Quicklime and Calcium Sulfoaluminate Cement Used as Mineral Accelerators to Improve the Properties of Cemented Paste Backfill with a High Volume of Fly Ash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparation
2.3. Testing Methods
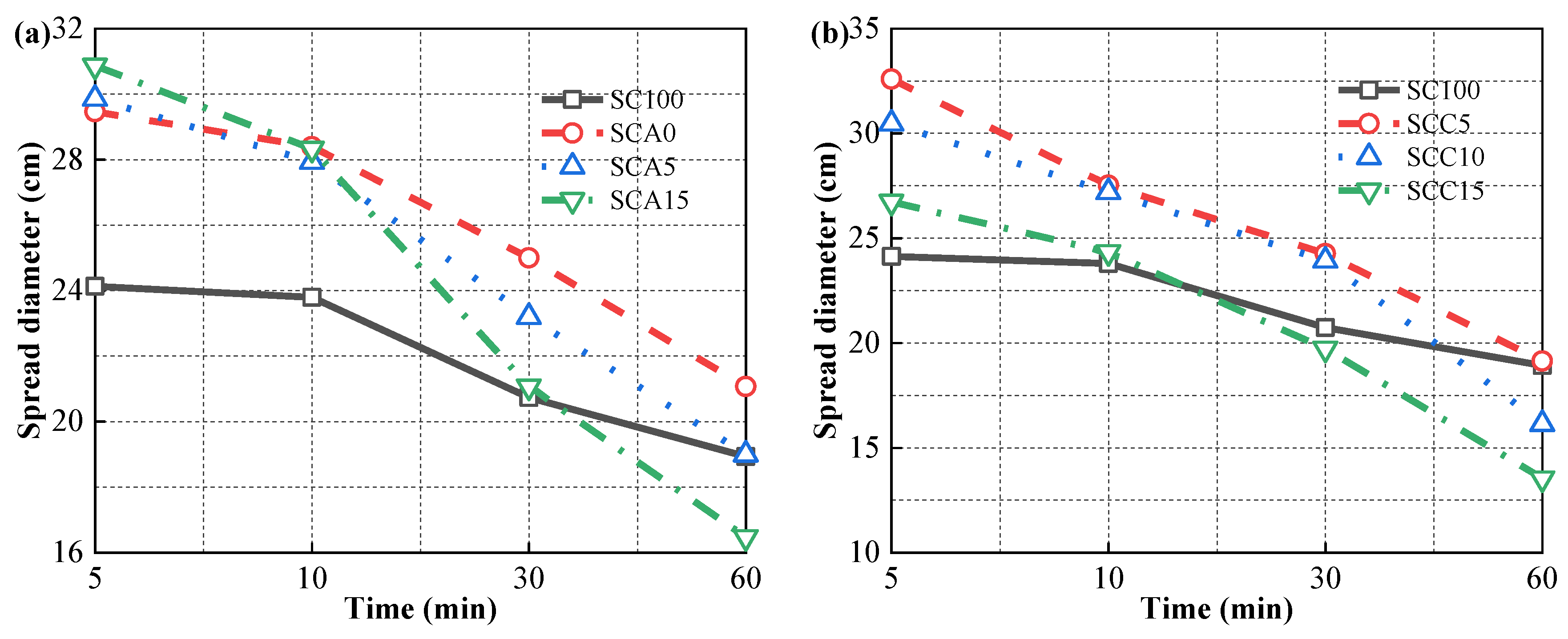
2.3.1. Spread Diameter
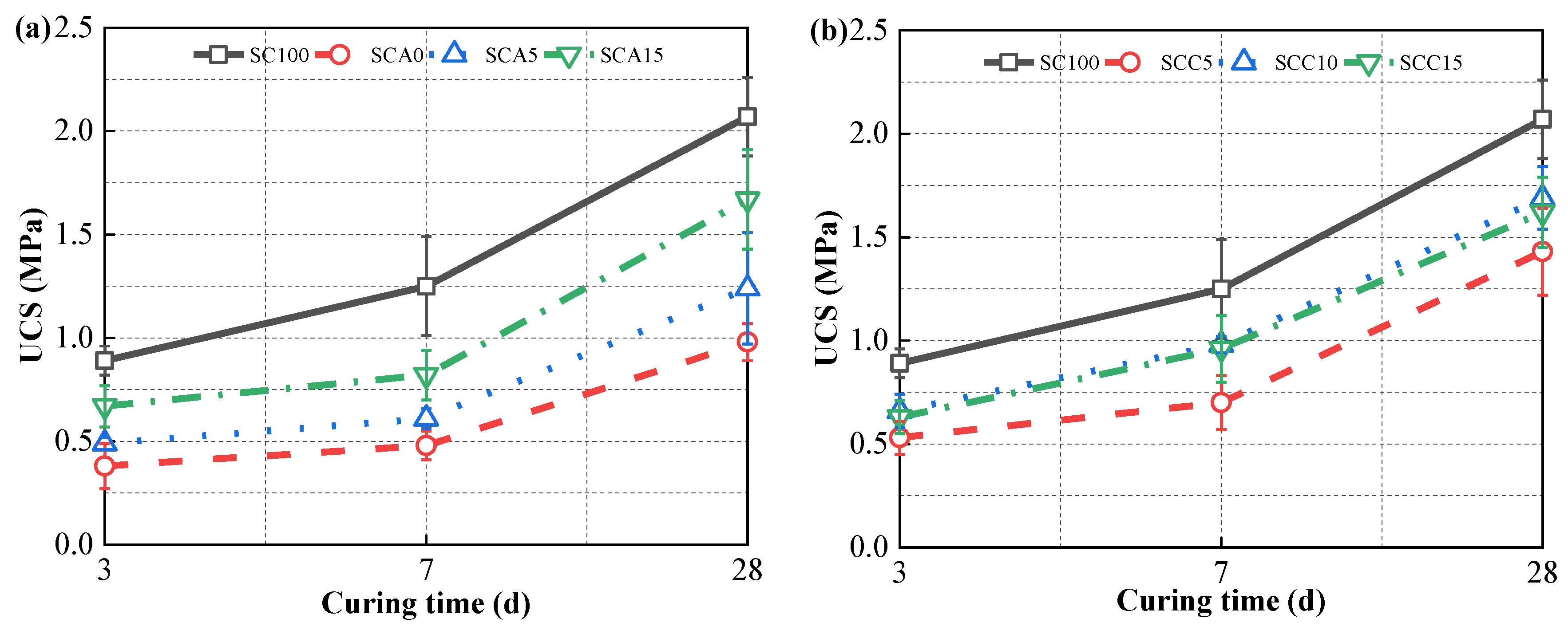
2.3.2. Unconfined Compressive Strength
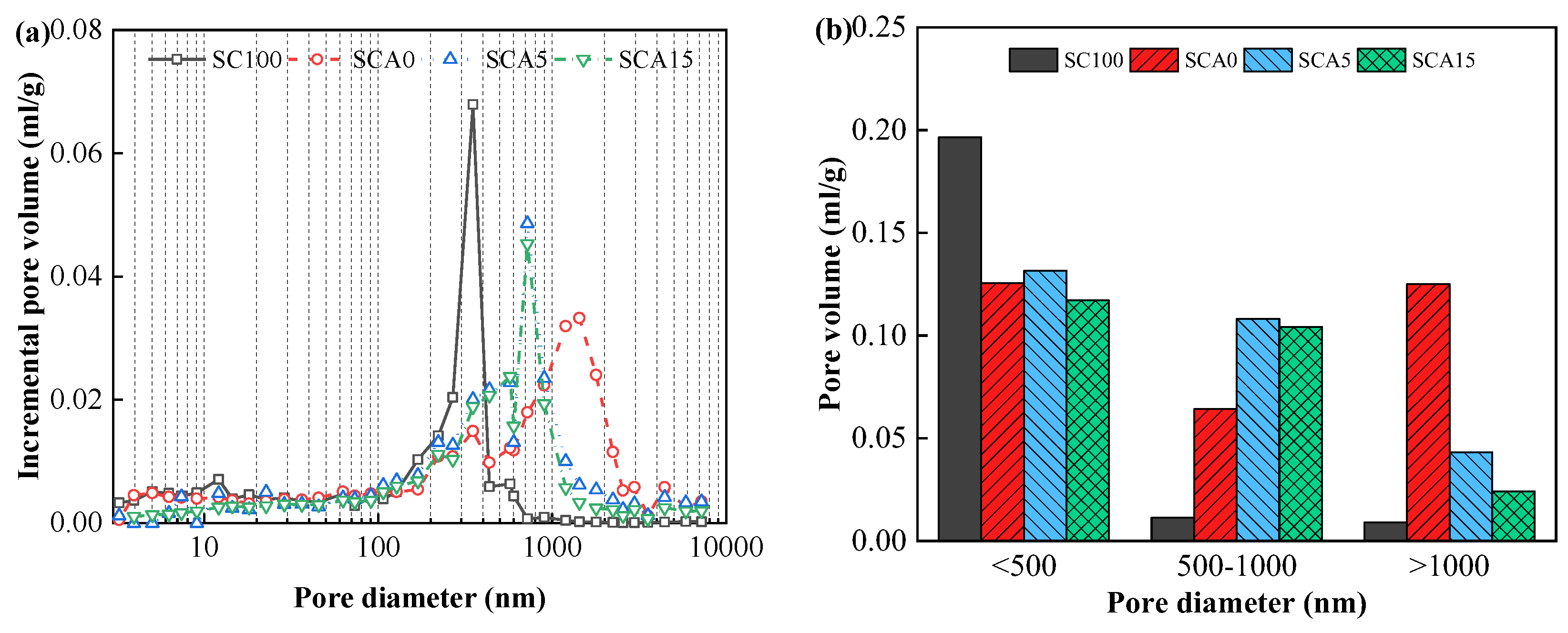
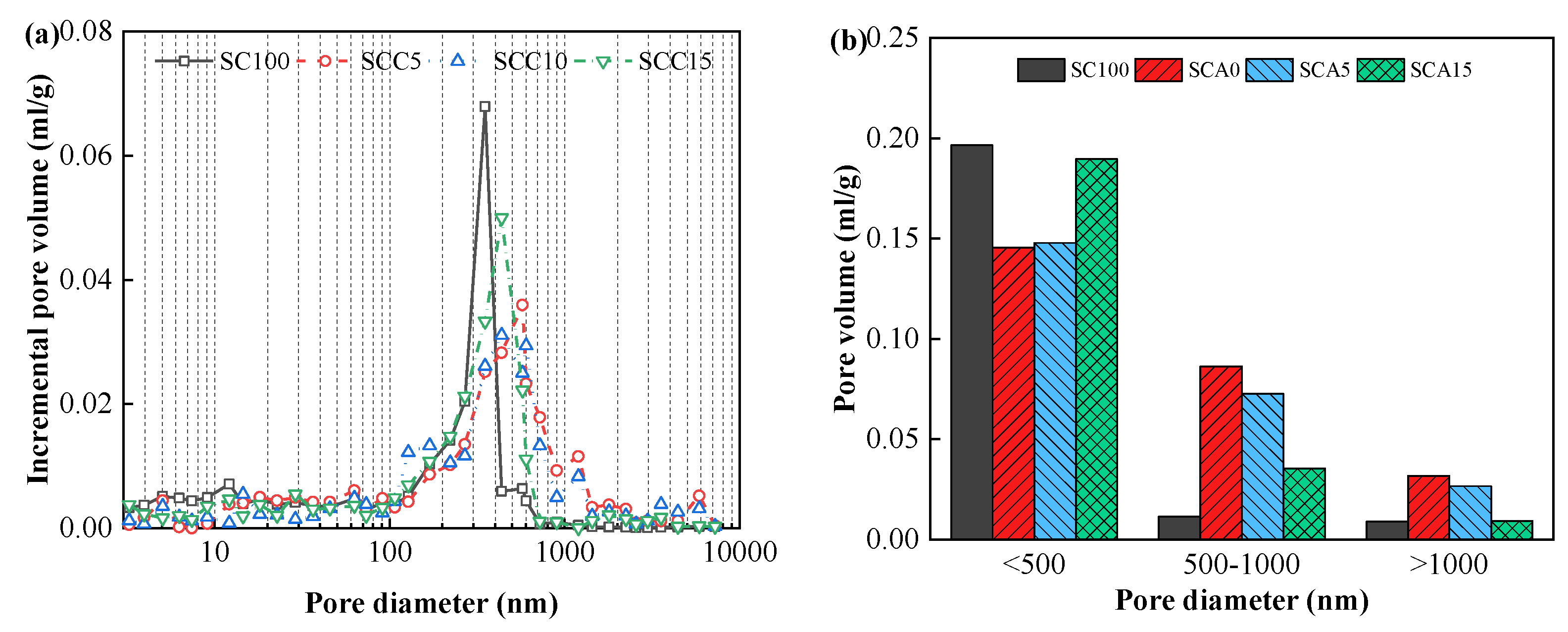
2.3.3. Pore Structure
2.3.4. Hydration Heat
2.3.5. Hydration Evolution
3. Results
3.1. Spread Diameter
3.2. Strength Development
3.3. Pore Structure
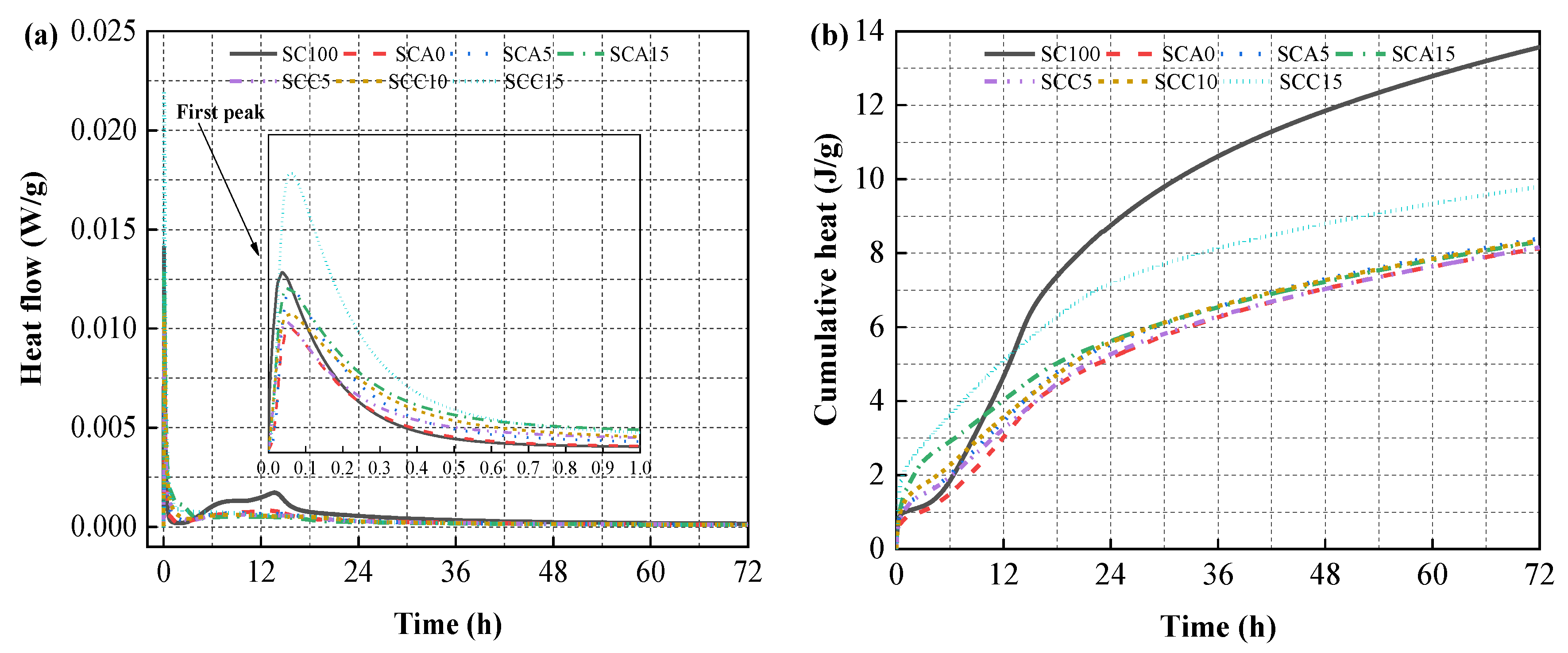
3.4. Hydration Heat
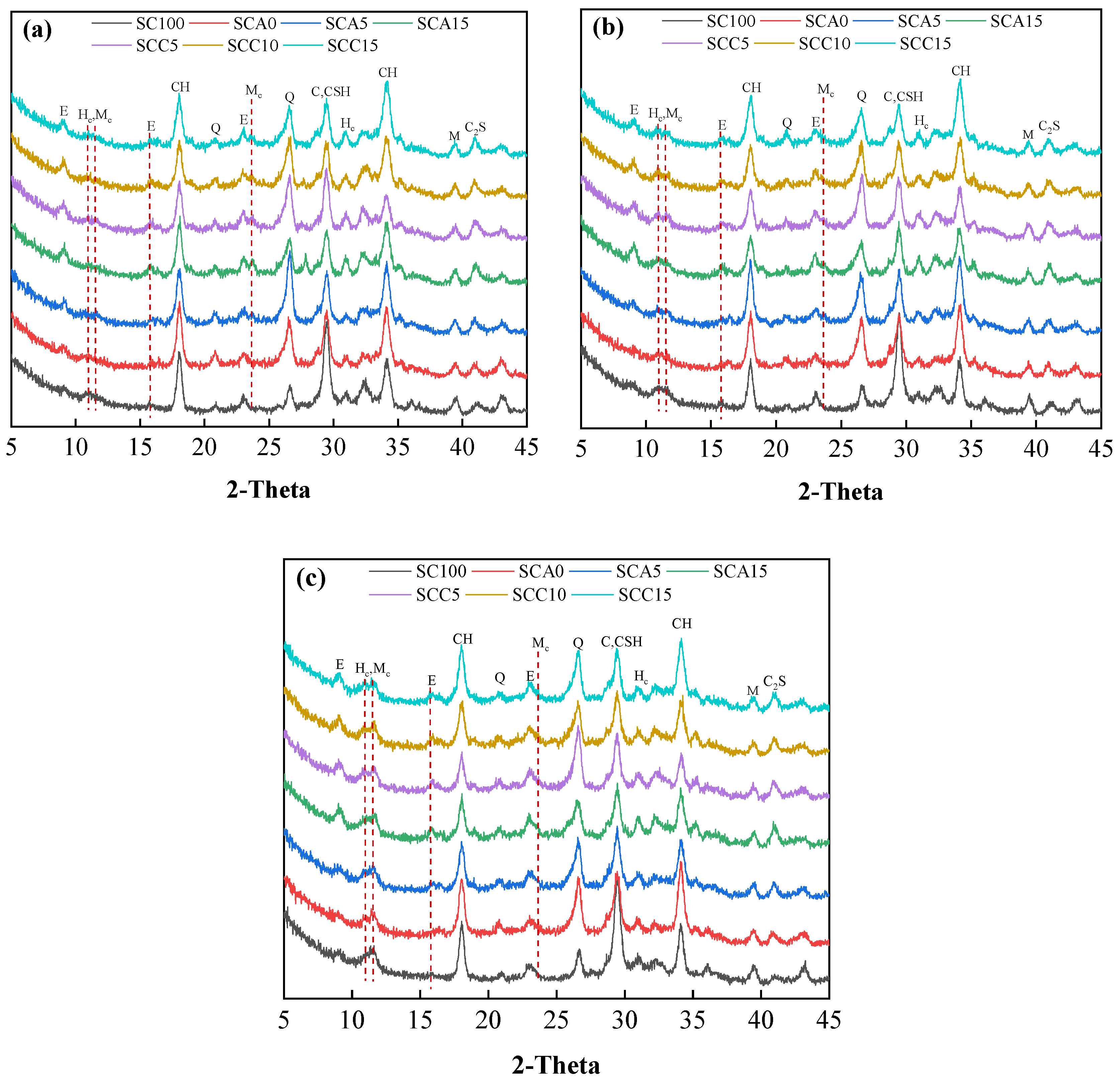
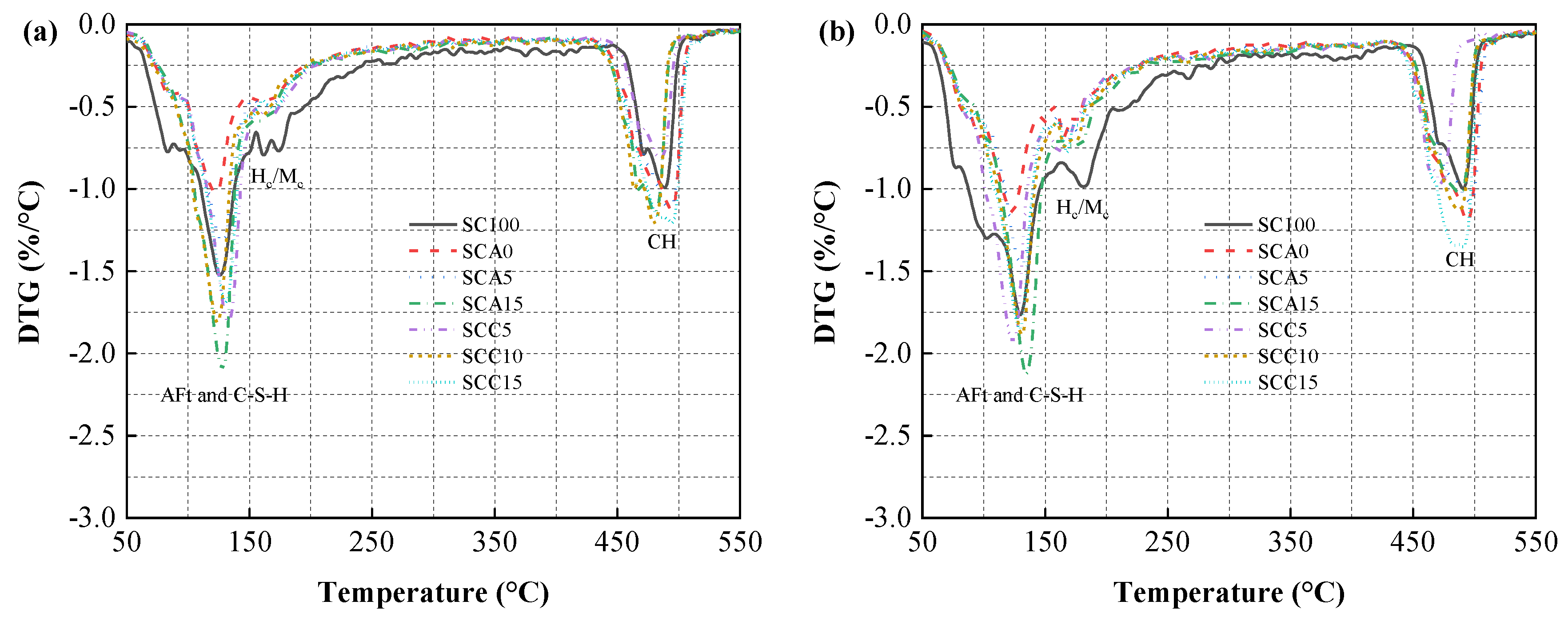
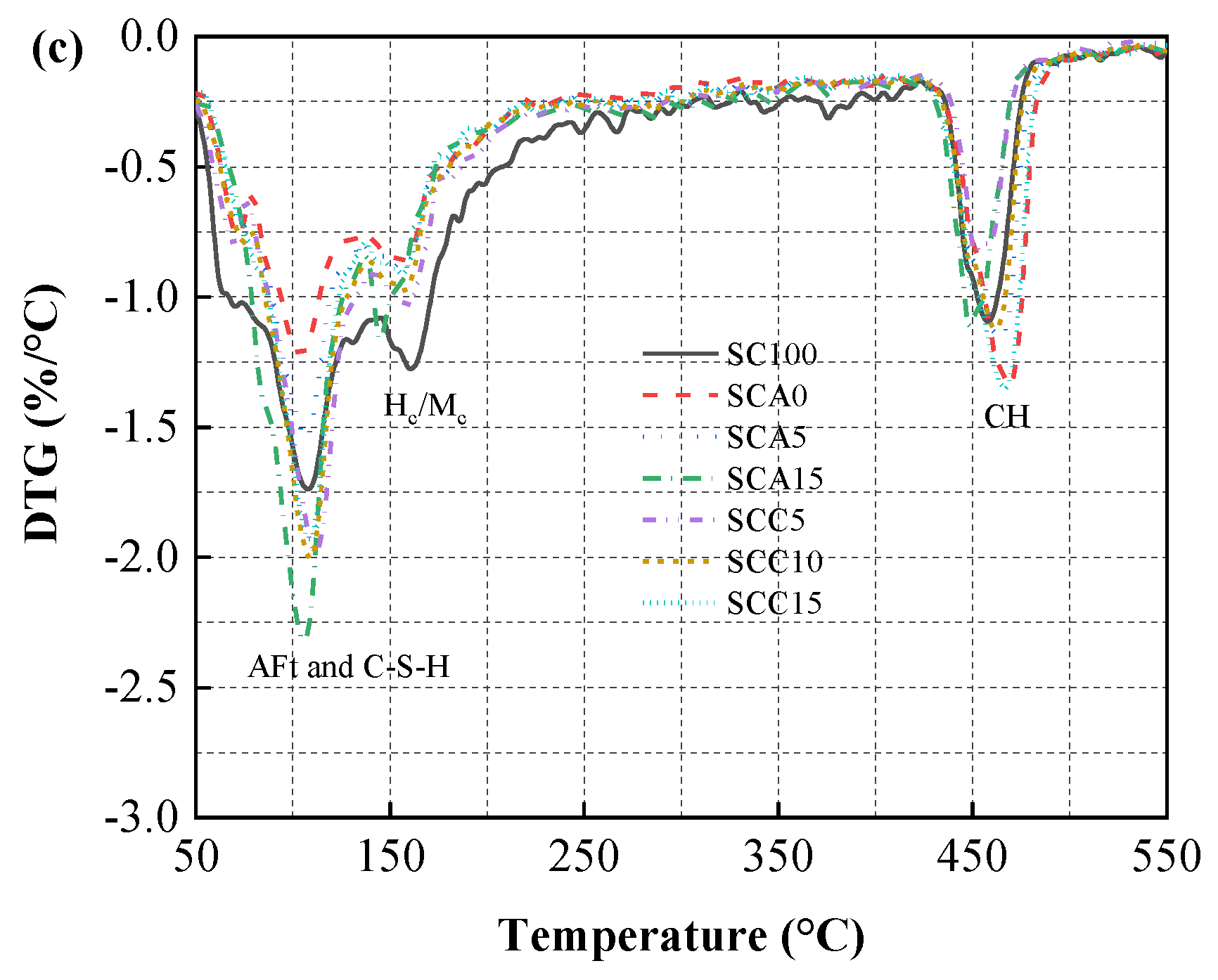
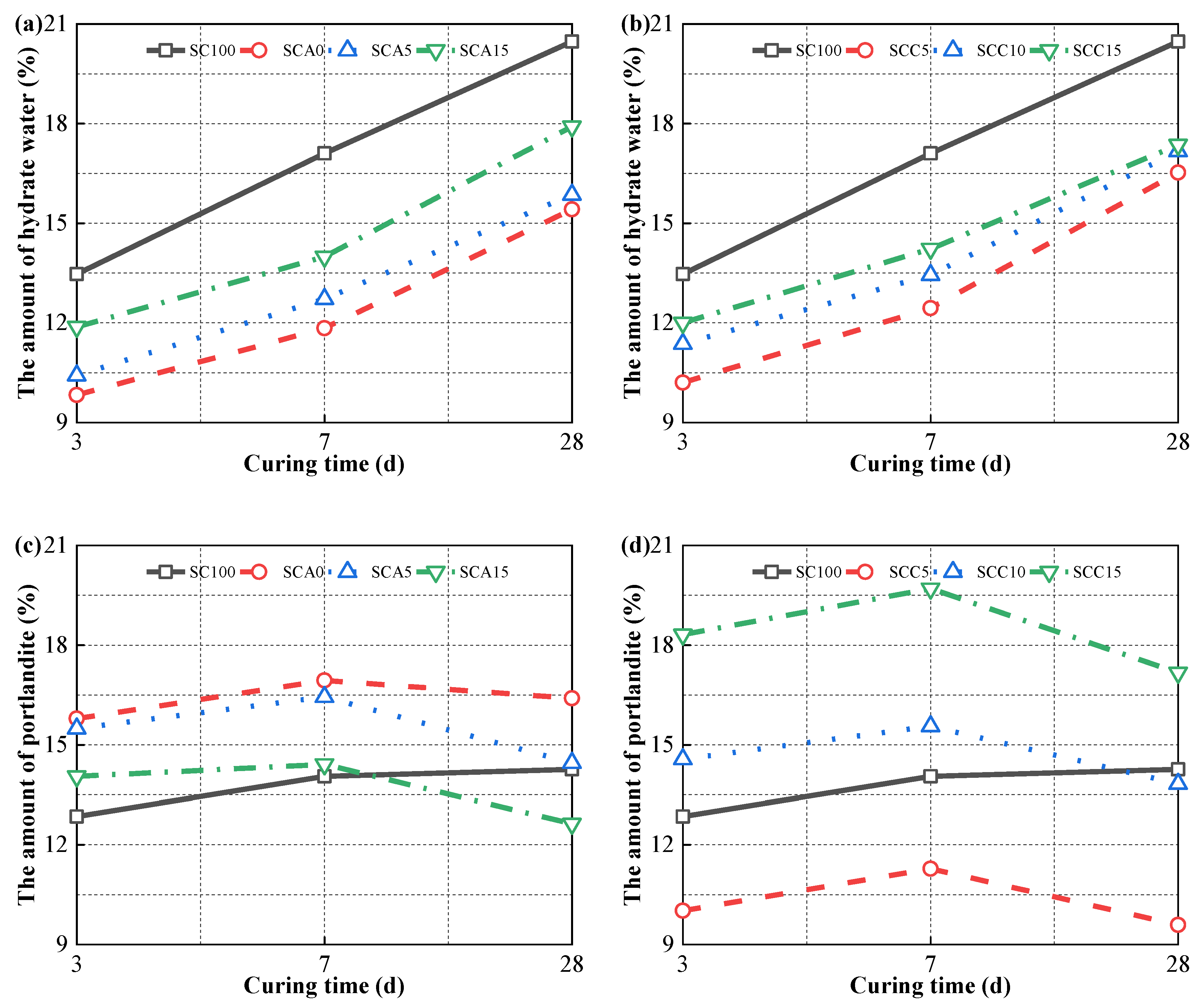
3.5. Hydration Evolution
3.5.1. XRD Patterns
3.5.2. TG Analysis
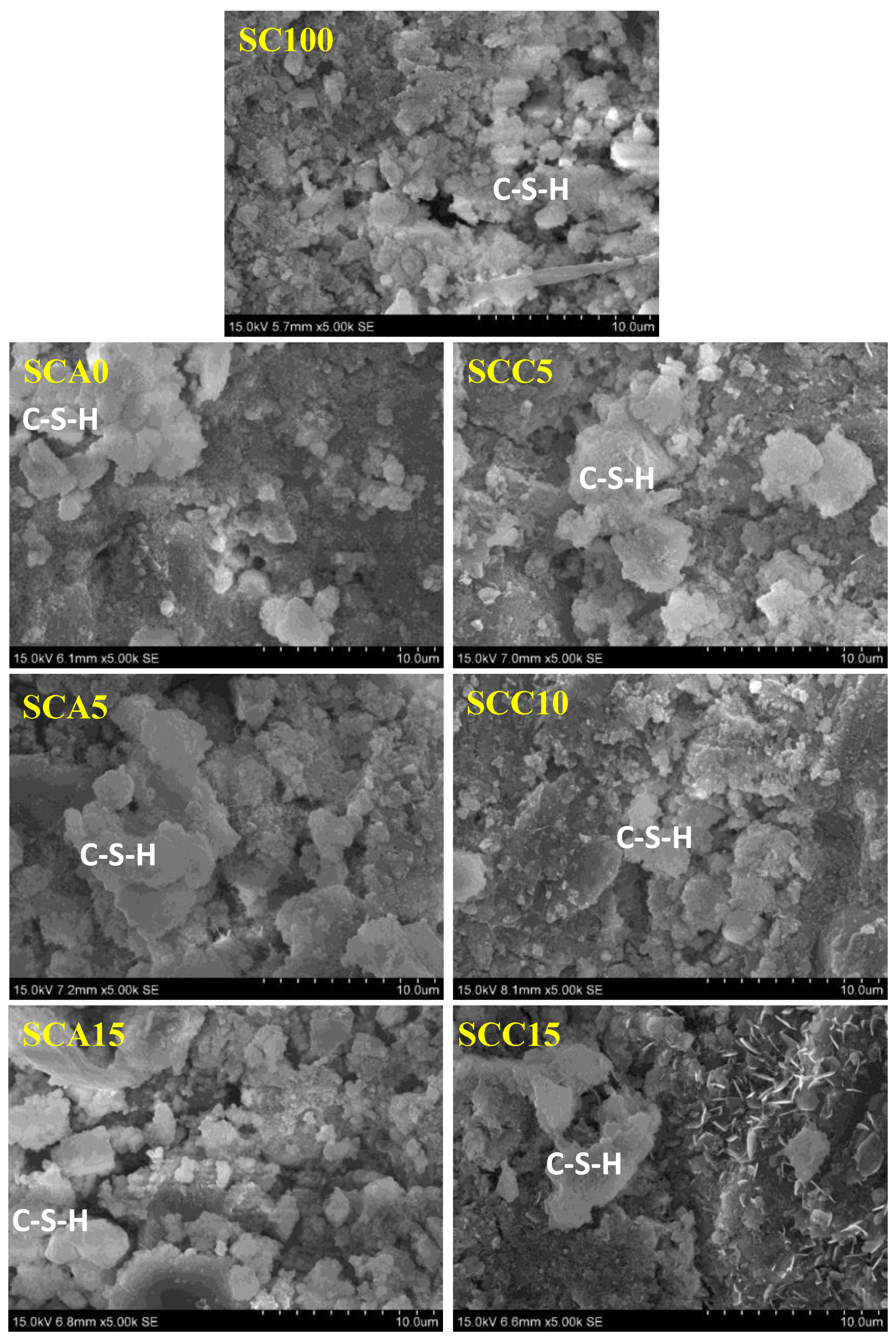
3.5.3. SEM Observation
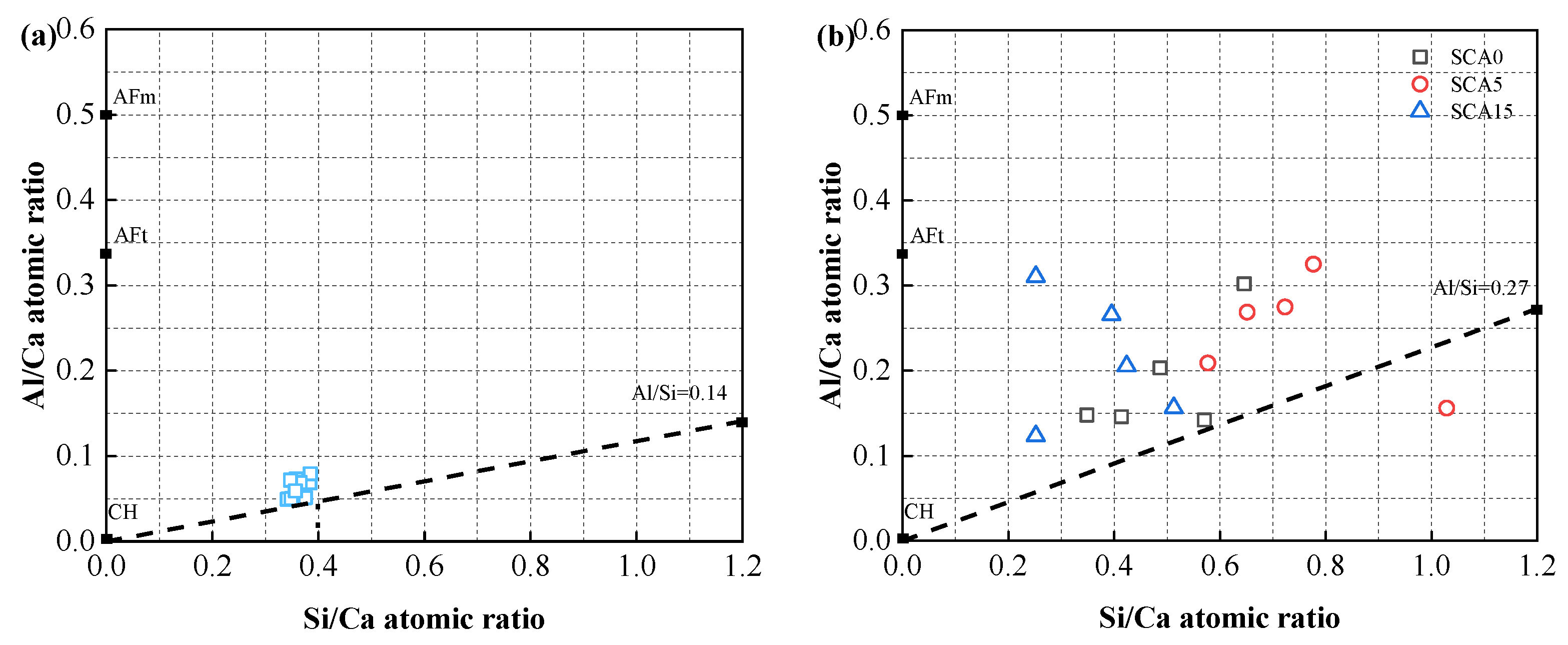
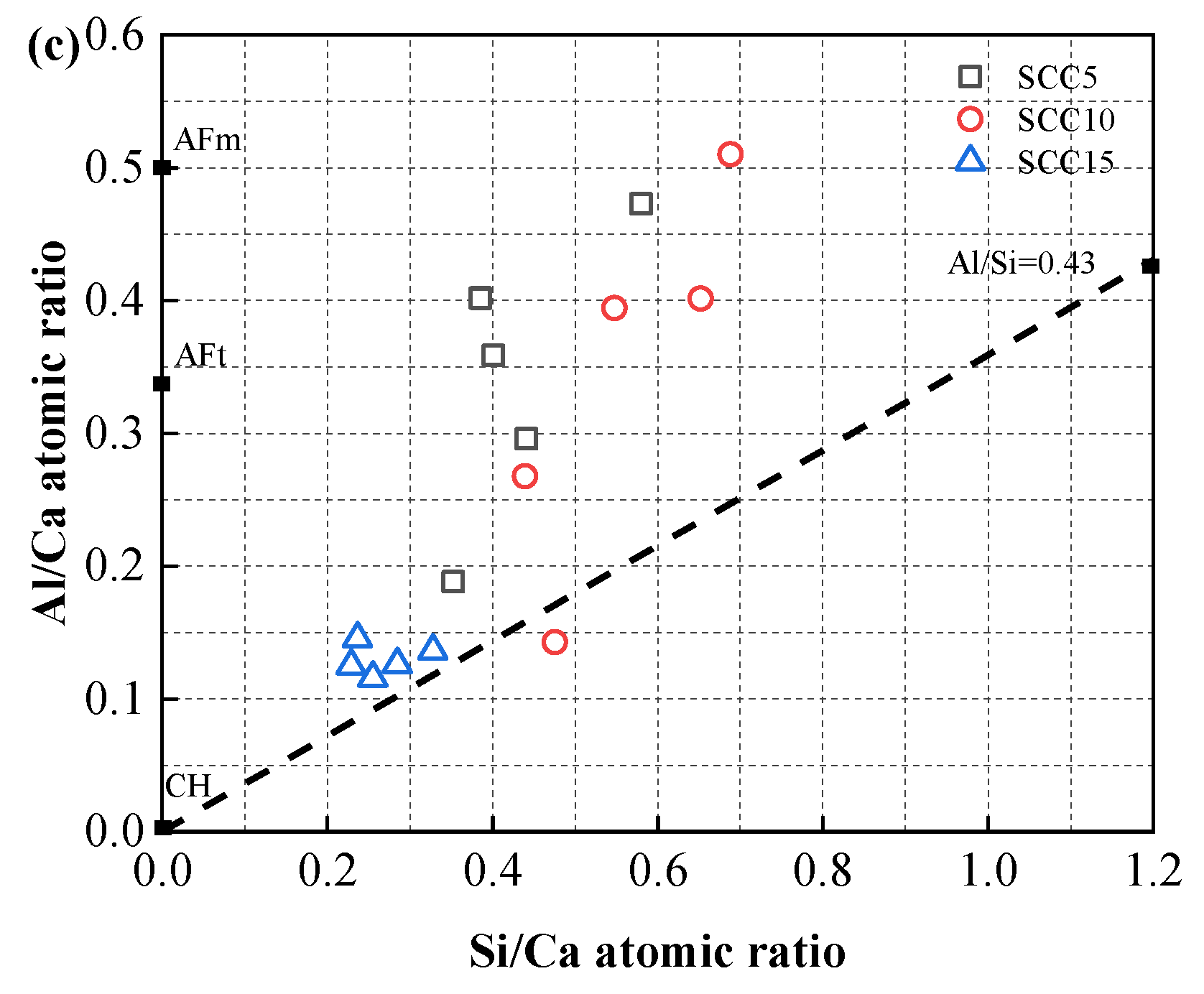
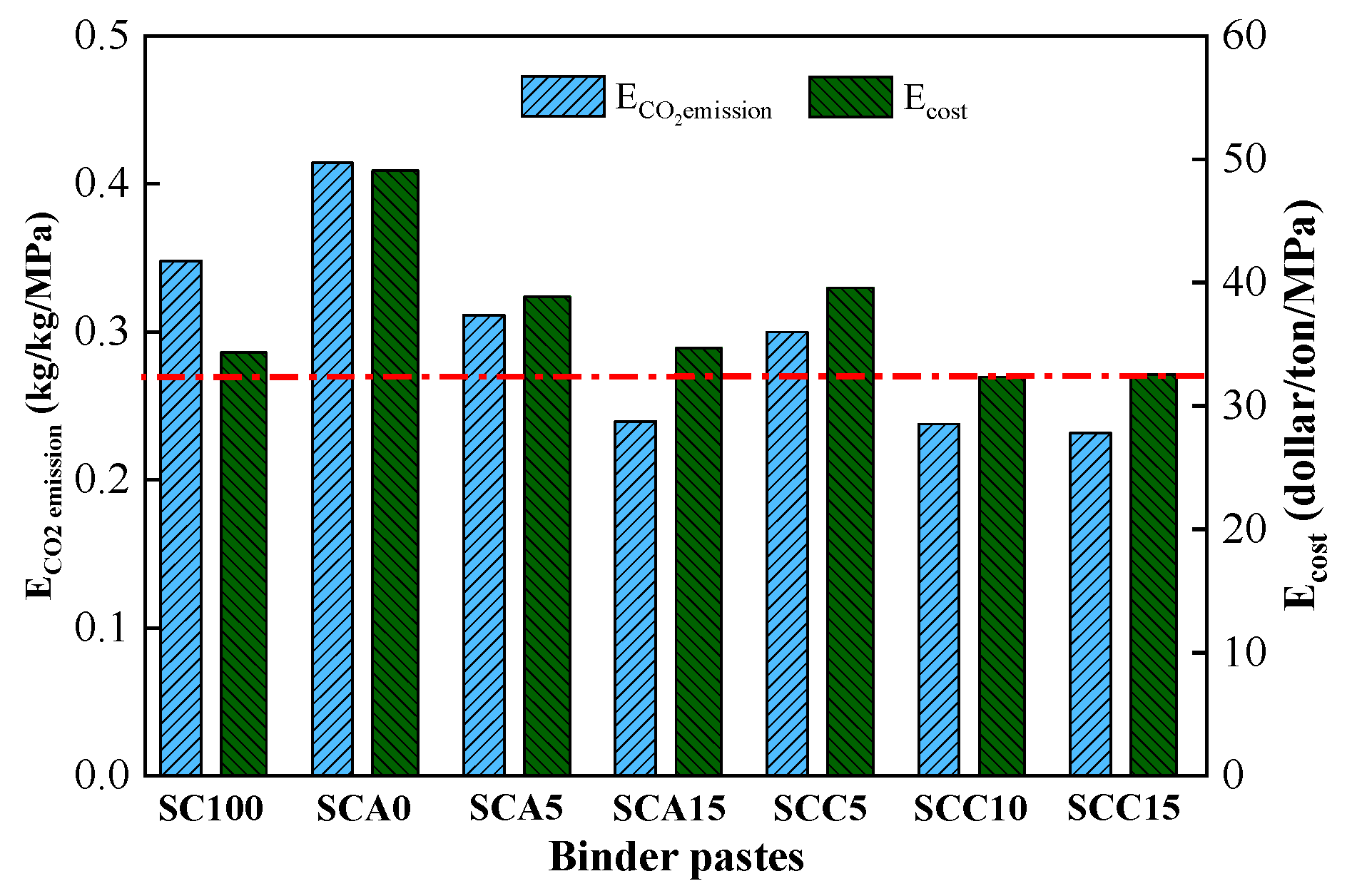
3.6. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Behera, S.K.; Ghosh, C.N.; Mishra, D.P.; Singh, P.; Mishra, K.; Buragohain, J.; Mandal, P.K. Strength development and microstructural investigation of lead-zinc mill tailings based paste backfill with fly ash as alternative binder. Cem. Concr. Compos. 2020, 109, 103553. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Fall, M.; Belem, T. A contribution to understanding the hardening process of cemented pastefill. Miner. Eng. 2004, 17, 141–152. [Google Scholar] [CrossRef]
- Fall, M.; Benzaazoua, M.; Ouellet, S. Experimental characterization of the influence of tailings fineness and density on the quality of cemented paste backfill. Miner. Eng. 2005, 18, 41–44. [Google Scholar] [CrossRef]
- Fall, M.; Célestin, J.; Pokharel, M.; Touré, M. A contribution to understanding the effects of curing temperature on the mechanical properties of mine cemented tailings backfill. Eng. Geol. 2010, 114, 397–413. [Google Scholar] [CrossRef]
