Microstructure and Composition Evolution of a Fused Slurry Silicide Coating on MoNbTaTiW Refractory High-Entropy Alloy in High-Temperature Oxidation Environment
Abstract
1. Introduction
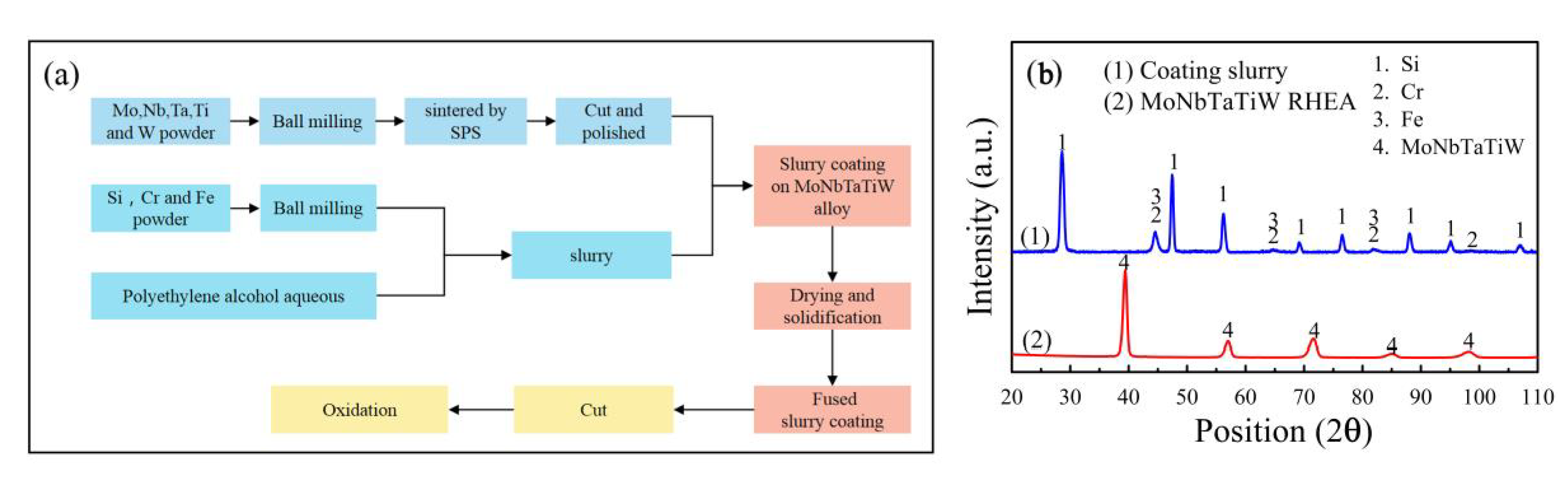
2. Materials and Methods
3. Results and Discussion
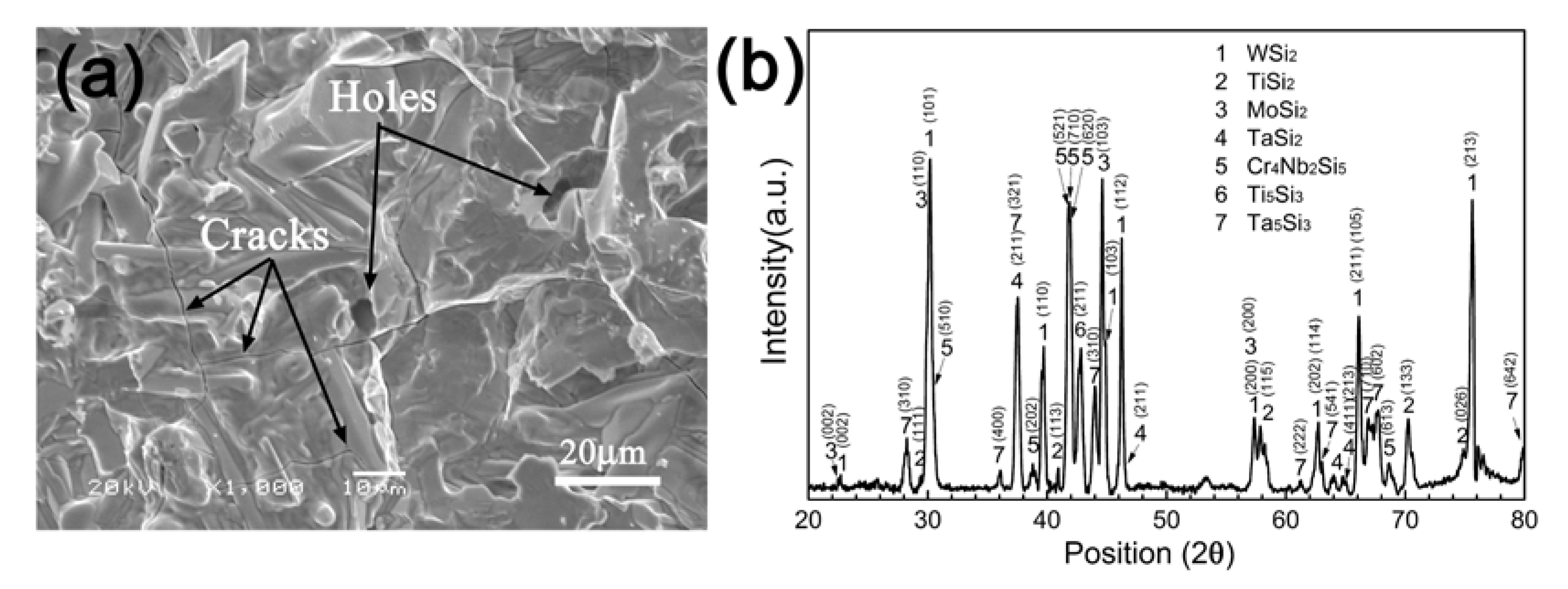
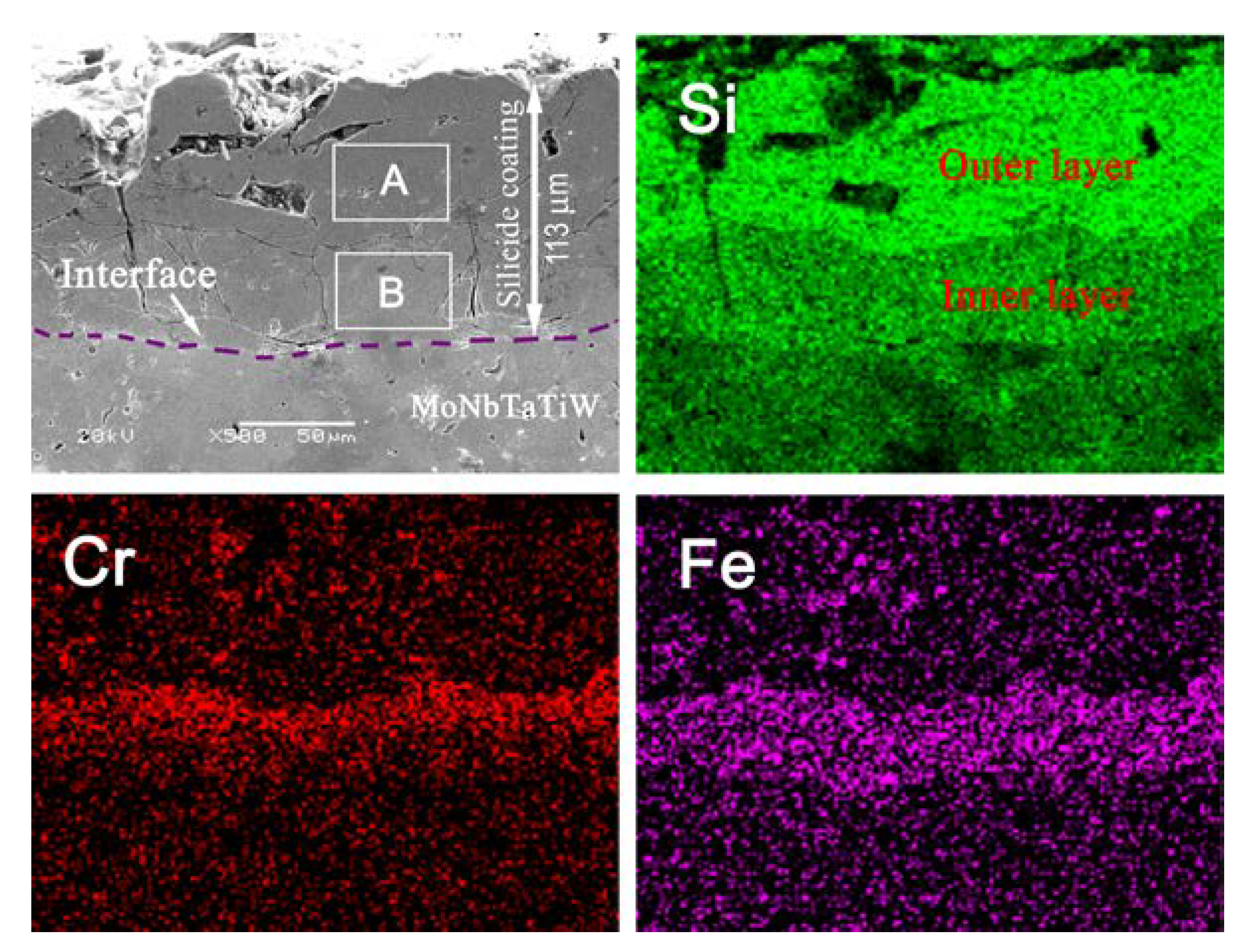
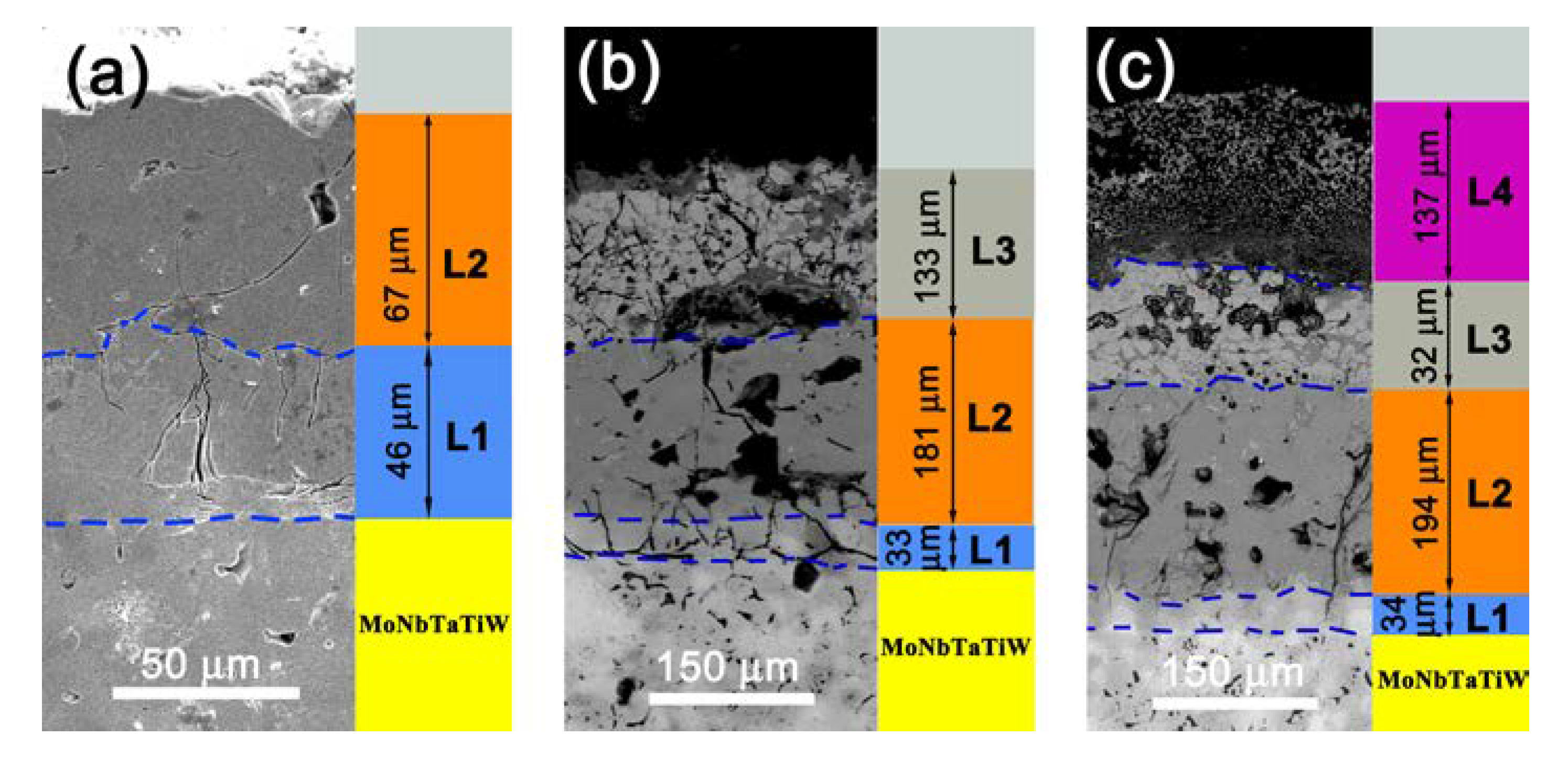
3.1. Morphology and Microstructure of the Silicide Coating
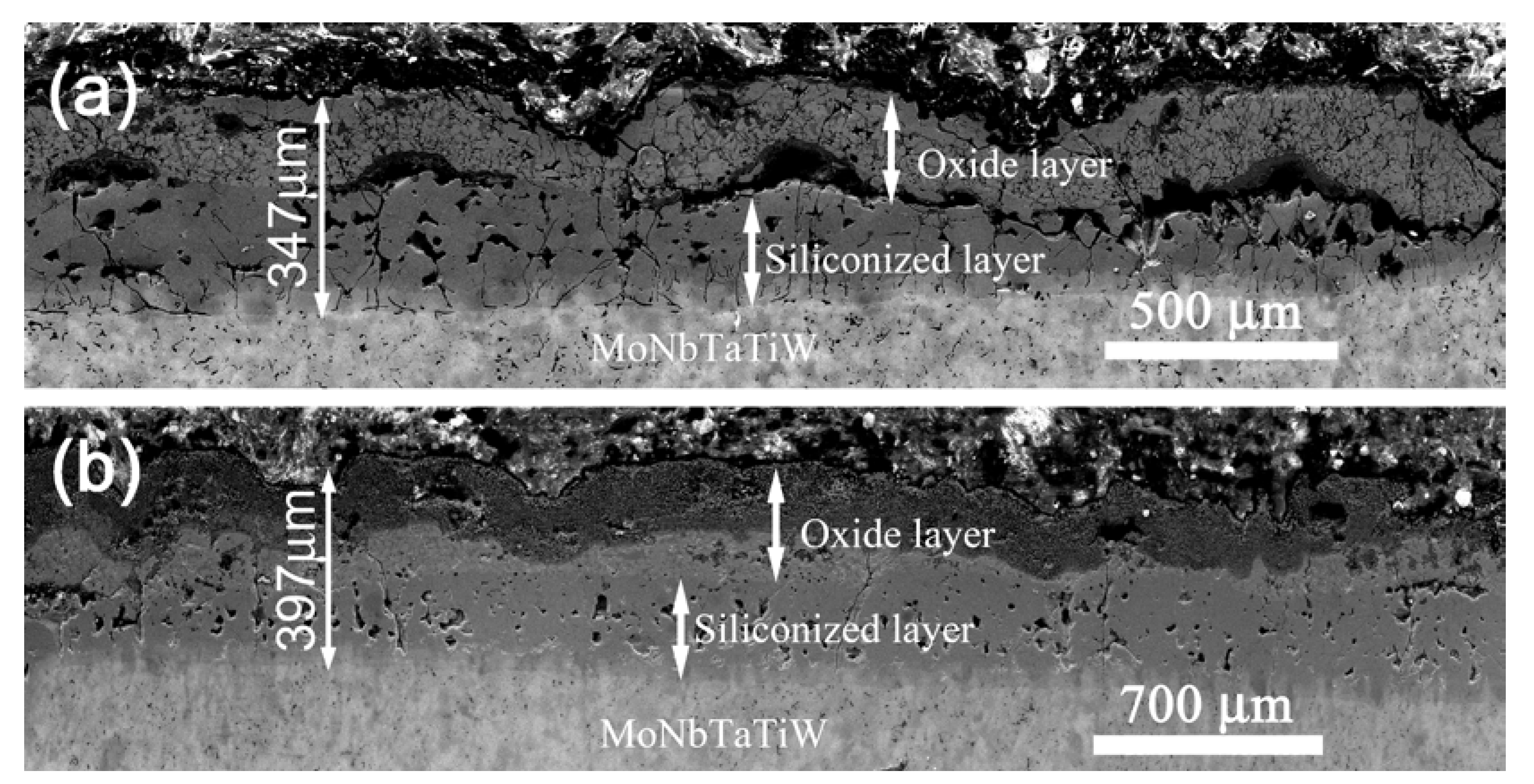
3.2. Oxidation Behavior of the Silicide Coating
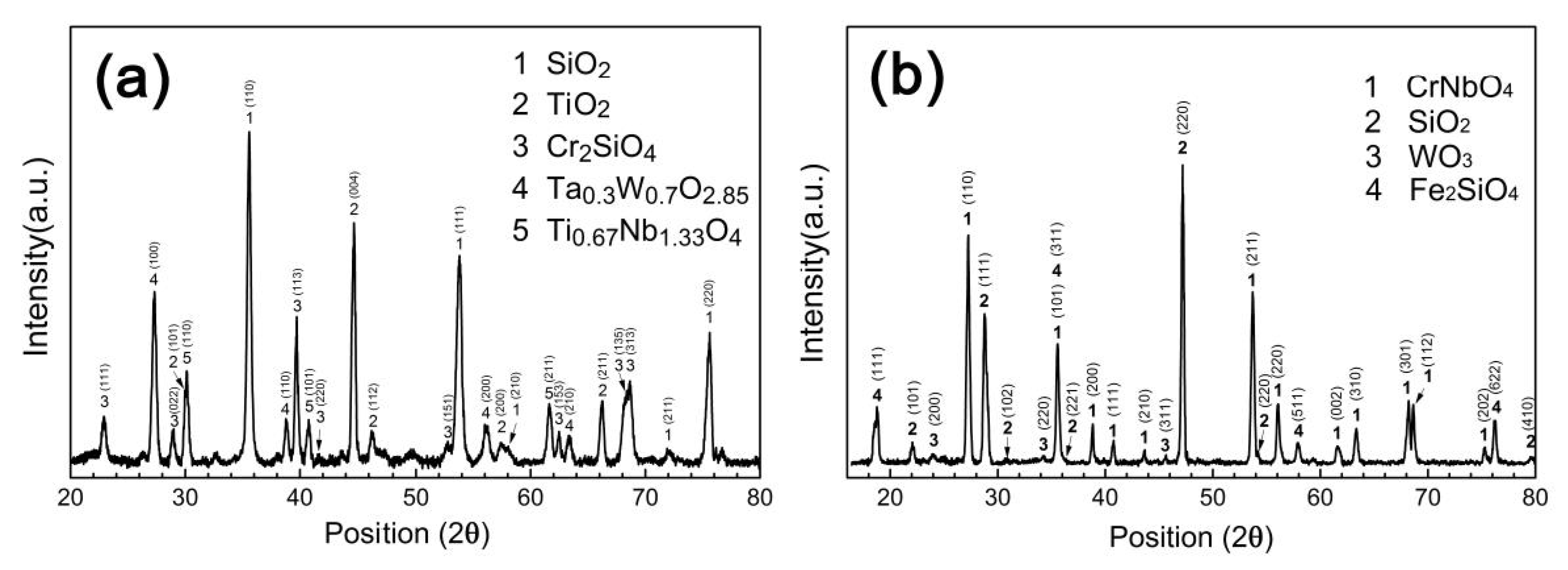
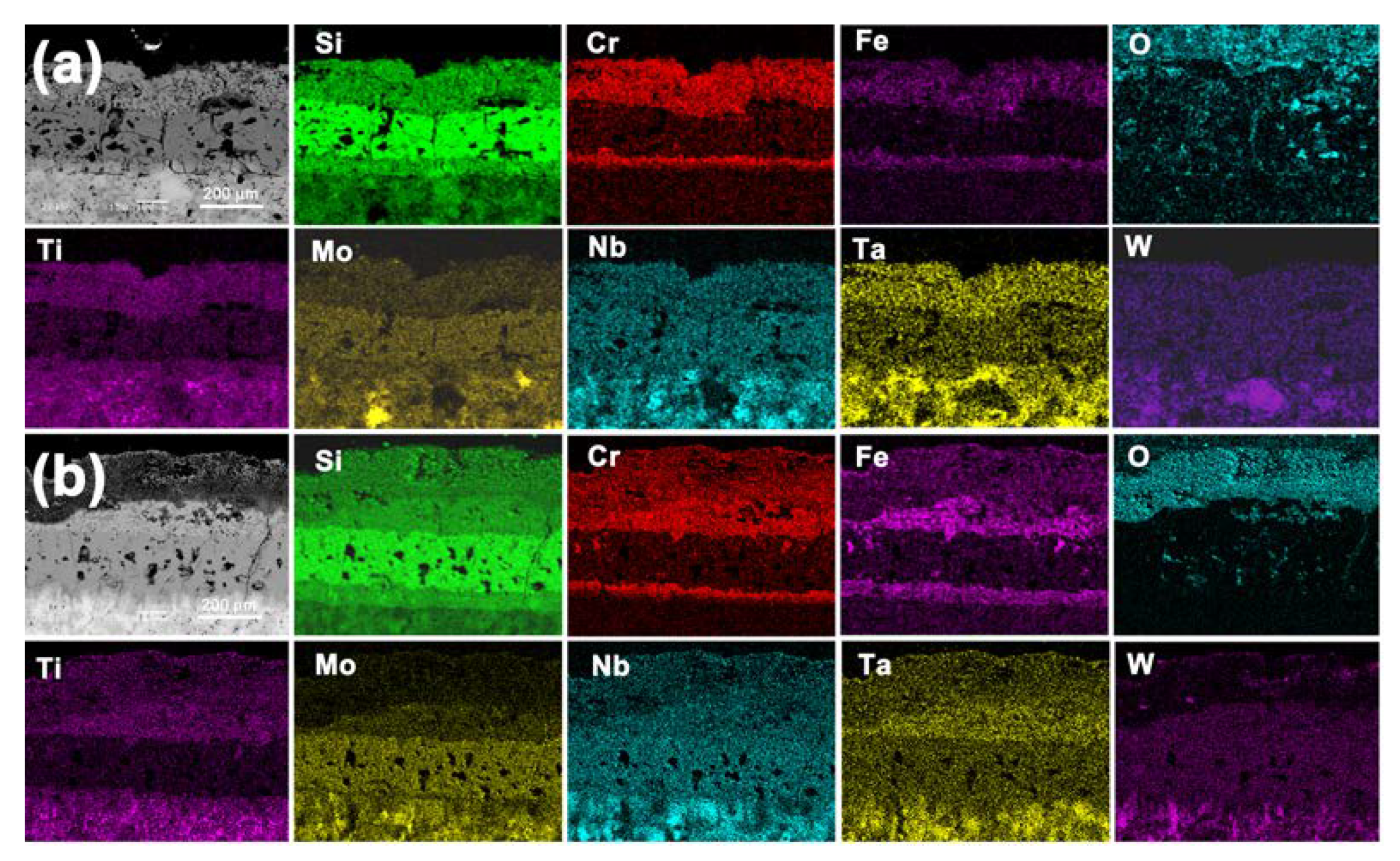
3.3. Evolution of the Structure and Compositions of Silicide Coating during the Oxidation Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tsai, M.H.; Yeh, J.W. High-Entropy Alloys: A Critical Review, Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Gao, M.C. Progress in High-Entropy Alloys. JOM 2014, 66, 1964–1965. [Google Scholar] [CrossRef]
