Novel Hybrid Biomass Anti-Aging Filler for Styrene-Butadiene Rubber Composites with Antioxidative and Reinforcing Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials
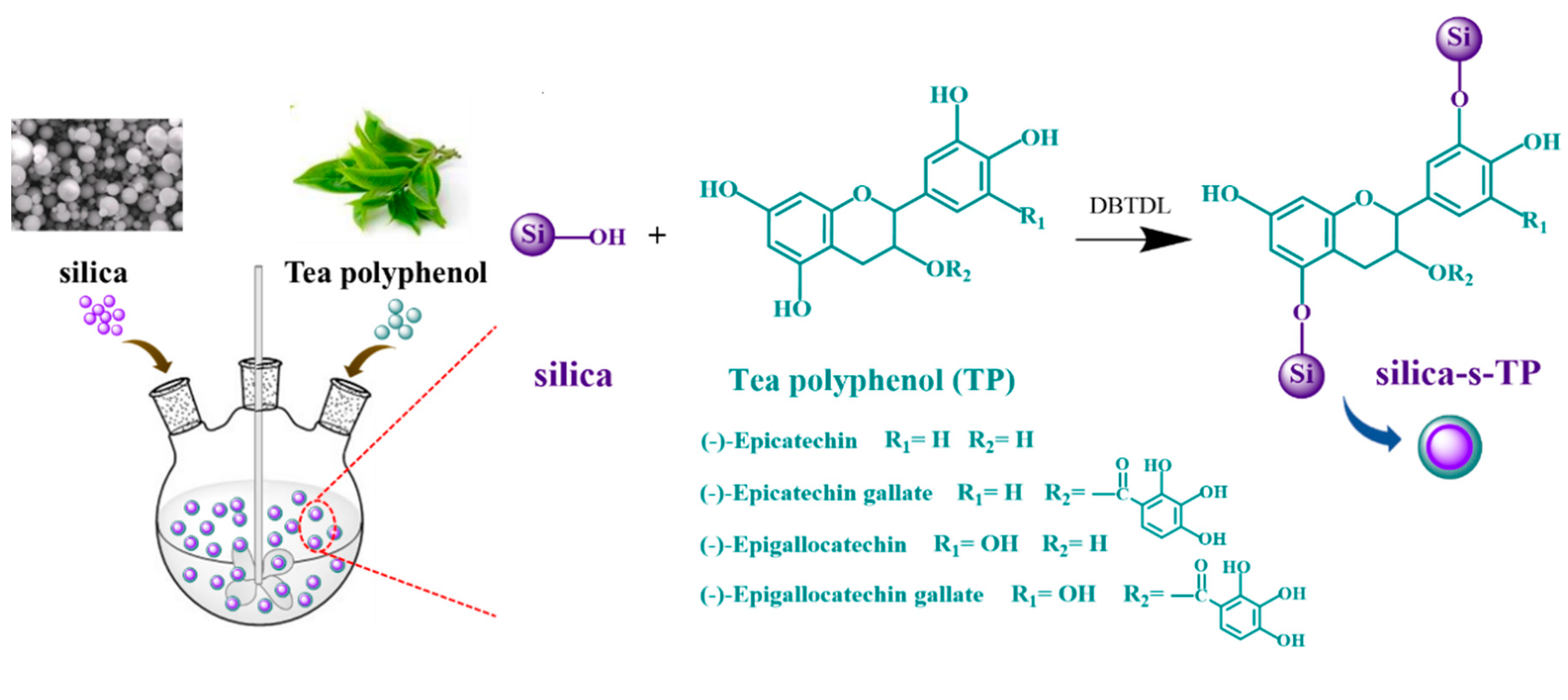
2.2. Preparation of an Organic-Inorganic Hybrid Biomass Anti-Aging Filler
2.3. Preparation of SBR/Silica-s-TP Composites
2.4. Characterization
3. Results and Discussion
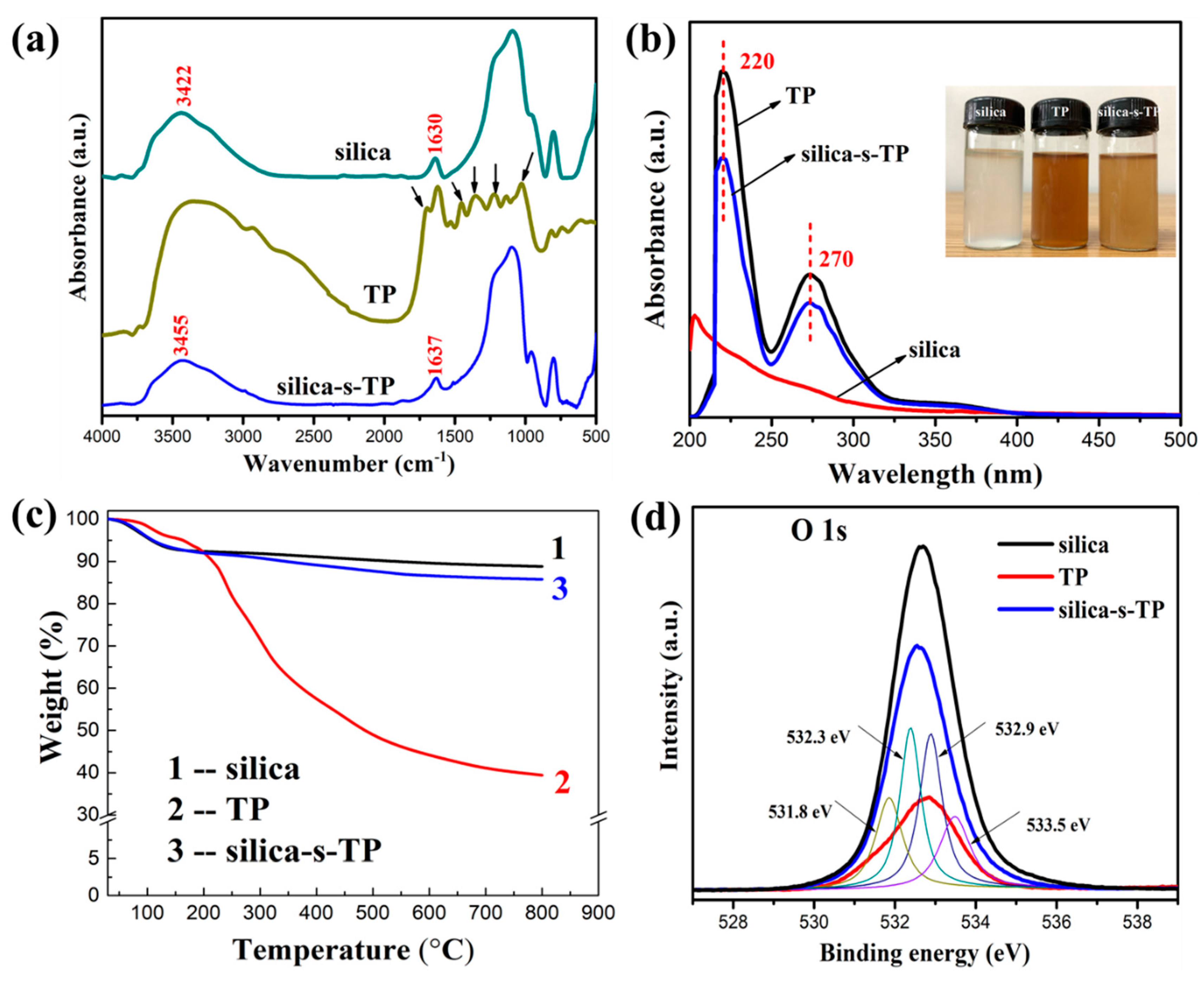
3.1. Characterization of Silica-s-TP
- LE: Loading Efficiency;
- WC: the weight of silica at 800 °C;
- WA-C: the weight of TP molecule loaded on silica (silica-s-TP) at 800 °C;
- WA: the weight of TP molecule at 800 °C.
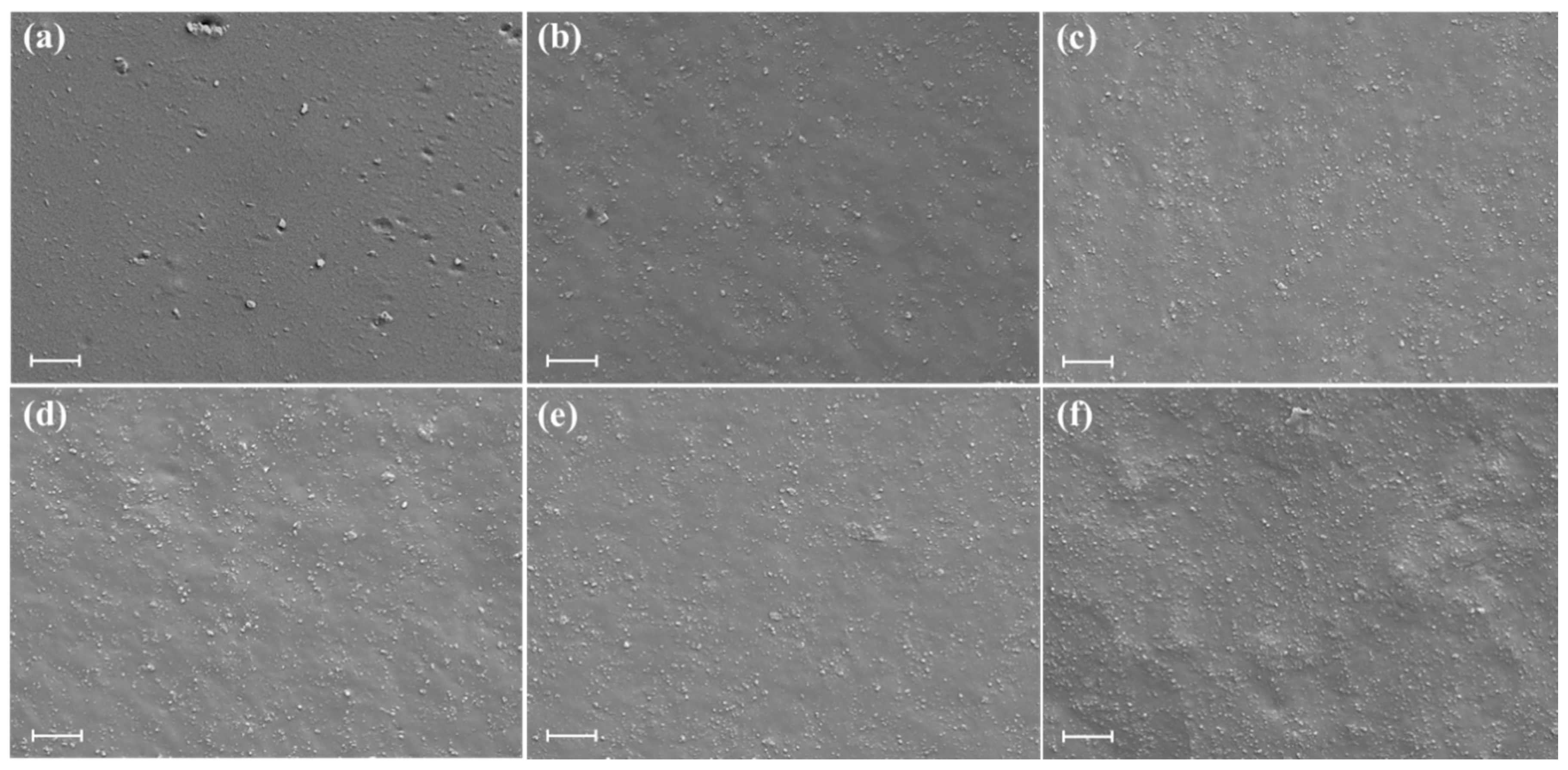
3.2. Morphology of SBR Composites
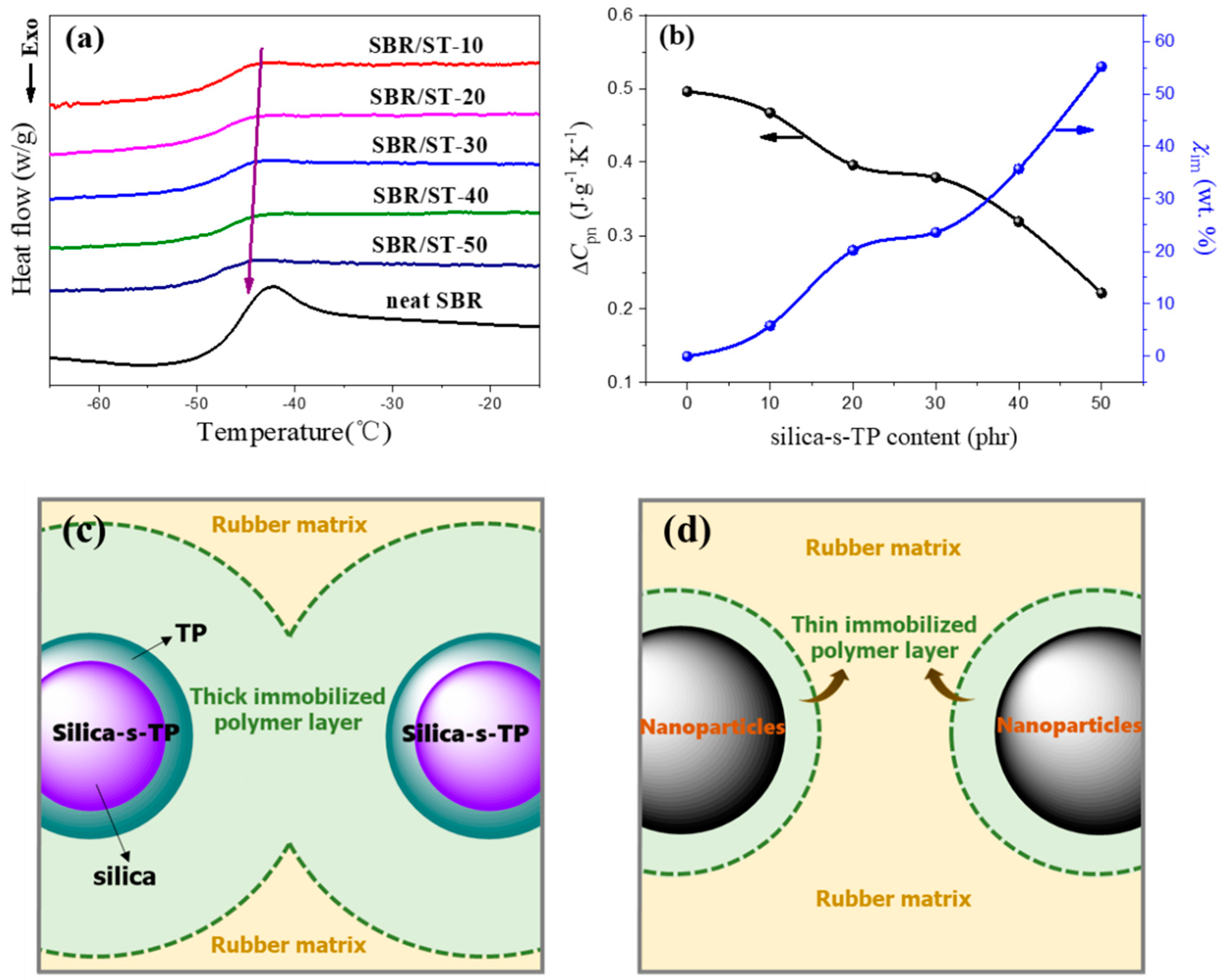
3.3. Interfacial Interaction between Biomass Anti-Aging Filler and Rubber
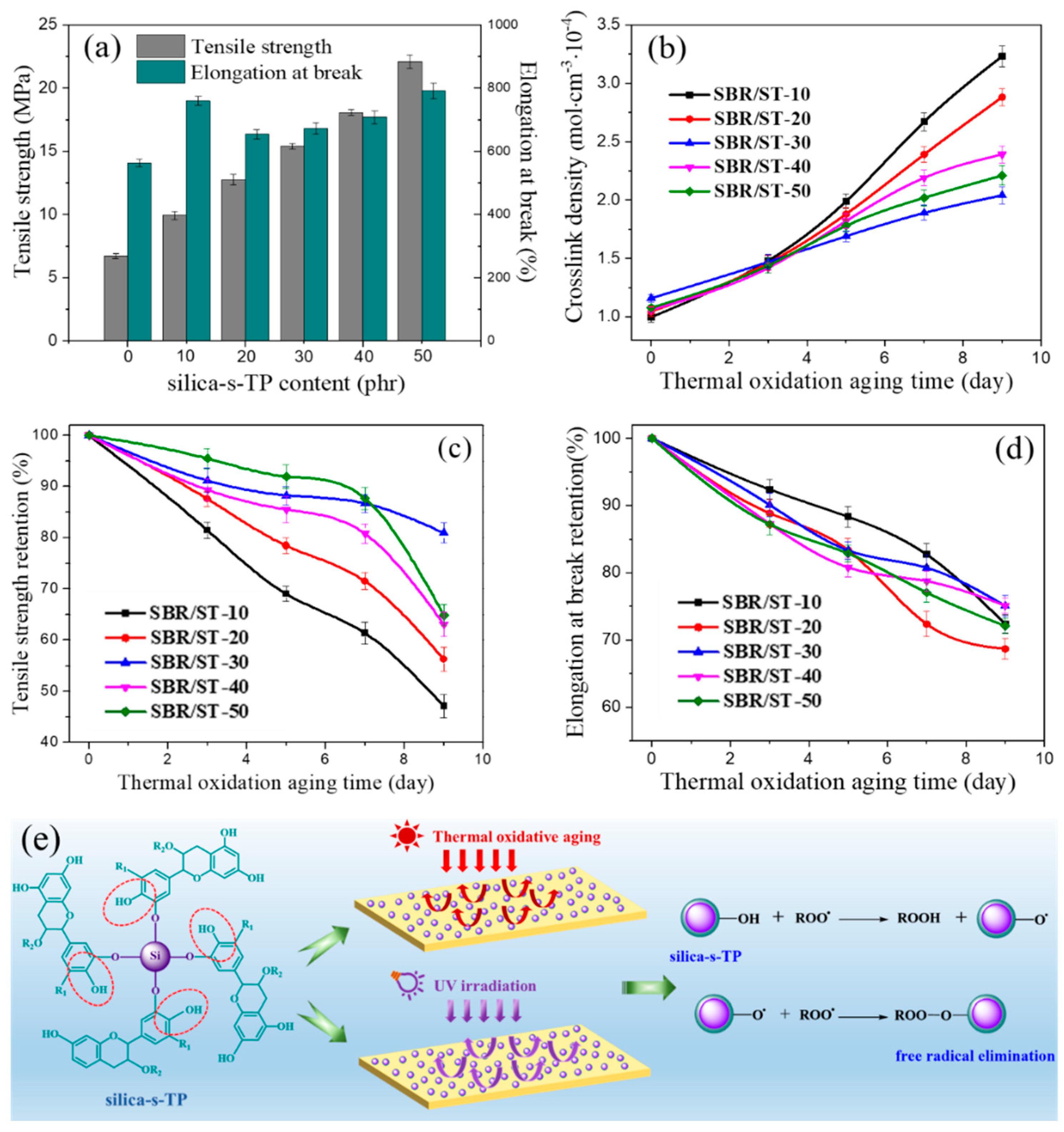
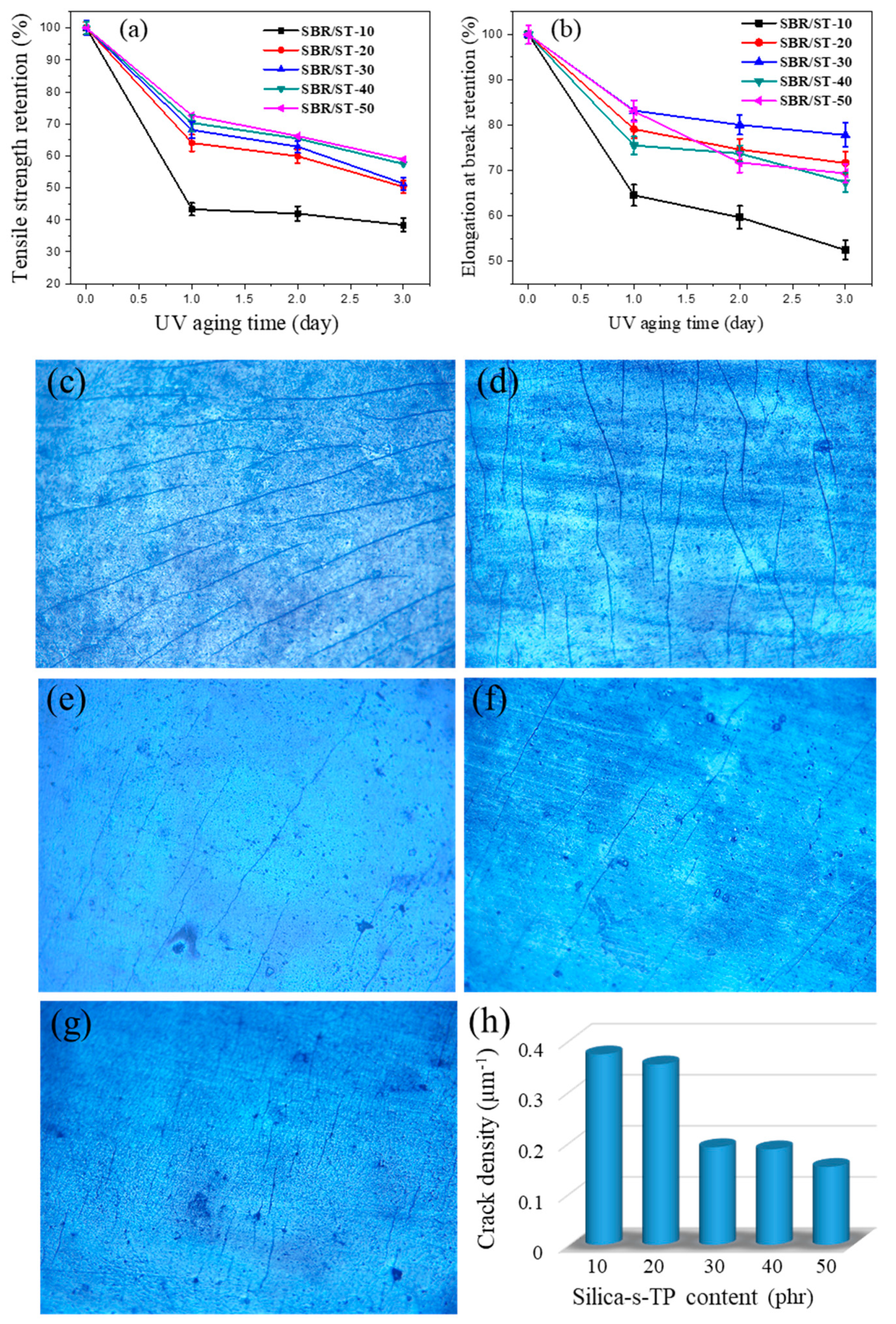
3.4. Aging Resistance of SBR Composites Filled with Anti-Aging Filler
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garbarczyk, M.; Kuhn, W.; Klinowski, J.; Jurga, S. Characterization of aged nitrile rubber elastomers by NMR spectroscopy and microimaging. Polymer 2002, 3169–3172. [Google Scholar] [CrossRef]
- Mitani, M.M.; Keller, A.A.; Bunton, C.A.; Rinker, R.G.; Sandall, O.C. Kinetics and products of reactions of MTBE with ozone and ozone/hydrogen peroxide in water. J. Hazard. Mater. 2002, 89, 197–212. [Google Scholar] [CrossRef]
- Mathew, N.M.; De, S.K. Thermo-oxidative ageing and its effect on the network structure and fracture mode of natural rubber vulcanizates. Polymer 1983, 24, 1042–1054. [Google Scholar] [CrossRef]
