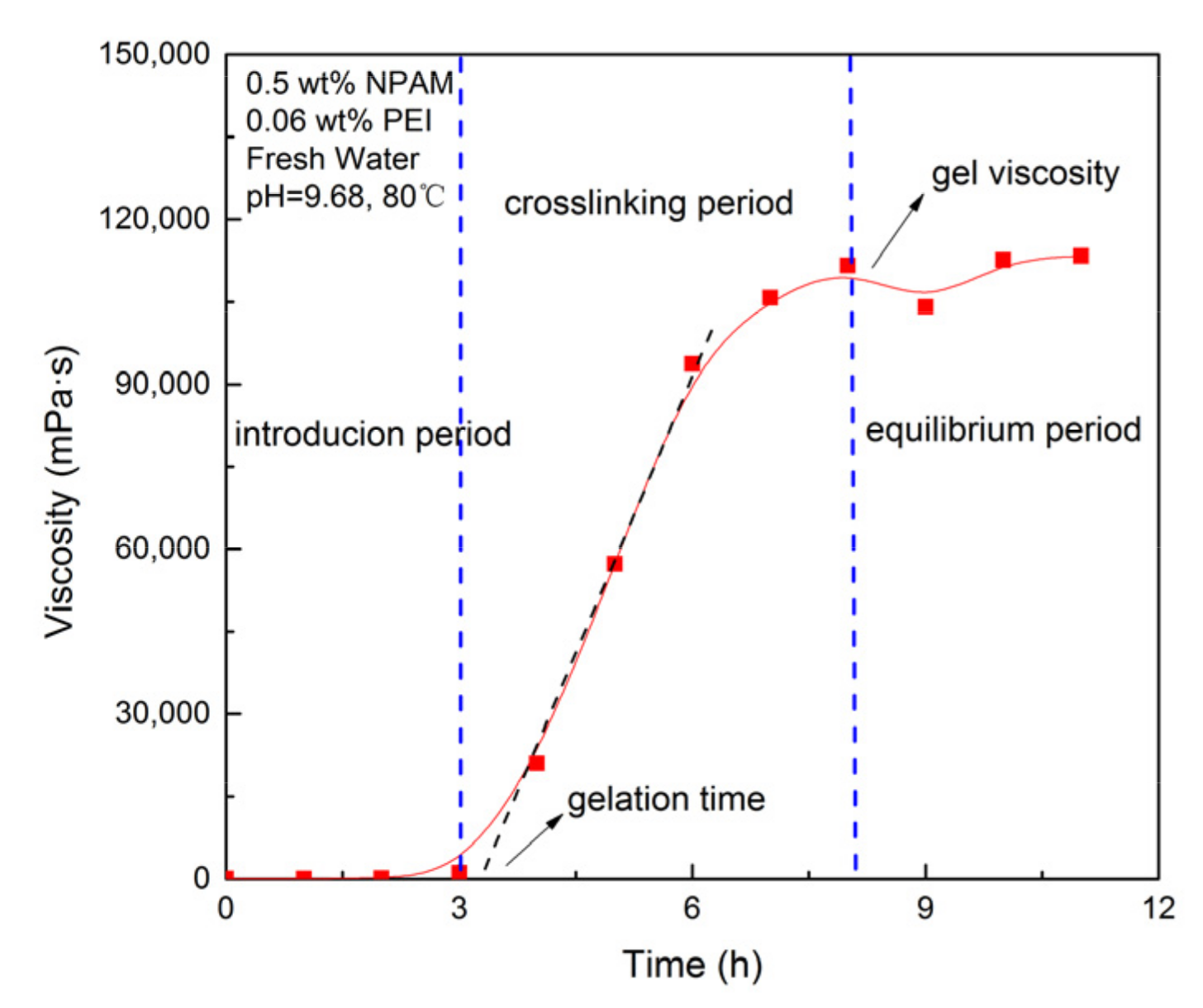
2.2.2. Determination of Gelation Time and Gel Viscosity of NPAM/PEI Gel System
2.2.3. Statistical Analysis
2.2.4. Scanning Electron Microscope (SEM)
2.2.5. Thermal Gravimetric Analysis (TGA)
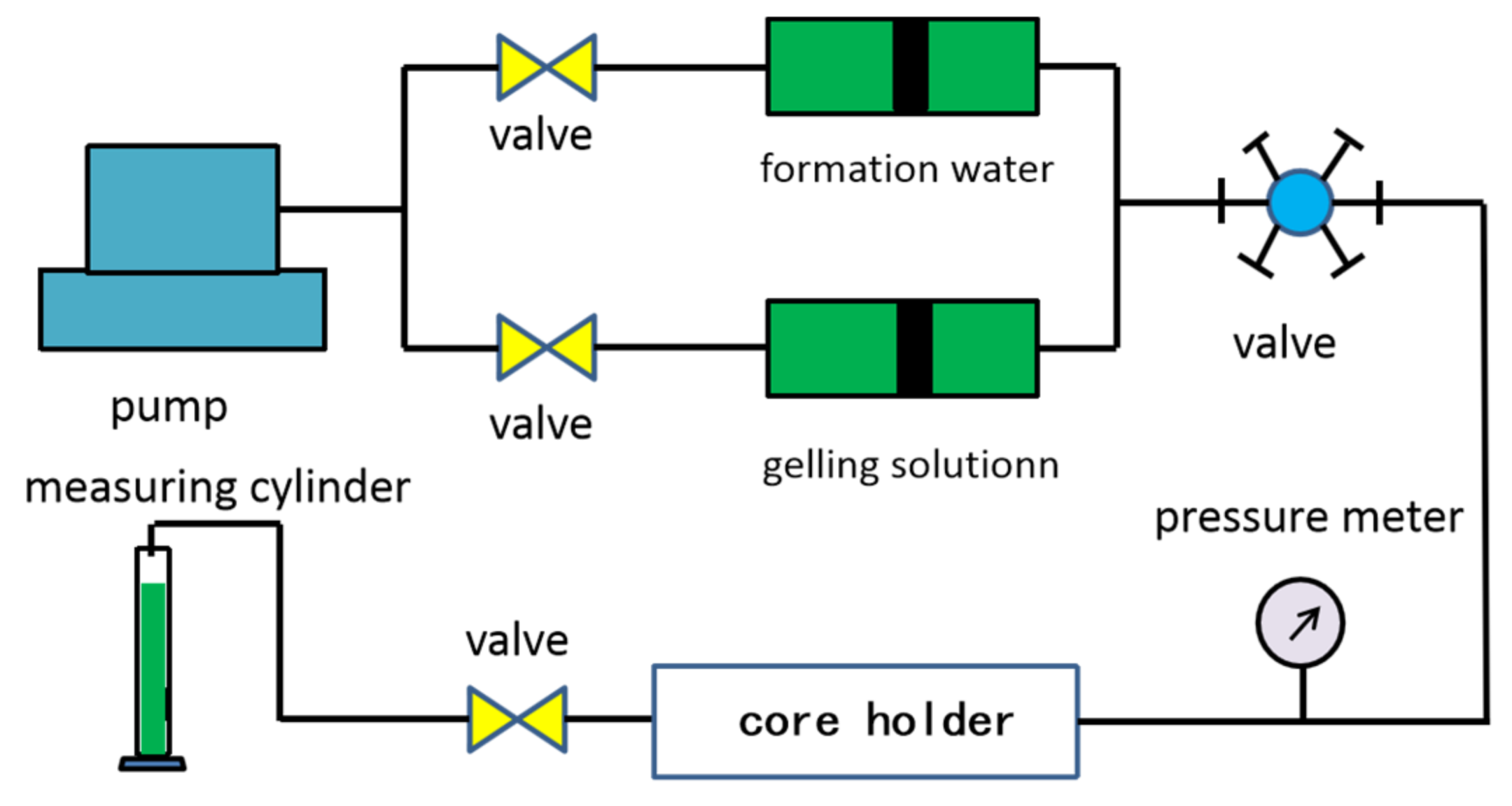
2.2.6. Core Flowing Experiment
- (1)
- The cores were washed, dried, and weighed. Injected water was used to saturate the cores in vacuum, and their pore volumes (PV) and porosity were determined using weight method.
