Durability Characterisation of Portland Cement–Carbon Nanotube Nanocomposites
Abstract
1. Introduction
- CNTs with and without a polycarboxylate-based superplasticiser to aid dispersion;
- CNT dosage rates of 0.05–0.25% by the weight of cement powder;
- Poor or adequate (i.e., agglomerated or well-dispersed) CNT dispersion quality.
2. Materials and Methods
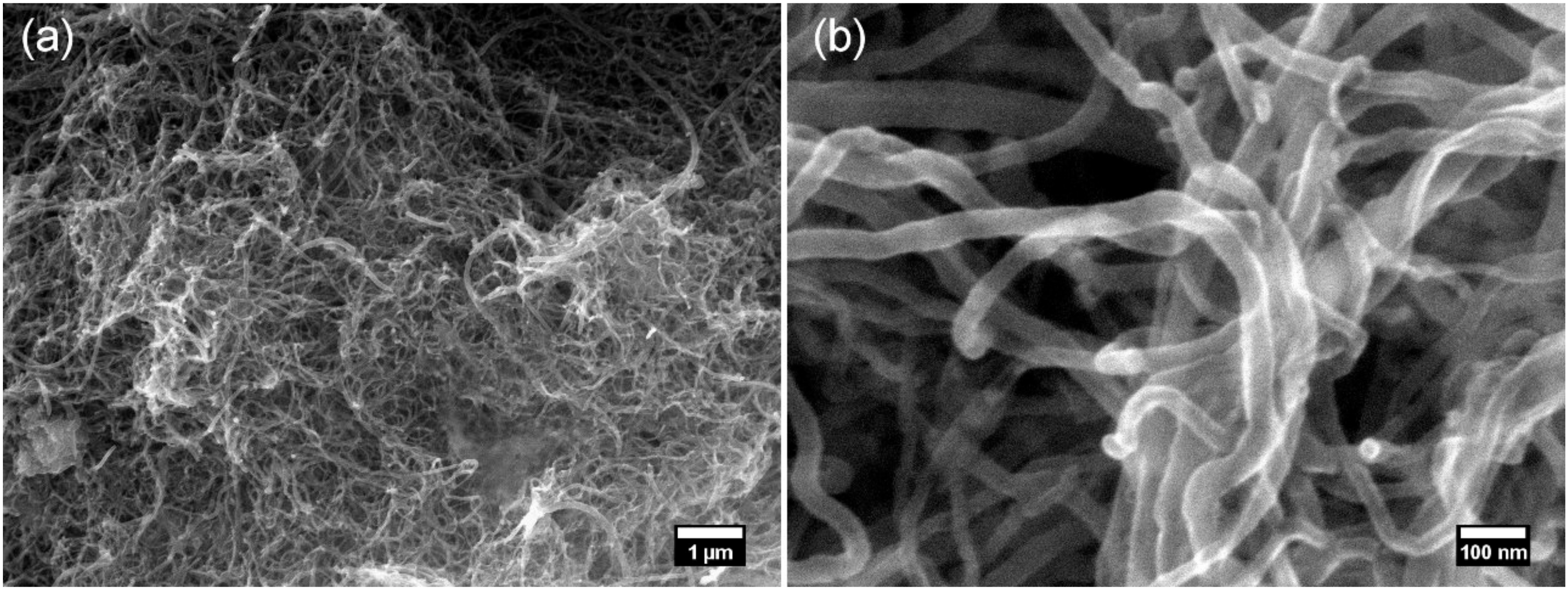
2.1. Materials
2.2. Nanocomposite Paste Preparation
- CNTs dispersed with and without the assistance of the superplasticiser—specimens C10, N10;
- CNT dose, between 0.05% and 0.25% (by the weight of cement powder)—specimens C05, C10 and C25;
- Intentionally poorly-dispersed (i.e., agglomerated) CNTs, with the superplasticiser—P10.
- Predispersion—the required dose of the superplasticiser was magnetically stirred with the mix (tap) water for 2 minutes. This step was not employed for specimens R0 and N10.