- Munteanu, S.B.; Brebu, M.; Vasile, C. Thermal and thermo-oxidative behavior of butadiene-styrene copolymers with different architectures. Polym. Degrad. Stab. 2005, 89, 501–512. [Google Scholar] [CrossRef]
- Chen, L.J.; Guo, X.H.; Luo, Y.F.; Jia, Z.X.; Chen, Y.J.; Jia, D.M. Inorganic and organic hybrid nanoparticles as multifunctional crosslinkers for rubber vulcanization with high-filler rubber interaction. Polymers 2018, 10, 1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagle, D.J.; Celina, M.; Rintoul, L.; Fredericks, P.M. Infrared microspectroscopic study of the thermo-oxidative degradation of hydroxy-terminated polybutadiene/isophorone diisocyanate polyurethane rubber. Polym. Degrad. Stab. 2007, 92, 1446–1454. [Google Scholar] [CrossRef] [Green Version]
- Abdelwahab, N.A.; El-Nashar, D.E.; El-Ghaffar, M.A. Polyfuran, polythiophene and their blend as novel antioxidants for styrene-butadiene rubber vulcanizates. Mater. Des. 2011, 32, 238–245. [Google Scholar] [CrossRef]
- Luo, K.Q.; You, G.H.; Zhao, X.Y.; Lu, L.; Wang, W.C.; Wu, S.Z. Synergistic effects of antioxidant and silica on enhancing thermo-oxidative resistance of natural rubber: Insights from experiments and molecular simulations. Mater. Des. 2019, 107944. [Google Scholar] [CrossRef]
- Yamano, T.; Shimizu, M. Skin sensitization potency and cross-reactivity of p-phenylenediamine and its derivatives evaluated by non-radioactive murine local lymph node assay and guinea-pig maximization test. Contact Dermat. 2010, 60, 193–198. [Google Scholar] [CrossRef]