- (2)
- The water flooding was carried out until the inlet pressure and output fluid reached a stable condition. The initial permeability of the core was calculated according to Darcy’s law in the form:where k is the permeability in mD, q is the flow rate in ml/min, is the viscosity of flooding water in mPa·s, L is the core length in cm, A is the cross-sectional area of core in cm2, and are the pressures of the core inlet and outlet in MPa, respectively.
- (3)
- The gelling solution (0.5 PV) was injected into the core, and water (0.5 PV) was injected to replace the gelling solution. After that, the core holder was sealed and placed at 80 °C until the gel matured.
- (4)
- Regained-permeability testing was conducted by water flooding as Step (2). The plugging rate () of NPAM/PEI gel system was evaluated using the following relation:
3. Results and Discussion
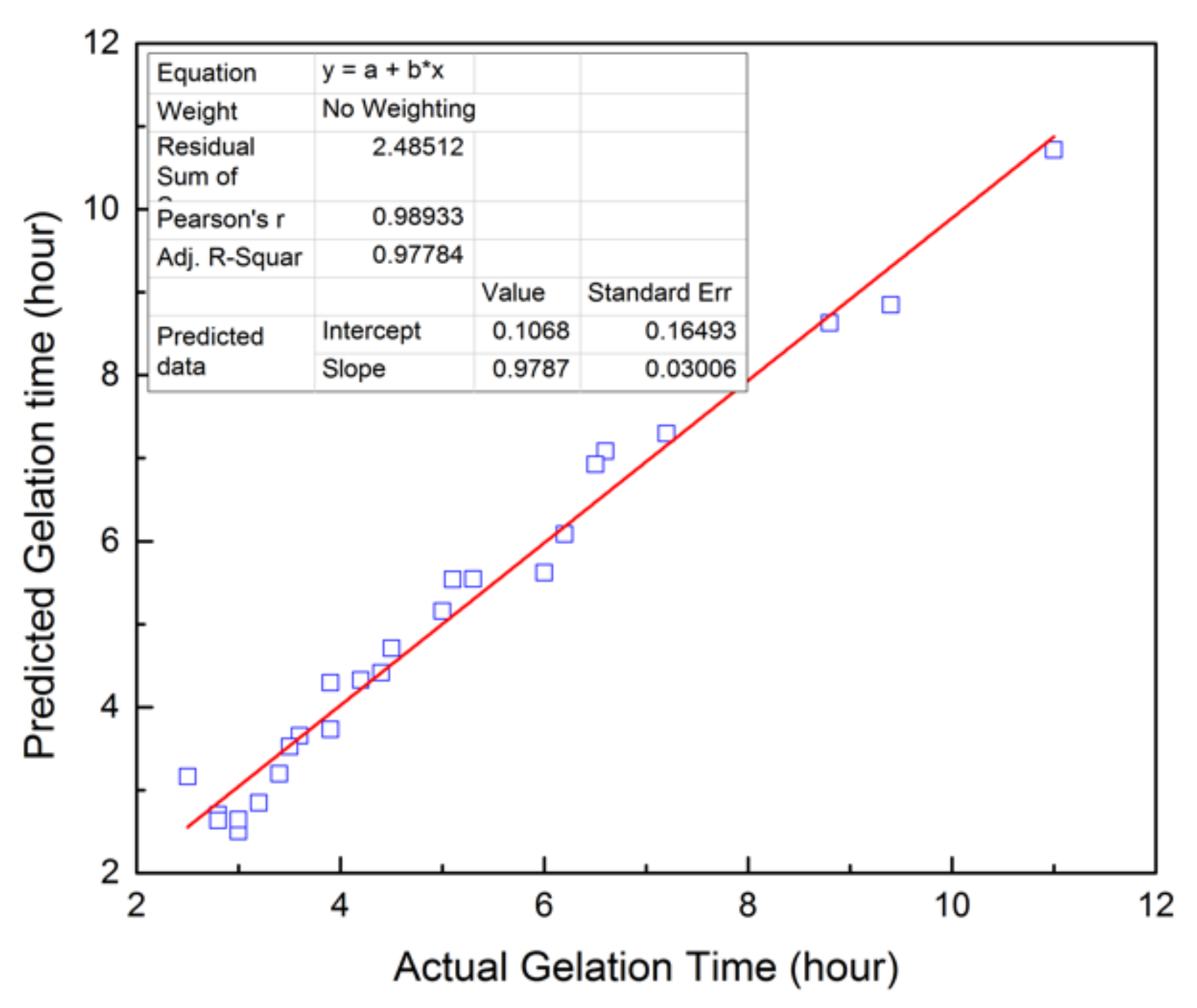
3.1. Mathematical Modeling of NPAM/PEI Gel System
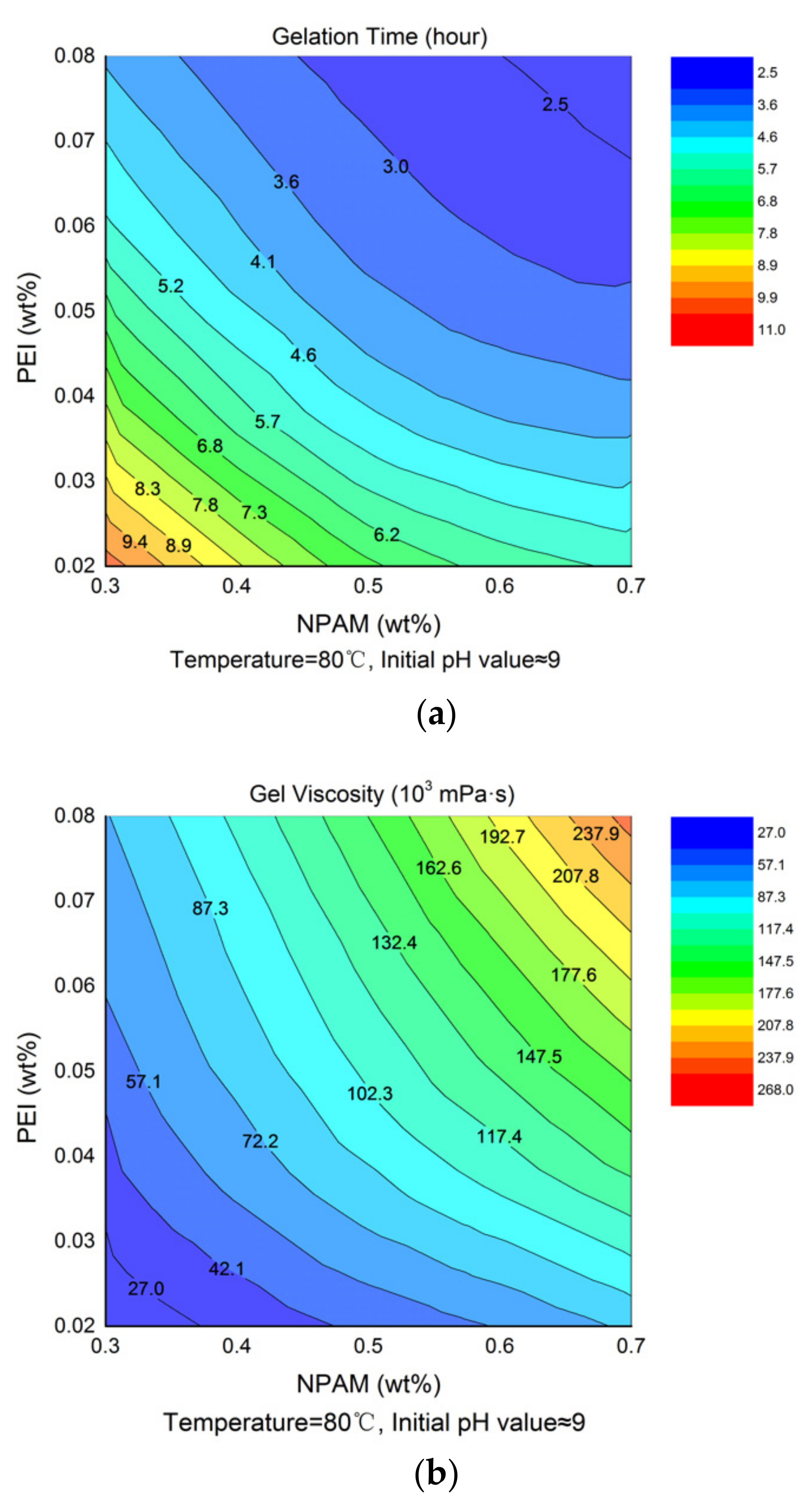
3.2. Effects of Polymer and Crosslinker Concentration
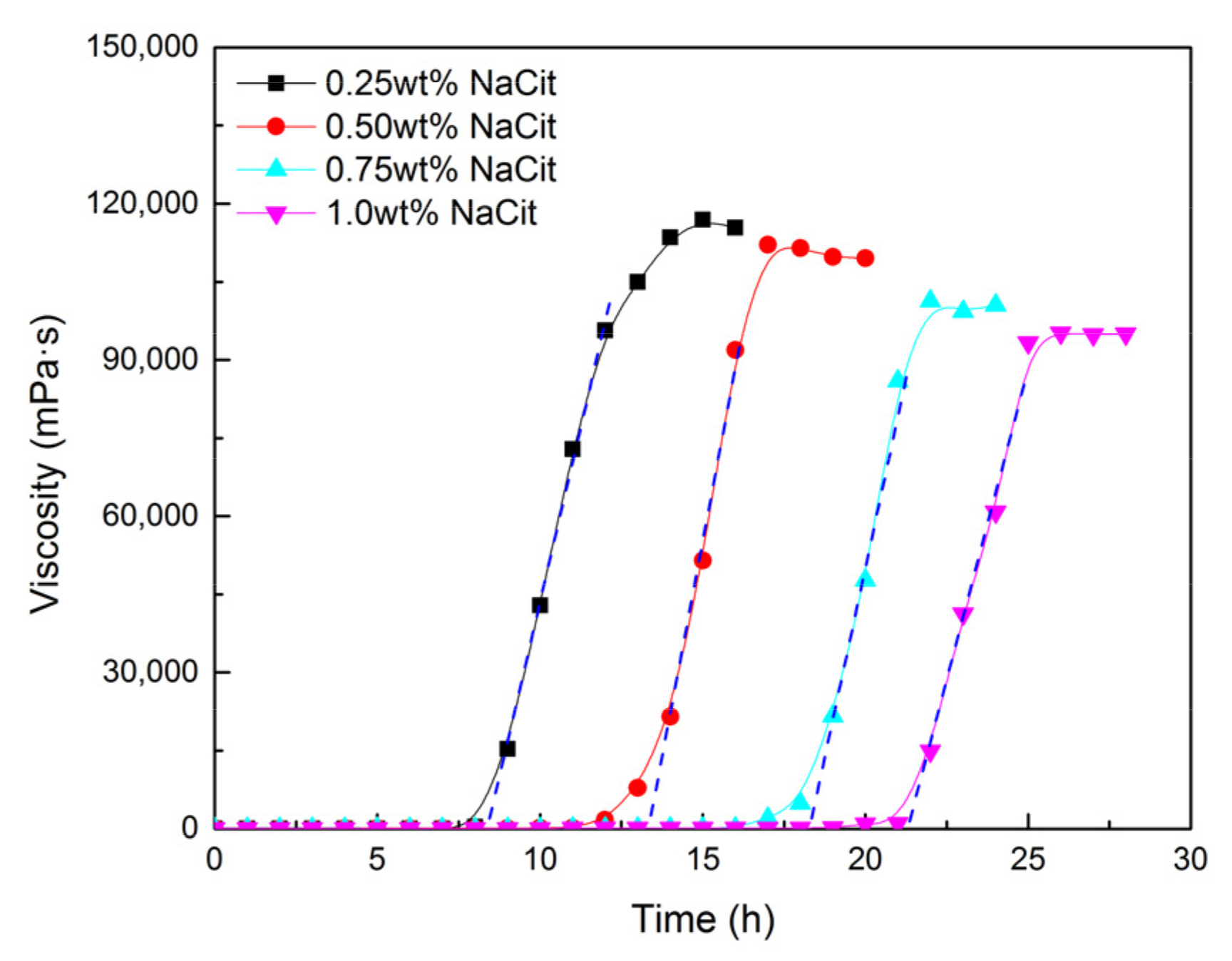
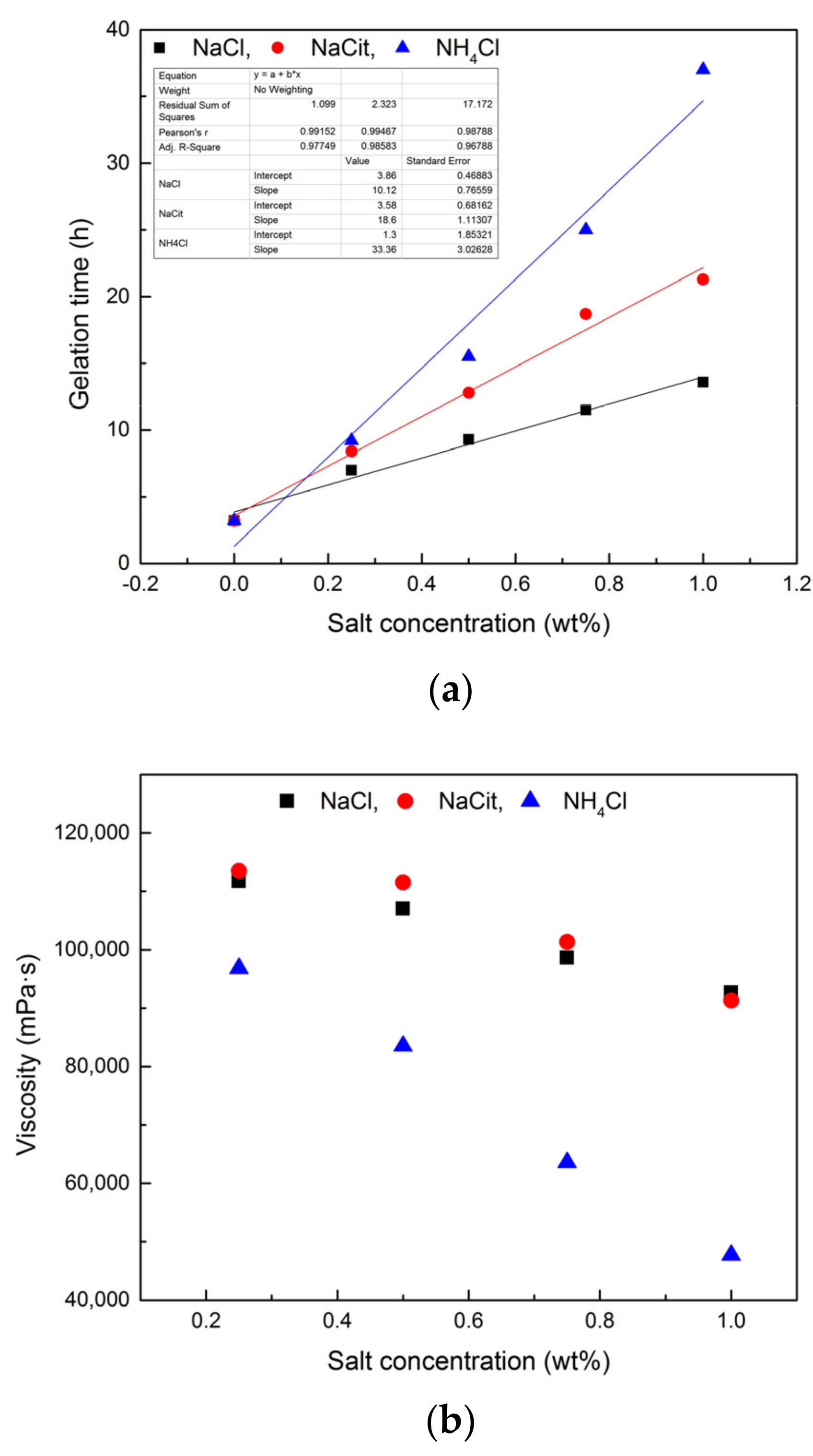
3.3. Effect of Retarder
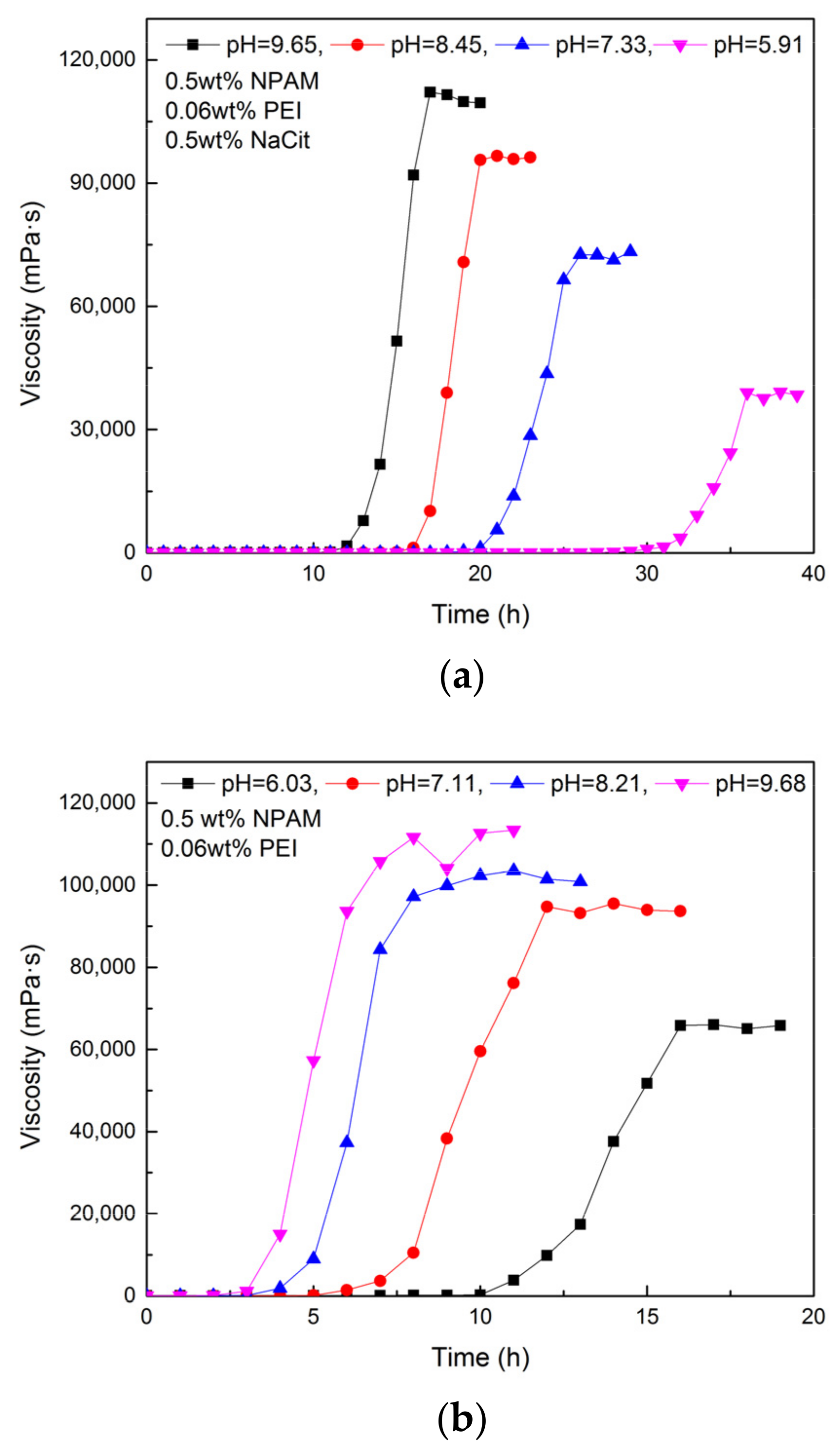
3.4. Effect of Initial pH Value
3.5. Effect of Temperature
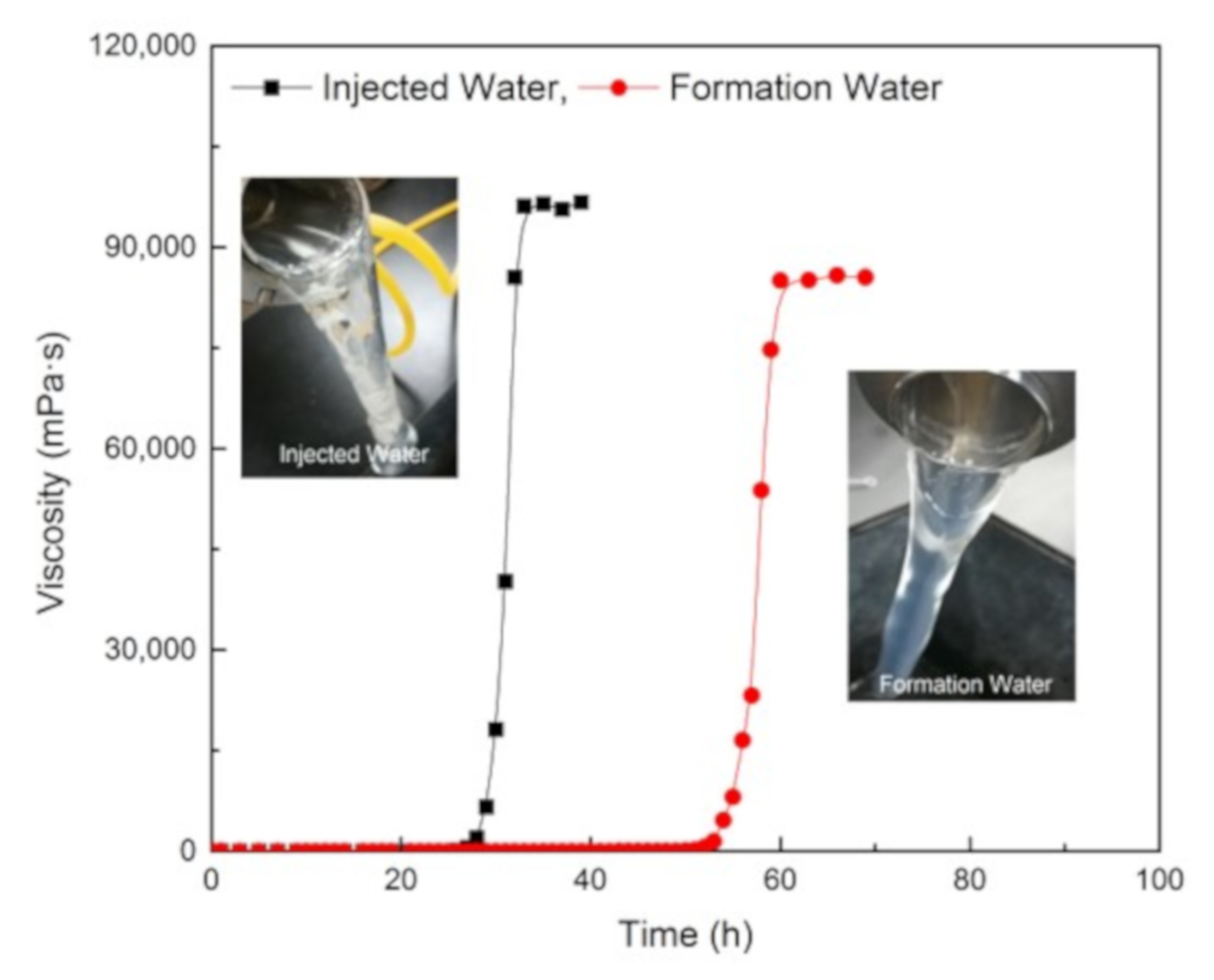
3.6. Compatibility with the Injection and Formation Water
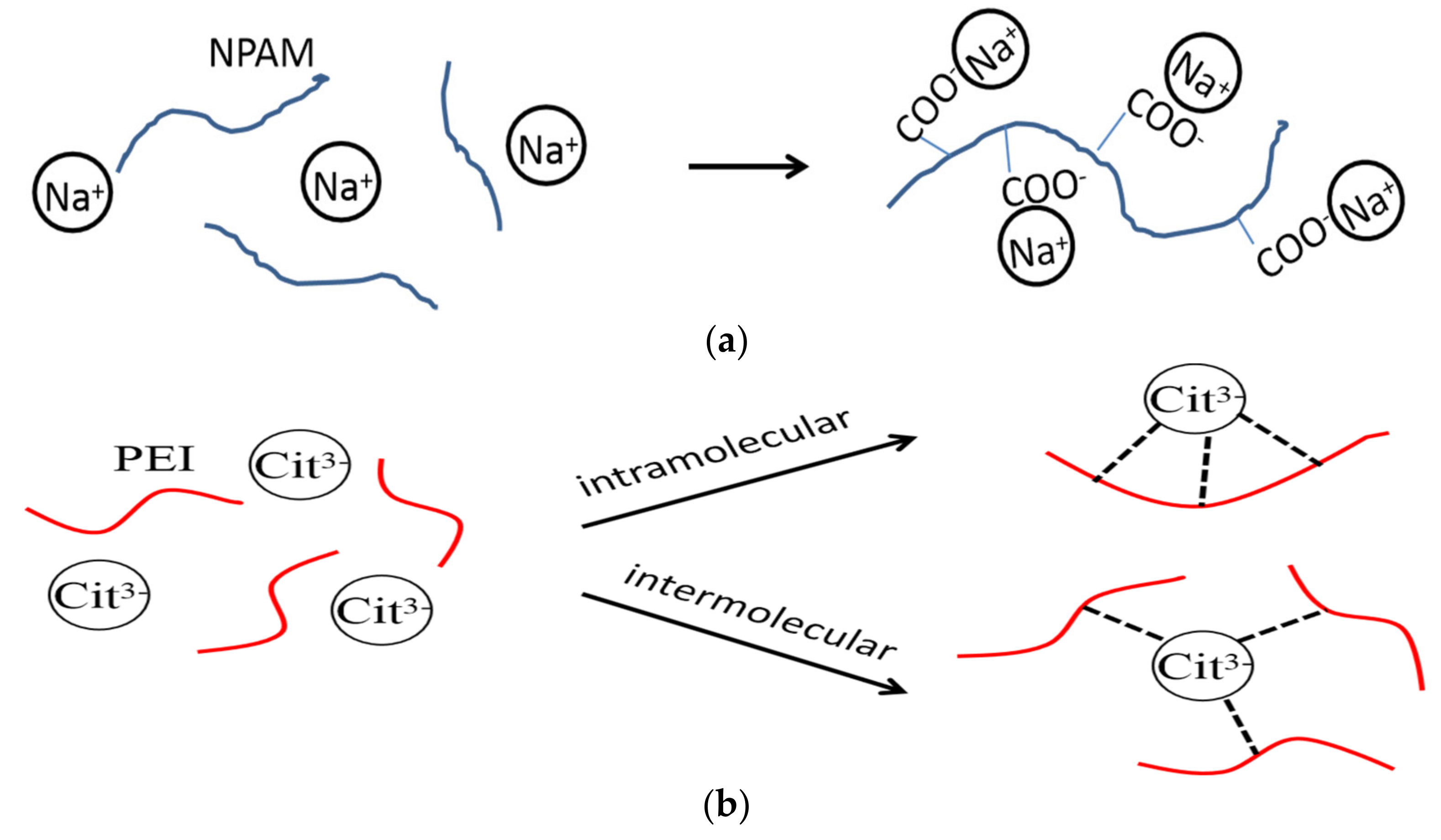
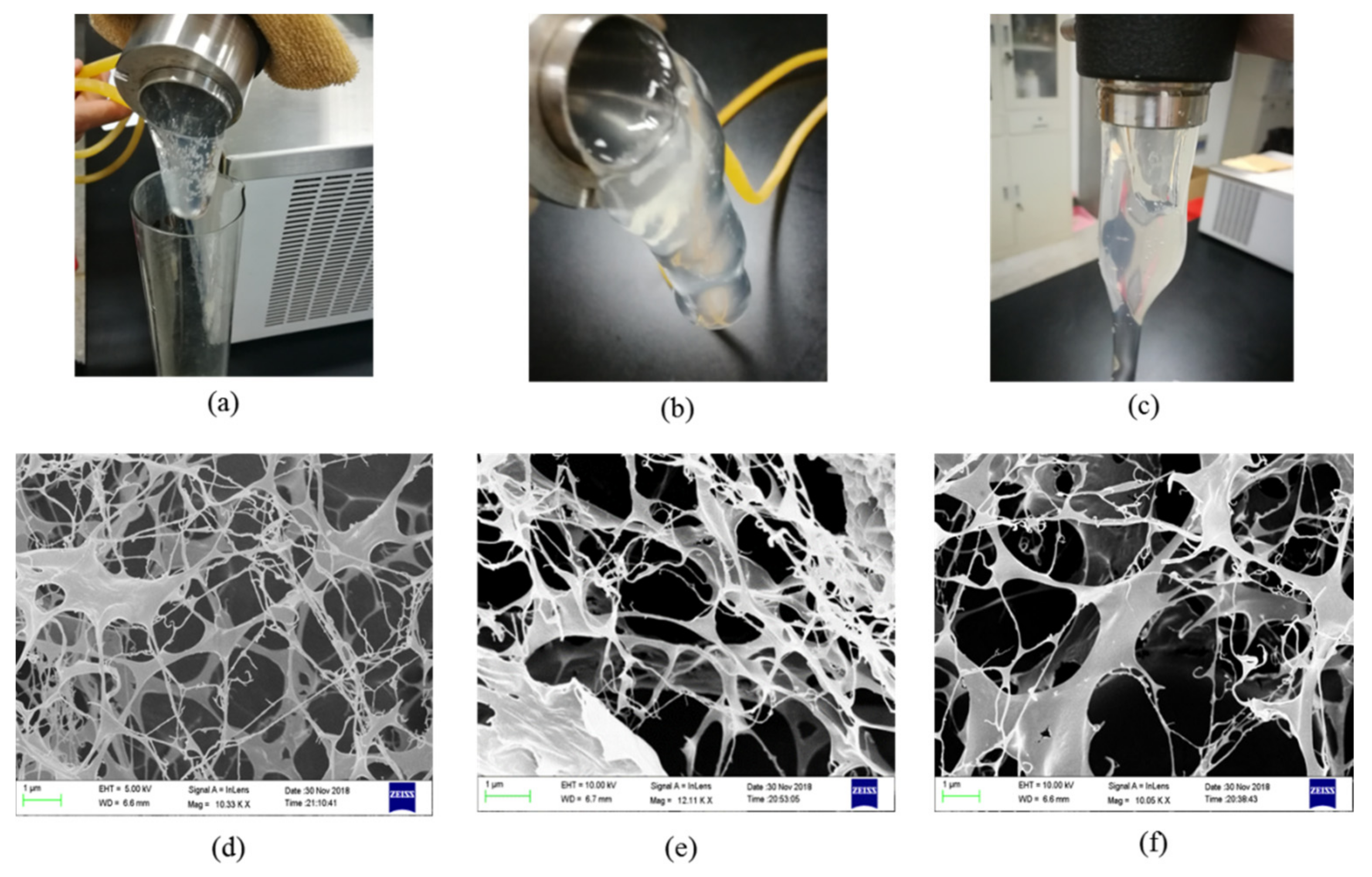
3.7. Microstructure of the Bulk Gel
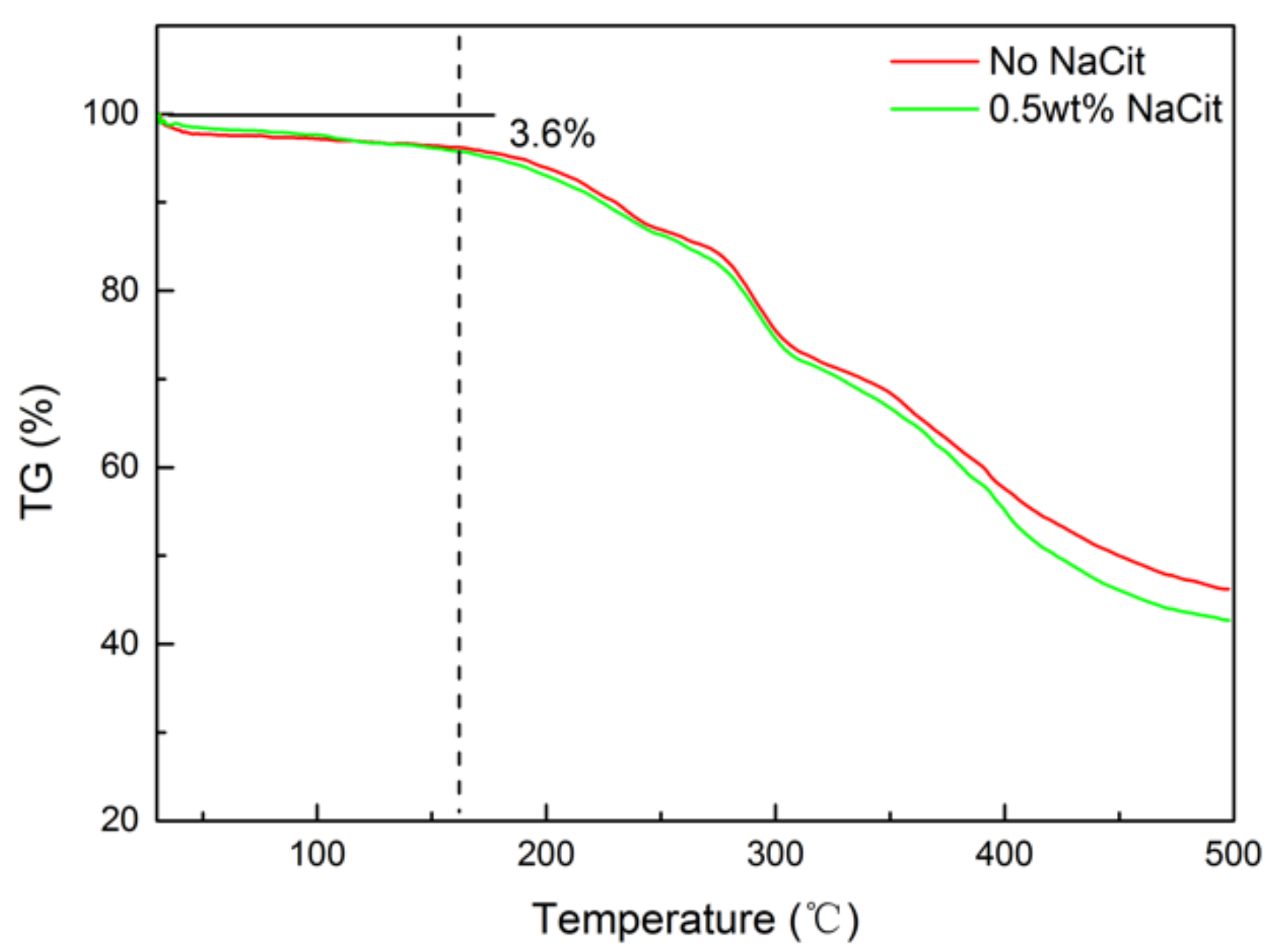
3.8. Thermal Stability of NPAM/PEI Gel System
3.9. Plugging Capacity of NPAM/PEI Gel System
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, L.; Han, Q.; Li, D.; Zhang, X.; Pu, W.; Tang, X.; Zhang, Y.; Bai, B. Water Plugging Performance of Preformed Particle Gel in Partially Filled Fractures. Ind. Eng. Chem. Res. 2019, 58, 6778–6784. [Google Scholar] [CrossRef]
