Mixed Oxide Layered Double Hydroxide Materials: Synthesis, Characterization and Efficient Application for Mn2+ Removal from Synthetic Wastewater
Abstract
1. Introduction
2. Materials and Methods
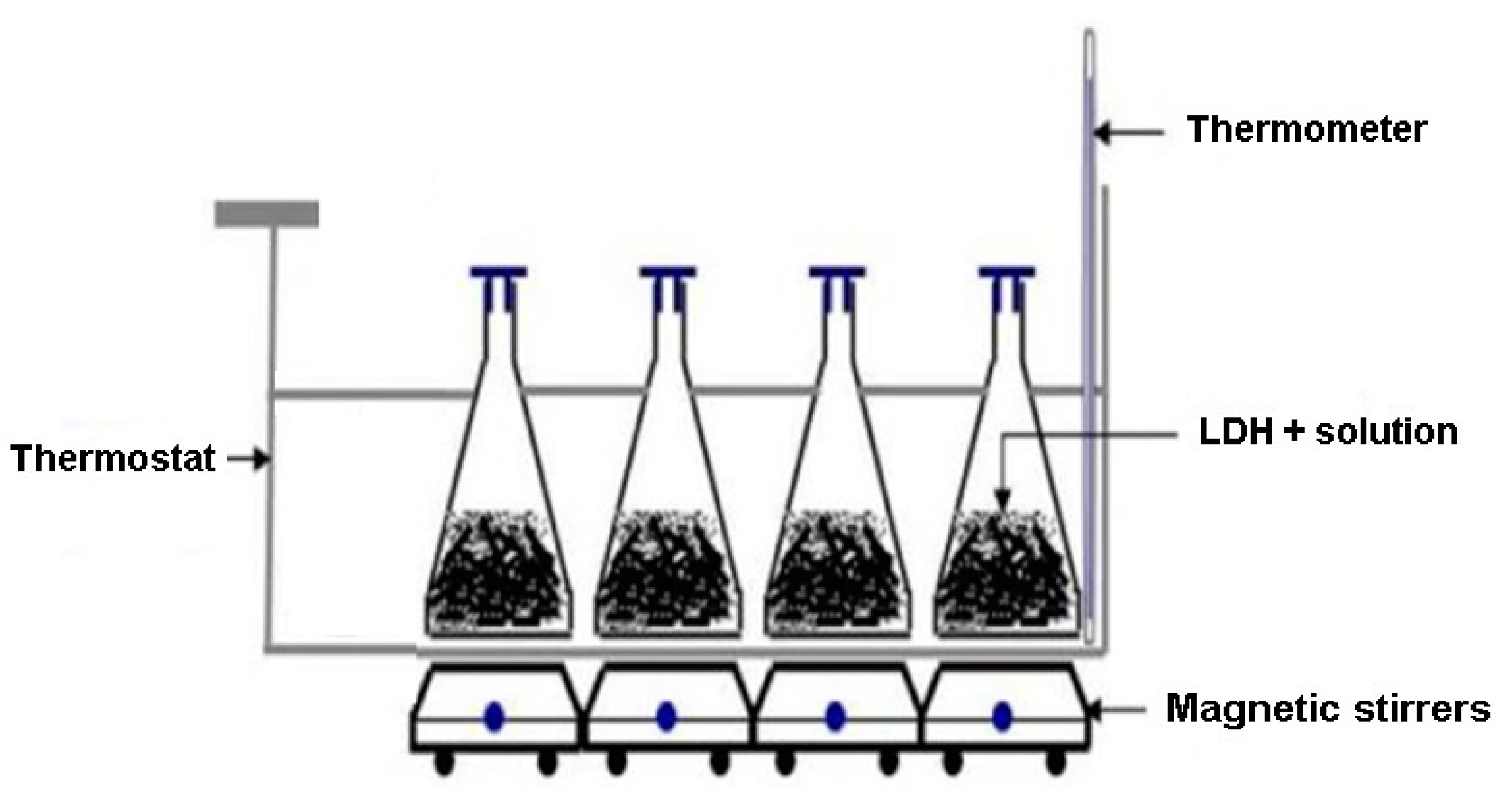
2.1. Synthesis of the Mg-Al- and Mg-Ni-Al LDH Materials
2.2. Characterization of the Synthesized Materials
2.3. Adsorption Study of Mn2+ Used LDHs
2.3.1. Adsorption Isotherms
2.3.2. Kinetics Studies
3. Results and Discussion
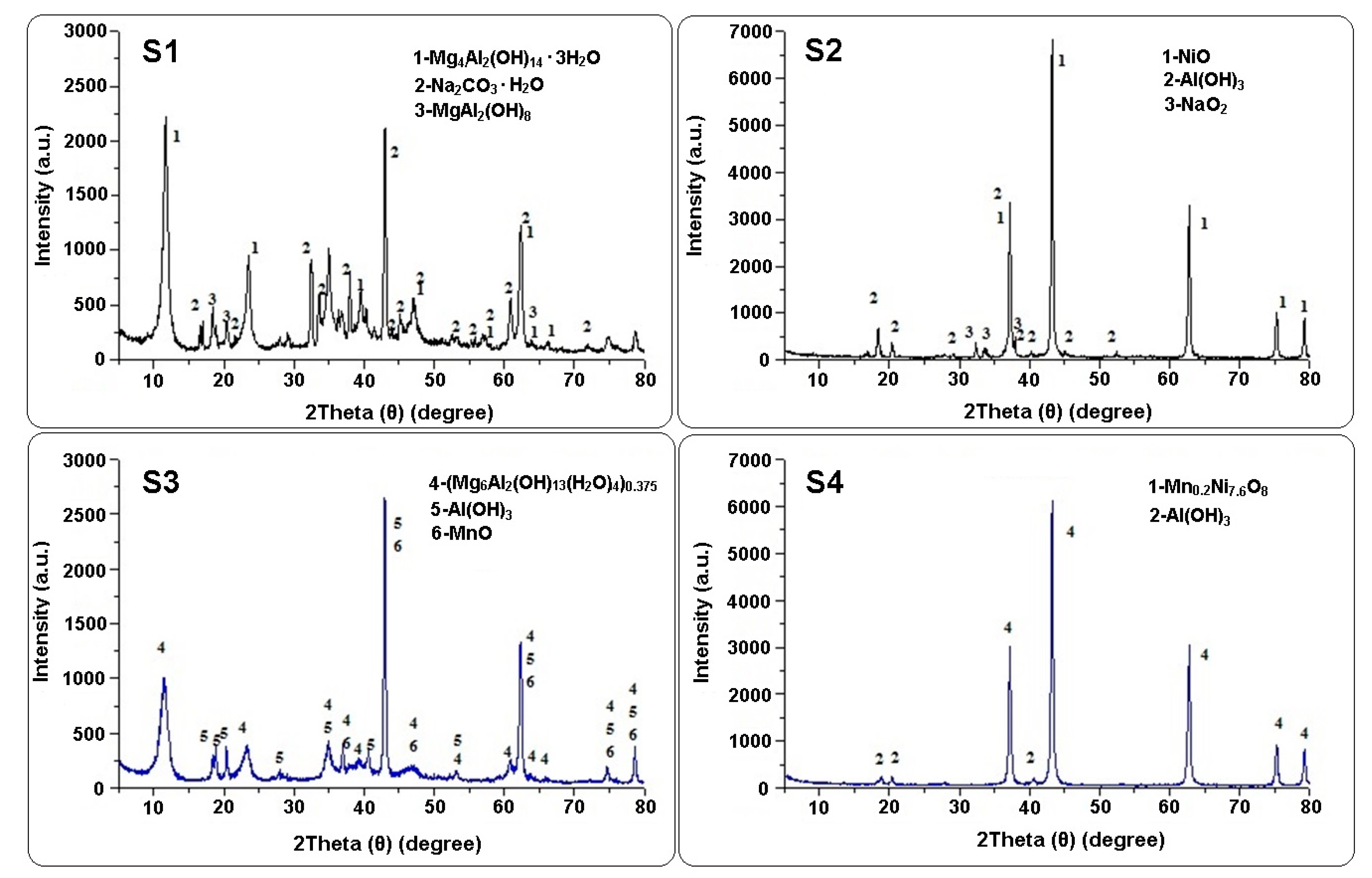
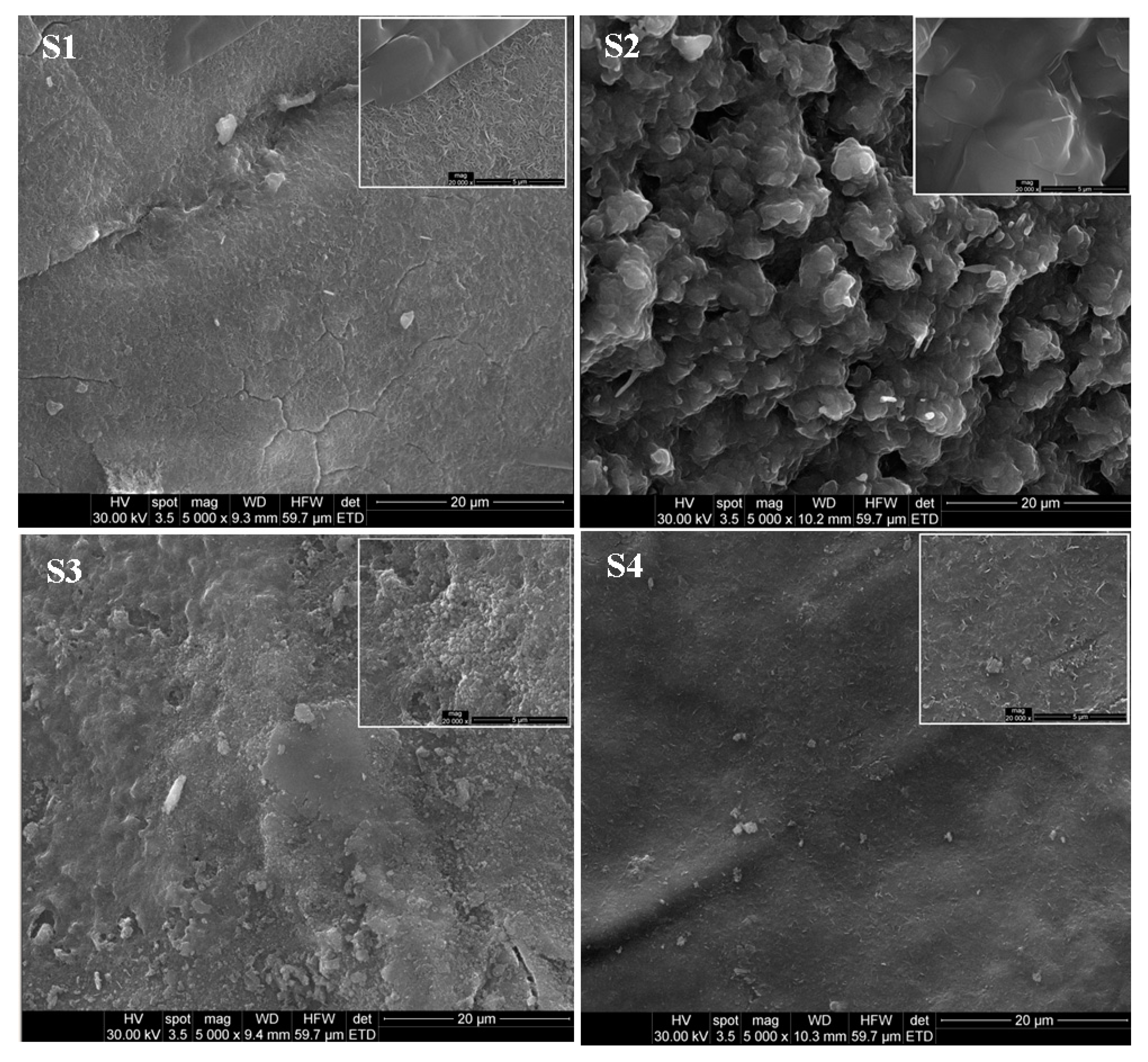
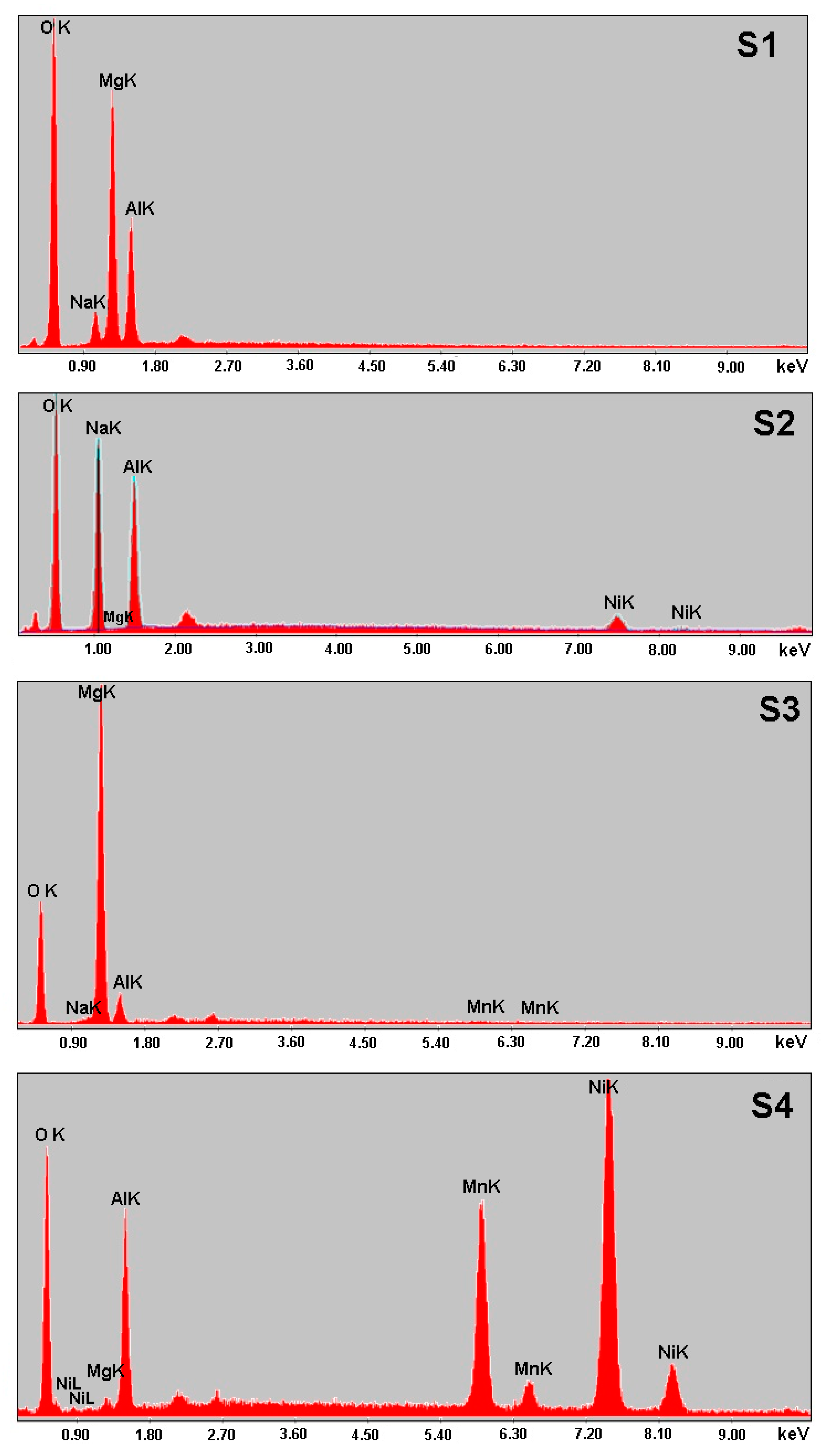
3.1. Characterization of LDHs
3.2. Adsorption Isotherms and Kinetics Evaluation
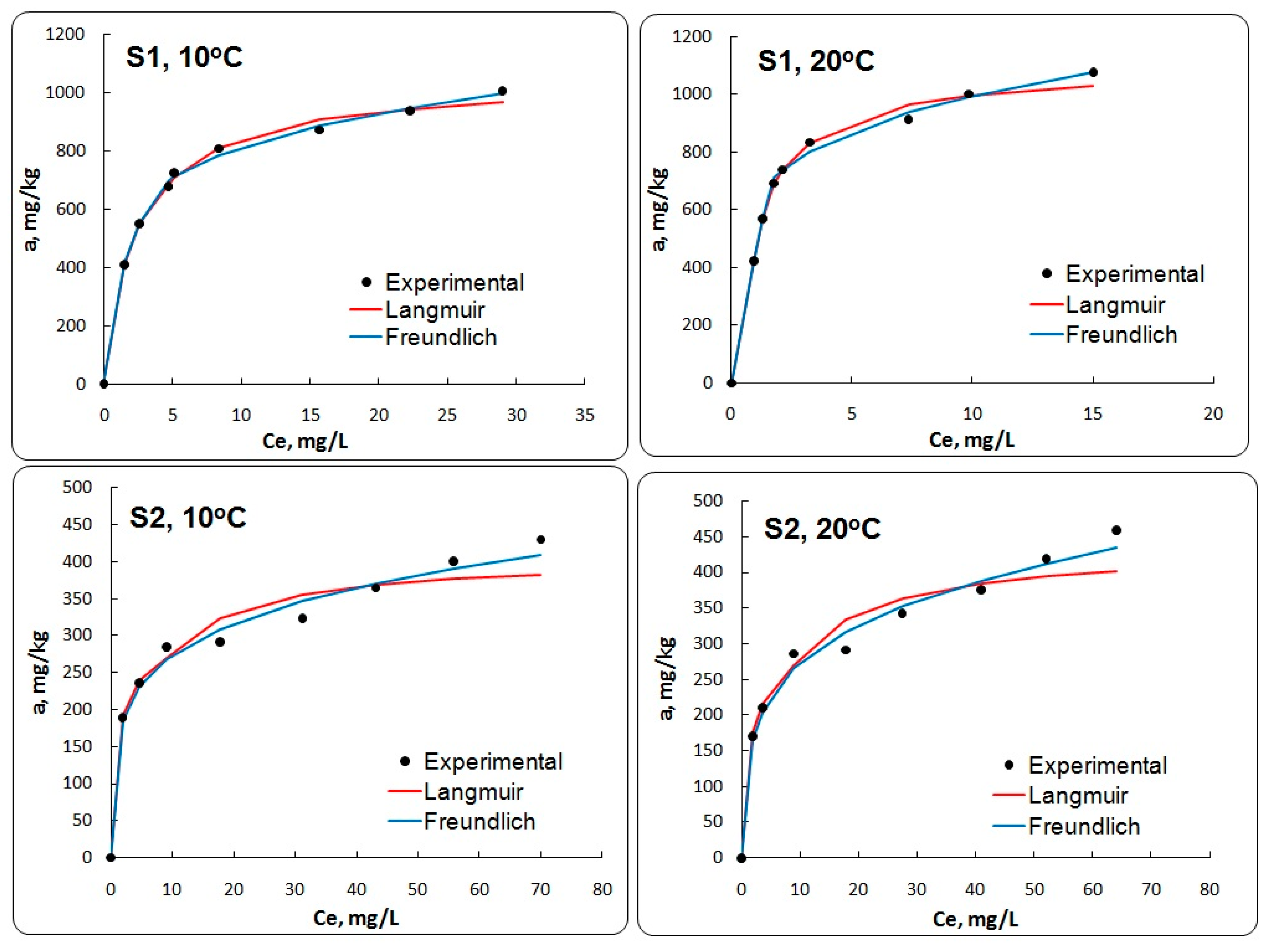
3.2.1. Adsorption Isotherm Evaluation
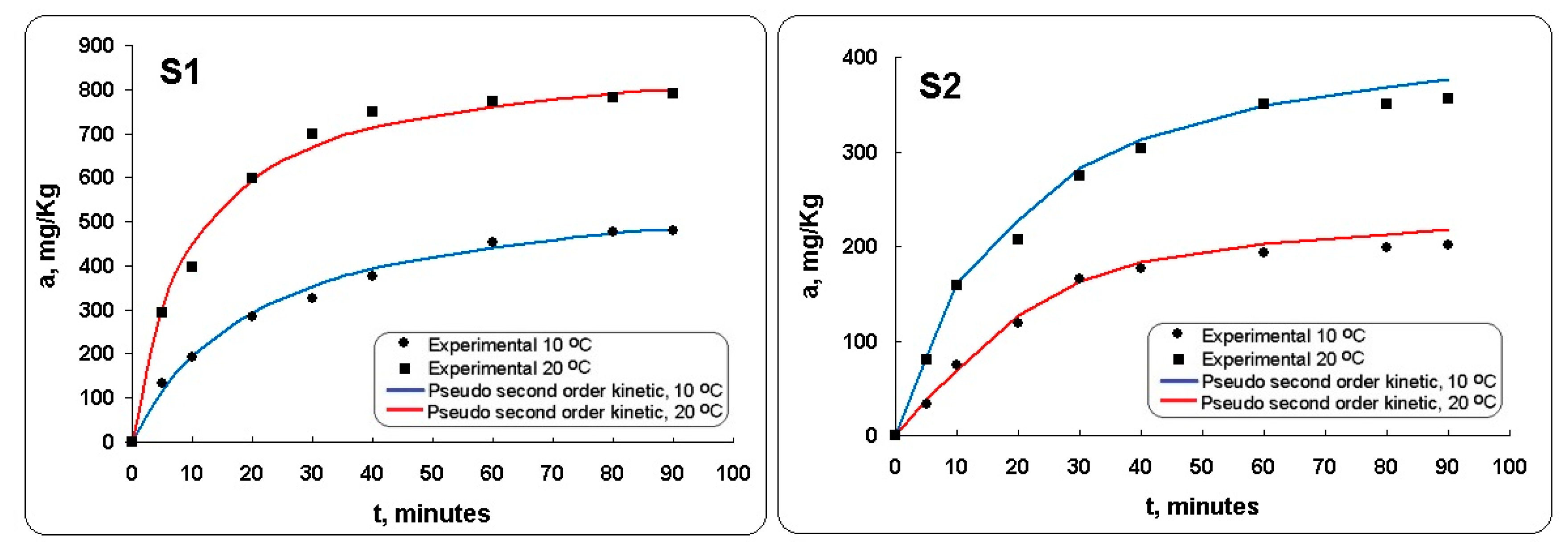
3.2.2. Kinetics Evaluation
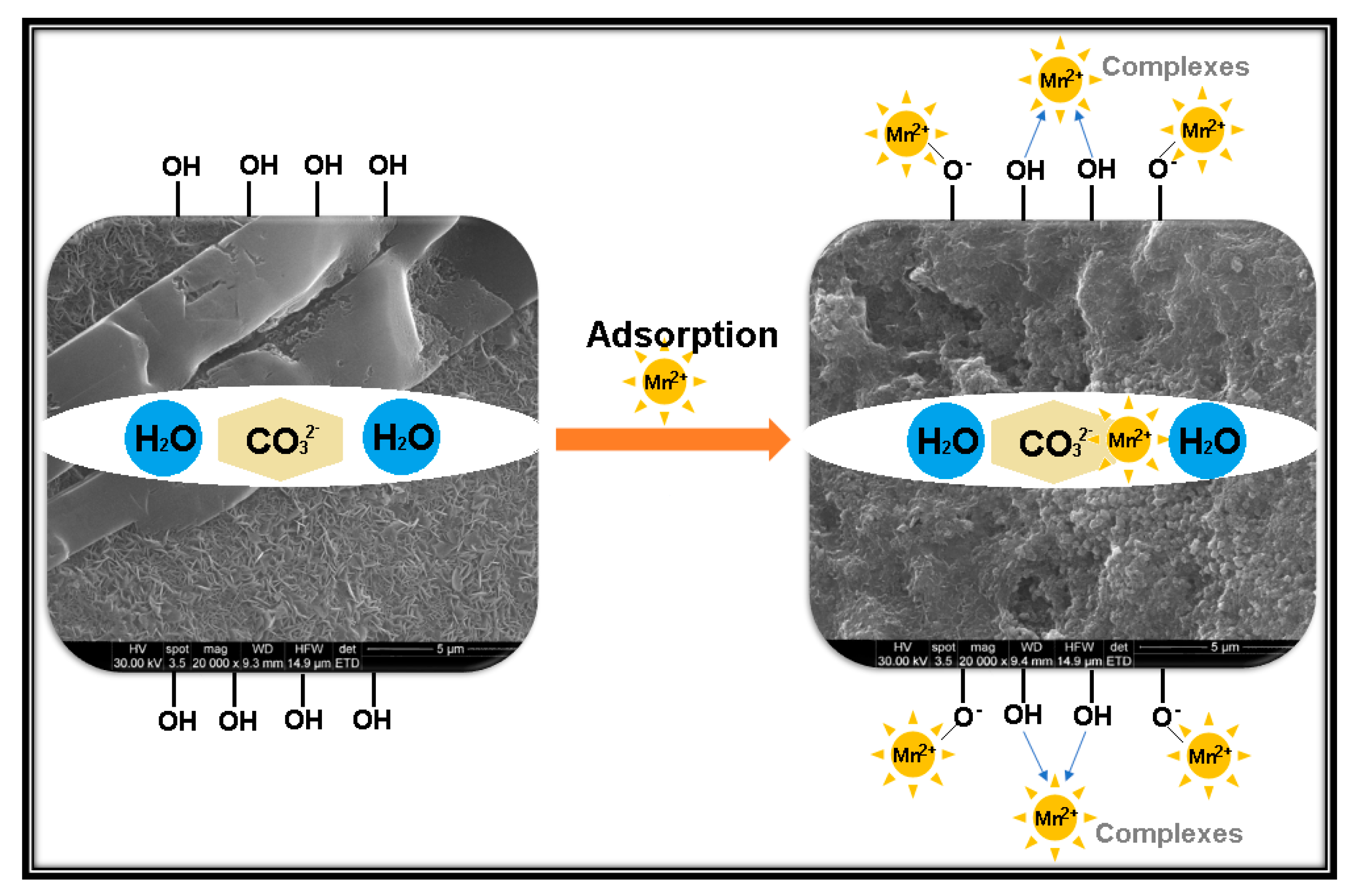
3.3. Adsorption Mechanisms
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, H.H.; Chen, L.J.; Yu, L.; Guo, Z.B.; Shan, C.Q.; Lin, J.Q.; Gu, Y.G.; Yang, Z.B.; Yang, Y.X.; Shao, J.R.; et al. Pollution characteristics and risk assessment of human exposure to oral bioaccessibility of heavy metals via urban street dusts from different functional areas in Chengdu, China. Sci. Total Environ. 2017, 586, 1076–1084. [Google Scholar] [CrossRef]
- Levy, B.S.; Nassetta, W.J. Neurologic Effects of Manganese in Humans: A Review. Int. J. Occup. Environ. Health 2003, 9, 153–163. [Google Scholar] [CrossRef]
- Dávila, O.F.; Torres, J.T.; Valdes, A.F. Effect of Mg Concentration on the Aluminothermic Reduction of Mn2O3 Particles Obtained from Cathodes of Discharged Alkaline Batteries: Mathematical Modeling and Experimental Results. Metals 2019, 9, 49. [Google Scholar] [CrossRef]
