Review of Plastic Surgery Biomaterials and Current Progress in Their 3D Manufacturing Technology
Abstract
1. Introduction
2. Classification of Plastic Cosmetic Materials
2.1. Plastic and Cosmetic Materials Classified by Source
2.1.1. Natural Biomaterials
2.1.2. Organic Polymer
- Good biological scaffold for cell growth. After implanting the ePTFE material, an histological observation showed that there was more tissue cells and giant cells on the surface of the material, the pores of the material were filled with a collagen matrix, the fibroblasts and functional capillaries attached to the surface of the material, and the tissue adheres to the surface of the material and grows in the pores of the material. Although the microporous structure of the material is firmly integrated into the tissue, it does not form a fibrous cystic structure and can be completely removed [43].
- Good physical properties. The ePTFE material is soft, with similar elasticity and hardness as a soft tissue, and has good tensile strength [45].
- Good clinical application effect. Clinical follow-up observations showed that there were no long-term complications such as chronic inflammation, absorption, atrophy, and capsule formation. The hardness cannot only meet the support requirements of the local tissue structure but also have a certain degree of toughness, so as to obtain a natural and real feeling. Many pieces of the literature report the application of ePTFE in plastic surgery [43].
2.1.3. Ceramic Materials
2.2. Classification According to Material Use
2.2.1. Prosthetic Materials
2.2.2. Injectable Materials
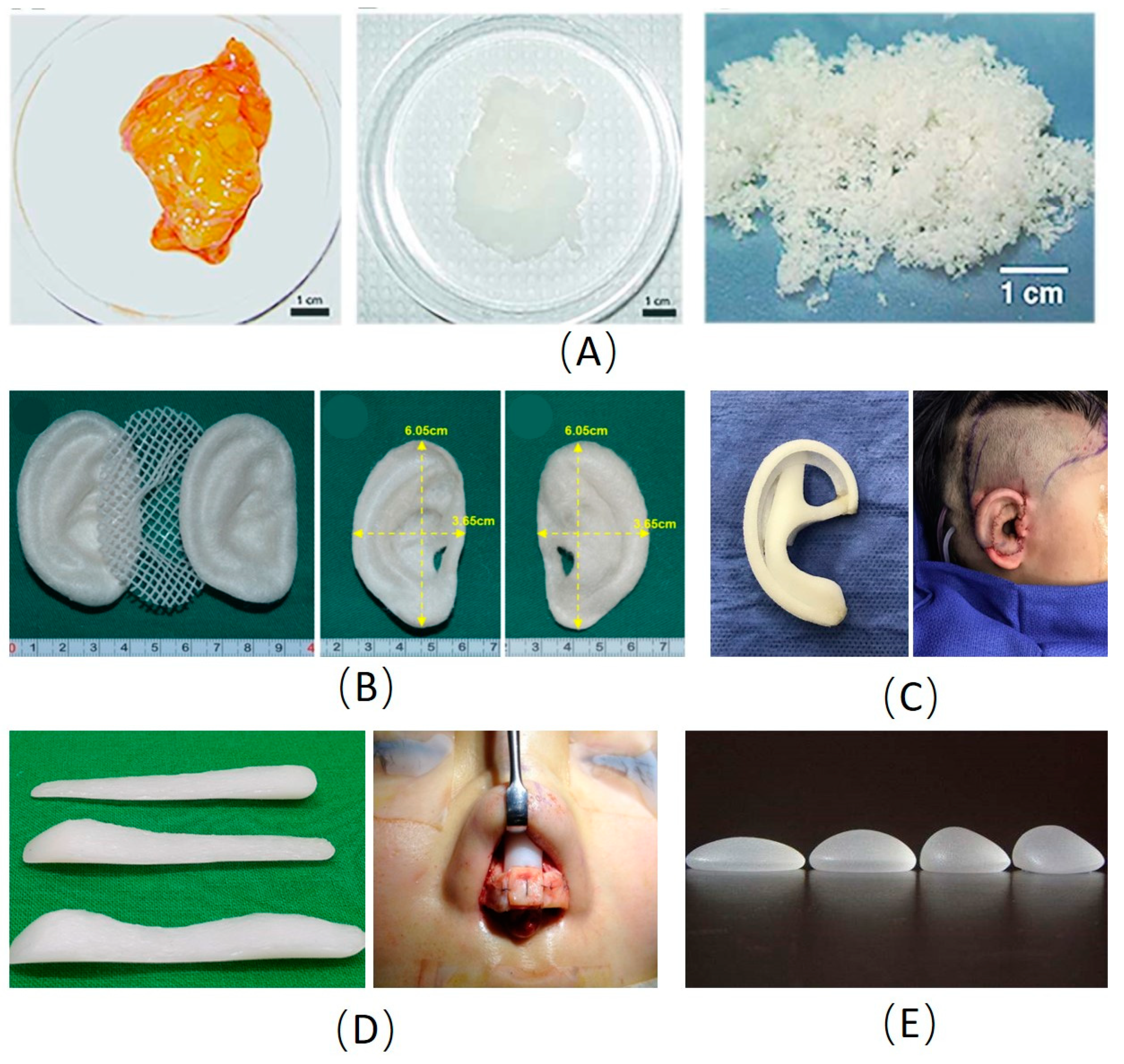
3. Development and Application of 3D Manufacturing Technology in Biomaterials for Plastic Surgery
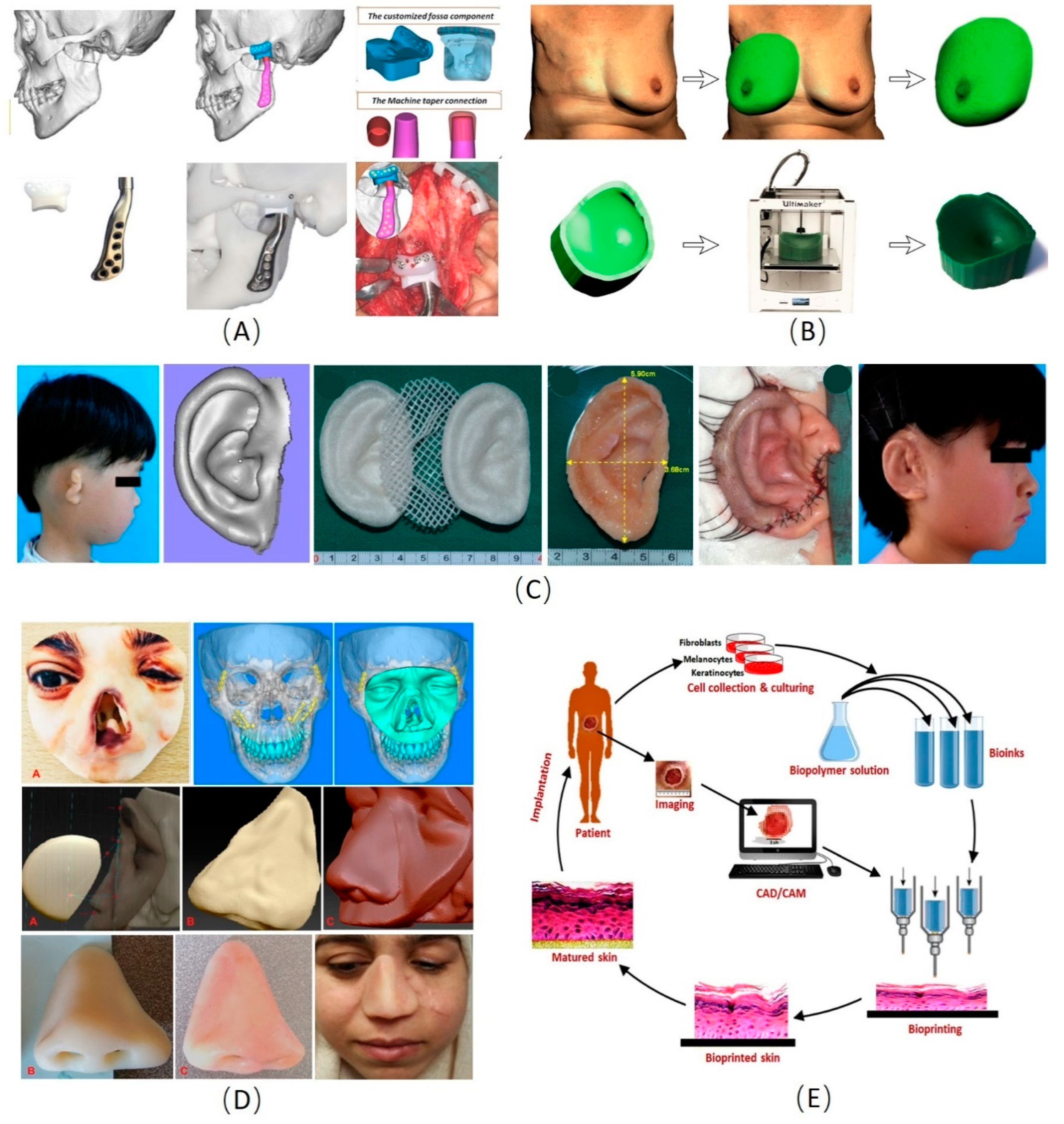
3.1. Cranio-Maxillofacial Reconstruction
3.2. Ear and Nose Reconstruction
3.3. Skin 3D Manufacturing
3.4. Breast Shaping and Reconstruction
4. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Sebastian, G. 150 years of the “Handbook of Plastic Surgery”--in memory of Eduard Zeis (1807–1868). Der Hautarzt Z. fur Dermatol. Venerol. Verwandte Geb. 1989, 40, 45–52. [Google Scholar] [PubMed]
- Wijsbek, H. The pursuit of beauty: The enforcement of aesthetics or a freely adopted lifestyle? J. Med. Ethics 2000, 26, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Haddock, N.T.; McCarthy, J.G. Key textbooks in the development of modern american plastic surgery: The first half of the twentieth century. Plast. Reconstr. Surg. 2013, 132, 130e–138e. [Google Scholar] [CrossRef] [PubMed]
- Battle, R. Plastic surgery in the two world wars and in the years between. J. R. Soc. Med. 1978, 71, 844–848. [Google Scholar] [PubMed]
- Al-Himdani, S.; Jessop, Z.M.; Al-Sabah, A.; Combellack, E.; Ibrahim, A.; Doak, S.H.; Hart, A.M.; Archer, C.W.; Thornton, C.A.; Whitaker, I.S. Tissue-Engineered Solutions in Plastic and Reconstructive Surgery: Principles and Practice. Front. Surg. 2017, 4, 4. [Google Scholar] [CrossRef]
- Egro, F.M.; Coleman, S.R. Facial Fat Grafting: The Past, Present, and Future. Clin. Plast. Surg. 2020, 47, 1–6. [Google Scholar] [CrossRef]
- Devgan, L.; Singh, P.; Durairaj, K. Minimally Invasive Facial Cosmetic Procedures. Otolaryngol. Clin. N. Am. 2019, 52, 443–459. [Google Scholar] [CrossRef]
- Helmy, Y. Non-surgical rhinoplasty using filler, Botox, and thread remodeling: Retro analysis of 332 cases. J. Cosmet. Laser Ther. Off. Publ. Eur. Soc. Laser Dermatol. 2018, 20, 293–300. [Google Scholar] [CrossRef]
- Yuan, Z.; Nie, H.; Wang, S.; Lee, C.H.; Li, A.; Fu, S.Y.; Zhou, H.; Chen, L.; Mao, J.J. Biomaterial selection for tooth regeneration. Tissue Eng. Part B Rev. 2011, 17, 373–388. [Google Scholar] [CrossRef]
- Amin, K.; Moscalu, R.; Imere, A.; Murphy, R.; Barr, S.; Tan, Y.; Wong, R.; Sorooshian, P.; Zhang, F.; Stone, J.; et al. The future application of nanomedicine and biomimicry in plastic and reconstructive surgery. Nanomedicine 2019, 14, 2679–2696. [Google Scholar] [CrossRef]
- Banyard, D.A.; Bourgeois, J.M.; Widgerow, A.D.; Evans, G.R.D. Regenerative biomaterials: A review. Plast. Reconstr. Surg. 2015, 135, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Panayi, A.C.; Orgill, D.P. Current Use of Biological Scaffolds in Plastic Surgery. Plast. Reconstr. Surg. 2019, 143, 209–220. [Google Scholar] [CrossRef]