- Torralba, J.M.; Alvaredo, P.; García-Junceda, A. High-entropy alloys fabricated via powder metallurgy. A critical review. Powder Metall. 2019, 62, 84–114. [Google Scholar] [CrossRef]
- Melnick, A.B.; Soolshenko, V.K. Thermodynamic design of high-entropy refractory alloys. J. Alloys Compd. 2017, 694, 223–227. [Google Scholar] [CrossRef]
- Yao, H.W.; Qiao, J.W.; Hawk, J.A.; Zhou, H.F.; Chen, M.W.; Gao, M.C. Mechanical properties of refractory high-entropy alloys: Experiments and modelling. J. Alloys Compd. 2017, 696, 1139–1150. [Google Scholar] [CrossRef]
- Gao, M.C.; Carney, C.S.; Doğan, Ö.N.; Jablonksi, P.D.; Hawk, J.A.; Alman, D.E. Design of Refractory High-Entropy Alloys. JOM 2015, 67, 2653–2669. [Google Scholar] [CrossRef]
- Senkov, O.; Isheim, D.; Seidman, D.; Pilchak, A. Development of a Refractory High Entropy Superalloy. Entropy 2016, 18, 102. [Google Scholar] [CrossRef]
- Park, J.M.; Kang, J.W.; Lee, W.H.; Lee, S.Y.; Min, S.H.; Ha, T.K.; Park, H.K. Preparation of spherical WTaMoNbV refractory high entropy alloy powder by inductively-coupled thermal plasma. Mater. Lett. 2019, 255, 126513. [Google Scholar] [CrossRef]
- Dobbelstein, H.; Gurevich, E.L.; George, E.P.; Ostendorf, A.; Laplanche, G. Laser metal deposition of compositionally graded TiZrNbTa refractory high-entropy alloys using elemental powder blends. Addit. Manuf. 2019, 25, 252–262. [Google Scholar] [CrossRef]
- Chen, S.Y.; Tong, Y.; Tseng, K.K.; Yeh, J.W.; Poplawsky, J.D.; Wen, J.G.; Gao, M.C.; Kim, G.; Chen, W.; Ren, Y.; et al. Phase transformations of HfNbTaTiZr high-entropy alloy at intermediate temperatures. Scr. Mater. 2019, 158, 50–56. [Google Scholar] [CrossRef]
