Influence of Rare Earth Element (Y) on Microstructure and Corrosion Behavior of Hot Extrusion AZ91 Magnesium Alloy
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Solutions
2.2. Microstructure Examination
2.3. Electrochemical Measurement
2.4. Weight Loss Measurement
3. Results
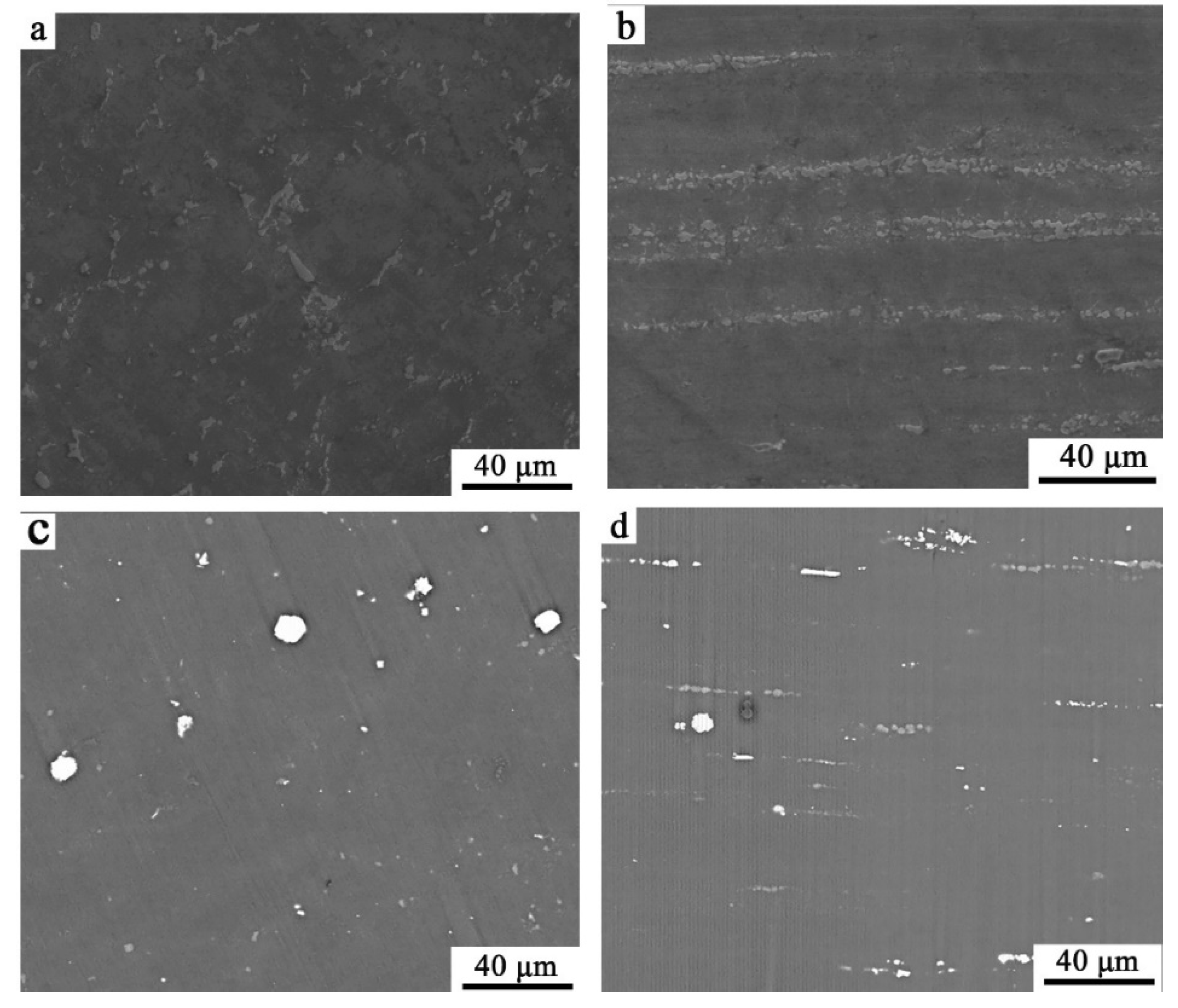
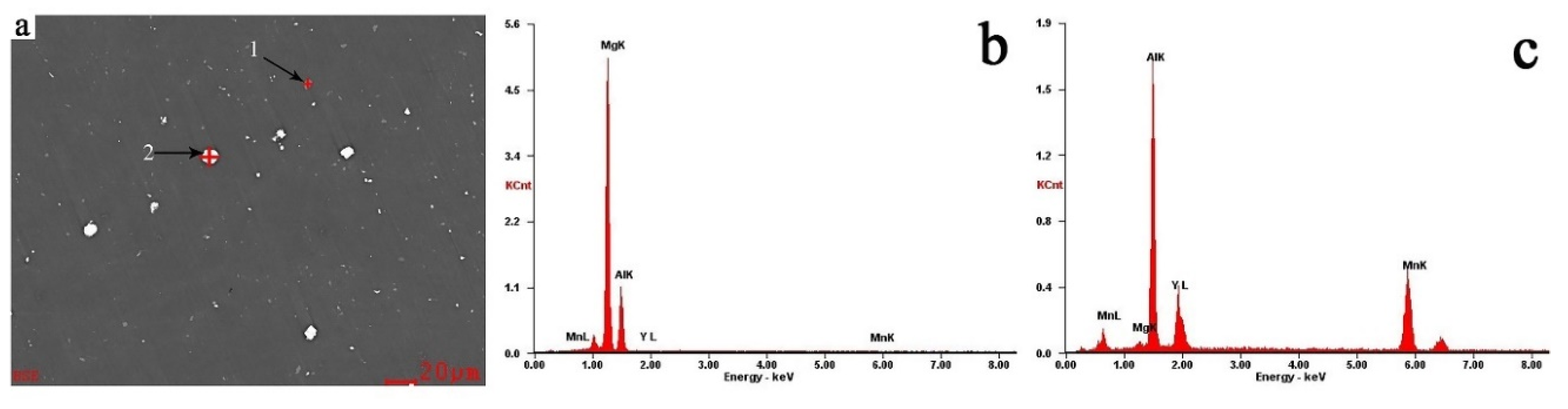
3.1. Scanning Electron Microscopy
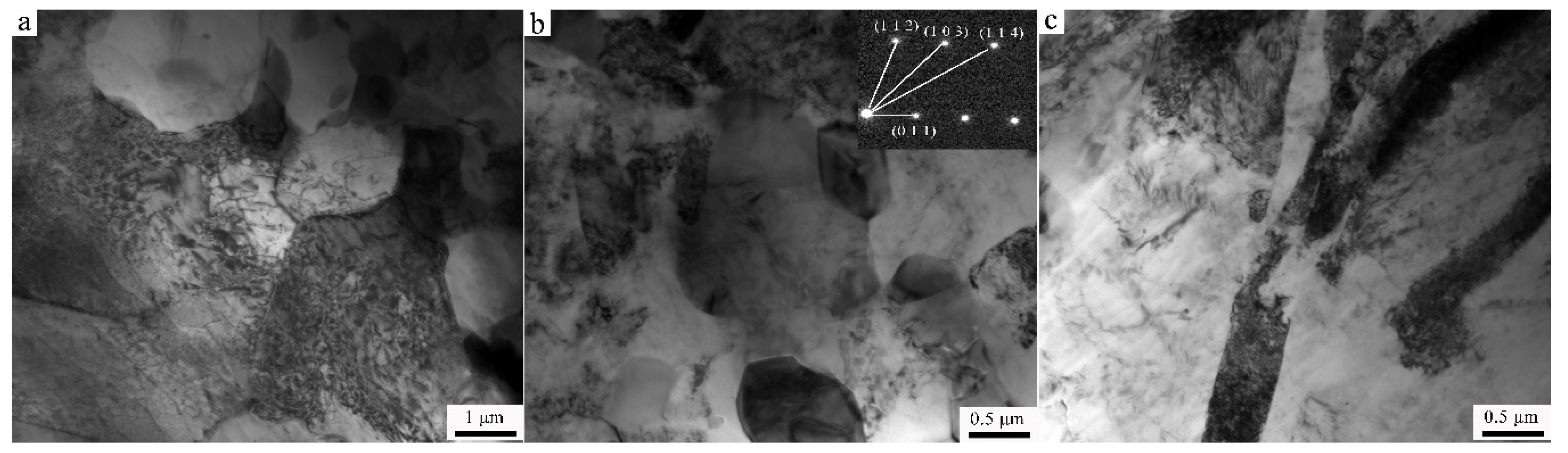
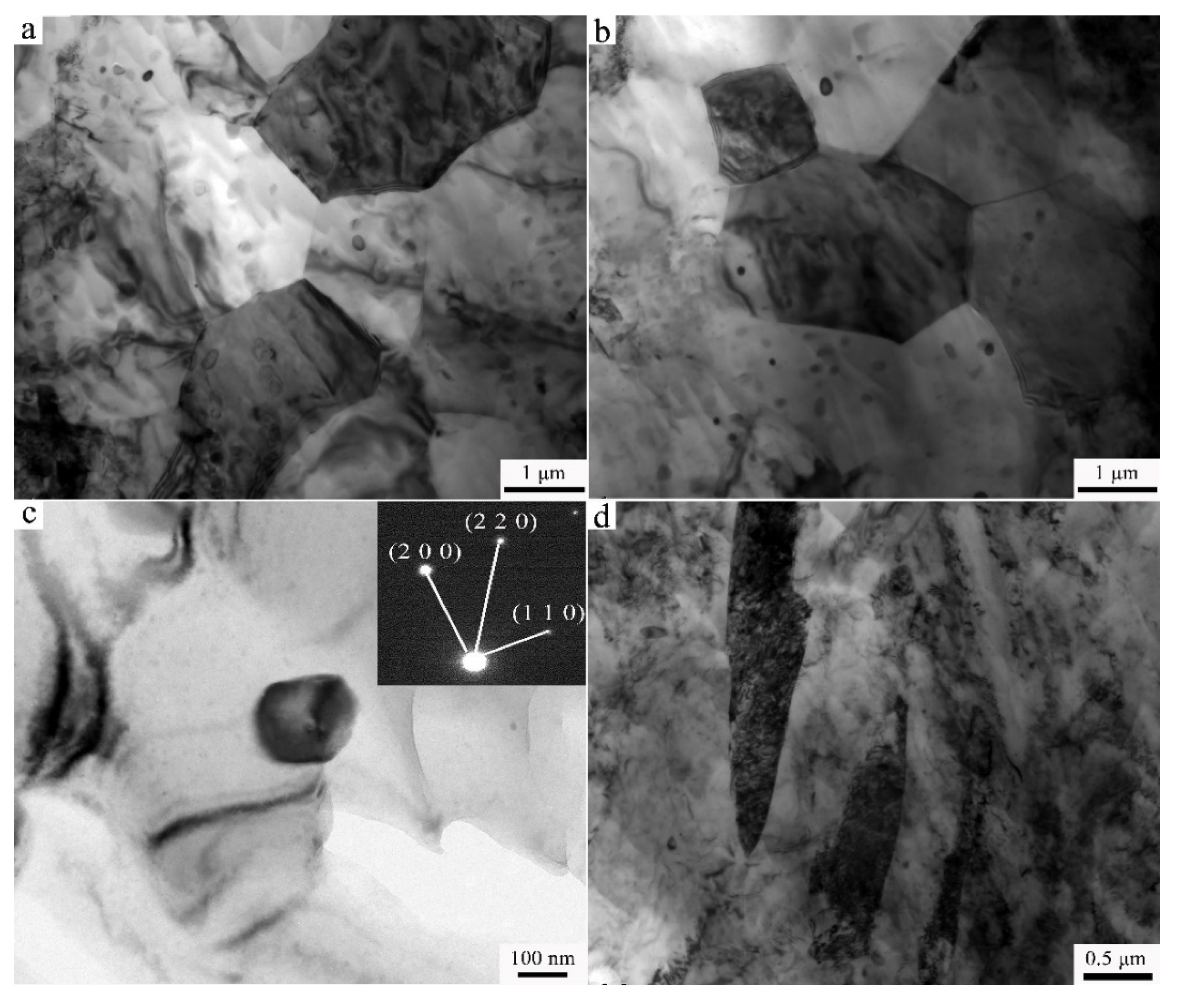