- Pfaff, M.J.; Steinbacher, D.M. Plastic Surgery Applications Using Three-Dimensional Planning and Computer-Assisted Design and Manufacturing. Plast. Reconstr. Surg. 2016, 137, 603e–616e. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.B.; Small, K.H.; Choi, M.; Karp, N.S. Three-dimensional surface imaging in plastic surgery: Foundation, practical applications, and beyond. Plast. Reconstr. Surg. 2015, 135, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Mooney, E.; Loh, C.; Pu, L.L. The use of fibrin glue in plastic surgery. Plast. Reconstr. Surg. 2009, 124, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Man, D.; Plosker, H.; Winland-Brown, J.E. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plast. Reconstr. Surg. 2001, 107, 229–237; discussion 238–239. [Google Scholar] [CrossRef]
- Bielecki, T.; Dohan Ehrenfest, D.M. Platelet-rich plasma (PRP) and Platelet-Rich Fibrin (PRF): Surgical adjuvants, preparations for in situ regenerative medicine and tools for tissue engineering. Curr. Pharm. Biotechnol. 2012, 13, 1121–1130. [Google Scholar] [CrossRef]
- Anitua, E.; Andia, I.; Ardanza, B.; Nurden, P.; Nurden, A.T. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb. Haemost. 2004, 91, 4–15. [Google Scholar] [CrossRef]
- Banyard, D.A.; Borad, V.; Amezcua, E.; Wirth, G.A.; Evans, G.R.D.; Widgerow, A.D. Preparation, Characterization, and Clinical Implications of Human Decellularized Adipose Tissue Extracellular Matrix: A Comprehensive Review. Aesthet Surg. J. 2016, 36, 349–357. [Google Scholar] [CrossRef]
- Dong, J.; Yu, M.; Zhang, Y.; Yin, Y.; Tian, W. Recent developments and clinical potential on decellularized adipose tissue. J. Biomed. Mater. Res. A 2018, 106, 2563–2574. [Google Scholar] [CrossRef]
- Golas, A.R.; Hernandez, K.A.; Spector, J.A. Tissue engineering for plastic surgeons: A primer. Aesthet. Plast. Surg. 2014, 38, 207–221. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.H.; Kim, S.W.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef]
- Saldin, L.T.; Cramer, M.C.; Velankar, S.S.; White, L.J.; Badylak, S.F. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomater. 2017, 49, 1–15. [Google Scholar] [CrossRef]
- Rana, D.; Zreiqat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of decellularized scaffolds for stem cell-driven tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 942–965. [Google Scholar] [CrossRef]
- Jackson, C.J.; Tønseth, K.A.; Utheim, T.P. Cultured epidermal stem cells in regenerative medicine. Stem. Cell Res. Ther. 2017, 8, 155. [Google Scholar] [CrossRef]
- Chua, I.L.S.; Kim, H.W.; Lee, J.H. Signaling of extracellular matrices for tissue regeneration and therapeutics. Tissue Eng. Regen. Med. 2016, 13, 1–12. [Google Scholar] [CrossRef]
- Jin, G.-Z.; Kim, H.-W. Effects of Type I Collagen Concentration in Hydrogel on the Growth and Phenotypic Expression of Rat Chondrocytes. Tissue Eng. Regen. Med. 2017, 14, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, Y.S.; Kim, S.M.; Kim, Y.J.; Rhie, J.W.; Jun, Y.J. Efficacy and safety of porcine collagen filler for nasolabial fold correction in Asians: A prospective multicenter, 12 months follow-up study. J. Korean Med. Sci. 2014, 29 (Suppl. 3), S217–S221. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef]
- Tan, H.; Li, H.; Rubin, J.P.; Marra, K.G. Controlled gelation and degradation rates of injectable hyaluronic acid-based hydrogels through a double crosslinking strategy. J. Tissue Eng. Regen Med. 2011, 5, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Korurer, E.; Kenar, H.; Doger, E.; Karaoz, E. Production of a composite hyaluronic acid/gelatin blood plasma gel for hydrogel-based adipose tissue engineering applications. J. Biomed. Mater. Res. A 2014, 102, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.M.A.; Silva, M.; Gershovich, P.; Betta, S.; Babo, P.; Caridade, S.G.; Mano, J.F.; Motta, A.; Reis, R.L.; Gomes, M.E. Development of Injectable Hyaluronic Acid/Cellulose Nanocrystals Bionanocomposite Hydrogels for Tissue Engineering Applications. Bioconjug. Chem. 2015, 26, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Ma, Y.; Zhang, Z.; Mao, J.; Tan, H.; Hu, X. Biodegradable hyaluronic acid hydrogels to control release of dexamethasone through aqueous Diels-Alder chemistry for adipose tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 56, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Segreto, F.; Marangi, G.F.; Cerbone, V.; Alessandri-Bonetti, M.; Caldaria, E.; Persichetti, P. Nonsurgical Rhinoplasty: A Graft-based Technique. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2241. [Google Scholar] [CrossRef]