- Lu, L.; Luo, K.Q.; Yang, W.; Zhang, S.D.; Wang, W.C.; Xu, H.Y.; Wu, S.Z. Insight into the anti-aging mechanisms of naturalphenolic antioxidants in natural rubber compositesusing a screening strategy based on molecularsimulation. RSC Adv. 2020, 10, 21318. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.Q.; Lai, X.J.; Wu, W.J.; Zeng, X.R. Hindered phenol functionalized graphene oxide for natural rubber. Mater. Lett. 2018, 210, 239–242. [Google Scholar] [CrossRef]
- Zhou, J.J.; Wei, L.Y.; Wei, H.T.; Zheng, J.; Huang, G.S. The synthesis of graphene-based antioxidants to promote anti-thermal properties of styrene-butadiene rubber. RSC Adv. 2017, 7, 53596. [Google Scholar] [CrossRef] [Green Version]
- Nie, J.D.; Huang, X.H.; Xu, C.H.; Ding, J.P.; Chen, Y.K. Antioxidant effects on curing/processing and thermo-oxidative aging of filled nitrile rubber. Mater. Chem. Phys. 2020, 253, 123403. [Google Scholar] [CrossRef]
- Bansal, S.; Syan, N.; Mathur, P.; Choudhary, S. Pharmacological profile of green tea and its polyphenols: A review. Med. Chem. Res. 2012, 21, 3347–3360. [Google Scholar] [CrossRef]
- Pasrija, D.; Ezhilarasi, P.N.; Indrani, D.; Anandharamakrishnan, C. Microencapsulation of green tea polyphenols and its effect on incorporated bread quality. LWT Food. Sci. Technol. 2015, 64, 289–296. [Google Scholar] [CrossRef]
- Chen, W.R.; Zhang, Z.Z.; Shen, Y.W.; Duan, X.W.; Jiang, Y.M. Effect of tea polyphenols on lipid peroxidation and antioxidant activity of litchi (Litchi chinensis Sonn.) fruit during cold storage. Molecules 2014, 19, 