- Yang, L.; Yilmaz, E.; Li, J.; Liu, H.; Jiang, H. Effect of superplasticizer type and dosage on fluidity and strength behavior of cemented tailings backfill with different solid contents. Constr. Build. Mater. 2018, 187, 290–298. [Google Scholar] [CrossRef]
- Li, W.; Fall, M. Strength and self-desiccation of slag-cemented paste backfill at early ages: Link to initial sulphate concentration. Cem. Concr. Compos. 2018, 89, 160–168. [Google Scholar] [CrossRef]
- Fall, M.; Pokharel, M. Coupled effects of sulphate and temperature on the strength development of cemented tailings backfills: Portland cement-paste backfill. Cem. Concr. Compos. 2010, 32, 819–828. [Google Scholar] [CrossRef]
- Pokharel, M.; Fall, M. Combined influence of sulphate and temperature on the saturated hydraulic conductivity of hardened cemented paste backfill. Cem. Concr. Compos. 2013, 38, 21–28. [Google Scholar] [CrossRef]
- Qi, C.; Fourie, A.; Chen, Q.; Zhang, Q. A strength prediction model using artificial intelligence for recycling waste tailings as cemented paste backfill. J. Clean. Prod. 2018, 183, 566–578. [Google Scholar] [CrossRef]
- Qi, C.; Liu, L.; He, J.; Chen, Q.; Yu, L.-J.; Liu, P. Understanding Cement Hydration of Cemented Paste Backfill: DFT Study of Water Adsorption on Tricalcium Silicate (111) Surface. Minerals 2019, 9, 202. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Guo, Z.; Yang, L.; Jiang, H.; Zhao, Y. Effects of packing density and water film thickness on the fluidity behaviour of cemented paste backfill. Powder Technol. 2020, 359, 27–35. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Bussière, B.; Demers, I.; Aubertin, M.; Fried, É.; Blier, A. Integrated mine tailings management by combining environmental desulphurization and cemented paste backfill: Application to mine Doyon, Quebec, Canada. Miner. Eng. 2008, 21, 330–340. [Google Scholar] [CrossRef]
- Fall, M.; Belem, T.; Samb, S.; Benzaazoua, M. Experimental characterization of the stress-strain behaviour of cemented paste backfill in compression. J. Mater. Sci. 2007, 42, 3914–3922. [Google Scholar] [CrossRef]
- Jiang, H.; Qi, Z.; Yilmaz, E.; Han, J.; Qiu, J.; Dong, C. Effectiveness of alkali-activated slag as alternative binder on workability and early age compressive strength of cemented paste backfills. Constr. Build. Mater. 2019, 218, 689–700. [Google Scholar] [CrossRef]