- Sengupta, B.; Sharma, V.P.; Udayabhanu, G. Gelation studies of an organically cross-linked polyacrylamide water shut-off gel system at different temperatures and pH. J. Pet. Sci. Eng. 2012, 81, 145–150. [Google Scholar] [CrossRef]
- Chung, T.; Bae, W.; Nguyen, N.T.B.; Dang, C.T.Q.; Lee, W.; Jung, B. A Review of Polymer Conformance Treatment: A Successful Guideline for Water Control in Mature Fields. Energy Sources Part A Recovery Uti. Environ. Eff. 2011, 34, 122–133. [Google Scholar] [CrossRef]
- El-Karsani, K.S.M.; Al-Muntasheri, G.A.; Hussein, I.A. Polymer System for Water shutoff and Profile Modification: A Review over the Last Decade. SPE J. 2014, 19, 135–149. [Google Scholar] [CrossRef]
- Bai, B.; Zhou, J.; Yin, M. A comprehensive review of polyacrylamide polymer gels for conformance control. Pet. Explor. Dev. 2015, 42, 525–532. [Google Scholar] [CrossRef]
- Zhang, L.; Khan, N.; Pu, C. A New Method of Plugging the Fracture to Enhance Oil Production for Fractured Oil Reservoir using Gel Particles and the HPAM/Cr3+ System. Polymers 2019, 11, 446. [Google Scholar] [CrossRef]
- Jia, H.; Chen, H. The potential of using Cr3+/salt-tolerant polymer gel for well workover in low-temperature reservoir: Laboratory investigation and pilot test. SPE Prod. Oper. 2018, 33, 569–582. [Google Scholar] [CrossRef]
- Zhang, L.; Khan, N.; Pu, C. Influence of salinity on the properties of the different HPAM/Al3+ systems. Oil Gas Sci. Technol. 2019, 74, 37. [Google Scholar] [CrossRef]
- Rose, J.; Chauveteau, G.; Tabary, R.; Renard, M.; Omari, A.; Toulhoat, H. Zirconium speciation in lactate solutions and polyacrylate gels. J. Synchrotron Radiat. 2001, 8, 686–688. [Google Scholar] [CrossRef]