16837–16850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Wu, W.B.; Chiang, H.S.; Fang, J.Y.; Chen, S.K.; Huang, C.C.; Hung, C.F. (+)-Catechin prevents ultraviolet B-induced human keratinocyte death via inhibition of JNK phosphorylation. Life Sci. 2006, 79, 801–807. [Google Scholar] [CrossRef]
- Liao, R.J.; Tang, Z.H.; Lei, Y.D.; Guo, B.C. Polyphenol-reduced graphene oxide: Mechanism and derivatization. J. Phys. Chem. C 2011, 115, 20740–20746. [Google Scholar] [CrossRef]
- Yan, Q.; Wang, M.Y.; Wu, Y.H.; Shen, Q. Tea polyphenol as environmentally friendly dopant and thermal stabilizer for polyaniline. Mater. Lett. 2016, 170, 202–204. [Google Scholar] [CrossRef]
- Yang, Z.J.; Xu, Z.C.; Zhang, L.Q.; Guo, B.C. Dispersion of graphene in chlorosulfonated polyethylene by slurry compounding. Compos. Sci. Technol. 2018, 162, 156–162. [Google Scholar] [CrossRef]
- Liao, R.J.; Tang, Z.H.; Lin, T.F.; Guo, B.C. Scalable and versatile graphene functionalized with the Mannich condensate. ACS Appl. Mater. Interfaces 2013, 5, 2174–2181. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.W.; Wang, B.B.; Chen, Z.H.; Zhao, J.Q. Reinforcement and antioxidation effects of antioxidant functionalized silica in styrene-butadiene rubber. Mater. Des. 2013, 50, 558–565. [Google Scholar] [CrossRef]
- Zhong, B.C.; Shi, Q.F.; Jia, Z.X.; Luo, Y.F.; Chen, Y.J.; Jia, D.M. Preparation of silica-supported 2-mercaptobenzimidazole and its antioxidative behavior in styrene-butadiene rubber. Polym. Degrad. Stab. 2014, 110, 260–267. [Google Scholar] [CrossRef]