- CNT dispersion—in a fume cupboard, the CNT powder quantity was added to the solution. The mixture was ultrasonicated with ice bath cooling for a total of 20 kJ (10 min) using a Sonics and Materials Vibra-Cell VCX 500 W ultrasonic processor (Sonics & Materials, Newton, CT, USA), equipped with a 19 mm diameter solid cylindrical probe, and operating continuously in energy set point mode. This step was not conducted for specimen P10.
- Paste mixing—the CNT mixture (or mix water, for specimen R0, or surfactant mixture, for specimens R1 and P10) was introduced to a CTE Model 7000 constant speed mixer (Cement Test Equipment, Tulsa, OH, USA); mixing was conducted following the procedure detailed in Section 9.5 of ASTM C1738 [35]. For specimen P10 only, CNTs (in a small portion of the mix water) were added to the paste mix immediately following this standard paste mixing procedure, thereby (intentionally) producing an inhomogeneous distribution of CNTs within the nanocomposite.
2.3. Experimental Methods
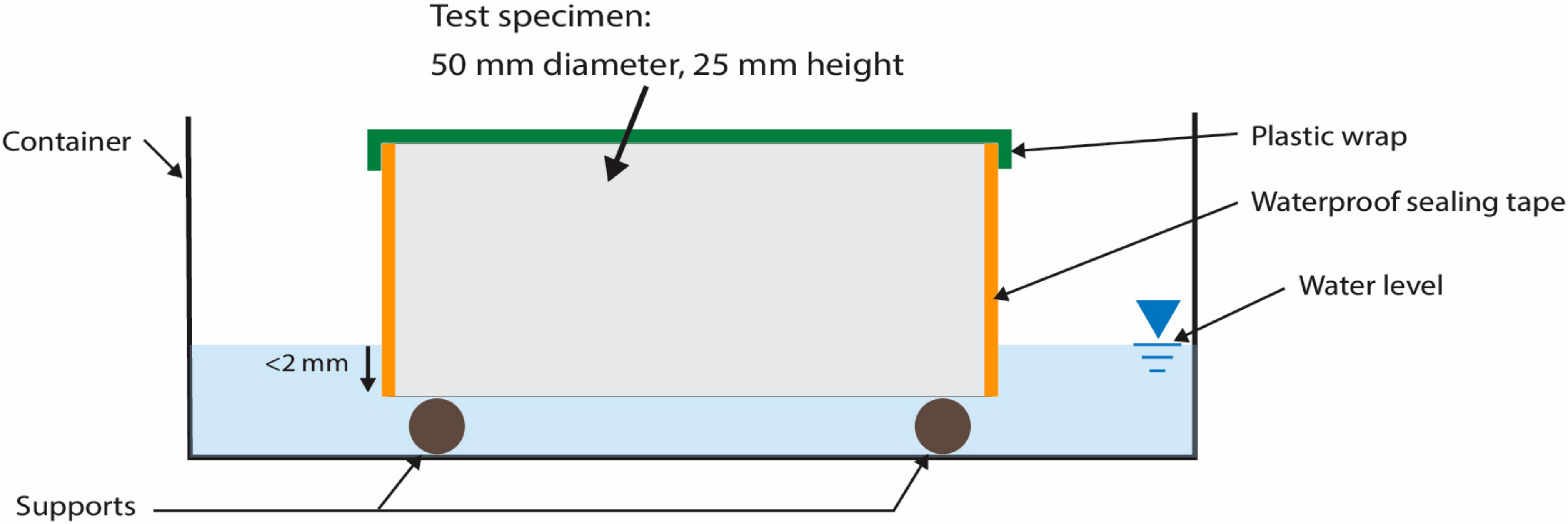
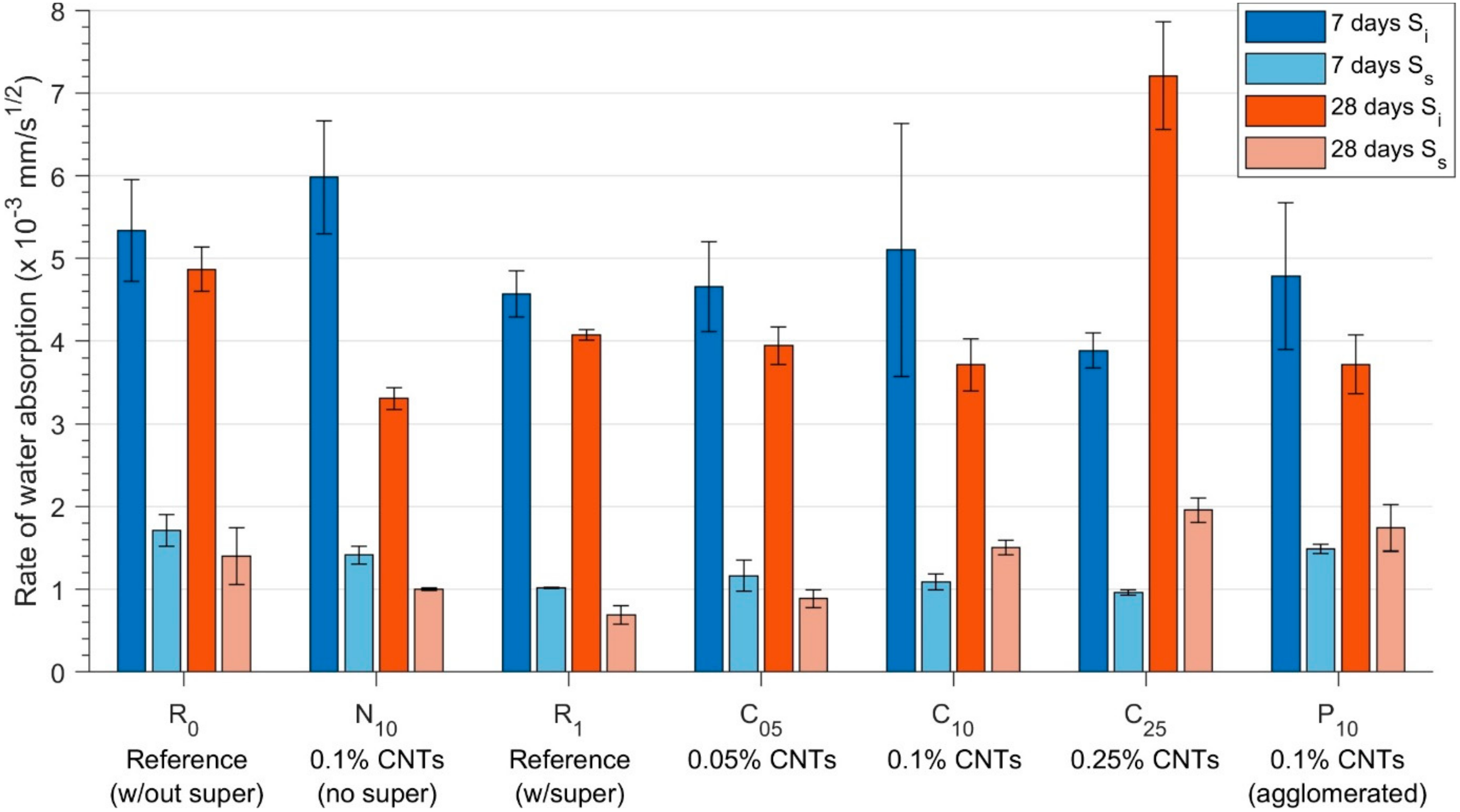
2.3.1. Water Sorptivity
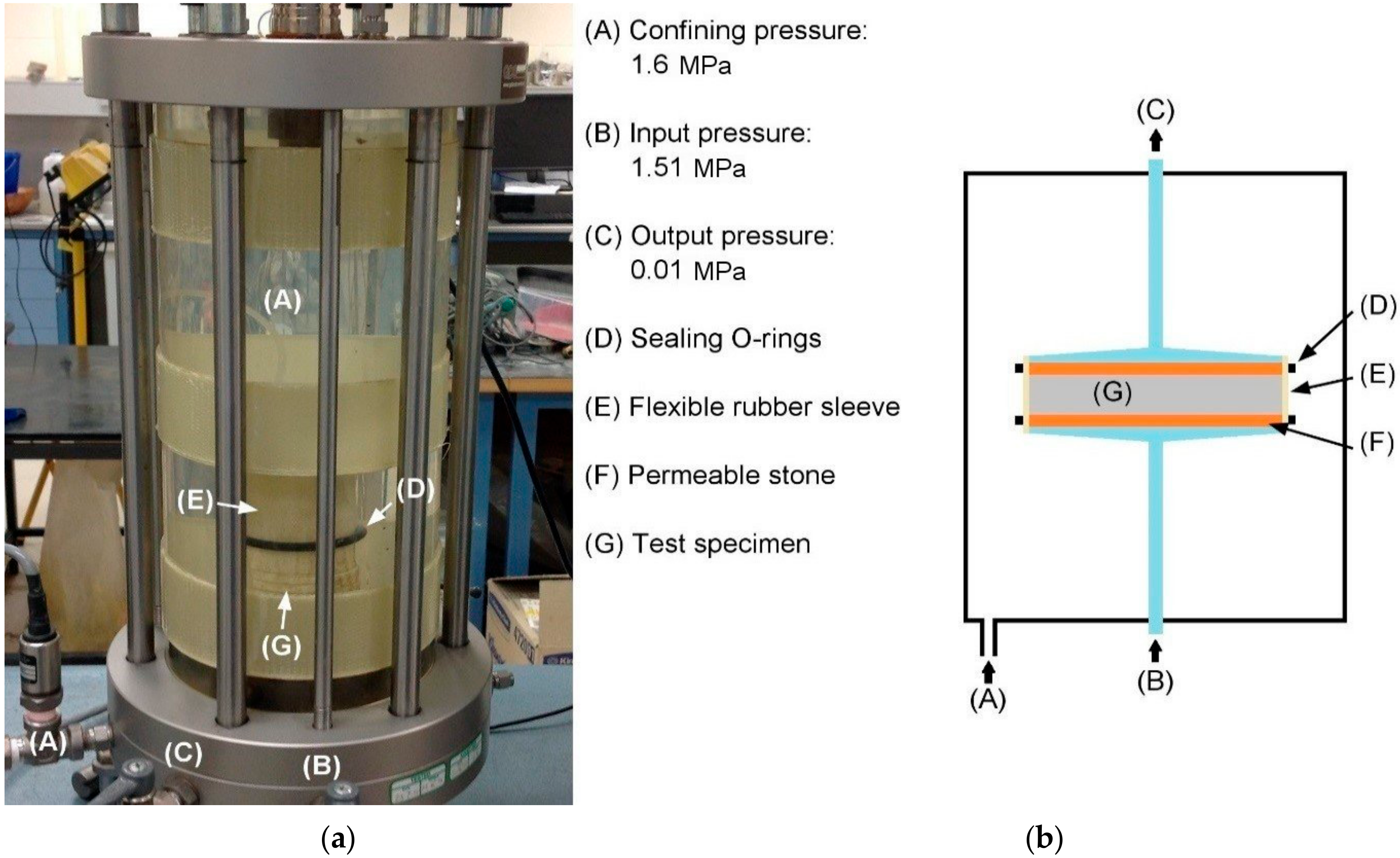
2.3.2. Water Permeability
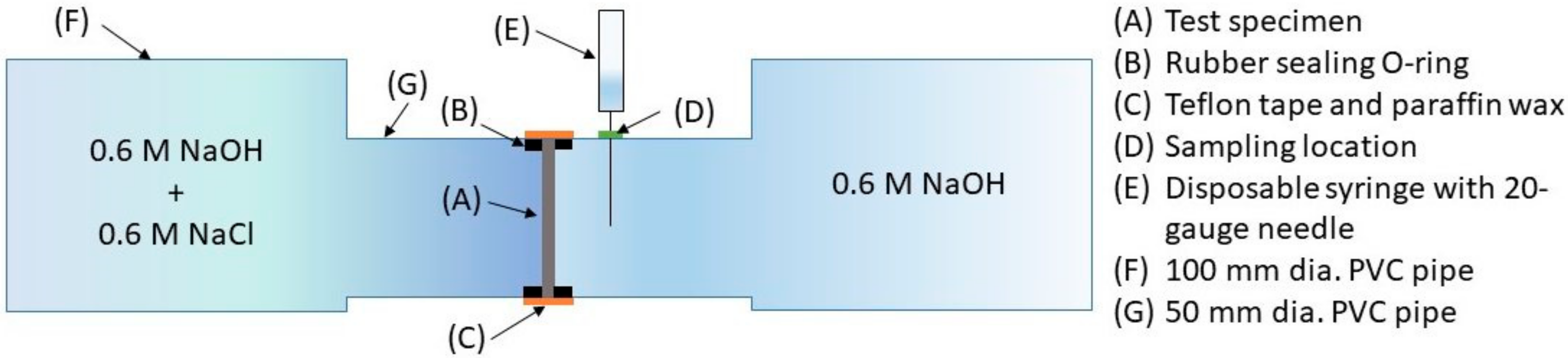
2.3.3. Steady-State Chloride Diffusivity
2.3.4. Nitrogen Adsorption Porosimetry
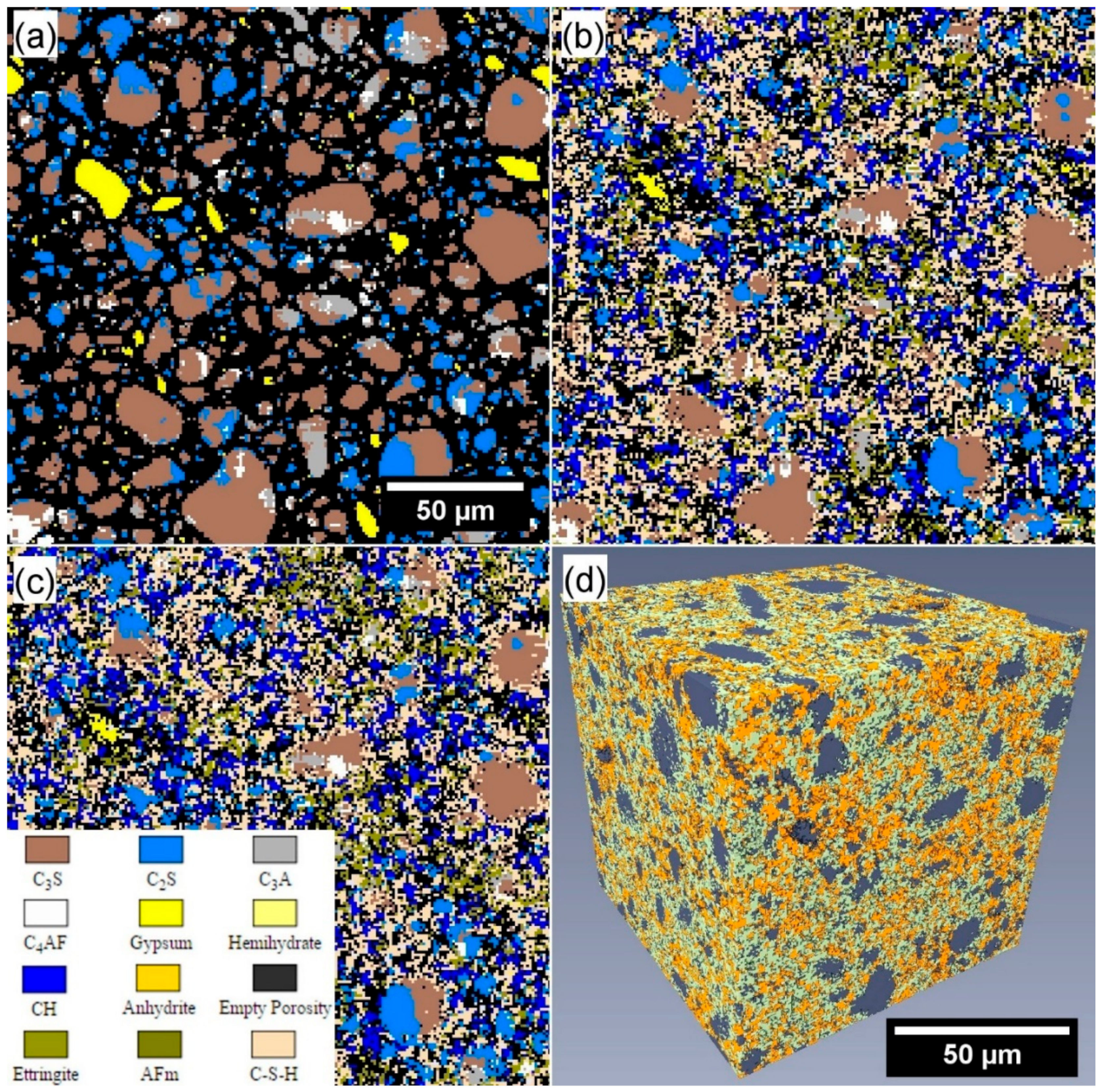
2.3.5. X-ray Computed Microtomography and Porosity Segmentation
2.4. Simulations
2.4.1. Simulated Cement Hydration and Microstructural Development
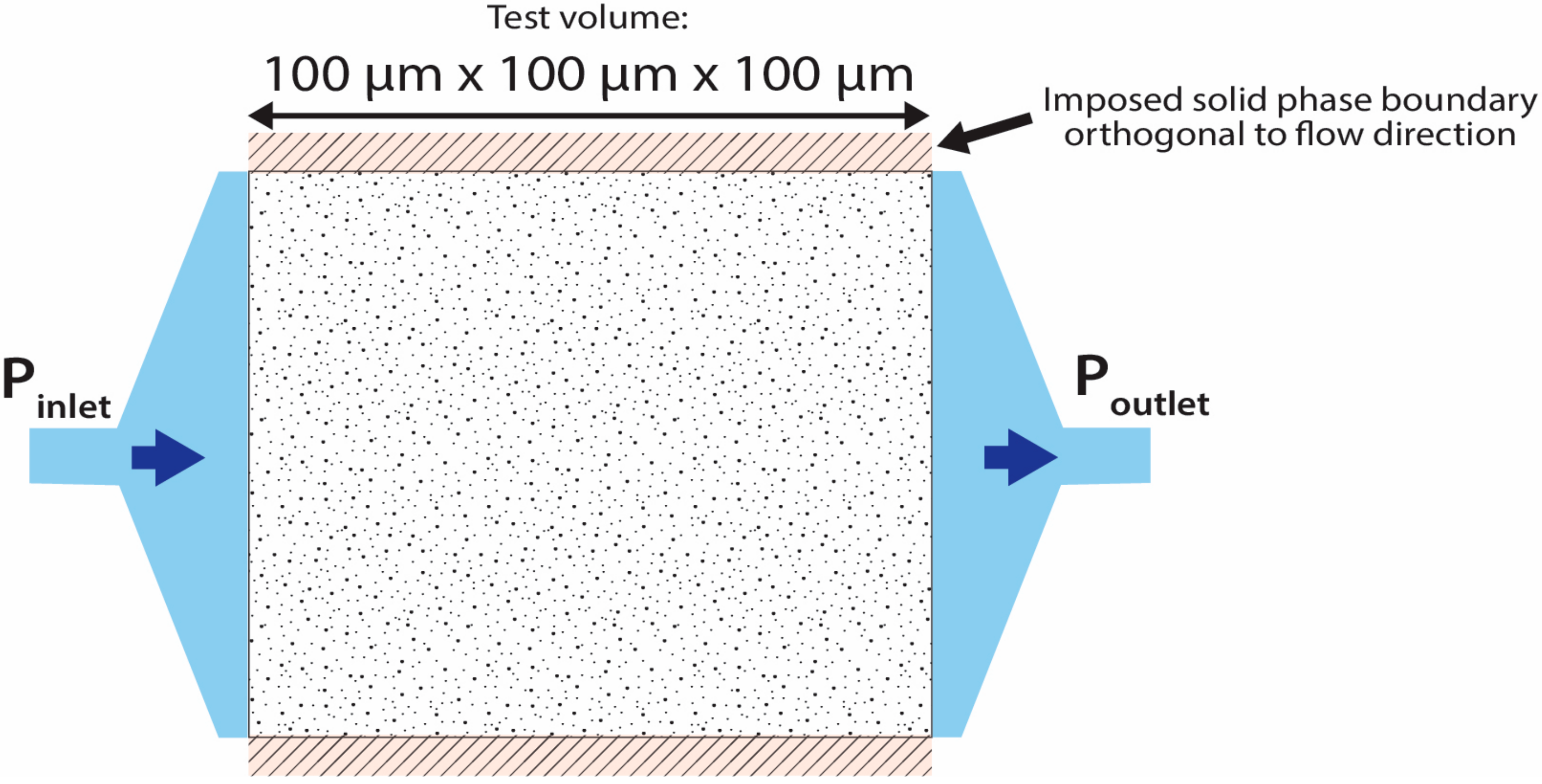
2.4.2. Fluid Flow Simulation
- Separate simulations were conducted in each of the three cardinal directions in turn for each of the VOI investigated. However, due to the limited 6-voxel pore connectivity applied for the simulations, each simulation was checked to ensure that the result corresponded to a continuous, connected pore network.