- Fallacara, A.; Manfredini, S.; Durini, E.; Vertuani, S. Hyaluronic Acid Fillers in Soft Tissue Regeneration. Facial Plast. Surg. 2017, 33, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Mehta, U.; Fridirici, Z. Advanced Techniques in Nonsurgical Rhinoplasty. Facial Plast. Surg. Clin. N. Am. 2019, 27, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Cammarata, M.J.; Wake, N.; Kantar, R.S.; Maroutsis, M.; Rifkin, W.J.; Hazen, A.; Brecht, L.E.; Bernstein, G.L.; Diaz-Siso, J.R.; Rodriguez, E.D. Three-Dimensional Analysis of Donor Masks for Facial Transplantation. Plast. Reconstr. Surg. 2019, 143, 1290e–1297e. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-S. Augmentation Rhinoplasty Using Silicone Implants. Facial Plast. Surg. Clin. N. Am. 2018, 26, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Hinderer, U.T. Malar implants for improvement of the facial appearance. Plast. Reconstr. Surg. 1975, 56, 157–165. [Google Scholar] [CrossRef]
- Hillard, C.; Fowler, J.D.; Barta, R.; Cunningham, B. Silicone breast implant rupture: A review. Gland Surg. 2017, 6, 163–168. [Google Scholar] [CrossRef]
- Pool, S.M.W.; Wolthuizen, R.; Mouës-Vink, C.M. Silicone breast prostheses: A cohort study of complaints, complications, and explantations between 2003 and 2015. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Tahiri, Y.; Reinisch, J. Porous Polyethylene Ear Reconstruction. Clin. Plast. Surg. 2019, 46, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Yu, W.; Jin, Y.; Chen, H.; Ma, G.; Chang, S.J.; Lin, X. Safety and Efficacy of Cosmetic Augmentation of the Nasal Tip and Nasal Dorsum With Expanded Polytetrafluoroethylene: A Randomized Clinical Trial. JAMA Facial Plast. Surg. 2018, 20, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Shadfar, S.; Farag, A.; Jarchow, A.M.; Shockley, W.W. Safety and Efficacy of Expanded Polytetrafluoroethylene Implants in the Surgical Management of Traumatic Nasal Deformity. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 710–715. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, S.; Lei, Z.; Li, X.; Fan, D. The use of expanded polytetrafluoroethylene in depressed deformities of the face. Exp. Ther. Med. 2016, 12, 3151–3154. [Google Scholar] [CrossRef][Green Version]
- Attenello, N.H.; Maas, C.S. Injectable fillers: Review of material and properties. Facial Plast. Surg. 2015, 31, 29–34. [Google Scholar] [CrossRef]
- Abu-Ghname, A.; Banuelos, J.; Oliver, J.D.; Vyas, K.; Daniels, D.; Sharaf, B. Outcomes and Complications of Pediatric Cranioplasty: A Systematic Review. Plast. Reconstr. Surg. 2019, 144, 433e–443e. [Google Scholar] [CrossRef]
- Boelch, S.P.; Rueckl, K.; Fuchs, C.; Jordan, M.; Knauer, M.; Steinert, A.; Rudert, M.; Luedemann, M. Comparison of Elution Characteristics and Compressive Strength of Biantibiotic-Loaded PMMA Bone Cement for Spacers: Copal® Spacem with Gentamicin and Vancomycin versus Palacos® R+G with Vancomycin. Biomed. Res. Int. 2018, 2018, 4323518. [Google Scholar] [CrossRef]
- Ahmad, S.B.; Hoellwarth, J.; Christie, N.; McGough, R. Radical resection of a giant rib osteosarcoma with complex chest wall reconstruction. Int. J. Surg. Case Rep. 2019, 62, 17–20. [Google Scholar] [CrossRef]
- Young, C.C.; Hanak, B.W.; Patel, A.P.; Sekhar, L.N. Rapid Intraoperative in Situ Synthetic Cranioplasty. World Neurosurg. 2018, 112, 161–165. [Google Scholar] [CrossRef]
- Lemperle, G.; Hazan-Gaúthier, N.; Lemperle, M. PMMA microspheres (Artecoll) for skin and soft-tissue augmentation. Part II: Clinical investigations. Plast. Reconstr. Surg. 1995, 96, 627–634. [Google Scholar] [CrossRef]