- Sun, Y.; Fang, Y.; Chen, A.; You, Q.; Dai, C.; Cheng, R.; Liu, Y. Gelation behavior study of a resorcinol–Hexamethyleneteramine crosslinked polymer gel for water shut-off treatment in low temperature and high salinity reservoirs. Energies 2017, 10, 913. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, X.; He, L.; Zhao, G.; Dai, C. Experimental research of hydroquinone (HQ)/hexamethylene tetramine (HMTA) gel for water plugging treatments in high-temperature and high-salinity reservoirs. J. Appl. Polym. Sci. 2017, 134, 44359. [Google Scholar] [CrossRef]
- Ghriga, M.A.; Grassl, B.; Gareche, M.; Khodja, M.; Lebouachera, S.E.; Andreu, N.; Drouiche, N. Review of recent advances in polyethylenimine crosslinked polymer gels used for conformance control applications. Polym. Bull. 2019, 76, 6001–6029. [Google Scholar] [CrossRef]
- Al-Muntasheri, G.A.; Hussein, I.A.; Nasr-El-Din, H.A.; Mohamed, B.A. Viscoelastic properties of a high temperature cross-linked water shut-off polymeric gel. J. Pet. Sci. Eng. 2007, 55, 56–66. [Google Scholar] [CrossRef]
- Tessarolli, F.G.C.; Souza, S.T.S.; Gomes, A.S.; Mansur, C.R.E. Influence of polymer structure on the gelation kinetics and gel strength of acrylamide-based copolymers, bentonite and polyethylenimine systems for conformance control of oil reservoirs. J. Appl. Polym. Sci. 2019, 136, 47556. [Google Scholar] [CrossRef]
- Zhu, D.; Hou, J.; Chen, Y.; Wei, Q.; Zhao, S.; Bai, B. Evaluation of terpolymer-gel systems crosslinked by polyethylenimine for conformance improvement in high-temperature reservoirs. SPE J. 2019, 24, 1726–1740. [Google Scholar] [CrossRef]
- Al-Muntasheri, G.A.; Nasr-El-Din, H.A.; Zitha, P.L.J. Gelation Kinetics and Performance Evaluation of an Organically Crosslinked Gel at High Temperature and Pressure. SPE J. 2008, 13, 337–345. [Google Scholar] [CrossRef]
- El-Karsani, K.S.M.; Al-Muntasheri, G.A.; Sultan, A.S.; Hussein, I. Gelation of a water-shutoff gel at high pressure and high temperature: Rheological investigation. SPE J. 2015, 20, 1103–1112. [Google Scholar] [CrossRef]