- A dynamic viscosity of 8.9 × 10−4 Pa.s, corresponding to water at 25 °C.
- As a result of the limited pore voxel connectivity used in the simulations, a refining coefficient of 2 was applied to intentionally oversample the VOI, to increase the precision of the evaluation of the unknowns, particularly for narrow pore throats.
- An inlet pressure of 1.51 MPa and an outlet pressure of 0.01 MPa were applied to each simulation.
3. Results and Discussion
3.1. Absorption—Water Sorptivity
3.2. Permeation—Water Permeability
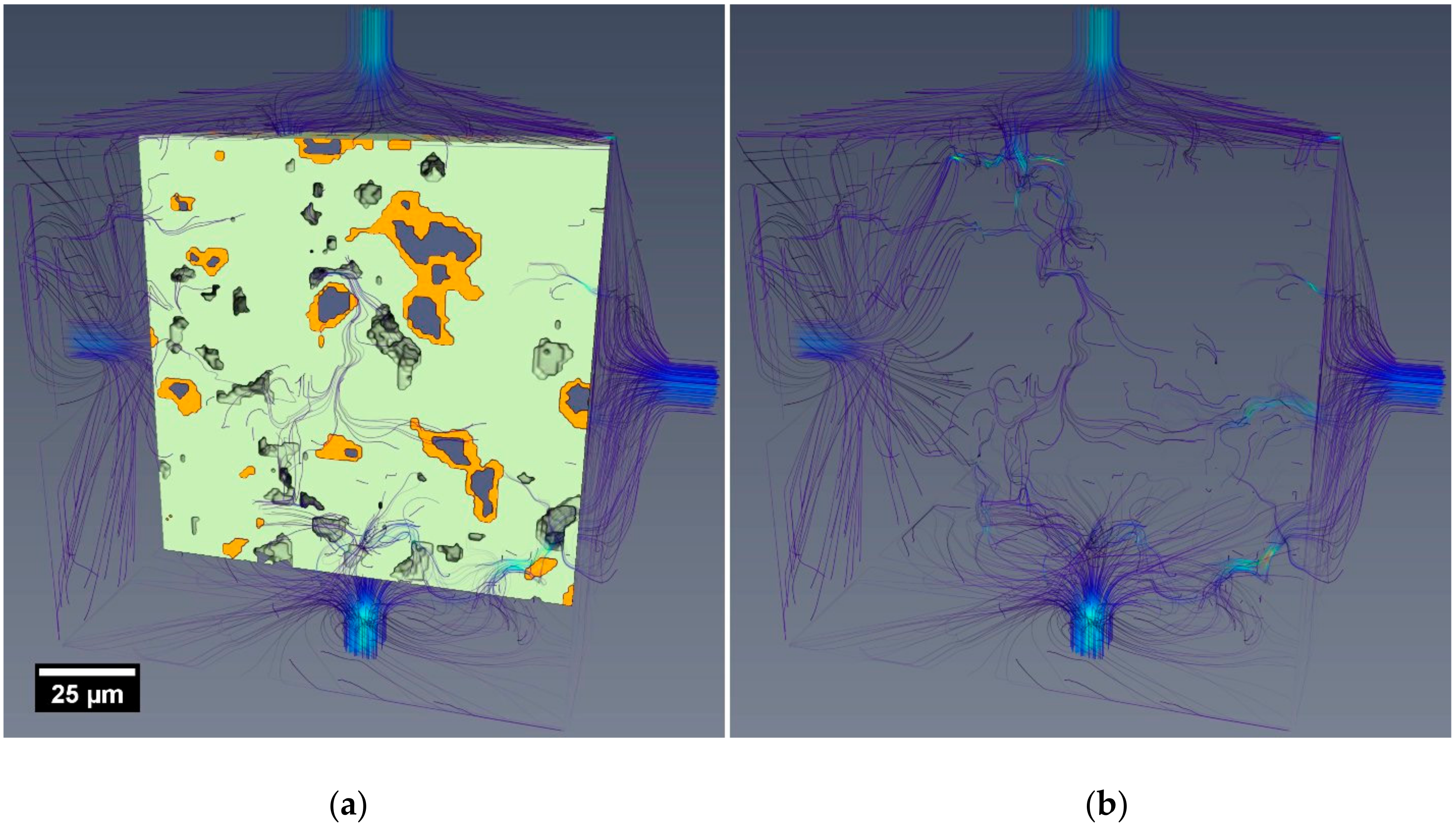
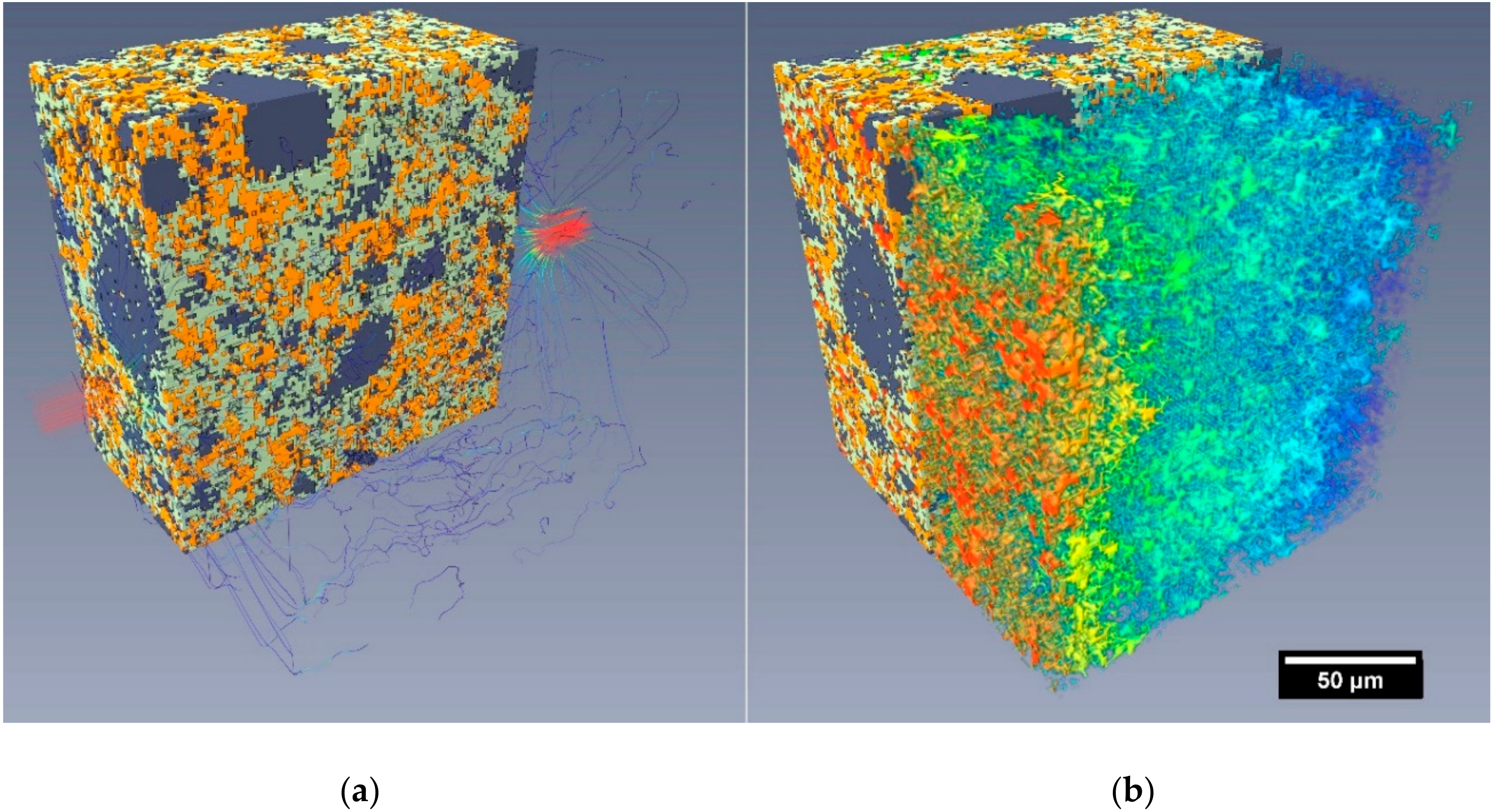
3.3. Fluid Flow Simulations in Reconstructed Microstructures
- Simulated flow was restricted to only the percolating pore network(s) within the segmented microstructures (i.e., no flow through the segmented C-S-H).
- The reconstructed microstructures (including the virtual samples V1 7 days and 28 days) were limited to a nominal resolution of around 1 µm. This factor, more than any other, would significantly alter the permeability of the reconstructed volumes compared to the experimental samples.
- The experimental test results were influenced by factors including C-S-H swelling, blockages and specimen microcracking, as discussed in Section 3.2.
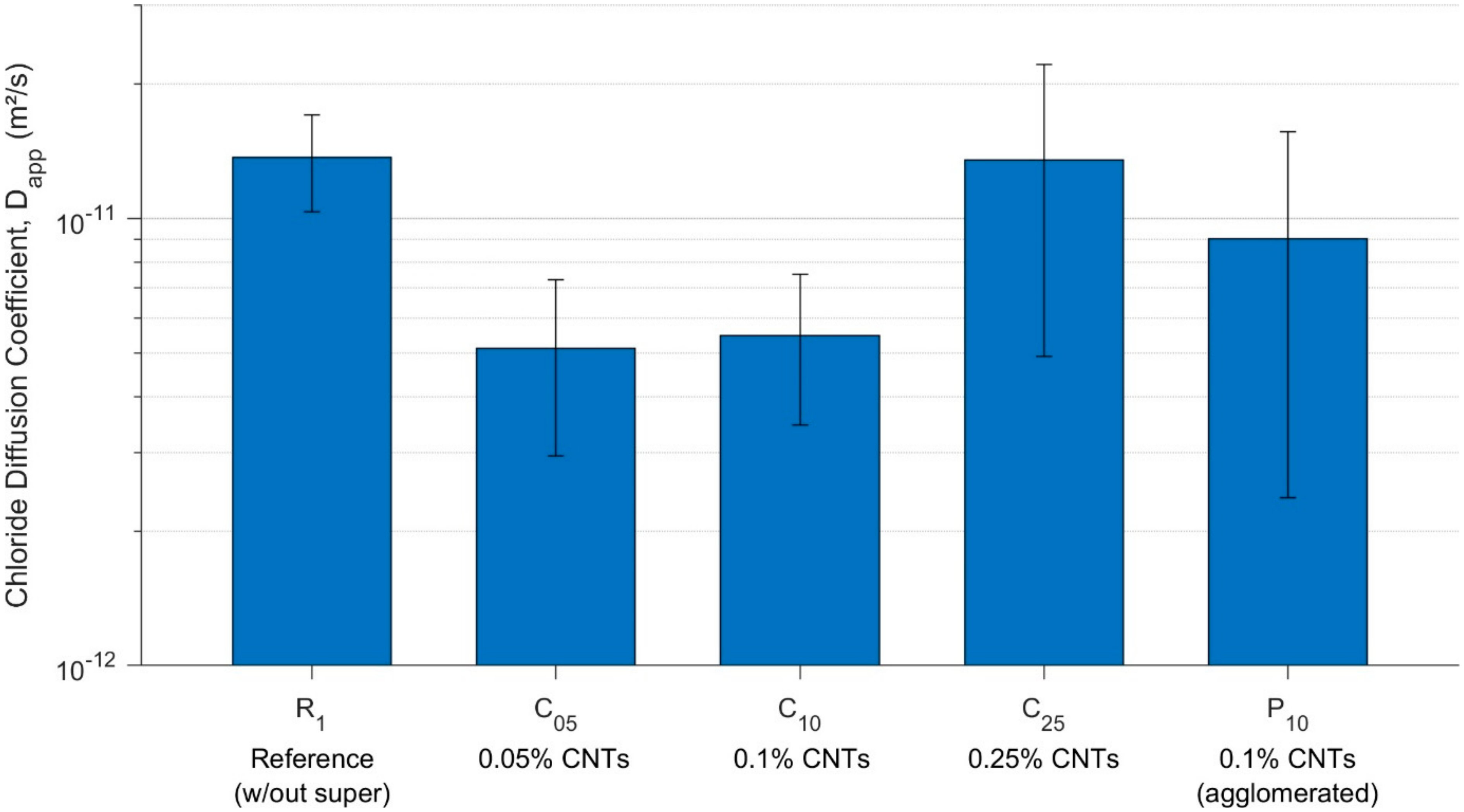
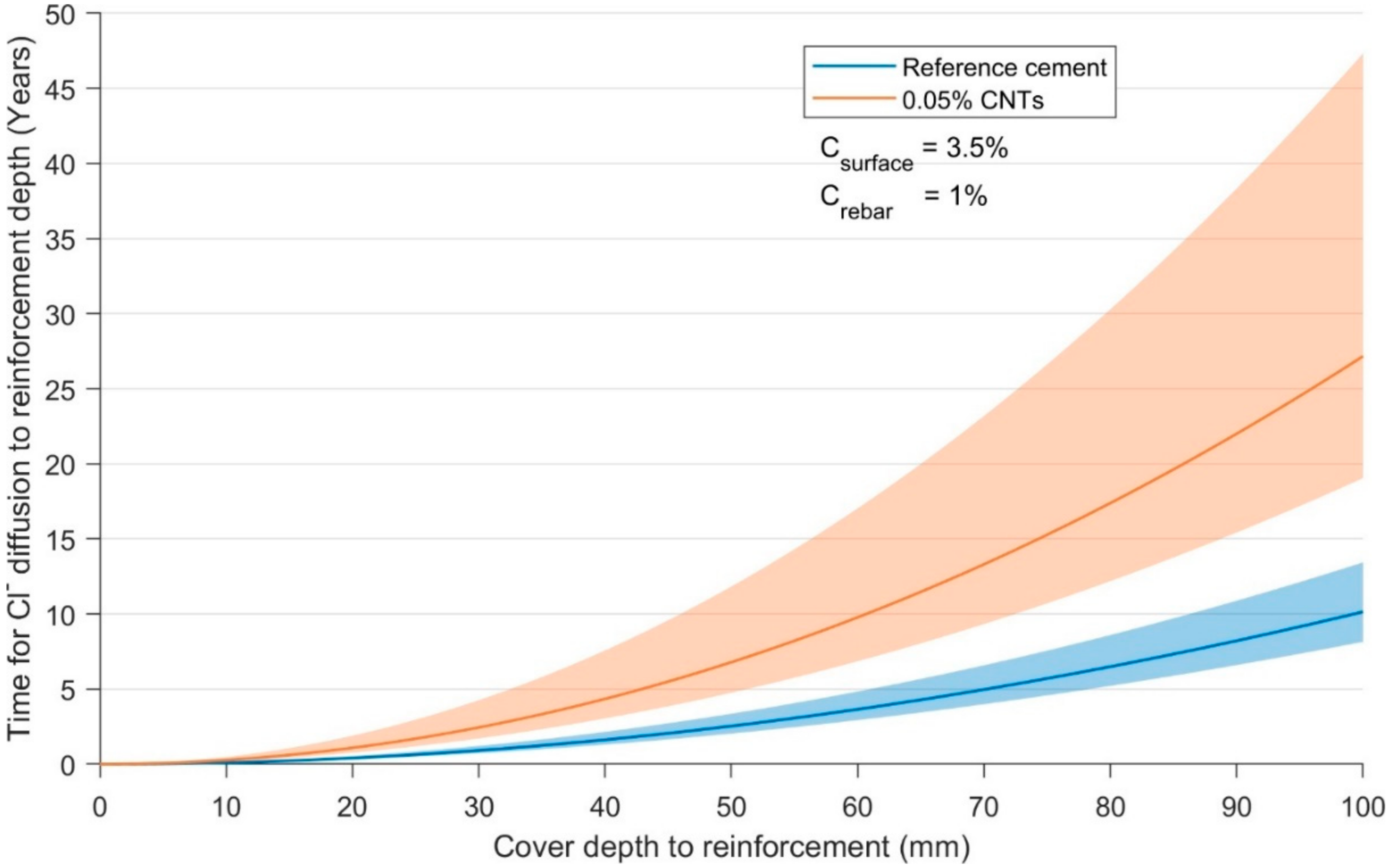
3.4. Diffusion—Chloride Diffusivity
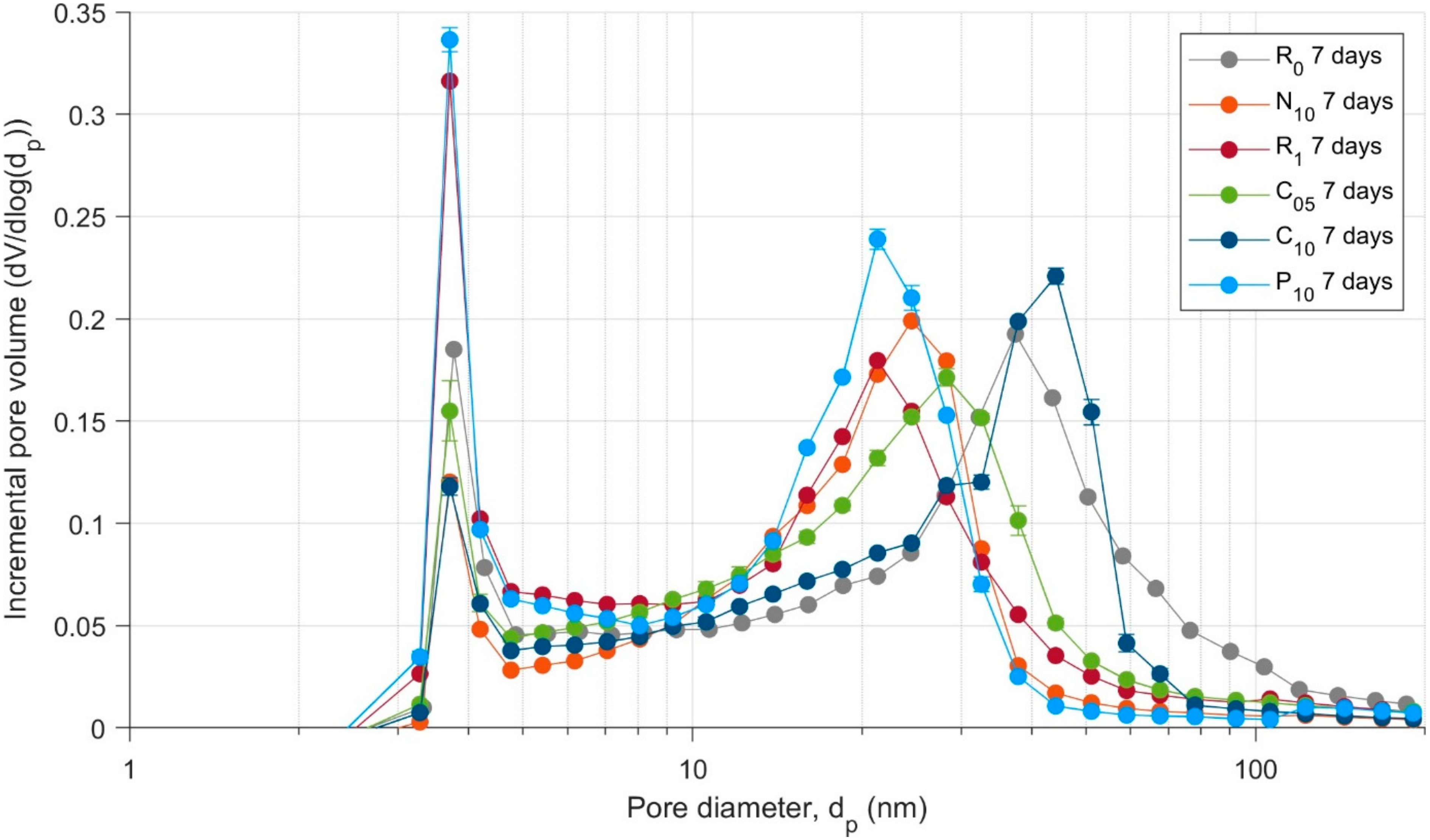
3.5. Porosity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Damme, H. Concrete material science: Past, present, and future innovations. Cem. Concr. Res. 2018, 112, 5–24. [Google Scholar] [CrossRef]
- Schneider, M. The cement industry on the way to a low-carbon future. Cem. Concr. Res. 2019, 124, 105792. [Google Scholar] [CrossRef]
- Monthioux, M.; Serp, P.; Flahaut, E.; Razafinimanana, M.; Laurent, C.; Peigney, A.; Bacsa, W.; Broto, J.-M. Introduction to Carbon Nanotubes. In Springer Handbook of Nanotechnology; Bhushan, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 47–118. [Google Scholar]
- Popov, V. Carbon nanotubes: Properties and application. Mater. Sci. Eng. R Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Chen, S.J.; Collins, F.G.; MacLeod, A.J.N.; Pan, Z.; Duan, W.H.; Wang, C.M. Carbon nanotube-cement composites: A retrospect. IES A Civ. Struct. Eng. 2011, 4, 254–265. [Google Scholar] [CrossRef]
- Mendoza Reales, O.A.; Dias Toledo Filho, R. A review on the chemical, mechanical and microstructural characterization of carbon nanotubes-cement based composites. Constr. Build. Mater. 2017, 154, 697–710. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Xu, S. Progress in Research on Carbon Nanotubes Reinforced Cementitious Composites. Adv. Mater. Sci. Eng. 2015. [Google Scholar] [CrossRef]
- Rashad, A.M. Effect of carbon nanotubes (CNTs) on the properties of traditional cementitious materials. Constr. Build. Mater. 2017, 153, 81–101. [Google Scholar] [CrossRef]