- Qiu, J.; Yang, L.; Sun, X.; Xing, J.; Li, S. Strength characteristics and failure mechanism of cemented super-fine unclassified tailings backfill. Minerals 2017, 7, 58. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.-P.; Yang, L.; Xing, J.; Sun, X.-G. Analytical solution for determining the required strength of mine backfill based on its damage constitutive model. Soil Mech. Found. Eng. 2018, 54, 371–376. [Google Scholar] [CrossRef]
- Yang, L.; Qiu, J.; Jiang, H.; Hu, S.; Li, H.; Li, S. Use of cemented super-fine unclassified tailings backfill for control of subsidence. Minerals 2017, 7, 216. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, E.; Belem, T.; Benzaazoua, M. Specimen size effect on strength behavior of cemented paste backfills subjected to different placement conditions. Eng. Geol. 2015, 185, 52–62. [Google Scholar] [CrossRef]
- Yilmaz, E.; Belem, T.; Bussière, B.; Benzaazoua, M. Relationships between microstructural properties and compressive strength of consolidated and unconsolidated cemented paste backfills. Cem. Concr. Compos. 2011, 33, 702–715. [Google Scholar] [CrossRef]
- Fall, M.; Benzaazoua, M. Modeling the effect of sulphate on strength development of paste backfill and binder mixture optimization. Cem. Concr. Res. 2005, 35, 301–314. [Google Scholar] [CrossRef]
- Fall, M.; Benzaazoua, M.; Saa, E. Mix proportioning of underground cemented tailings backfill. Tunn. Undergr. Space Technol. 2008, 23, 80–90. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, L.; Ren, F.; Qiu, J.; Ding, H. Rheological and mechanical properties of cemented foam backfill: Effect of mineral admixture type and dosage. Cem. Concr. Compos. 2020, 112, 103689. [Google Scholar] [CrossRef]
- De Belie, N.; Soutsos, M.; Gruyaert, E. Limestone Powder. In Properties of Fresh and Hardened Concrete Containing Supplementary Cementitious Materials; Springer: Cham, Switzerland, 2018; Volume 25, pp. 123–151. [Google Scholar] [CrossRef]