3.2. Transmission Electron Microscopy
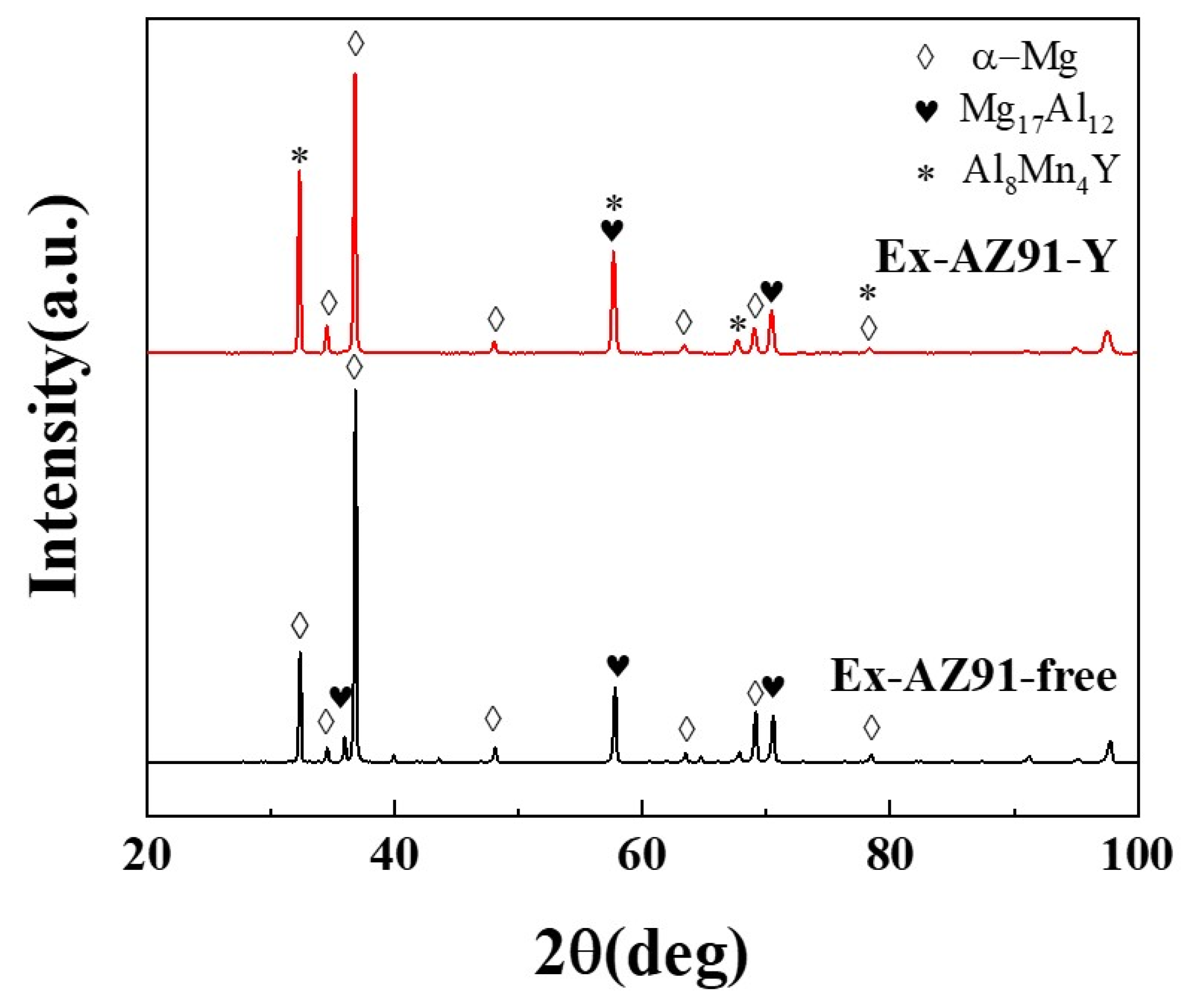
3.3. X-ray Diffraction Spectrometry
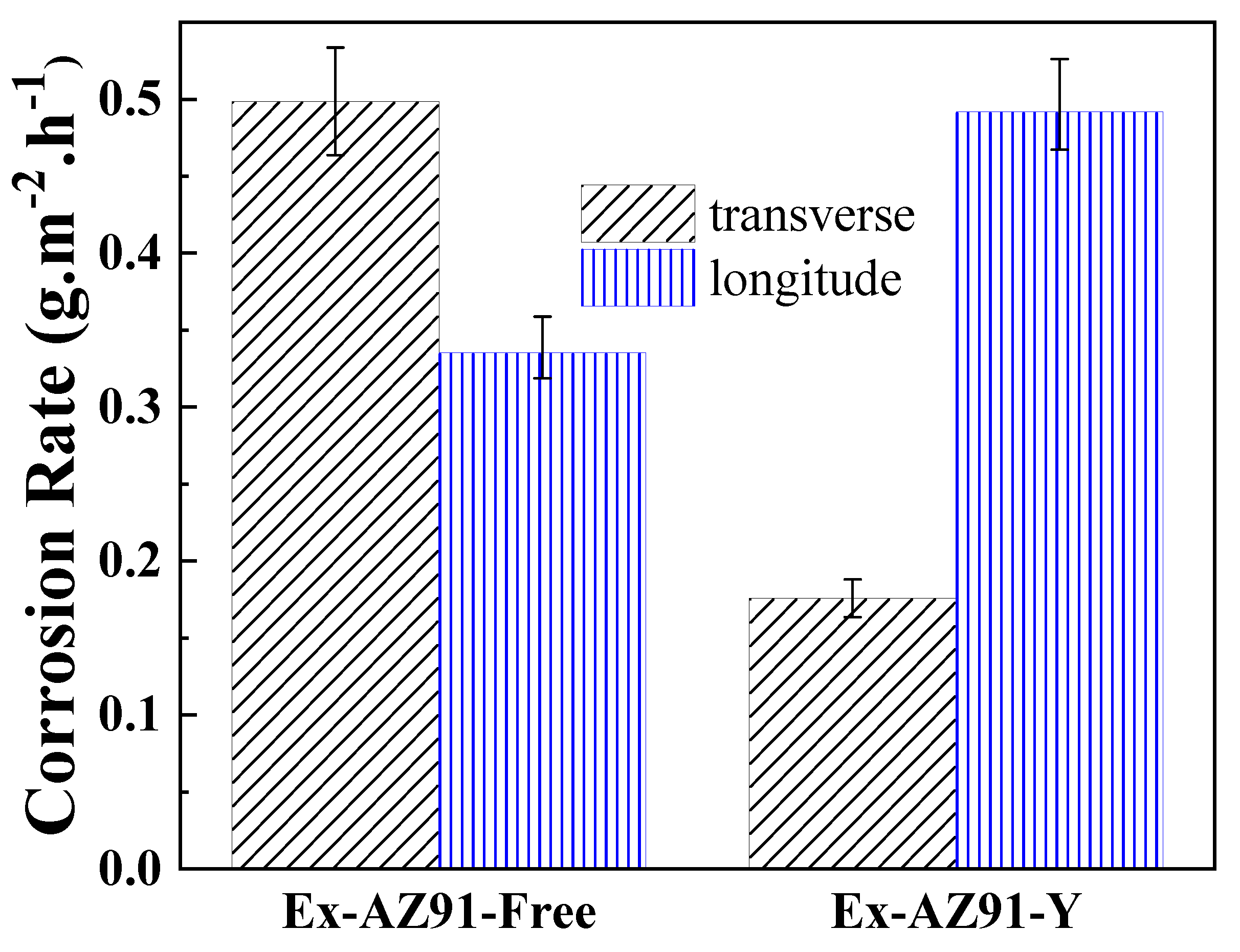
3.4. Weight Loss Measurement
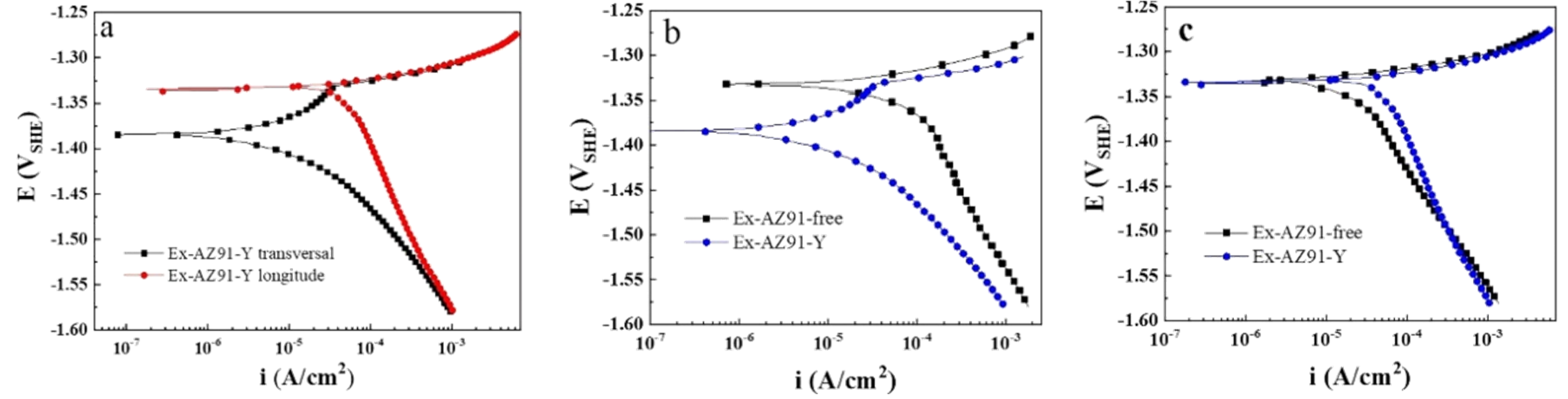
3.5. Potentiodynamic Polarization Curve
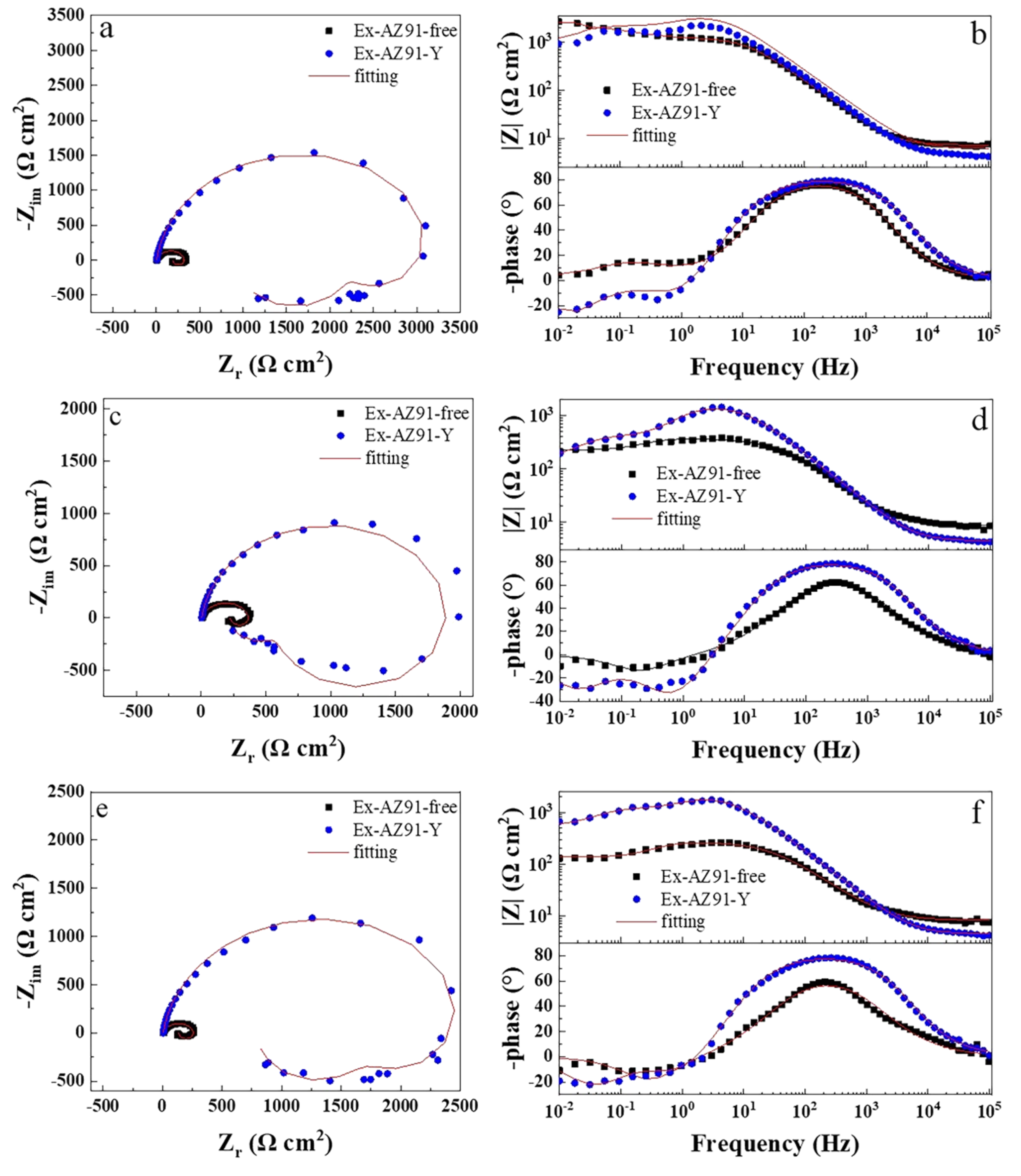
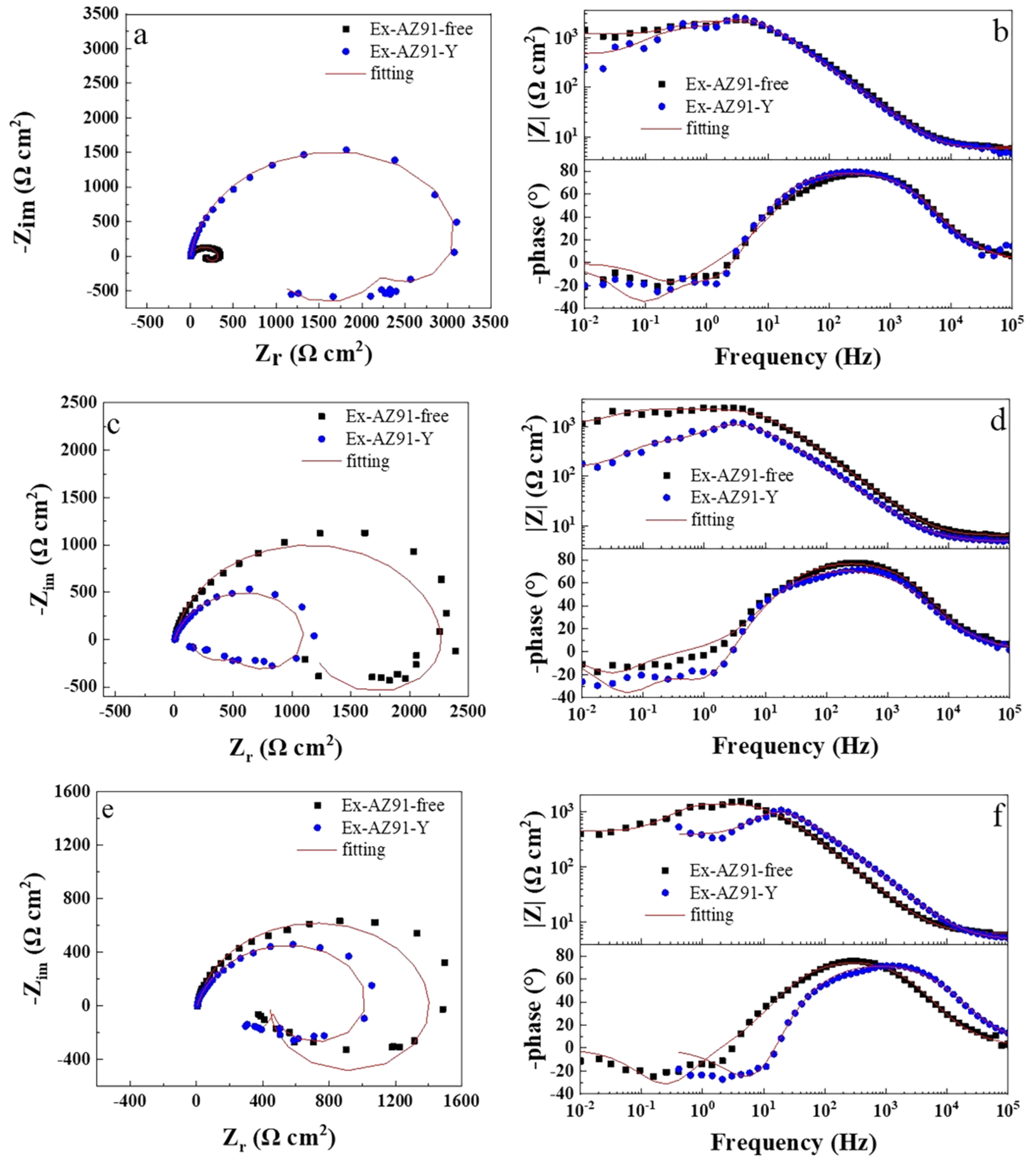
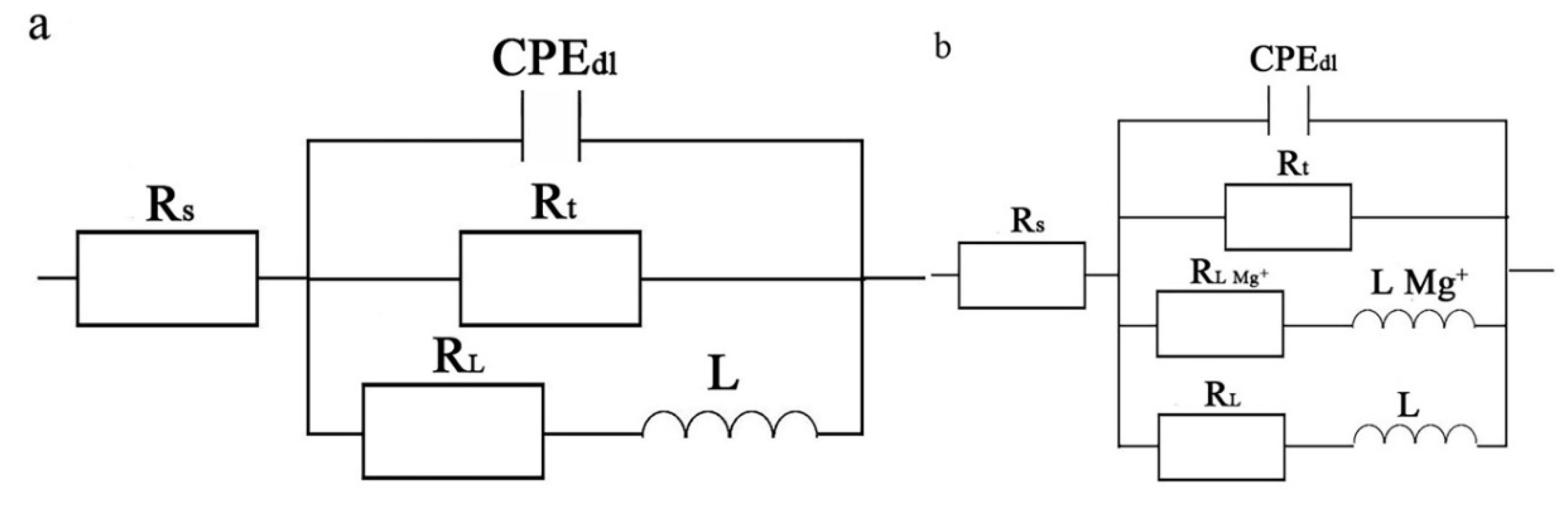
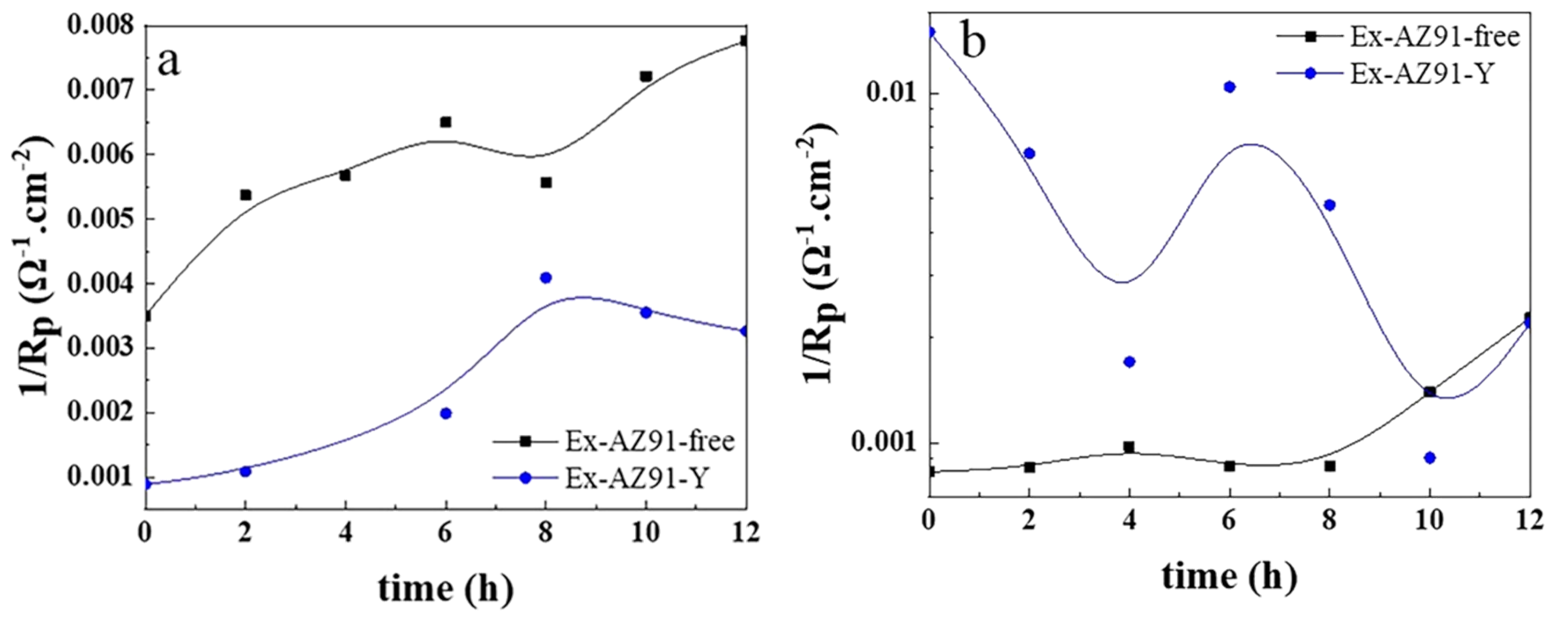
3.6. Electrochemical Impedance Spectroscopy
3.7. Corrosion Morphology