- Patel, K.; Brandstetter, K. Solid Implants in Facial Plastic Surgery: Potential Complications and How to Prevent Them. Facial Plast. Surg. 2016, 32, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, O.J.; Christiano, L.D.; Arnaout, O.; Adel, J.G.; Liu, J.K. Reconstruction of pterional defects after frontotemporal and orbitozygomatic craniotomy using Medpor Titan implant: Cosmetic results in 98 patients. Clin. Neurol. Neurosurg. 2013, 115, 1716–1720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zeng, X.; Yang, X. Clinical applications of ear reconstruction with Medpor. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2019, 44, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Trost, J.G.; Truong, T.A.; Harshbarger, R.J., 3rd. Total Ear Reconstruction Using Porous Polyethylene. Semin. Plast. Surg. 2017, 31, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hwang, K. Causes of the Removal of High-Density Polyethylene Sheets (Medpor) in Revision Rhinoplasty. J. Craniofac. Surg. 2018, 29, 1730–1732. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, R.; Bass, L.M.; Goldberg, D.J.; Graivier, M.H.; Lorenc, Z.P. Physiochemical Characteristics of Poly-L-Lactic Acid (PLLA). Aesthet. Surg. J. 2018, 38, S13–S17. [Google Scholar] [CrossRef]
- Zhou, G.; Jiang, H.; Yin, Z.; Liu, Y.; Zhang, Q.; Zhang, C.; Pan, B.; Zhou, J.; Zhou, X.; Sun, H.; et al. In Vitro Regeneration of Patient-specific Ear-shaped Cartilage and Its First Clinical Application for Auricular Reconstruction. EBioMedicine 2018, 28, 287–302. [Google Scholar] [CrossRef]
- Saito, N.; Okada, T.; Horiuchi, H.; Murakami, N.; Takahashi, J.; Nawata, M.; Ota, H.; Miyamoto, S.; Nozaki, K.; Takaoka, K. Biodegradable poly-D,L-lactic acid-polyethylene glycol block copolymers as a BMP delivery system for inducing bone. J. Bone Jt. Surg. Am. Vol. 2001, 83 (Suppl. 1), S92–S98. [Google Scholar] [CrossRef]
- Mayer, M.; Hollinger, J.; Ron, E.; Wozney, J. Maxillary alveolar cleft repair in dogs using recombinant human bone morphogenetic protein-2 and a polymer carrier. Plast. Reconstr. Surg. 1996, 98, 247–259. [Google Scholar] [CrossRef]
- Ramesh, N.; Moratti, S.C.; Dias, G.J. Hydroxyapatite-polymer biocomposites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2046–2057. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.H. The Case for Synthetic Injectables. Facial Plast. Surg. Clin. N. Am. 2015, 23, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Rho, N.-K.; Chang, Y.-Y.; Chao, Y.Y.-Y.; Furuyama, N.; Huang, P.Y.C.; Kerscher, M.; Kim, H.-J.; Park, J.-Y.; Peng, H.L.P.; Rummaneethorn, P.; et al. Consensus Recommendations for Optimal Augmentation of the Asian Face with Hyaluronic Acid and Calcium Hydroxylapatite Fillers. Plast. Reconstr. Surg. 2015, 136, 940–956. [Google Scholar] [CrossRef] [PubMed]
- Fouad, H.; AlFotawi, R.; Alothman, O.Y.; Alshammari, B.A.; Alfayez, M.; Hashem, M.; Mahmood, A. Porous Polyethylene Coated with Functionalized Hydroxyapatite Particles as a Bone Reconstruction Material. Materials 2018, 11, 521. [Google Scholar] [CrossRef]
- Singh, B.N.; Veeresh, V.; Mallick, S.P.; Jain, Y.; Sinha, S.; Rastogi, A.; Srivastava, P. Design and evaluation of chitosan/chondroitin sulfate/nano-bioglass based composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 817–830. [Google Scholar] [CrossRef]
- Lizzi, F.; Villat, C.; Attik, N.; Jackson, P.; Grosgogeat, B.; Goutaudier, C. Mechanical characteristic and biological behaviour of implanted and restorative bioglasses used in medicine and dentistry: A systematic review. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2017, 33, 702–712. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol-gel reversible hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 37–51. [Google Scholar] [CrossRef]
- Singelyn, J.M.; Christman, K.L. Injectable materials for the treatment of myocardial infarction and heart failure: The promise of decellularized matrices. J. Cardiovasc. Transl. Res. 2010, 3, 478–486. [Google Scholar] [CrossRef]
- Cho, K.-H.; Uthaman, S.; Park, I.-K.; Cho, C.-S. Injectable Biomaterials in Plastic and Reconstructive Surgery: A Review of the Current Status. Tissue Eng. Regen. Med. 2018, 15, 559–574. [Google Scholar] [CrossRef]
- Zelken, J.A.; Hong, J.P.; Chang, C.S.; Hsiao, Y.C. Silicone-Polytetrafluoroethylene Composite Implants for Asian Rhinoplasty. Ann. Plast. Surg. 2017, 78, 131–137. [Google Scholar] [CrossRef]