- Silva, A.M.; Cunha, E.C.; Silva, F.D.R.; Leão, V.A. Treatment of high-manganese mine water with limestone and sodium carbonate. J. Clean. Prod. 2012, 29, 11–19. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, C.Y.; Pranolo, Y. Investigation of methods for removal and recovery of manganese in hydrometallurgical processes. Hydrometallurgy 2010, 101, 58–63. [Google Scholar] [CrossRef]
- Aziz, H.A.; Smith, P.G. Removal of manganese from water using crushed dolomite filtration technique. Water Res. 1996, 30, 489–492. [Google Scholar] [CrossRef]
- Han, M.; Zhao, Z.; Gao, W.; Cui, F. Study on the factors affecting simultaneous removal of ammonia and manganese by pilot-scale biological aerated filter (BAF) for drinking water pre-treatment. Bioresour. Technol. 2013, 145, 17–24. [Google Scholar] [CrossRef]
- Wimonsong, P.; Nitisoravut, R.; Llorca, J. Application of Fe–Zn–Mg–Al–O hydrotalcites supported Au as active nano-catalyst for fermentative hydrogen production. Chem. Eng. J. 2014, 253, 148–154. [Google Scholar] [CrossRef]
- Lin, J.; Jia, H.; Liang, H.; Chen, S.; Cai, Y.; Qi, J.; Qu, C.; Cao, J.; Fei, W.; Feng, J. Hierarchical CuCo2S4@NiMn-layered double hydroxide core-shell hybrid arrays as electrodes for supercapacitors. Chem. Eng. J. 2018, 336, 562–569. [Google Scholar] [CrossRef]
- Kameda, T.; Konda, E.; Yoshioka, T. Equilibrium and kinetics studies on As(V) and Sb(V) removal by Fe2+-doped Mg–Al layered double hydroxides. J. Environ. Manag. 2015, 151, 303–309. [Google Scholar] [CrossRef]
- Sepehr, M.N.; Al-Musawi, T.J.; Ghahramani, E.; Kazemian, H.; Zarrabi, M. Adsorption performance of magnesium/aluminum layered double hydroxide nanoparticles for metronidazole from aqueous solution. Arab. J. Chem. 2017, 10, 611–623. [Google Scholar] [CrossRef]
- Gilea, D.; Lutic, D.; Carja, G. Heterostructures of silver and zinc based layered double hydroxides for pollutant removal under simulated solar light. Environ. Eng. Manag. J. 2019, 18, 1765–1772. [Google Scholar]