4. Discussions
- The results of TEM manifest the size of the second phase to be about 0.5 μm in the Ex-AZ91-free alloy and about 100 nm in the Ex-AZ91-Y alloy. The size of the second phase of Ex-AZ91-free alloy is five times that of Ex-AZ91-Y alloy. Hence, the second phase of Ex-AZ91-Y alloy is considered as a signal piece and the second phase of Ex-AZ91-free alloy is assumed as five pieces.
- It is supposed that the corrosion potential of the α and second phase, the polarization resistivity of the α phase in Ex-AZ91-free alloy and Ex-AZ91-Y alloy are same, and the ΔE is equal to the difference value between the second phase and α phase.
- L is named as the distance between the piece of the second phase(β1) and α phase. Therefore, 2L, 3L, 4L, and 5L are the distances between the α phase and the piece of the second phase (β2, β3, β4, and β5), respectively.
5. Conclusions
- With the addition of Y, the β phase of Ex-AZ91 alloy is reduced and replaced by the Al-Mn-RE phase. Moreover, the volume fraction of the second phase in Ex-AZ91-Y alloy is lower than that of Ex-AZ91-free alloy.
- The grains of Ex-AZ91 alloy were refined, the dislocation was not observed, and twin crystals reduced after adding Y.
- The corrosion resistance of the transversal section of Ex-AZ91-Y alloy was improved compared with the transversal section of Ex-AZ91-free alloy and the corrosion resistance of the longitudinal section of Ex-AZ91-Y alloy was reduced compared with the longitudinal section of Ex-AZ91-free alloy. The improved corrosion resistance of the transversal section of Ex-AZ91-Y alloy can be attributed to the decreased volume fraction of the second phase and the decreased cathodic rate. The volume fraction of the longitudinal section of Ex-AZ91-Y alloy also reduced, but the accelerated cathodic corrosion rate of the longitudinal section had a more significant influence on the corrosion resistance, leading to decreased corrosion resistance of the longitudinal section.