- Reddy, B.R.R.; Crespo, F.; Eoff, L.S. Water shutoff at ultralow temperatures using organically crosslinked polymer gels. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar] [CrossRef]
- Jia, H.; Pu, W.F.; Zhao, J.Z.; Jin, F.Y. Research on the gelation performance of low toxic PEI crosslinking PHPAM gel systems as water shutoff agents in low temperature reservoirs. Ind. Eng. Chem. Res. 2010, 49, 9618–9624. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, J.Z.; Jin, F.Y.; Pu, W.F.; Li, Y.M.; Li, K.X. New insights into the gelation behavior of polyethyleneimine cross-linking partially hydrolyzed polyacrylamide gels. Ind. Eng. Chem. Res. 2012, 51, 12155–12166. [Google Scholar] [CrossRef]
- El-Karsani, K.S.M.; Al-Muntasheri, G.A.; Sultan, A.S.; Hussein, I.A. Impact of salts on polyacrylamide hydrolysis and gelation: New insights. J. Appl. Polym. Sci. 2014, 131, 41185. [Google Scholar] [CrossRef]
- Ghriga, M.A.; Gareche, M.; Khodja, M.; Andreu, N.; Lebouachera, S.E.I.; Khoukh, A.; Drouiche, A.; Grassl, B. Structure–property relationships of the thermal gelation of partially hydrolyzed polyacrylamide/polyethylenimine mixtures in a semidilute regime. Polym. Bull. 2020, 77, 1465–1488. [Google Scholar] [CrossRef]
- Jayakumar, S.; Lane, R.H. Delayed crosslink polymer flowing gel system for water shutoff in conventional and unconventional oil and gas reservoirs. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 15–17 February 2012. [Google Scholar] [CrossRef]
- Jayakumar, S.; Lane, R.H. Delayed Crosslink Polymer Gel System for Water Shutoff in Conventional and Unconventional Oil and Gas Reservoirs. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 8–10 April 2013. [Google Scholar] [CrossRef]
- Zhao, G.; Dai, C.; Chen, A.; Yan, Z.; Zhao, M. Experimental study and application of gels formed by nonionic polyacrylamide and phenolic resin for in-depth profile control. J. Pet. Sci. Eng. 2015, 135, 552–560. [Google Scholar] [CrossRef]