- Gu, P.; Zhang, S.; Li, X.; Wang, X.; Wen, T.; Jehan, R.; Alsaedi, A.; Hayat, T.; Wang, X. Recent advances in layered double hydroxide-based nanomaterials for the removal of radionuclides from aqueous solution. Environ. Pollut. 2018, 240, 493–505. [Google Scholar] [CrossRef]
- Seftel, E.M.; Cool, P.; Lutic, D. Mg–Al and Zn–Fe layered double hydroxides used for organic species storage and controlled release. Mat. Sci. Eng. C 2013, 33, 5071–5078. [Google Scholar] [CrossRef]
- Figueiredo, M.P.; Cunha, V.R.R.; Leroux, F.; Taviot-Gueho, C.; Nakamae, M.N.; Kang, Y.R.; Souza, R.B.; Martins, A.M.C.R.P.F.; Koh, I.H.J.; Constantino, V.R.L. Iron-Based Layered Double Hydroxide Implants: Potential Drug Delivery Carriers with Tissue Biointegration Promotion and Blood Microcirculation Preservation. ACS Omega 2018, 3, 18263–18274. [Google Scholar] [CrossRef]
- Benício, L.P.; Eulálio, D.; Guimarães, L.D.M.; Pinto, F.G.; Marciano da Costa, L.; Tronto, J. Layered Double Hydroxides as Hosting Matrices for Storage and Slow Release of Phosphate Analyzed by Stirred-Flow Method. Mat. Res. 2018, 21, 1–13. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Xu, S.; Shao, M.; Zhang, Q.; Wei, F.; Ma, J.; Wei, M.; Evans, D.G.; Duan, X. Hierarchical NiMn Layered Double Hydroxide/Carbon Nanotubes Architecture with Superb Energy Density for Flexible Supercapacitors. Adv. Funct. Mater. 2014, 20, 2921–3099. [Google Scholar] [CrossRef]
- Gutierrez Moreno, J.J.; Pan, K.; Wang, Y.; Li, W. A computational study of APTES surface functionalization of diatom-like amorphous SiO2 surfaces for heavy metal adsorption. Langmuir 2020, 36, 5680–5689. [Google Scholar] [CrossRef]
- Hernández-Ávila, J.; Salinas-Rodríguez, E.; Cerecedo-Sáenz, E.; Reyes-Valderrama, M.; Arenas-Flores, A.; Román-Gutiérrez, A.; Rodríguez-Lugo, V. Diatoms and Their Capability for Heavy Metal Removal by Cationic Exchange. Metals 2017, 7, 169. [Google Scholar] [CrossRef]