- Zhong, B.C.; Jia, Z.X.; Hu, D.C.; Luo, Y.F.; Jia, D.M. Reinforcement and reinforcing mechanism of styrene-butadiene rubber by antioxidant-modified silica. Compos. Part A Appl. Sci. Manuf. 2015, 78, 303–310. [Google Scholar] [CrossRef]
- Guo, L.L.; Lei, H.X.; Zheng, J.; Huang, G.S. Synthesis of nanosilica-based immobile antioxidant and its antioxidative efficiency in SBR composites. Polym. Compos. 2013, 34, 1856–1862. [Google Scholar] [CrossRef]
- Chen, L.J.; Guo, X.H.; Jia, Z.X.; Tang, Y.H.; Wu, L.H.; Luo, Y.F.; Jia, D.M. High reactive sulphide chemically supported on silica surface to prepare functional nanoparticle. Appl. Surf. Sci. 2018, 442, 673–681. [Google Scholar] [CrossRef]
- Gao, X.W.; Hu, G.J.; Qian, Z.Z.; Ding, Y.F.; Zhang, S.M.; Wang, D.J.; Yang, M.S. Immobilization of antioxidant on nanosilica and the antioxidative behavior in low density polyethylene. Polymer 2007, 48, 7309–7315. [Google Scholar] [CrossRef]
- Sun, Y.K.; He, J.W.; Zhong, B.C.; Zhu, L.X.; Liu, F. A synthesized multifunctional rubber additive and its improvements on the curing and antioxidative properties of styrene-butadiene rubber/silica composites. Polym. Degrad. Stab. 2019, 170. [Google Scholar] [CrossRef]
- Rooj, S.; Das, A.; Stöckelhuber, K.W.; Wang, D.Y. Understanding the reinforcing behavior of expanded clay particles in natural rubber compounds. Soft Matter 2013, 9, 3798. [Google Scholar] [CrossRef]
- Li, Y.Q.; Ishida, H. A Study of Morphology and Intercalation Kinetics of Polystyrene-Organoclay Nanocomposites. Macromolecules 2005, 38, 6513–6519. [Google Scholar] [CrossRef]