- Fernandez, A.; Calvo, J.G.; Alonso, M. Ordinary Portland Cement composition for the optimization of the synergies of supplementary cementitious materials of ternary binders in hydration processes. Cem. Concr. Compos. 2018, 89, 238–250. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Xing, J.; Sun, X. Chemical activation of binary slag cement with low carbon footprint. J. Clean. Prod. 2020, 267, 121455. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Zhang, S.; Guo, Z.; Ma, Z.; Sun, X.; Xing, J. Effect of sodium sulfate on the hydration and mechanical properties of lime-slag based eco-friendly binders. Constr. Build. Mater. 2020, 250, 118603. [Google Scholar] [CrossRef]
- Deschner, F.; Winnefeld, F.; Lothenbach, B.; Seufert, S.; Schwesig, P.; Dittrich, S.; Goetz-Neunhoeffer, F.; Neubauer, J. Hydration of Portland cement with high replacement by siliceous fly ash. Cem. Concr. Res. 2012, 42, 1389–1400. [Google Scholar] [CrossRef]
- Schöler, A.; Lothenbach, B.; Winnefeld, F.; Zajac, M. Hydration of quaternary Portland cement blends containing blast-furnace slag, siliceous fly ash and limestone powder. Cem. Concr. Compos. 2015, 55, 374–382. [Google Scholar] [CrossRef]
- Criado, M.; Fernández-Jiménez, A.; Palomo, A. Alkali activation of fly ash: Effect of the SiO2/Na2O ratio. Microporous Mesoporous Mater. 2007, 106, 180–191. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar] [CrossRef] [Green Version]
- Shi, C. Studies on Several Factors Affecting Hydration and Properties of Lime-Pozzolan Cements. J. Mater. Civ. Eng. 2001, 13, 441–445. [Google Scholar] [CrossRef]
- Antiohos, S.; Papageorgiou, A.; Tsimas, S. Activation of fly ash cementitious systems in the presence of quicklime. Part II: Nature of hydration products, porosity and microstructure development. Cem. Concr. Res. 2006, 36, 2123–2131. [Google Scholar] [CrossRef]
- Antiohos, S.; Tsimas, S. Activation of fly ash cementitious systems in the presence of quicklime: Part I. Compressive strength and pozzolanic reaction rate. Cem. Concr. Res. 2004, 34, 769–779. [Google Scholar] [CrossRef]
- Li, G.; Zhang, J.; Song, Z.; Shi, C.; Zhang, A. Improvement of workability and early strength of calcium sulphoaluminate cement at various temperature by chemical admixtures. Constr. Build. Mater. 2018, 160, 427–439. [Google Scholar] [CrossRef]