- Torres, D.; Ayala, L.; Jeldres, R.I.; Cerecedo-Sáenz, E.; Salinas-Rodríguez, E.; Robles, P.; Toro, N. Leaching Chalcopyrite with High MnO2 and Chloride Concentrations. Metals 2020, 10, 107. [Google Scholar] [CrossRef]
- Saeki, K. Adsorption of Fe2+ and Mn2+ on silica, gibbsite, and humic acids. Soil Sci. 2004, 169, 832–840. [Google Scholar] [CrossRef]
- Bakr, A.A.; Sayed, N.A.; Salama, T.M.; Ali, I.O.; Abdel Gayed, R.R.; Negm, N.A. Kinetics and thermodynamics of Mn(II) removal from aqueous solutions onto Mg-Zn-Al LDH/montmorillonite nanocomposite. Egypt. J. Pet. 2018, 27, 1215–1220. [Google Scholar] [CrossRef]
- Bakr, A.A.; Sayed, N.A.; Salama, T.M.; Ali, I.O.; Abdel Gayed, R.R.; Negm, N.A. Potential of Mg–Zn–Al layered double hydroxide (LDH)/montmorillonite nanocomposite in remediation of wastewater containing manganese ions. Res. Chem. Intermed. 2018, 44, 389–405. [Google Scholar] [CrossRef]
- Khokhar, I.; Ahmed, M.; Tariq, M. Biosorption of Chromium by Baker’s Yeast: Freundlich and Langmuir Adsorption Models; LAP LAMBERT Academic Publishing: Norwich, UK, 2011; p. 96. ISBN 10 9783847328568. [Google Scholar]
- Teixeira, M.A.; Mageste, A.B.; Dias, A.; Virtuoso, L.S.; Siqueira, K.P.F. Layered double hydroxides for remediation of industrial wastewater containing manganese and fluoride. J. Clean. Prod. 2018, 171, 275–284. [Google Scholar] [CrossRef]
- Yang, K.; Yan, L.; Yang, Y.; Yu, S.; Shan, R.; Yu, H.; Zhu, B.B.D. Adsorptive removal of phosphate by Mg–Al and Zn–Al layered double hydroxides: Kinetics, isotherms and mechanisms. Sep. Purif. Technol. 2014, 124, 36–42. [Google Scholar] [CrossRef]
- Modrogan, C.; Orbuleț, O.D.; Orbeci, C.; Bobiricǎ, C.; Dancila, A.M. Removal of manganese from groundwater by adsorption on gellan gum/Fe3O4 composite. In Proceedings of the 18th International Multidisciplinary Scientific Geoconferences SGEM 2018, Ecology, Economics, Eduction and Legislation Conference Proceedings, Albena, Bulgaria, 2–8 July 2018. [Google Scholar]