- Feng, L.; Li, J.F.; Ye, J.R.; Song, W.; Jia, J.; Shen, Q. Enhancing the mechanical and thermal properties of polyacrylonitrile through blending with tea polyphenol. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Chen, L.J.; Jia, Z.X.; Tang, Y.H.; Wu, L.H.; Luo, Y.F.; Jia, D.M. Novel functional silica nanoparticles for rubber vulcanization and reinforcement. Compos. Sci. Technol. 2017, 144, 11–17. [Google Scholar] [CrossRef]
- Chen, L.J.; Jia, Z.X.; Guo, X.H.; Zhong, B.C.; Chen, Y.J.; Luo, Y.F.; Jia, D.M. Functionalized HNTs nanocluster vulcanized natural rubber with high filler-rubber interaction. Chem. Eng. J. 2018, 336, 748–756. [Google Scholar] [CrossRef]
- Sileika, T.S.; Barrett, D.G.; Zhang, R.; Lau, K.H.A.; Messersmith, P.B. Colorless multifunctional coatings inspired by polyphenols found in tea, chocolate, and wine. Angew. Chem. Int. Ed. 2013, 52, 10766–10770. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.C.; Li, Q.; Li, H.Y.; Hu, Y.L. Polyethylene-modified nano silica and its fine dispersion in polyethylene. Ind. Eng. Chem. Res. 2017, 56, 5892–5898. [Google Scholar] [CrossRef]
- Yan, F.H.; Zhang, X.B.; Liu, F.; Li, X.H.; Zhang, Z.J. Adjusting the properties of silicone rubber filled with nanosilica by changing the surface organic groups of nanosilica. Compos. Part B Eng. 2015, 75, 47–52. [Google Scholar] [CrossRef]
- Rahman, I.A.; Jafarzadeh, M.; Sipaut, C.S. Synthesis of organo-functionalized nanosilica via a co-condensation modification using γ-aminopropyltriethoxysilane (APTES). Ceram. Int. 2009, 35, 1883–1888. [Google Scholar] [CrossRef]