- Gastaldini, A.L.G.; Isaia, G.C.; Saciloto, A.P.; Missau, F.; Hoppe, T.F. Influence of curing time on the chloride penetration resistance of concrete containing rice husk ash: A technical and economical feasibility study. Cem. Concr. Compos. 2010, 32, 783–793. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, Y.; Yang, H.; Li, H. Calcium sulphoaluminate cement used as mineral accelerator to improve the property of Portland cement at sub-zero temperature. Cem. Concr. Compos. 2020, 106, 103452. [Google Scholar] [CrossRef]
- ASTM C39. Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Zhao, Y.; Qiu, J.; Xing, J.; Sun, X. Recycling of quarry dust for supplementary cementitious materials in low carbon cement. Constr. Build. Mater. 2020, 237, 117608. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, F.; Guo, Z.; Qiu, J.; Ding, H. Strength and deformation behavior of cemented foam backfill in sub-zero environment. J. Mater. Res. Technol. 2020, 9, 9219–9231. [Google Scholar] [CrossRef]
- De Weerdt, K.; Haha, M.B.; Le Saout, G.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Ye, H.; Gao, X.; Wang, R.; Wang, H. Relationship among particle characteristic, water film thickness and flowability of fresh paste containing different mineral admixtures. Constr. Build. Mater. 2017, 153, 193–201. [Google Scholar] [CrossRef]
- Nguyen, H.-A.; Chang, T.-P.; Shih, J.-Y.; Chen, C.-T. Influence of low calcium fly ash on compressive strength and hydration product of low energy super sulfated cement paste. Cem. Concr. Compos. 2019, 99, 40–48. [Google Scholar] [CrossRef]
- Baquerizo, L.G.; Matschei, T.; Scrivener, K.L.; Saeidpour, M.; Wadsö, L. Hydration states of AFm cement phases. Cem. Concr. Res. 2015, 73, 143–157. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The AFm phase in Portland cement. Cem. Concr. Res. 2007, 37, 118–130. [Google Scholar] [CrossRef]
- Jeon, D.; Yum, W.S.; Jeong, Y.; Oh, J.E. Properties of quicklime(CaO)-activated Class F fly ash with the use of CaCl2. Cem. Concr. Res. 2018, 111, 147–156. [Google Scholar] [CrossRef]
- Wang, X.-Y. Effect of fly ash on properties evolution of cement based materials. Constr. Build. Mater. 2014, 69, 32–40. [Google Scholar] [CrossRef]