- Pérez-Ramírez, J.; Mul, G.; Kapteijn, F.; Moulijn, J.A. In situ investigation of the thermal decomposition of Co–Al hydrotalcite in different atmospheres. J. Mat. Chem. 2001, 11, 821–830. [Google Scholar] [CrossRef]
- Shi, M.; Zhao, Z.; Song, Y.; Xu, M.; Li, J.; Yao, L. A novel heat-treated humic acid/MgAl-layered double hydroxide composite for efficient removal of cadmium: Fabrication, performance and mechanisms. Appl. Clay Sci. 2020, 187, 105482. [Google Scholar] [CrossRef]
- Fu, F.; Ma, J.; Xie, L.; Tang, B.; Han, W.; Lin, S. Chromium removal using resin supported nanoscale zero-valent iron. J. Environ. Manage. 2013, 128, 822–827. [Google Scholar] [CrossRef]
- Yavuz, O.; Altunkaynak, Y.; Guze, F. Removal of copper, nickel, cobalt and manganese from aqueous solution by kaolinite. Water. Res. 2003, 37, 948–952. [Google Scholar] [CrossRef]
- Chojnacka, K. Bioaccumulation of Cr (III) ions by blue-green alga Spirulina sp. Part I. A comparison with biosorption. Am. J. Agric. Biol. Sci. 2007, 2, 218–223. [Google Scholar] [CrossRef]
- Emmanuel, K.A.; Rao, A.V. Comparative Study on Adsorption of Mn(II) from Aqueous Solutions on Various Activated Carbons. E-J. Chem. 2009, 6, 693–704. [Google Scholar] [CrossRef]
- Li, S.; Guo, Y.; Xiao, M.; Zhang, T.; Yao, S.; Zang, S.; Fan, H.; Shen, Y.; Zhang, Z.; Li, W. Enhanced arsenate removal from aqueous solution by Mn-doped MgAl-layered double hydroxides. Environ. Sci. Pollut. Res. Int. 2019, 26, 12014–12024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kano, N.; Mishima, K.; Okawa, H. Adsorption and Desorption Mechanisms of Rare Earth Elements (REEs) by Layered Double Hydroxide (LDH) Modified with Chelating Agents. Appl. Sci. 2019, 9, 4805. [Google Scholar] [CrossRef]