- Li, Y.; Han, B.Y.; Wen, S.P.; Lu, Y.L.; Yang, H.B.; Zhang, L.Q.; Liu, L. Effect of the temperature on surface modification of silica and properties of modified silica filled rubber composites. Compos. Part A Appl. Sci. Manuf. 2014, 62, 52–59. [Google Scholar] [CrossRef]
- Li, Y.; Han, B.Y.; Liu, L.; Zhang, F.Z.; Zhang, L.Q.; Wen, S.P.; Lu, Y.L.; Yang, H.B.; Shen, J. Surface modification of silica by two-step method and properties of solution styrene butadiene rubber (SSBR) nanocomposites filled with modified silica. Compos. Sci. Technol. 2013, 88, 69–75. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, S.H.; Zhang, X.Y.; Li, X.L.; Bai, Y. Preparation, structure, and properties of solution-polymerized styrene-butadiene rubber with functionalized end-groups and its silica-filled composites. Polymer 2014, 55, 1964–1976. [Google Scholar] [CrossRef]
- Zhang, X.G.; Loo, L.S. Study of glass transition and reinforcement mechanism in polymer/layered silicate nanocomposites. Macromolecules 2009, 42, 5196–5207. [Google Scholar] [CrossRef]
- Zou, Y.K.; Sun, Y.K.; He, J.W.; Tang, Z.H.; Zhu, L.X.; Luo, Y.F.; Liu, F. Enhancing mechanical properties of styrene–butadiene rubber/silica nanocomposites by in situ interfacial modification with a novel rare-earth complex. Compos. Part A Appl. Sci. Manuf. 2016, 87, 297–309. [Google Scholar] [CrossRef]
- Begum, P.M.S. Use of antioxidant-modified precipitated silica in natural rubber. Prog. Rubber. Plast. Recycl. Technol. 2011, 27, 215–228. [Google Scholar] [CrossRef]
- Wu, S.W.; Qiu, M.; Guo, B.C.; Zhang, L.Q. Nanodot-loaded clay nanotubes as green and sustained radical scavengers for elastomer. ACS Sustain. Chem. Eng. 2017, 5, 1775–1783. [Google Scholar] [CrossRef]