- Wang, Y.; Shui, Z.; Wang, L.; Gao, X.; Huang, Y.; Song, Q.; Liu, K. Alumina-rich pozzolan modification on Portland-limestone cement concrete: Hydration kinetics, formation of hydrates and long-term performance evolution. Constr. Build. Mater. 2020, 258, 119712. [Google Scholar] [CrossRef]
- Yingliang, Z.; Jingping, Q.; Zhengyu, M.A.; Zhenbang, G.; Hui, L. Effect of superfine blast furnace slags on the binary cement containing high-volume fly ash. Powder Technol. 2020, 375, 539–548. [Google Scholar] [CrossRef]
- Everett, D.H. IUPAC Manual of Symbols and Terminology, appendix 2, Part 1, Colloid and Surface Chemistry. Pure Appl. Chem. 1972, 31, 578–621. [Google Scholar] [CrossRef]
- Ho, L.S.; Nakarai, K.; Duc, M.; Le Kouby, A.; Maachi, A.; Sasaki, T. Analysis of strength development in cement-treated soils under different curing conditions through microstructural and chemical investigations. Constr. Build. Mater. 2018, 166, 634–646. [Google Scholar] [CrossRef]
- Nakarai, K.; Ishida, T.; Kishi, T.; Maekawa, K. Enhanced thermodynamic analysis coupled with temperature-dependent microstructures of cement hydrates. Cem. Concr. Res. 2007, 37, 139–150. [Google Scholar] [CrossRef]
- Horpibulsuk, S.; Rachan, R.; Chinkulkijniwat, A.; Raksachon, Y.; Suddeepong, A. Analysis of strength development in cement-stabilized silty clay from microstructural considerations. Constr. Build. Mater. 2010, 24, 2011–2021. [Google Scholar] [CrossRef]
- Berodier, E.; Scrivener, K. Evolution of pore structure in blended systems. Cem. Concr. Res. 2015, 73, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Chindaprasirt, P.; Jaturapitakkul, C.; Sinsiri, T. Effect of fly ash fineness on compressive strength and pore size of blended cement paste. Cem. Concr. Compos. 2005, 27, 425–428. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, J.; Han, S. Fractal analysis of relation between strength and pore structure of hardened mortar. Constr. Build. Mater. 2017, 135, 1–7. [Google Scholar] [CrossRef]
- Fernando, P.-T.; Joao, C.-G.; Said, J. Alkali-activated binders: A review Part 1. Historical background, terminology, reaction mechanisms and hydration products. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar]