- Sadeek, S.A.; Negm, N.A.; Hefni, H.H.H.; Abdel Wahab, M.M. Metal adsorption by agricultural biosorbents: Adsorption isotherm, kinetic and biosorbents chemical structures. Int. J. Biol. Macromol. 2015, 81, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Soliman, E.R.; Kotp, Y.H.; Souaya, E.R.; Guindy, K.A.; Ibrahim, R.G.M. Development the sorption behavior of nanocomposite Mg/Al LDH by chelating with different monomers. Compos. Part B 2019, 175, 107131. [Google Scholar] [CrossRef]

| Samples | Notation |
|---|---|
| Mg-Al LDH blank | S1 |
| Mg-Al-Ni LDH blank | S2 |
| Mg-Al LDH—Mn2+ | S3 |
| Mg-Al-Ni LDH—Mn2+ | S4 |
| Sample | Phase, System, Lattice Parameters | Pos. (2θ°) | d [Å] | Phase, System, Lattice Parameters | Pos. (2θ°) | d [Å] | Phase, System, Lattice Parameters | Pos. (2θ°) | d [Å] |
|---|---|---|---|---|---|---|---|---|---|
| S1 | Mg4Al2(OH)14 3H2O | 11.66 | 7.581 | Na2CO3H2O | 16.58 | 5.340 | MgAl2(OH)18 | 18.36 | 4.828 |
| Rhombohedral | |||||||||
| a (Å) = 3.0380 | 23.42 | 3.794 | Orthorhombic | 21.73 | 4.083 | Monoclinic | 20.39 | 4.350 | |
| b (Å) = 3.08380 | 39.48 | 2.280 | a (Å) = 10.7000 | 32.35 | 2.764 | a (Å) = 5.9900 | 36.84 | 2.437 | |
| b (Å) = 3.08380 | 46.97 | 1.932 | b (Å) = 6.4580 | 32.53 | 2.749 | b (Å) = 7.9700 | 63.88 | 1.456 | |
| c (Å) = 22.6200 | 62.32 | 1.488 | c (Å) = 5.2540 | 37.94 | 2.369 | c (Å) = 4.3700 | |||
| α (°) = 90 | α (°) = 90 | 42.95 | 2.104 | α (°) = 90 | |||||
| β (°) = 90 | β (°) = 90 | 60.87 | 1.520 | β (°) = 91.73 | |||||
| γ (°) = 120 | γ (°) = 90 | 62.32 | 1.488 | γ (°) = 90 | |||||
| S2 | NiO | 37.23 | 2.412 | Al(OH)3 | 18.41 | 4.814 | NaO2 | 32.42 | 2.758 |
| Cubic | Monoclinic | Orthorhombic | |||||||
| a (Å) = 4.1800 | a (Å) = 8.6410 | a (Å) = 4.3350 | |||||||
| b (Å) = 4.1800 | b (Å) = 5.0700 | b (Å) = 5.5370 | |||||||