- Shi, T.; Li, Z.; Guo, J.; Gong, H.; Gu, C. Research progress on CNTs/CNFs-modified cement-based composites—A review. Constr. Build. Mater. 2019, 202, 290–307. [Google Scholar] [CrossRef]
- Girifalco, L.A.; Hodak, M.; Lee, R.S. Carbon nanotubes, buckyballs, ropes, and a universal graphitic potential. Phys. Rev. B 2000, 62, 13104–13110. [Google Scholar] [CrossRef]
- Collins, F.; Lambert, J.; Duan, W.H. The influences of admixtures on the dispersion, workability, and strength of carbon nanotube—OPC paste mixtures. Cem. Concr. Compos. 2012, 34, 201–207. [Google Scholar] [CrossRef]
- MacLeod, A.J.N.; Collins, F.G.; Duan, W.H. Carbon nanotube dispersion using Portland cement-compatible surfactants. In Proceedings of the 6th International Composites Conference (ACUN-6) Composite and Nanocomposites in Civil, Offshore and Mining Infrastructure, Melbourne, Australia, 14–16 November 2012; pp. 199–205. [Google Scholar]
- Makar, J.M.; Chan, G.W. Growth of cement hydration products on single-walled carbon nanotubes. J. Am. Ceram. Soc. 2009, 92, 1303–1310. [Google Scholar] [CrossRef]
- Carriço, A.; Bogas, J.A.; Hawreen, A.; Guedes, M. Durability of multi-walled carbon nanotube reinforced concrete. Constr. Build. Mater. 2018, 164, 121–133. [Google Scholar] [CrossRef]
- Konsta-Gdoutos, M.S.; Metaxa, Z.S.; Shah, S.P. Multi-scale mechanical and fracture characteristics and early-age strain capacity of high performance carbon nanotube/cement nanocomposites. Cem. Concr. Compos. 2010, 32, 110–115. [Google Scholar] [CrossRef]
- MacLeod, A.J.N.; Collins, F.G.; Duan, W.; Gates, W.P. Quantitative microstructural characterisation of Portland cement-carbon nanotube composites using electron and X-ray microscopy. Cem. Concr. Res. 2019, 123, 105767. [Google Scholar] [CrossRef]
- Fehervari, A.; MacLeod, A.J.N.; Garcez, E.O.; Aldridge, L.; Gates, W.P.; Yang, Y.; Collins, F. On the mechanisms for improved strengths of carbon nanofiber-enriched mortars. Cem. Concr. Res. 2020, 136, 106178. [Google Scholar] [CrossRef]
- Nochaiya, T.; Chaipanich, A. Behaviour of multi-walled carbon nanotubes on the porosity and microstructure of cement-based materials. Appl. Surf. Sci. 2011, 257, 1941–1945. [Google Scholar] [CrossRef]
- Basheer, P.A.M.; Barbhuiya, S. Pore structure and transport processes. In Concrete Durability: A Practical Guide to the Design of Durable Concrete Structures; Soutsos, M., Ed.; Thomas Telford: London, UK, 2009; pp. 14–34. [Google Scholar]
- Han, B.; Yang, Z.; Shi, X.; Yu, X. Transport Properties of Carbon-Nanotube/Cement Composites. J. Mater. Eng. Perform. 2013, 22, 184–189. [Google Scholar] [CrossRef]
- Li, L.; Jing, H.; Gao, Y.; Hu, T.; Meng, Y.; Fu, G. Influence of methylcellulose on the impermeability properties of carbon nanotube-based cement pastes at different water-to-cement ratios. Constr. Build. Mater. 2020, 244, 118403. [Google Scholar] [CrossRef]