- Jansen, D.; Goetz-Neunhoeffer, F.; Stabler, C.; Neubauer, J. A remastered external standard method applied to the quantification of early OPC hydration. Cem. Concr. Res. 2011, 41, 602–608. [Google Scholar] [CrossRef]
- De Rojas, M.I.S.; Luxan, M.P.; Frias, M.; Garcia, N. The influence of different additions on portland cement hydration heat. Cem. Concr. Res. 1993, 23, 46–54. [Google Scholar] [CrossRef]
- Dyer, T.D.; Dhir, R.K. Hydration reactions of cement combinations containing vitrified incinerator fly ash. Cem. Concr. Res. 2004, 34, 849–856. [Google Scholar] [CrossRef]
- Bullard, J.W.; Jennings, H.M.; Livingston, R.A.; Nonat, A.; Scherer, G.W.; Schweitzer, J.S.; Scrivener, K.L.; Thomas, J.J. Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Briendl, L.G.; Mittermayr, F.; Baldermann, A.; Steindl, F.R.; Sakoparnig, M.; Letofsky-Papst, I.; Galan, I. Early hydration of cementitious systems accelerated by aluminium sulphate: Effect of fine limestone. Cem. Concr. Res. 2020, 134, 106069. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Damidot, D.; Lothenbach, B.; Herfort, D.; Glasser, F.P. Thermodynamics and cement science. Cem. Concr. Res. 2011, 41, 679–695. [Google Scholar] [CrossRef]
- Duchesne, J.; Be’rubé, M.A. Effect of supplementary cementing materials on the composition of cement hydration products. Adv. Cem. Based Mater. 1995, 2, 43–52. [Google Scholar] [CrossRef]
- Girão, A.V.; Richardson, I.G.; Taylor, R.; Brydson, R.M.D. Composition, morphology and nanostructure of C–S–H in 70% white Portland cement–30% fly ash blends hydrated at 55 °C. Cem. Concr. Res. 2010, 40, 1350–1359. [Google Scholar] [CrossRef]
- Richardson, I.G.; Groves, G.W. The incorporation of minor and trace elements into calcium silicate hydrate (C S H) gel in hardened cement pastes. Cem. Concr. Res. 1993, 23, 131–138. [Google Scholar] [CrossRef]
- Chen, J.J.; Thomas, J.J.; Taylor, H.F.W.; Jennings, H.M. Solubility and structure of calcium silicate hydrate. Cem. Concr. Res. 2004, 34, 1499–1519. [Google Scholar] [CrossRef] [Green Version]
- Hwang, C.; Shen, D. The effects of blast-furnace slag and fly ash on the hydration of Portland cement. Cem. Concr. Res. 1991, 21, 410–425. [Google Scholar] [CrossRef]