- Chang, E.I.; Hammond, D.C. Clinical Results on Innovation in Breast Implant Design. Plast. Reconstr. Surg. 2018, 142, 31s–38s. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.R.; Meecham, W.J.; Seiff, S.R. Silicone frontalis slings for the correction of blepharoptosis: Indications and efficacy. Ophthalmology 1996, 103, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Senderoff, D.M. Revision Buttock Implantation: Indications, Procedures, and Recommendations. Plast. Reconstr. Surg. 2017, 139, 327–335. [Google Scholar] [CrossRef]
- Coleman, K.M.; Pozner, J. Combination Therapy for Rejuvenation of the Outer Thigh and Buttock: A Review and Our Experience. Dermatol. Surg. 2016, 42 (Suppl. 2), S124–S130. [Google Scholar] [CrossRef]
- Brie, J.; Chartier, T.; Chaput, C.; Delage, C.; Pradeau, B.; Caire, F.; Boncoeur, M.P.; Moreau, J.J. A new custom made bioceramic implant for the repair of large and complex craniofacial bone defects. J. Cranio Maxillofac. Surg. 2013, 41, 403–407. [Google Scholar] [CrossRef]
- Bogdan Allemann, I.; Baumann, L. Hyaluronic acid gel (Juvéderm) preparations in the treatment of facial wrinkles and folds. Clin. Interv. Aging 2008, 3, 629–634. [Google Scholar] [CrossRef]
- Negredo, E.; Puig, J.; Ornelas, A.; Echeverría, P.; Bonjoch, A.; Estany, C.; Higueras, C.; Gonzalez-Mestre, V.; Clotet, B. Ten-Year Safety with Polyacrylamide Gel Used to Correct Facial Lipoatrophy in HIV-Infected Patients. AIDS Res. Hum. Retrovir. 2015, 31, 817–821. [Google Scholar] [CrossRef]
- Patrick, T. Polyacrylamide gel in cosmetic procedures: Experience with Aquamid. Semin. Cutan. Med. Surg. 2004, 23, 233–235. [Google Scholar] [CrossRef]
- Lemperle, G.; Gauthier-Hazan, N.; Lemperle, M. PMMA-Microspheres (Artecoll) for long-lasting correction of wrinkles: Refinements and statistical results. Aesthetic. Plast. Surg. 1998, 22, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Luo, S.K.; Wang, Y.C.; Lemperle, G. Facial Volume Restoration with Permanent Dermal Filler (Artecoll-4) in Chinese Women. Facial Plast. Surg. 2017, 33, 537–544. [Google Scholar] [CrossRef]
- Coleman, S.R.; Saboeiro, A.P. Fat grafting to the breast revisited: Safety and efficacy. Plast. Reconstr. Surg. 2007, 119, 775–787. [Google Scholar] [CrossRef]
- Malvezzi, M.; Carioli, G.; Bertuccio, P.; Rosso, T.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2016 with focus on leukaemias. Ann. Oncol. Off. J. Eur. Soci. Med. Oncol. 2016, 27, 725–731. [Google Scholar] [CrossRef]
- Cohen, S.R.; Hewett, S.; Ross, L.; Delaunay, F.; Goodacre, A.; Ramos, C.; Leong, T.; Saad, A. Regenerative Cells For Facial Surgery: Biofilling and Biocontouring. Aesthet. Surg. J. 2017, 37, S16–S32. [Google Scholar] [CrossRef] [PubMed]
- Luca, A.; Butnaru, M.; Maier, S.S.; Knieling, L.; Bredetean, O.; Verestiuc, L.; Dimitriu, D.C.; Popa, M. Atelocollagen-based Hydrogels Crosslinked with Oxidised Polysaccharides as Cell Encapsulation Matrix for Engineered Bioactive Stromal Tissue. Tissue Eng. Regen. Med. 2017, 14, 539–556. [Google Scholar] [CrossRef] [PubMed]
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H. Collagen from Marine Biological Sources and Medical Applications. Chem. Biodivers. 2018, 15, e1700557. [Google Scholar] [CrossRef] [PubMed]
- Kastellorizios, M.; Tipnis, N.; Burgess, D.J. Foreign Body Reaction to Subcutaneous Implants. In Immune Responses to Biosurfaces; Springer: Cham, Switzerland, 2015; Volume 865, pp. 93–108. [Google Scholar]
- Kim, B.S.; Choi, J.S.; Kim, J.D.; Yoon, H.I.; Choi, Y.C.; Cho, Y.W. Human collagen isolated from adipose tissue. Biotechnol. Prog. 2012, 28, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Báez, J.; Olsen, D.; Polarek, J.W. Recombinant microbial systems for the production of human collagen and gelatin. Appl. Microbiol. Biotechnol. 2005, 69, 245–252. [Google Scholar] [CrossRef]
- Beierlein, W.; Scheule, A.M.; Antoniadis, G.; Braun, C.; Schosser, R. An immediate, allergic skin reaction to aprotinin after reexposure to fibrin sealant. Transfusion 2000, 40, 302–305. [Google Scholar] [CrossRef]
- Carless, P.A.; Henry, D.A. Systematic review and meta-analysis of the use of fibrin sealant to prevent seroma formation after breast cancer surgery. Br. J. Surg. 2006, 93, 810–819. [Google Scholar] [CrossRef]
- Jones, B.M.; Grover, R. Early postoperative efficacy of fibrin glue in face lifts: A prospective randomized trial. Plast. Reconstr. Surg. 2007, 119, 433–434. [Google Scholar] [CrossRef]