- Sun, G.-L. Morphological, Mechanical and Durability Properties of Cement Mortar Reinforced with Multi-Walled Carbon Nanotubes. J. Adv. Microsc. Res. 2015, 10, 60–64. [Google Scholar] [CrossRef]
- Wang, X.; Rhee, I.; Wang, Y.; Xi, Y. Compressive Strength, Chloride Permeability, and Freeze-Thaw Resistance of MWNT Concretes under Different Chemical Treatments. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Alafogianni, P.; Dassios, K.; Tsakiroglou, C.D.; Matikas, T.E.; Barkoula, N.M. Effect of CNT addition and dispersive agents on the transport properties and microstructure of cement mortars. Constr. Build. Mater. 2019, 197, 251–261. [Google Scholar] [CrossRef]
- Konsta-Gdoutos, M.S.; Batis, G.; Danoglidis, P.A.; Zacharopoulou, A.K.; Zacharopoulou, E.K.; Falara, M.G.; Shah, S.P. Effect of CNT and CNF loading and count on the corrosion resistance, conductivity and mechanical properties of nanomodified OPC mortars. Constr. Build. Mater. 2017, 147, 48–57. [Google Scholar] [CrossRef]
- Lee, H.S.; Balasubramanian, B.; Gopalakrishna, G.V.T.; Kwon, S.-J.; Karthick, S.P.; Saraswathy, V. Durability performance of CNT and nanosilica admixed cement mortar. Constr. Build. Mater. 2018, 159, 463–472. [Google Scholar] [CrossRef]
- ASTM. C1202-17 Standard Test Method for Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration; ASTM International: West Conshohocken, PA, USA, 2017; p. 8. [Google Scholar]
- Andrade, C.; Whiting, D. A comparison of chloride ion diffusion coefficients derived from concentration gradients and non-steady state accelerated ionic migration. Mater. Struct. 1996, 29, 476–484. [Google Scholar] [CrossRef]
- ASTM. C1556-11a (2016) Standard Test Method for Determining the Apparent Chloride Diffusion Coefficient of Cementitious Mixtures by Bulk Diffusion; ASTM International: West Conshohocken, PA, USA, 2011; p. 7. [Google Scholar]
- ASTM. C876 Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete; ASTM International: West Conshohocken, PA, USA, 2015; p. 8. [Google Scholar]
- Del Carmen Camacho, M.; Galao, O.; Baeza, F.; Zornoza, E.; Garcés, P. Mechanical Properties and Durability of CNT Cement Composites. Materials 2014, 7, 1640–1651. [Google Scholar] [CrossRef] [PubMed]
- Standards Australia. AS 3972:2010 General Purpose and Blended Cements; Standards Australia: Sydney, Australia, 2010; p. 29. [Google Scholar]
- Standards Australia. AS 1478.1-2000 (R2018): Chemical Admixtures for Concrete, Mortar and Grout; Standards Australia: Sydney, Australia, 2018; p. 61. [Google Scholar]
- Konsta-Gdoutos, M.S.; Metaxa, Z.S.; Shah, S.P. Highly dispersed carbon nanotube reinforced cement based materials. Cem. Concr. Res. 2010, 40, 1052–1059. [Google Scholar] [CrossRef]
- ASTM. C1738-14 Standard Practice for High-Shear Mixing of Hydraulic Cement Pastes; ASTM International: West Conshohocken, PA, USA, 2014; p. 3. [Google Scholar]