- Han, Z.D.; Luan, H.W.; Liu, X.; Chen, N.; Li, X.Y.; Shao, Y.; Yao, K.F. Microstructures and mechanical properties of Ti NbMoTaW refractory high-entropy alloys. J. Mater. Eng. A 2018, 712, 380–385. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.P. Development and exploration of refractory high entropy alloys—A review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef]
- Senkov, O.N.; Gorsse, S.; Miracle, D.B. High temperature strength of refractory complex concentrated alloys. Acta Mater. 2019, 175, 394–405. [Google Scholar] [CrossRef]
- Sheikh, S.; Bijaksana, M.K.; Motallebzadeh, A.; Shafeie, S.; Lozinko, A.; Gan, L.; Tsao, T.K.; Klement, U.; Canadinc, D.; Murakami, H.; et al. Accelerated oxidation in ductile refractory high-entropy alloys. Intermetallics 2018, 97, 58–66. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Gasson, P. Encyclopedia of Aerospace Engineering. In Materials Technology Aeronaut; Cambridge University Press: Cambridge, UK, 2016; Volume 116, pp. 326–328. [Google Scholar] [CrossRef]
- Gorr, B.; Mueller, F.; Christ, H.J.; Chen, H.; Kauffmann, A.; Schweiger, R.; Szabó, D.V.; Heilmaier, M. Development of Oxidation Resistant Refractory High Entropy Alloys for High Temperature Applications: Recent Results and Development Strategy. In TMS 2018 147th Annual Meeting Exhibition Supplemental Proceedings; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Chen, Z.; Zhang, J.; Shen, B. Oxidation response of a vacuum arc melted NbZrTiCrAl refractory high entropy alloy at 800–1200 °C. Vacuum 2019, 162, 20–27. [Google Scholar] [CrossRef]
- Kanyane, L.R.; Popoola, A.P.; Malatji, N. Development of spark plasma sintered TiAlSiMoW multicomponent alloy: Microstructural evolution, corrosion and oxidation resistance. Results Phys. 2019, 12, 1754–1761. [Google Scholar] [CrossRef]