- Rahhal, V.; Talero, R. Influence of two different fly ashes on the hydration of Portland cements. J. Therm. Anal. Calorim. 2004, 78, 191–205. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Zuo, L.; Wang, L.; Zhu, Q.; Wang, M. Investigation into the effect of calcium on the existence form of geopolymerized gel product of fly ash based geopolymers. Cem. Concr. Compos. 2019, 103, 279–292. [Google Scholar] [CrossRef]




| Element | SC | CṠA | FA | ST |
|---|---|---|---|---|
| CaO | 55.24 | 53.91 | 4.08 | 0.03 |
| SiO2 | 26.15 | 13.60 | 55.51 | 96.46 |
| Al2O3 | 8.83 | 15.31 | 30.82 | 1.87 |
| MgO | 2.93 | 3.01 | 0.63 | – |
| Fe2O3 | 3.02 | 2.03 | 4.02 | 0.07 |
| Na2O | 0.02 | 0.34 | 0.04 | 0.01 |
| K2O | 1.27 | 0.81 | 2.49 | 1.30 |
| SO2 | 1.77 | 10.35 | 0.79 | 0.03 |
| SSA a (m2/kg) | 528.42 | 496.93 | 297.91 | 227.27 |
| Blended Binder | SC | CṠA | FA | Quicklime |
|---|---|---|---|---|
| SC100 | 100 | 0 | 0 | 0 |
| SCA0 | 50 | 0 | 40 | 10 |
| SCA5 | 45 | 5 | 40 | 10 |
| SCA15 | 35 | 15 | 40 | 10 |
| SCC5 | 45 | 10 | 40 | 5 |
| SCC10 | 40 | 10 | 40 | 10 |
| SCC15 | 35 | 10 | 40 | 15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.; Zhang, S. Quicklime and Calcium Sulfoaluminate Cement Used as Mineral Accelerators to Improve the Properties of Cemented Paste Backfill with a High Volume of Fly Ash. Materials 2020, 13, 4018. https://doi.org/10.3390/ma13184018
Ding H, Zhang S. Quicklime and Calcium Sulfoaluminate Cement Used as Mineral Accelerators to Improve the Properties of Cemented Paste Backfill with a High Volume of Fly Ash. Materials. 2020; 13(18):4018. https://doi.org/10.3390/ma13184018
Chicago/Turabian StyleDing, Hangxing, and Shiyu Zhang. 2020. "Quicklime and Calcium Sulfoaluminate Cement Used as Mineral Accelerators to Improve the Properties of Cemented Paste Backfill with a High Volume of Fly Ash" Materials 13, no. 18: 4018. https://doi.org/10.3390/ma13184018
APA StyleDing, H., & Zhang, S. (2020). Quicklime and Calcium Sulfoaluminate Cement Used as Mineral Accelerators to Improve the Properties of Cemented Paste Backfill with a High Volume of Fly Ash. Materials, 13(18), 4018. https://doi.org/10.3390/ma13184018