- Gu, C.; Lv, Y.; Fan, X.; Zhao, C.; Dai, C.; Zhao, G. Study on rheology and microstructure of phenolic resin cross-linked nonionic polyacrylamide (NPAM) gel for profile control and water shutoff treatments. J. Pet. Sci. Eng. 2018, 169, 546–552. [Google Scholar] [CrossRef]
- Ghriga, M.A.; Hasanzadeh, M.; Gareche, M.; Lebouachera, S.E.I.; Drouiche, N.; Grassl, B. Thermal gelation of partially hydrolysed polyacrylamide/polyethyleneimine mixtures using design of experiments approach. Mater. Today Commun. 2019, 21, 100685. [Google Scholar] [CrossRef]
- Al-Anazi, A.; Al-Kaidar, Z.; Wang, J. Modeling Gelation Time of Organically Crosslinked Polyacrylamide Gel System for Conformance Control Applications. In Proceedings of the SPE Russian Petroleum Technology Conference, Moscow, Russia, 22–24 October 2019. [Google Scholar] [CrossRef]
- Singh, R.; Mahto, V. Study of the polymer concentration and polymer/crosslinker ratio effect on gelation time of a novel grafted polymer gel for water shutoff using a central composite design method. Polym. Adv. Technol. 2016, 27, 204–212. [Google Scholar] [CrossRef]
- Avadiar, L.; Leong, Y.K. Interactions of PEI (polyethylenimine)–silica particles with citric acid in dispersions. Colloid Polym. Sci. 2011, 289, 237–245. [Google Scholar] [CrossRef]
- Johnson, S.; Trejo, J.; Veisi, M. Effects of divalent cations, seawater, and formation brine on positively charged polyethylenimine/dextran sulfate/chromium(III) polyelectrolyte complexes and partially hydrolyzed polyacrylamide/chromium(III) gelation. J. Appl. Polym. Sci. 2010, 115, 1008–1014. [Google Scholar] [CrossRef]
- Suh, J.; Paik, H.; Hwang, B.K. Ionization of Poly(ethylenimine) and Poly(allylamine) at Various pH′ s. Bioorg. Chem. 1994, 22, 318–327. [Google Scholar] [CrossRef]