Author Contributions
Funding
Conflicts of Interest
References
- Seifzadeh, D.; Bezaatpour, A.; Asadpour Joghani, R. Corrosion inhibition effect of N, N′-bis (2-pyridylmethylidene)-1,2-diiminoethane on AZ91D magnesium alloy in acidic media. Trans. Nonferr. Met. Soc. China 2014, 24, 3441–3451. [Google Scholar] [CrossRef]
- Gao, H.; Li, Q.; Dai, Y.; Luo, F.; Zhang, H.X. High efficiency corrosion inhibitor 8-hydroxyquinoline and its synergistic effect with sodium dodecylbenzenesulphonate on AZ91D magnesium alloy. Corros. Sci. 2010, 52, 1603–1609. [Google Scholar] [CrossRef]
- Gray, J.E.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Funatani, K. Emerging technology in surface modification of light metals. Surf. Coat. Technol. 2000, 133, 264–272. [Google Scholar] [CrossRef]
- Sudagar, J.; Ling, J.; Chen, X.; Liang, P.; Liang, Y. High corrosion resistance of electroless Ni-P with chromium-free conversion pre-treatments on AZ91D magnesium alloy. Trans. Nonferr. Met. Soc. China 2011, 21, 921–928. [Google Scholar] [CrossRef]
- Hua, J.; Zeng, D.; Zhang, Z.; Shi, T.; Song, G.; Guo, X. 2-hydroxy-4-methoxy-acetophenone as an environment-friendly corrosion inhibitor for AZ91D magnesium alloy. Corros. Sci. 2013, 74, 35–43. [Google Scholar] [CrossRef]
- Pu, Z.; Song, G.L.; Yang, S.; Outeiro, J.C.; Dillon, O.W., Jr.; Puleo, D.A.; Jawahir, I.S. Grain refined and basal textured surface produced by burnishing for improved corrosion performance of AZ31B Mg alloy. Corros. Sci. 2012, 57, 192–201. [Google Scholar] [CrossRef]
- Song, G.L.; Xu, Z. Crystal orientation and electrochemical corrosion of polycrystalline Mg. Corros. Sci. 2012, 54, 97–105. [Google Scholar] [CrossRef]
- Cui, Z.; Ge, F.; Lin, Y.; Wang, L.; Lei, L.; Tian, H.Y.; Yu, M.D.; Wang, X. Corrosion behavior of AZ31 magnesium alloy in the chloride solution containing ammonium nitrate. Electrochim. Acta 2018, 278, 421–437. [Google Scholar] [CrossRef]
- Liu, X.; Hou, L.; Wang, H.; Li, Y.; Wei, H.; Liu, B.; Du, H.; Wei, Y. Synergistic inhibition behavior of sodium diethyldithiocarbamate and sodium acetate for AZ31B magnesium alloy in NaCl solution. Mater. Express 2019, 9, 28–41. [Google Scholar] [CrossRef]
- Chen, X.B.; Birbilis, N.; Abbott, T.B. Review of corrosion-resistant conversion coatings for magnesium and its alloys. Corrosion 2011, 67, 1–16. [Google Scholar] [CrossRef]
- Saji, V.S. Organic conversion coatings for magnesium and its alloys. J. Ind. Eng. Chem. 2019, 75, 20–37. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Vaghefinazari, B.; Mei, D.; Petrauskas, R.P.; Höche, D.; Zheludkevich, M.L. Comprehensive screening of Mg corrosion inhibitors. Corros. Sci. 2017, 128, 224–240. [Google Scholar] [CrossRef]
- Lia, Z.; Yu, Q.; Zhang, C.; Liua, Y.; Liang, J.; Wang, D.; Zhou, F. Synergistic effect of hydrophobic film and porous MAO membrane containing alkynol inhibitor for enhanced corrosion resistance of magnesium alloy. Surf. Coat. Technol. 2019, 357, 515–525. [Google Scholar] [CrossRef]
- Muhammad, J.A.; Zhao, J.; Vahdat, Z.A.; Ghulam, Y.; Wang, W.; Wei, S.; Zhao, Z.; Waheed, Q.K. In-situ intercalation of 8-hydroxyquinoline in Mg-Al LDH coating to improve the corrosion resistance of AZ31. Corros. Sci. 2019, 157, 1–10. [Google Scholar]
- Baek, S.M.; Kang, J.S.; Shin, H.J.; Chang, D.Y.; You, B.S. Role of alloyed Y in improving the corrosion resistance of extruded Mg-Al-Ca-based alloy. Corros. Sci. 2017, 118, 227–232. [Google Scholar] [CrossRef]
- Zhang, T.; Meng, G.; Shao, Y.; Cui, Z.; Wang, F. Corrosion of hot extrusion AZ91 magnesium alloy. Part II: Effect of rare earth element neodymium (Nd) on the corrosion behavior of extruded alloy. Corros. Sci. 2011, 53, 2934–2942. [Google Scholar] [CrossRef]
- Yamasaki, M.; Hayashi, N.; Izumi, S.; Kawamura, Y. Corrosion behavior of rapidly solidified Mg-Zn-rare earth element alloys in NaCl solution. Corros. Sci. 2007, 49, 255–262. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, Y.; Wei, Z.; Chen, Q.; Gan, F. Improvement of corrosion resistance of AZ91D magnesium alloy by holmium addition. Corros. Sci. 2006, 48, 4223–4233. [Google Scholar] [CrossRef]
- Guo, X.; Chang, J.; Fu, S.P.; Peng, L.; Ding, W. Effect of heat treatment on corrosion and electrochemical behaviour of Mg-3Nd-0.2Zn-0.4Zr (wt.%) alloy. Electrochim. Acta 2007, 52, 3160–3167. [Google Scholar]
- Liu, X.; Shan, D.; Song, Y.; Han, E. Influence of yttrium element on the corrosion behaviors of Mg-Y binary magnesium alloy. J. Magnes. Alloy. 2017, 5, 26–34. [Google Scholar] [CrossRef]
- Liu, M.; Schmutz, P.; Uggowitzer, P.; Song, G.; Atrens, A. The influence of yttrium (Y) on the corrosion of Mg-Y binary alloys. Corros. Sci. 2010, 52, 3687–3701. [Google Scholar] [CrossRef]
- Takenaka, T.; Ono, T.; Narazaki, Y.; Naka, Y.; Kawakami, M. Improvement of corrosion resistance of magnesium metal by rare earth elements. Electrochim. Acta 2007, 53, 117–121. [Google Scholar] [CrossRef]
- Rosalbino, F.; Angelini, E.; De Negri, S.; Saccone, A.; Delfino, S. Effect of erbium addition on the corrosion behavior of Mg-Al alloys. Intermetallics 2005, 13, 55–60. [Google Scholar] [CrossRef]