- Foster, K.; Greenhalgh, D.; Gamelli, R.L.; Mozingo, D.; Gibran, N.; Neumeister, M.; Abrams, S.Z.; Hantak, E.; Grubbs, L.; Ploder, B.; et al. Efficacy and safety of a fibrin sealant for adherence of autologous skin grafts to burn wounds: Results of a phase 3 clinical study. J. Burn Care Res. Off. Publ. Am. Burn Assoc. 2008, 29, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Ma, L.; Zhang, B.; Sun, J.; Sun, Y.; Fan, Y.; Gou, Z.; Zhou, C.; Zhang, X. Creating hierarchical porosity hydroxyapatite scaffolds with osteoinduction by three-dimensional printing and microwave sintering. Biofabrication 2017, 9, 045008. [Google Scholar] [CrossRef]
- Zhang, B.; Pei, X.; Zhou, C.; Fan, Y.; Jiang, Q.; Ronca, A.; D’Amora, U.; Chen, Y.; Li, H.; Sun, Y.; et al. The biomimetic design and 3D printing of customized mechanical properties porous Ti6Al4V scaffold for load-bearing bone reconstruction. Mater. Des. 2018, 152, 30–39. [Google Scholar] [CrossRef]
- Zhang, B.; Pei, X.; Song, P.; Sun, H.; Li, H.; Fan, Y.; Jiang, Q.; Zhou, C.; Zhang, X. Porous bioceramics produced by inkjet 3D printing: Effect of printing ink formulation on the ceramic macro and micro porous architectures control. Compos. Part B 2018, 155, 112–121. [Google Scholar] [CrossRef]
- Zhao, L.; Pei, X.; Jiang, L.; Hu, C.; Sun, J.; Xing, F.; Zhou, C.; Fan, Y.; Zhang, X. Bionic design and 3D printing of porous titanium alloy scaffolds for bone tissue repair. Compos. Part B Eng. 2018, 162, 154–161. [Google Scholar] [CrossRef]
- Song, P.; Hu, C.; Pei, X.; Sun, J.; Sun, H.; Wu, L.; Jiang, Q.; Fan, H.; Yang, B.; Zhou, C.; et al. Dual modulation of crystallinity and macro-/microstructures of 3D printed porous titanium implants to enhance stability and osseointegration. J. Mater. Chem. B 2019, 7, 2865–2877. [Google Scholar] [CrossRef]
- Peng, Z.; Tang, P.; Zhao, L.; Wu, L.; Xu, X.; Lei, H.; Zhou, M.; Zhou, C.; Li, Z. Advances in biomaterials for adipose tissue reconstruction in plastic surgery. Nanotechnol. Rev. 2020, 9, 385–395. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef]
- Chhaya, M.P.; Melchels, F.P.W.; Holzapfel, B.M.; Baldwin, J.G.; Hutmacher, D.W. Sustained regeneration of high-volume adipose tissue for breast reconstruction using computer aided design and biomanufacturing. Biomaterials 2015, 52, 551–560. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C. 3D Bioprinting of Adipose-Derived Stem Cells for Organ Manufacturing. In Cutting-Edge Enabling Technologies for Regenerative Medicine; Springer: Cham, Switzerland, 2018; Volume 1078, pp. 3–14. [Google Scholar]
- Do, A.V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef]
- Gu, B.K.; Choi, D.J.; Park, S.J.; Kim, Y.J.; Kim, C.H. 3D Bioprinting Technologies for Tissue Engineering Applications. Adv. Exp. Med. Biol. 2018, 1078, 15–28. [Google Scholar] [CrossRef]
- Chen, M.; Le, D.Q.; Baatrup, A.; Nygaard, J.V.; Hein, S.; Bjerre, L.; Kassem, M.; Zou, X.; Bünger, C. Self-assembled composite matrix in a hierarchical 3-D scaffold for bone tissue engineering. Acta Biomater. 2011, 7, 2244–2255. [Google Scholar] [CrossRef]
- Peter, S.J.; Miller, M.J.; Yasko, A.W.; Yaszemski, M.J.; Mikos, A.G. Polymer concepts in tissue engineering. J. Biomed. Mater. Res. 1998, 43, 422–427. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, H.; Wu, L.; Ma, L.; Xing, F.; Kong, Q.; Fan, Y.; Zhou, C.; Zhang, X. 3D printing of calcium phosphate bioceramic with tailored biodegradation rate for skull bone tissue reconstruction. Bio Des. Manuf. 2019, 2, 161–171. [Google Scholar] [CrossRef]
- Sun, H.; Hu, C.; Zhou, C.; Wu, L.; Sun, J.; Zhou, X.; Xing, F.; Long, C.; Kong, Q.; Liang, J.J.M.; et al. 3D printing of calcium phosphate scaffolds with controlled release of antibacterial functions for jaw bone repair. Mater. Des. 2020, 189, 108540. [Google Scholar] [CrossRef]
- Tao, O.; Kort-Mascort, J.; Lin, Y.; Pham, H.M.; Charbonneau, A.M.; ElKashty, O.A.; Kinsella, J.M.; Tran, S.D. The Applications of 3D Printing for Craniofacial Tissue Engineering. Micromachines 2019, 10, 480. [Google Scholar] [CrossRef]