- TTsao, K.; Yeh, A.C.; Kuo, C.M.; Murakami, H. High Temperature Oxidation and Corrosion Properties of High Entropy Superalloys. Entropy 2016, 18, 62. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Dimiduk, D.M.; Woodward, C.; Miracle, D.B. Oxidation behavior of a refractory NbCrMo0.5Ta0.5TiZr alloy. J. Mater. Sci. 2012, 47, 6522–6534. [Google Scholar] [CrossRef]
- Liu, C.M.; Wang, H.M.; Zhang, S.Q.; Tang, H.B.; Zhang, A.L. Microstructure and oxidation behavior of new refractory high entropy alloys. J. Alloys Compd. 2014, 583, 162–169. [Google Scholar] [CrossRef]
- Gorr, B.; Mueller, F.; Christ, H.-J.; Mueller, T.; Chen, H.A.; Kauffmann, M. Heilmaier: High temperature oxidation behavior of an equimolar refractory metal-based alloy 20Nb-20Mo-20Cr-20Ti-20Al with and without Si addition. J. Alloys Compd. 2016, 688, 468. [Google Scholar] [CrossRef]
- Priceman, S.; Sama, L. Technical Report TR-68-210, Development of Fused Slurry Silicide Coatings for the Elevated Temperature Oxidation Protection of Columbium and Tantalum Alloys. 1968. Available online: https://core.ac.uk/display/109028321 (accessed on 5 July 2020).
- Griffiths, V. Technical Report NGR-27-003-001, A Metallographic Evaluation of Some Coated Columbium Alloys, NASA. 1972. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19720022815.pdf (accessed on 7 August 2013).
- Levine, S.R.; Merutka, J.P. Technical Report TN D-7617, Performance of Coated Columbium ad Tantalum Alloys in Plasma Arc Reentry Simulation Tests, NASA. 1974. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19740015000.pdf (accessed on 3 September 2013).
- Glass, D.E. Technical Report 201753, Oxidation and Emittance Studies of Coated Mo-Re, NASA. 1997. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19980000283.pdf (accessed on 7 September 2013).