| c (Å) = 4.2110 | 43.25 | 2.089 | c (Å) = 9.7190 | 20.42 | 4.345 | c (Å) = 3.3630 | 33.39 | 2.683 | |
| α (°) = 90 | 62.79 | 1.478 | α (°) = 90 | 37.23 | 2.412 | α (°) = 90 | 37.29 | 2.412 | |
| β (°) = 90 | 75.28 | 1.261 | β (°) = 94.58 | 45.17 | 2.005 | β (°) = 90 | |||
| γ (°) = 90 | 79.25 | 1.207 | γ (°) = 90 | γ (°) = 90 | |||||
| S3 | ((Mg6Al2)(OH) | 11.42 | 7.740 | Al(OH)3 | 18.35 | 4.830 | MnO | 36.96 | 2.429 |
| 18(H2O)4)0.375 | |||||||||
| Rhombohedral | Hexagonal | Cubic | |||||||
| a (Å) = 3.0463 | a (Å) = 5.0470 | a (Å) = 4.2110 | |||||||
| b (Å) = 3.0463 | b (Å) = 5.0470 | b (Å) = 4.2110 | |||||||
| c (Å) = 22.9300 | 23.15 | 3.838 | c (Å) = 4.7300 | 18.81 | 4.713 | c (Å) = 4.2110 | 42.95 | 2.103 | |
| α (°) = 90 | 34.91 | 2.567 | α (°) = 90 | 20.38 | 4.352 | α (°) = 90 | 46.59 | 1.947 | |
| β (°) = 90 | 46.59 | 1.947 | β (°) = 90 | 42.95 | 2.103 | β (°) = 90 | 62.32 | 1.488 | |
| γ (°) = 120 | 60.89 | 1.520 | γ (°) = 120 | 62.32 | 1.488 | γ (°) = 90 | |||
| S4 | Mn0.2Ni7.6O8 | 37.23 | 2.413 | β-Al(OH)3 | 18.70 | 4.740 | |||
| Cubic | Monoclinic | ||||||||
| a (Å) = 8.3495 | a (Å) = 5.0100 | ||||||||
| b (Å) = 8.3495 | b (Å) = 8.6800 | ||||||||
| c (Å) = 8.3495 | c (Å) = 4.7600 | ||||||||
| α (°) = 90 | 43.25 | 2.098 | α (°) = 90 | 20.40 | 4.348 | ||||
| β (°) = 90 | 62.79 | 1.478 | β (°) = 90 | 40.62 | 2.219 | ||||
| γ (°) = 90 | 75.28 | 1.261 | γ (°) = 90 | ||||||
| Temperature (°C) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| KL (L/mg) | amax (mg/kg) | R2 | KF ((mg/g)/(mg/L)n | m | R2 | |
| Sample S1 | ||||||
| 10 | 0.4061 ± 0.004 | 1048.320 ± 0.277 | 0.9759 | 0.5178 ± 0.237 | 0.1948 ± 0.004 | 0.9782 |
| 20 | 0.9529 ± 0.007 | 1100.607 ± 0.656 | 0.9586 | 0.6356 ± 0.311 | 0.1946 ± 0.004 | 0.9808 |
| Sample S2 | ||||||
| 10 | 0.2178 ± 0.003 | 407.537 ± 0.189 | 0.7282 | 0.1691 ± 0.614 | 0.2083 ± 0.005 | 0.9096 |