- Nyberg, E.L.; Farris, A.L.; Hung, B.P.; Dias, M.; Garcia, J.R.; Dorafshar, A.H.; Grayson, W.L. 3D-Printing Technologies for Craniofacial Rehabilitation, Reconstruction, and Regeneration. Ann. Biomed. Eng. 2017, 45, 45–57. [Google Scholar] [CrossRef]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, X.; Jiang, W.; Zhang, S.; Chen, M.; Yang, C. An innovative total temporomandibular joint prosthesis with customized design and 3D printing additive fabrication: A prospective clinical study. J. Transl. Med. 2019, 17, 4. [Google Scholar] [CrossRef]
- Yi, H.G.; Choi, Y.J.; Jung, J.W.; Jang, J.; Song, T.H.; Chae, S.; Ahn, M.; Choi, T.H.; Rhie, J.W.; Cho, D.W. Three-dimensional printing of a patient-specific engineered nasal cartilage for augmentative rhinoplasty. J. Tissue Eng. 2019, 10, 2041731418824797. [Google Scholar] [CrossRef]
- Nuseir, A.; Hatamleh, M.M.; Alnazzawi, A.; Al-Rabab’ah, M.; Kamel, B.; Jaradat, E. Direct 3D Printing of Flexible Nasal Prosthesis: Optimized Digital Workflow from Scan to Fit. J. Prosthodont. 2019, 28, 10–14. [Google Scholar] [CrossRef]
- Liao, J.; Chen, Y.; Chen, J.; He, B.; Qian, L.; Xu, J.; Wang, A.; Li, Q.; Xie, H.; Zhou, J. Auricle shaping using 3D printing and autologous diced cartilage. Laryngoscope 2019, 129, 2467–2474. [Google Scholar] [CrossRef]
- Kim, D.H.; Yun, W.S.; Shim, J.H.; Park, K.H.; Choi, D.; Park, M.I.; Hwang, S.H.; Kim, S.W. Clinical Application of 3-Dimensional Printing Technology for Patients With Nasal Septal Deformities: A Multicenter Study. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 1145–1152. [Google Scholar] [CrossRef]
- Mussi, E.; Furferi, R.; Volpe, Y.; Facchini, F.; McGreevy, K.S.; Uccheddu, F. Ear Reconstruction Simulation: From Handcrafting to 3D Printing. Bioengineering 2019, 6, 14. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef]
- Kim, B.S.; Kwon, Y.W.; Kong, J.S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.B.; Lee, H.; Kim, J.H.; Cho, D.W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53. [Google Scholar] [CrossRef]
- Tarassoli, S.P.; Jessop, Z.M.; Al-Sabah, A.; Gao, N.; Whitaker, S.; Doak, S.; Whitaker, I.S. Skin tissue engineering using 3D bioprinting: An evolving research field. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 615–623. [Google Scholar] [CrossRef]
- Lee, V.; Singh, G.; Trasatti, J.P.; Bjornsson, C.; Xu, X.; Tran, T.N.; Yoo, S.S.; Dai, G.; Karande, P. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. Part C Methods 2014, 20, 473–484. [Google Scholar] [CrossRef]
- Augustine, R. Skin bioprinting: A novel approach for creating artificial skin from synthetic and natural building blocks. Prog. Biomater. 2018, 7, 77–92. [Google Scholar] [CrossRef]
- Cleversey, C.; Robinson, M.; Willerth, S.M. 3D Printing Breast Tissue Models: A Review of Past Work and Directions for Future Work. Micromachines 2019, 10, 501. [Google Scholar] [CrossRef]
- Chen, K.; Feng, C.J.; Ma, H.; Hsiao, F.Y.; Tseng, L.M.; Tsai, Y.F.; Lin, Y.S.; Huang, L.Y.; Yu, W.C.; Perng, C.K. Preoperative breast volume evaluation of one-stage immediate breast reconstruction using three-dimensional surface imaging and a printed mold. J. Chin. Med. Assoc. JCMA 2019, 82, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Tytgat, L.; Van Damme, L.; Ortega Arevalo, M.D.P.; Declercq, H.; Thienpont, H.; Otteveare, H.; Blondeel, P.; Dubruel, P.; Van Vlierberghe, S. Extrusion-based 3D printing of photo-crosslinkable gelatin and κ-carrageenan hydrogel blends for adipose tissue regeneration. Int. J. Biol. Macromol. 2019, 140, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Hummelink, S.; Verhulst, A.C.; Maal, T.J.J.; Ulrich, D.J.O. Applications and limitations of using patient-specific 3D printed molds in autologous breast reconstruction. Eur. J. Plast. Surg. 2018, 41, 571–576. [Google Scholar] [CrossRef]
- DeFazio, M.V.; Arribas, E.M.; Ahmad, F.I.; Le-Petross, H.T.; Liu, J.; Chu, C.K.; Santiago, L.; Clemens, M.W. Application of Three-Dimensional Printed Vascular Modeling as a Perioperative Guide to Perforator Mapping and Pedicle Dissection during Abdominal Flap Harvest for Breast Reconstruction. J. Reconstr. Microsurg. 2020, 36, 325–338. [Google Scholar] [CrossRef]
| Name | Source | Application Mode | Application Site | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Natural Biomaterials | |||||
| Bio-protein glue [15,16,17,18] | Blood from animals | Injectable liquid | Hemostasis of venule hemorrhage in burn patients | Good biocompatibility | The application range is narrow |