- Stechman, C.; Lawson, C. Historical Evolution of the Space Shuttle Primary and Vernier Reaction Control Rocket Engine Designs. In Proceedings of the 42nd AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Sacramento, CA, USA, 9–12 July 2006. [Google Scholar] [CrossRef]
- Sankar, M.; Prasad, V.V.S.; Baligidad, R.G.; Alam, M.Z.; Das, D.K.; Gokhale, A.A. Microstructure, oxidation resistance and tensile properties of silicide coated Nb-alloy C-103. Mater. Sci. Eng. A 2015, 645, 339–346. [Google Scholar] [CrossRef]
- Majumdar, S.; Sengupta, P.; Kale, G.B.; ISharma, G. Development of multilayer oxidation resistant coatings on niobium and tantalum. Surf. Coat. Tech. 2006, 200, 3713–3718. [Google Scholar] [CrossRef]
- Goward, G.W. Protective Coatings—Purpose, Role, and Design. Mater. Sci. Technol. 1986, 2, 194–200. [Google Scholar] [CrossRef]
- Novak, M.D.; Levi, C.G. Oxidation and Volatilization of Silicide Coatings for Refractory Niobium Alloys. In Proceedings of the ASME 2007 International Mechanical Engineering Congress and Exposition. Advances in Aerospace Technology, Seattle, DC, USA, 11–15 November 2007; pp. 261–267. [Google Scholar] [CrossRef]
- Mueller, A.; Ge, W.; Rapp, R.A.; Courtright, E.L.; Kircher, T.A. Oxidation Behavior of Tungsten and Germanium-Alloyed Molybdenum Disilicide Coatings. Mater. Sci. Eng. A 1992, 155, 199–207. [Google Scholar] [CrossRef]
- Samsonov, G.V.; Vinitskii, I.M. Handbook of Refractory Compounds; Plenum: New York, NY, USA, 1980. [Google Scholar]


| Chemical Composition, at.% | Density (g/cm3) | Hardness (GPa) | Yield Strength Rp0.2 (MPa) | Peak Stress Rmc (MPa) | Fracture Strain εtc (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mo | Nb | Ta | Ti | W | |||||
| 20.0 | 18.5 | 22.3 | 21.1 | 18.1 | 11.6 ± 0.1 | 4.27 ± 0.07 | 1547 ± 23 | 1911 ± 115 | 11.5 ± 2.6 |
| Layer | Initial Silicide Coating | Oxidized at 1000 °C | Oxidized at 1300 °C | Possible Phase | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | Si | O | M | Si | O | M | Si | O | ||
| L1 | 62.2 | 37.8 | - | 58.1 | 41.9 | - | 59.2 | 40.8 | - | M5Si3 |
| L2 | 33.8 | 66.2 | - | 34.2 | 65.8 | - | 34.6 | 65.4 | - | MSi2 |
| L3 | - | - | - | 31.3 | 48.1 | 20.6 | 56.9 | 38.8 | 4.3 | SiO2, Ti2O, Cr2SiO4, Ta0.3W0.7O2.85, Ti0.67Nb1.33O4 |
| L4 | - | - | - | - | - | - | 14.3 | 18.1 | 67.6 | SiO2, WO3, CrNbO4, Fe2SiO4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Su, B.; Meng, J.; Zhang, A.; Wu, Y. Microstructure and Composition Evolution of a Fused Slurry Silicide Coating on MoNbTaTiW Refractory High-Entropy Alloy in High-Temperature Oxidation Environment. Materials 2020, 13, 3592. https://doi.org/10.3390/ma13163592
Han J, Su B, Meng J, Zhang A, Wu Y. Microstructure and Composition Evolution of a Fused Slurry Silicide Coating on MoNbTaTiW Refractory High-Entropy Alloy in High-Temperature Oxidation Environment. Materials. 2020; 13(16):3592. https://doi.org/10.3390/ma13163592
Chicago/Turabian StyleHan, Jiesheng, Bo Su, Junhu Meng, Aijun Zhang, and Youzhi Wu. 2020. "Microstructure and Composition Evolution of a Fused Slurry Silicide Coating on MoNbTaTiW Refractory High-Entropy Alloy in High-Temperature Oxidation Environment" Materials 13, no. 16: 3592. https://doi.org/10.3390/ma13163592
APA StyleHan, J., Su, B., Meng, J., Zhang, A., & Wu, Y. (2020). Microstructure and Composition Evolution of a Fused Slurry Silicide Coating on MoNbTaTiW Refractory High-Entropy Alloy in High-Temperature Oxidation Environment. Materials, 13(16), 3592. https://doi.org/10.3390/ma13163592