| Water Type | CO32− (mg/L) | HCO3− (mg/L) | Cl− (mg/L) | SO42− (mg/L) | Ca2+ (mg/L) | Mg2+ (mg/L) | Na+/K+ (mg/L) | TDS (mg/L) |
|---|---|---|---|---|---|---|---|---|
| Injected water | 1216.25 | — | 274.38 | 27.03 | 95.65 | 15.27 | 984.6 | 2613.18 |
| Formation water | 6792.4 | 146.7 | 52.44 | 71.69 | 43.36 | 27.64 | 10,468.7 | 17,602.93 |
| NPAM Concentration (wt%) | PEI Concentration (wt%) | Initial Viscosity (mPa·s) | Gelation Time (hour) | Gel Viscosity (×103 mPa·s) |
|---|---|---|---|---|
| 0.3 | 0.02 | 11.8 | 11 | 27.02 |
| 0.03 | 11.4 | 9.4 | 33.726 | |
| 0.04 | 11.4 | 7.2 | 40.081 | |
| 0.06 | 10.7 | 5 | 69.547 | |
| 0.08 | 10.5 | 3.9 | 55.242 | |
| 0.4 | 0.02 | 16.0 | 8.8 | 32.254 |
| 0.03 | 15.8 | 6.5 | 38.274 | |
| 0.04 | 15.3 | 5.1 | 70.508 | |
| 0.06 | 16.5 | 3.9 | 80.832 | |
| 0.08 | 14.8 | 3.4 | 102.587 | |
| 0.5 | 0.02 | 22.3 | 6.6 | 46.676 |
| 0.03 | 22.5 | 5.3 | 70.641 | |
| 0.04 | 23.4 | 4.2 | 102.708 | |
| 0.06 | 25.2 | 3.2 | 111.609 | |
| 0.08 | 22.6 | 3 | 137.169 | |
| 0.6 | 0.02 | 46.9 | 6.2 | 49.249 |
| 0.03 | 43.2 | 4.5 | 87.003 | |
| 0.04 | 43.9 | 3.6 | 123.668 | |
| 0.06 | 45.4 | 3 | 135.19 | |
| 0.08 | 44.8 | 2.8 | 203.426 | |
| 0.7 | 0.02 | 73.0 | 6 | 53.054 |
| 0.03 | 73.1 | 4.4 | 105.372 | |
| 0.04 | 72.7 | 3.5 | 160.43 | |
| 0.06 | 74.3 | 2.8 | 182.256 | |
| 0.08 | 71.4 | 2.5 | 267.564 |
| ANOVA | Sum of Square | DOF | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| Regression | 117.04 | 5 | 23.41 | 175.16 | <0.0001 |
| Residual | 2.54 | 19 | 0.1336 | ||
| Total | 119.58 | 24 | |||
| Data fit | R2 | Ra2 | Residual error | ||
| 0.9788 | 0.9732 | 0.3656 |
| Gel Systems | Temperature (°C) | Gelation Time (hour) | Viscosity (×103 mPa·s) | R2 | Ea (kJ/mol) |
|---|---|---|---|---|---|
| Formula 1: 0.5 wt% NPAM + 0.06 wt% PEI | 50 | 17.6 | 57.686 | 0.9529 | 51.91 |
| 60 | 10.5 | 74.852 | |||