- Rosalbino, F.; Angelini, E.; De Negri, S.; Saccone, A.; Delfino, S. Electrochemical behaviour assessment of novel Mg-rich Mg–Al–RE alloys (RE = Ce, Er). Intermetallics 2006, 14, 1487–1492. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, K.; Hu, K.; Wang, Z.; Du, J. Influence of mixed rare earth elements (Y and Ce) on the microstructure and corrosion behaviour of Mg-4Al-3Ca alloy. Mater. Res. Express 2019, 6, 016555. [Google Scholar] [CrossRef]
- Wu, P.P.; Xu, F.J.; Deng, K.K.; Han, F.Y.; Zhang, Z.Z.; Gao, R. Effect of extrusion on corrosion properties of Mg-2Ca-χAl (χ = 0, 2, 3, 5) alloys. Corros. Sci. 2017, 127, 280–290. [Google Scholar] [CrossRef]
- Orlov, D.; Ralston, K.D.; Birbilis, N.; Estrin, Y. Enhanced corrosion resistance of Mg alloy ZK60 after processing by integrated extrusion and equal channel angular pressing. Acta Mater. 2011, 59, 6176–6186. [Google Scholar] [CrossRef]
- Luo, Y.; Deng, Y.; Guan, L.; Ye, L.; Guo, X.; Luo, A. Effect of grain size and crystal orientation on the corrosion behavior of as-extruded Mg-6Gd-2Y-0.2Zr alloy. Corros. Sci. 2020, 164, 108338. [Google Scholar] [CrossRef]
- Jeong, Y.S.; Kim, W.J. Enhancement of mechanical properties and corrosion resistance of Mg-Ca alloys through microstructural refinement by indirect extrusion. Corros. Sci. 2014, 82, 392–403. [Google Scholar] [CrossRef]
- Ben-Haroush, M.; Ben-Hamu, G.; Eliezer, D.; Wagner, L. The relation between microstructure and corrosion behavior of AZ80 Mg alloy following different extrusion temperatures. Corros. Sci. 2008, 50, 1766–1778. [Google Scholar] [CrossRef]
- Ben-Hamu, G.; Eliezer, D.; Wagner, L. The relation between severe plastic deformation microstructure and corrosion behavior of AZ31 magnesium alloy. J. Alloys Compd. 2009, 468, 222–229. [Google Scholar] [CrossRef]
- Hu, J.; Huang, D.; Zhang, G.; Song, G.; Guo, X. Research on the inhibition mechanism of tetraphenylporphyrin on AZ91D magnesium alloy. Corros. Sci. 2012, 63, 367–378. [Google Scholar] [CrossRef]
- Zhang, T.; Shao, Y.; Meng, G.; Cui, Z.; Wang, F. Corrosion of hot extrusion AZ91 magnesium alloy: I-relation between the microstructure and corrosion behavior. Corros. Sci. 2011, 53, 1960–1968. [Google Scholar] [CrossRef]
- Poorqasemi, E.; Abootalebi, O.; Peikari, M.; Haqdar, F. Investigating accuracy of the Tafel extrapolation method in HCl solutions. Corros. Sci. 2009, 51, 1043–1054. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Han, E.; Dong, J.; Ke, W. AC impedance spectroscopy study of the corrosion behavior of an AZ91 magnesium alloy in 0.1 M sodium sulfate solution. Electrochim. Acta 2007, 52, 3299–3309. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Understanding magnesium corrosion. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Song, G. Potential and current distributions of one-dimensional galvanic corrosion systems. Corros. Sci. 2010, 52, 455–480. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, T.; Wang, Y.; Wei, F.; Shao, Y.; Meng, G.; Wang, F.; Wu, K. Effect of SiC particulates on the corrosion behavior of extruded AZ91/SICp composites during the early stage of exposure. J. Electrochem. Soc. 2015, 162, 754–766. [Google Scholar] [CrossRef]


| Element | Al | Zn | Mn | Fe | Si | Y | Mg |
|---|---|---|---|---|---|---|---|
| Ex-AZ91-Free | 8.98 | 0.98 | 0.205 | 0.003 | 0.002 | 0 | Bal |
| Ex-AZ91-Y | 8.98 | 0.98 | 0.205 | 0.003 | 0.002 | 1.5 | Bal |
| Specimen | Ex-AZ91-Free Alloy (%) | Ex-AZ91-Y Alloy (%) |
|---|---|---|
| transverse | 2.65 | 0.80 |
| longitude | 6.59 | 1.32 |
| Element | Point 1 | Point 2 | ||
|---|---|---|---|---|
| Wt% | At% | Wt% | At% | |
| Mg K | 62.77 | 65.45 | 0.86 | 1.46 |
| Al K | 36.43 | 34.23 | 39.65 | 60.34 |
| Y L | 0.25 | 0.07 | 21.94 | 10.14 |
| Mn K | 0.55 | 0.25 | 37.55 | 28.07 |
| Matrix | correction | ZAF | correction | ZAF |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Wang, Y.; Cui, Z.; Qi, W.; Wang, J.; Ju, P.; Zhao, Y.; Liu, B.; Zhang, T.; Wang, F. Influence of Rare Earth Element (Y) on Microstructure and Corrosion Behavior of Hot Extrusion AZ91 Magnesium Alloy. Materials 2020, 13, 3651. https://doi.org/10.3390/ma13163651
Cui Y, Wang Y, Cui Z, Qi W, Wang J, Ju P, Zhao Y, Liu B, Zhang T, Wang F. Influence of Rare Earth Element (Y) on Microstructure and Corrosion Behavior of Hot Extrusion AZ91 Magnesium Alloy. Materials. 2020; 13(16):3651. https://doi.org/10.3390/ma13163651
Chicago/Turabian StyleCui, Yanan, Yonghai Wang, Zhongyu Cui, Wenlong Qi, Jidong Wang, Pengfei Ju, Yang Zhao, Bin Liu, Tao Zhang, and Fuhui Wang. 2020. "Influence of Rare Earth Element (Y) on Microstructure and Corrosion Behavior of Hot Extrusion AZ91 Magnesium Alloy" Materials 13, no. 16: 3651. https://doi.org/10.3390/ma13163651
APA StyleCui, Y., Wang, Y., Cui, Z., Qi, W., Wang, J., Ju, P., Zhao, Y., Liu, B., Zhang, T., & Wang, F. (2020). Influence of Rare Earth Element (Y) on Microstructure and Corrosion Behavior of Hot Extrusion AZ91 Magnesium Alloy. Materials, 13(16), 3651. https://doi.org/10.3390/ma13163651