- Standards Australia. AS 1012.8.1:2014 Methods of Testing Concrete—Method 8.1: Method for Making and Curing Concrete—Compression and Indirect Tensile Test Specimens; Standards Australia: Sydney, Australia, 2014; p. 13. [Google Scholar]
- ASTM. C1585-13 Standard Test Method for Measurement of Rate of Absorption of Water by Hydraulic-Cement Concretes; ASTM International: West Conshohocken, PA, USA, 2013; p. 6. [Google Scholar]
- ASTM. D5084-10 Standard Test Methods for Measurement of Hydraulic Conductivity of Saturated Porous Materials Using a Flexible Wall Permeameter; ASTM International: West Conshohocken, PA, USA, 2010; p. 23. [Google Scholar]
- Darcy, H. Les Fontaines Publiques de la ville de Dijon; V Dalmont: Paris, France, 1856. [Google Scholar]
- Fick, A. Ueber Diffusion. Ann. der Phys. 1855, 170, 59–86. [Google Scholar] [CrossRef]
- Dyer, T. Corrosion of Steel Reinforcement in Concrete. In Concrete Durability; CRC Press: Boca Raton, FL, USA, 2014; pp. 183–243. [Google Scholar]
- Diamond, S. Mercury porosimetry: An inappropriate method for the measurement of pore size distributions in cement-based materials. Cem. Concr. Res. 2000, 30, 1517–1525. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Jennings, H.M. A model for the microstructure of calcium silicate hydrate in cement paste. Cem. Concr. Res. 2000, 30, 101–116. [Google Scholar] [CrossRef]
- Bullard, J.W.; Garboczi, E.J. A model investigation of the influence of particle shape on portland cement hydration. Cem. Concr. Res. 2006, 36, 1007–1015. [Google Scholar] [CrossRef]
- Bullard, J.W.; Stutzman, P.E. Analysis of CCRL proficiency cements 151 and 152 using the Virtual Cement and Concrete Testing Laboratory. Cem. Concr. Res. 2006, 36, 1548–1555. [Google Scholar] [CrossRef]
- Valentini, L.; Parisatto, M.; Russo, V.; Ferrari, G.; Bullard, J.W.; Angel, R.J.; Dalconi, M.C.; Artioli, G. Simulation of the hydration kinetics and elastic moduli of cement mortars by microstructural modelling. Cem. Concr. Compos. 2014, 52, 54–63. [Google Scholar] [CrossRef]
- Bentz, D.P. Three-Dimensional Computer Simulation of Portland Cement Hydration and Microstructure Development. J. Am. Ceram. Soc. 1997, 80, 3–21. [Google Scholar] [CrossRef]
- FEI Company. Avizo 3D Software Version 90.1.; FEI Company: Hillsboro, OR, USA, 2015. [Google Scholar]
- FEI Company Avizo Software XLab extension. Available online: https://www.thermofisher.com/au/en/home/industrial/electron-microscopy/electron-microscopy-instruments-workflow-solutions/3d-visualization-analysis-software/avizo-software-extensions.html (accessed on 11 July 2020).
- Van Der Wegen, G.; Bijen, J.; Van Selst, R. Behaviour of concrete affected by sea-water under high pressure. Mater. Struct. 1993, 26, 549–556. [Google Scholar] [CrossRef]