| 70 | 7.2 | 104.703 | |||
| 80 | 3.2 | 111.609 | |||
| Formula 2: 0.5 wt% NPAM + 0.06 wt% PEI + 0.5 wt% NaCit | 50 | 71.5 | 53.62 | 0.9880 | 55.45 |
| 60 | 45 | 81.86 | |||
| 70 | 22.5 | 101.435 | |||
| 80 | 12.8 | 109.523 |
| Gel System | Core | Size | Porosity % | Permeability (×10−3 μm2) | Plugging Rate (%) | RRF | ||
|---|---|---|---|---|---|---|---|---|
| Length (cm) | Diameter (cm) | Before Gel Injection | After Gel Injection | |||||
| 0.5 wt% NPAM + 0.06 wt% PEI + 0.5% NaCit | Core 1 | 4.74 | 2.54 | 28.73 | 66.88 | 2.35 | 96.49 | 28.45 |
| 0.7 wt% NPAM + 0.08 wt% PEI + 0.5% NaCit | Core 2 | 4.88 | 2.54 | 28.50 | 68.82 | 1.58 | 97.70 | 43.56 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Liao, R.; Luo, S.; Li, J. The Thermal Gelation Behavior and Performance Evaluation of High Molecular Weight Nonionic Polyacrylamide and Polyethyleneimine Mixtures for In-Depth Water Control in Mature Oilfields. Materials 2020, 13, 4142. https://doi.org/10.3390/ma13184142
Qin Y, Liao R, Luo S, Li J. The Thermal Gelation Behavior and Performance Evaluation of High Molecular Weight Nonionic Polyacrylamide and Polyethyleneimine Mixtures for In-Depth Water Control in Mature Oilfields. Materials. 2020; 13(18):4142. https://doi.org/10.3390/ma13184142
Chicago/Turabian StyleQin, Yi, Ruiquan Liao, Shunshe Luo, and Junliang Li. 2020. "The Thermal Gelation Behavior and Performance Evaluation of High Molecular Weight Nonionic Polyacrylamide and Polyethyleneimine Mixtures for In-Depth Water Control in Mature Oilfields" Materials 13, no. 18: 4142. https://doi.org/10.3390/ma13184142
APA StyleQin, Y., Liao, R., Luo, S., & Li, J. (2020). The Thermal Gelation Behavior and Performance Evaluation of High Molecular Weight Nonionic Polyacrylamide and Polyethyleneimine Mixtures for In-Depth Water Control in Mature Oilfields. Materials, 13(18), 4142. https://doi.org/10.3390/ma13184142