| Decellularized tissue [19,20,21,22,23,24,25,26] | Dermis, valve, and other tissue from human or animals | Biological patch | Skin transplantation in burn patients | Good biocompatibility | High cost |
| Collagen [27,28] | Connective tissue of animals, such as bovine achilles tendon | Injectable liquid | Facial soft tissue filler for crow’s feet, periorbital wrinkles, and deep scars | Good biocompatibility; can interact with cells; good mechanical properties; moisture retention; low immunogenicity; biodegradability | Rejection reaction; possible disease transfection |
| HA [29,30,31,32,33,34,35,36] | cockscomb, umbilical cord, eyeballs, and cartilage | Injectable liquid | Fillers for wrinkles of the decree lines, corners of the mouth, forehead, eyebrows, nose, lips, chin, acne, and chickenpox crypt scar | Minimal rejection reaction | Mild swelling; rapid degradation |
| Name | Application Mode | Application Site | Advantages | Disadvantages | Molecular Structure/Formula |
|---|---|---|---|---|---|
| Organic Polymer Materials | |||||
| Silicone [37,38,39,40,41] | Prosthesis | Rhinoplasty, breast enhancement, plumping forehead and lumping buttock | Heat resistance, cold resistance, non-toxicity, biological aging resistance, physiological inertia, little response to human tissues, and good physical and mechanical properties | Strong hydrophobicity, poor antibacterial properties, and aging problems |  |
| ePTFE [42,43,44,45] | Prosthesis | Ear reconstruction, rhinoplasty | Good biocompatibility, good tensile strength | Non-biodegradable, poor antibacterial properties |  |
| PMMA [46,47,48,49,50,51] | Injectable liquid | Facial wrinkles, fill in acne scars, correct nipple depressions, highlight chins, and swell cheeks | long-lasting | Bead protrusions, allergic reactions, telangiectasia, and granuloma formation | 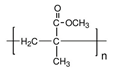 |
| High-density polyethylene [52,53,54,55,56] | Prosthesis | Repair of cheeks, orbital arch, orbital floor, upper and lower jaws, cheekbones, temporal, and ears | Good biocompatibility, rough, convergent, and porous structure | High risk of infection, poor mechanical properties | 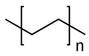 |
| PLA and PLGA [57,58,59,60] | Prosthesis; sutures | Surgical absorbable sutures, bone fixation plates, screws | Good biocompatibility, biodegradability | Rapid biodegradation | 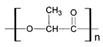 & 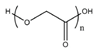 |
| Ceramic Materials | |||||
| Hydroxyapatite [61,62,63,64] | Prosthesis | Substitute for large area bone defects such as oral and maxillofacial regions, substitutes for teeth | Good biocompatibility, biodegradability, low toxicity | High brittleness, low strength, and poor toughness |  |
| Bioglass materials [65,66] | Prosthesis | Bone repair and regeneration | Good biocompatibility and bone binding ability | Low mechanical strength, high brittleness, poor bending strength, and compressive strength | E.g. 45S5 Bioglass® (developed by L.L. Hench): 45 wt% SiO2, 24.5 wt% CaO, 24.5 wt% Na2O, and 6.0 wt% P2O5. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, W.; Peng, Z.; Tang, P.; Sun, H.; Lei, H.; Li, Z.; Hui, D.; Du, C.; Zhou, C.; Wang, Y. Review of Plastic Surgery Biomaterials and Current Progress in Their 3D Manufacturing Technology. Materials 2020, 13, 4108. https://doi.org/10.3390/ma13184108
Peng W, Peng Z, Tang P, Sun H, Lei H, Li Z, Hui D, Du C, Zhou C, Wang Y. Review of Plastic Surgery Biomaterials and Current Progress in Their 3D Manufacturing Technology. Materials. 2020; 13(18):4108. https://doi.org/10.3390/ma13184108
Chicago/Turabian StylePeng, Wei, Zhiyu Peng, Pei Tang, Huan Sun, Haoyuan Lei, Zhengyong Li, Didi Hui, Colin Du, Changchun Zhou, and Yongwei Wang. 2020. "Review of Plastic Surgery Biomaterials and Current Progress in Their 3D Manufacturing Technology" Materials 13, no. 18: 4108. https://doi.org/10.3390/ma13184108
APA StylePeng, W., Peng, Z., Tang, P., Sun, H., Lei, H., Li, Z., Hui, D., Du, C., Zhou, C., & Wang, Y. (2020). Review of Plastic Surgery Biomaterials and Current Progress in Their 3D Manufacturing Technology. Materials, 13(18), 4108. https://doi.org/10.3390/ma13184108