- Tennis, P.D.; Jennings, H.M. A model for two types of calcium silicate hydrate in the microstructure of Portland cement pastes. Cem. Concr. Res. 2000, 30, 855–863. [Google Scholar] [CrossRef]
- Brown, L.; Sanchez, F. Influence of carbon nanofiber clustering on the chemo-mechanical behavior of cement pastes. Cem. Concr. Compos. 2016, 65, 101–109. [Google Scholar] [CrossRef]

| Constituent 1 | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | TiO2 | K2O | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| OPC (%) | 19.90 | 4.70 | 3.38 | 1.30 | 63.93 | 0.17 | 0.245 | 0.446 | 2.54 | 2.97 |
| Parameter | R0 | N10 | R1 | C05 | C10 | C25 | P10 |
|---|---|---|---|---|---|---|---|
| Cement (g) | 741.4 | 741.4 | 741.4 | 741.4 | 741.4 | 741.4 | 741.4 |
| Water (g) | 296.6 | 295.9 | 296.6 | 296.2 | 295.9 | 294.7 | 295.9 |
| CNT dose (wt.% OPC) | - | 0.1 | - | 0.05 | 0.1 | 0.25 | 0.1 |
| Superplasticiser (wt.% OPC) | - | - | 0.275 a | 0.2 | 0.4 | 1.0 | 0.4 |
| Ultrasonication (20 kJ) | N | Y | Y | Y | Y | Y | Y b |
| Composition and Mix Proportions | Mass Fraction (%) | ||
|---|---|---|---|
| Cement 116 | GP Cement | ||
| OPC Principal Phases | C3S | 66.86 | 61.3 |
| C2S | 22.41 | 22.2 | |
| C3A | 6.596 | 4.6 | |
| C4AF | 4.134 | 3.4 | |
| Binder | 71.43 | 71.43 | |
| Water | 28.57 | 28.57 | |
| Specimen | Water Permeability Coefficient × 10–13 m/s |
|---|---|
| Aged 7 Days | |
| R0: Reference (w/out super) | 11.18 (0.09) |
| N10: 0.1% CNTs (no super) | 2.67 (0.02) |
| R1: Reference (w/super) | 2.22 (0.02) |
| C05: 0.05% CNTs | 6.25 (0.05) |
| C10: 0.1% CNTs | 10.03 (0.08) |
| C25: 0.25% CNTs | 7.80 (0.06) |
| P10: 0.1% CNTs (agglomerated) | 10.62 (0.08) |
| ×10–8 m/s | R1 a 7 Days | C10 a 7 Days | C10 a 28 Days | V1 b 7 Days | V1 b 28 Days |
|---|---|---|---|---|---|
| X direction | 3.3 (0.5) | 10.9 (3.3) | 4.9 (0.4) | 17.8 (1.0) | 15.1 (1.1) |
| Y direction | 3.3 (0.8) | 11.7 (3.8) | 4.7 (0.4) | 17.7 (1.7) | 14.9 (1.7) |
| Z direction | 3.0 (0.2) | 10.8 (4.1) | 4.9 (0.7) | 17.7 (0.8) | 14.9 (1.1) |
| Mean | 3.2 | 11.1 | 4.8 | 17.7 | 15.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacLeod, A.J.N.; Gates, W.P.; Collins, F. Durability Characterisation of Portland Cement–Carbon Nanotube Nanocomposites. Materials 2020, 13, 4097. https://doi.org/10.3390/ma13184097
MacLeod AJN, Gates WP, Collins F. Durability Characterisation of Portland Cement–Carbon Nanotube Nanocomposites. Materials. 2020; 13(18):4097. https://doi.org/10.3390/ma13184097
Chicago/Turabian StyleMacLeod, Alastair J. N., Will P. Gates, and Frank Collins. 2020. "Durability Characterisation of Portland Cement–Carbon Nanotube Nanocomposites" Materials 13, no. 18: 4097. https://doi.org/10.3390/ma13184097
APA StyleMacLeod, A. J. N., Gates, W. P., & Collins, F. (2020). Durability Characterisation of Portland Cement–Carbon Nanotube Nanocomposites. Materials, 13(18), 4097. https://doi.org/10.3390/ma13184097