- Vinod, V.S.; Varghese, S.; Kuriakose, B. Degradation behaviour of natural rubber-aluminium powder composites: Effect of heat, ozone and high energy radiation. Polym. Degrad. Stab. 2002, 75, 405–412. [Google Scholar] [CrossRef]

| SBR | Silica-s-TP | ZnO | CBS | SA | S | |
|---|---|---|---|---|---|---|
| Neat SBR | 100 | 0 | 4 | 2 | 2 | 1.6 |
| SBR/ST-10 | 100 | 10 | 4 | 2 | 2 | 1.6 |
| SBR/ST-20 | 100 | 20 | 4 | 2 | 2 | 1.6 |
| SBR/ST-30 | 100 | 30 | 4 | 2 | 2 | 1.6 |
| SBR/ST-40 | 100 | 40 | 4 | 2 | 2 | 1.6 |
| SBR/ST-50 | 100 | 50 | 4 | 2 | 2 | 1.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Luo, Y.; Chen, Y.; Chen, L.; Jia, D. Novel Hybrid Biomass Anti-Aging Filler for Styrene-Butadiene Rubber Composites with Antioxidative and Reinforcing Properties. Materials 2020, 13, 4045. https://doi.org/10.3390/ma13184045
Guo X, Luo Y, Chen Y, Chen L, Jia D. Novel Hybrid Biomass Anti-Aging Filler for Styrene-Butadiene Rubber Composites with Antioxidative and Reinforcing Properties. Materials. 2020; 13(18):4045. https://doi.org/10.3390/ma13184045
Chicago/Turabian StyleGuo, Xiaohui, Yuanfang Luo, Yongjun Chen, Lijuan Chen, and Demin Jia. 2020. "Novel Hybrid Biomass Anti-Aging Filler for Styrene-Butadiene Rubber Composites with Antioxidative and Reinforcing Properties" Materials 13, no. 18: 4045. https://doi.org/10.3390/ma13184045
APA StyleGuo, X., Luo, Y., Chen, Y., Chen, L., & Jia, D. (2020). Novel Hybrid Biomass Anti-Aging Filler for Styrene-Butadiene Rubber Composites with Antioxidative and Reinforcing Properties. Materials, 13(18), 4045. https://doi.org/10.3390/ma13184045