| 20 | 0.1819 ± 0.004 | 436.972 ± 0.260 | 0.7568 | 0.1556 ± 0.338 | 0.2468 ± 0.006 | 0.9206 |
| Kinetic Model | Parameters | Sample S1 | Sample S2 | Sample S1 | Sample S2 |
|---|---|---|---|---|---|
| 10 °C | 10 °C | 20 °C | 20 °C | ||
| Experimental | qe,exp. | 483.33 | 205.50 | 804.37 | 350.14 |
| Pseudo-first-order | k1, min−1 | 0.1320 | 0.1550 | 0.1430 | 0.1470 |
| R2 | 0.8832 | 0.8119 | 0.8973 | 0.8580 | |
| Pseudo-second-order | qe,calc. | 500.00 | 314.40 | 810.00 | 395.20 |
| k2, g mg−1 min−1 | 0.0040 | 0.0045 | 0.0017 | 0.0021 | |
| R2 | 0.9917 | 0.9671 | 0.9958 | 0.9123 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modrogan, C.; Cǎprǎrescu, S.; Dǎncilǎ, A.M.; Orbuleț, O.D.; Vasile, E.; Purcar, V. Mixed Oxide Layered Double Hydroxide Materials: Synthesis, Characterization and Efficient Application for Mn2+ Removal from Synthetic Wastewater. Materials 2020, 13, 4089. https://doi.org/10.3390/ma13184089
Modrogan C, Cǎprǎrescu S, Dǎncilǎ AM, Orbuleț OD, Vasile E, Purcar V. Mixed Oxide Layered Double Hydroxide Materials: Synthesis, Characterization and Efficient Application for Mn2+ Removal from Synthetic Wastewater. Materials. 2020; 13(18):4089. https://doi.org/10.3390/ma13184089
Chicago/Turabian StyleModrogan, Cristina, Simona Cǎprǎrescu, Annette Madelene Dǎncilǎ, Oanamari Daniela Orbuleț, Eugeniu Vasile, and Violeta Purcar. 2020. "Mixed Oxide Layered Double Hydroxide Materials: Synthesis, Characterization and Efficient Application for Mn2+ Removal from Synthetic Wastewater" Materials 13, no. 18: 4089. https://doi.org/10.3390/ma13184089
APA StyleModrogan, C., Cǎprǎrescu, S., Dǎncilǎ, A. M., Orbuleț, O. D., Vasile, E., & Purcar, V. (2020). Mixed Oxide Layered Double Hydroxide Materials: Synthesis, Characterization and Efficient Application for Mn2+ Removal from Synthetic Wastewater. Materials, 13(18), 4089. https://doi.org/10.3390/ma13184089









