A New Insight into Coating’s Formation Mechanism Between TiO2 and Alendronate on Titanium Dental Implant
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Chemicals, and Solutions
2.2. Alendronate Coating Formation on the Implant Surface
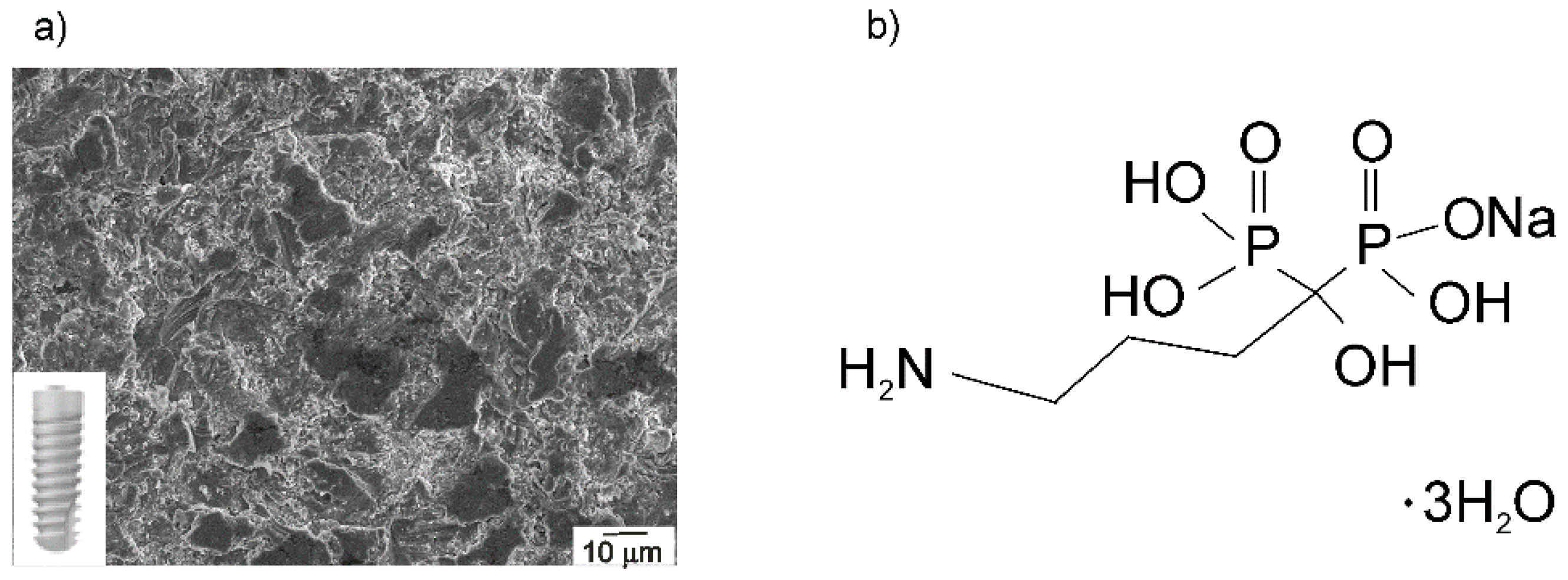
2.3. Characterization of Implant Samples
2.4. Computational Study
3. Results and Discussion
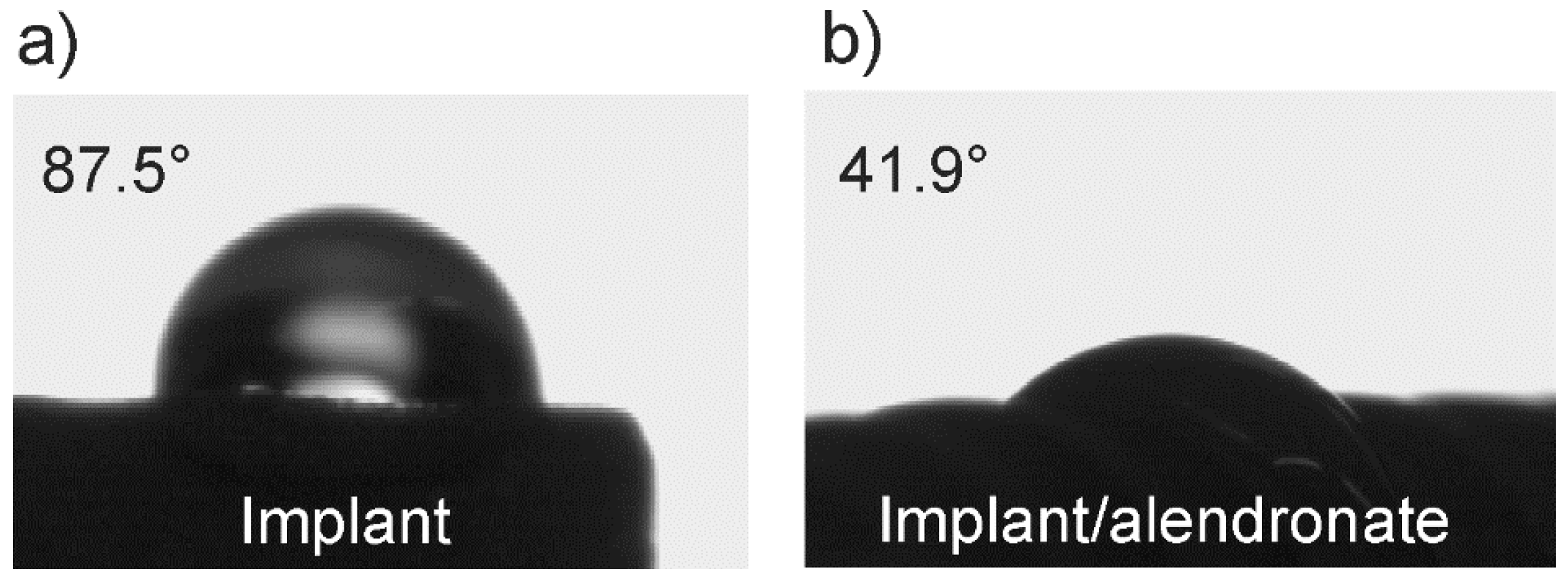
3.1. The Wetting Properties of Implant Samples
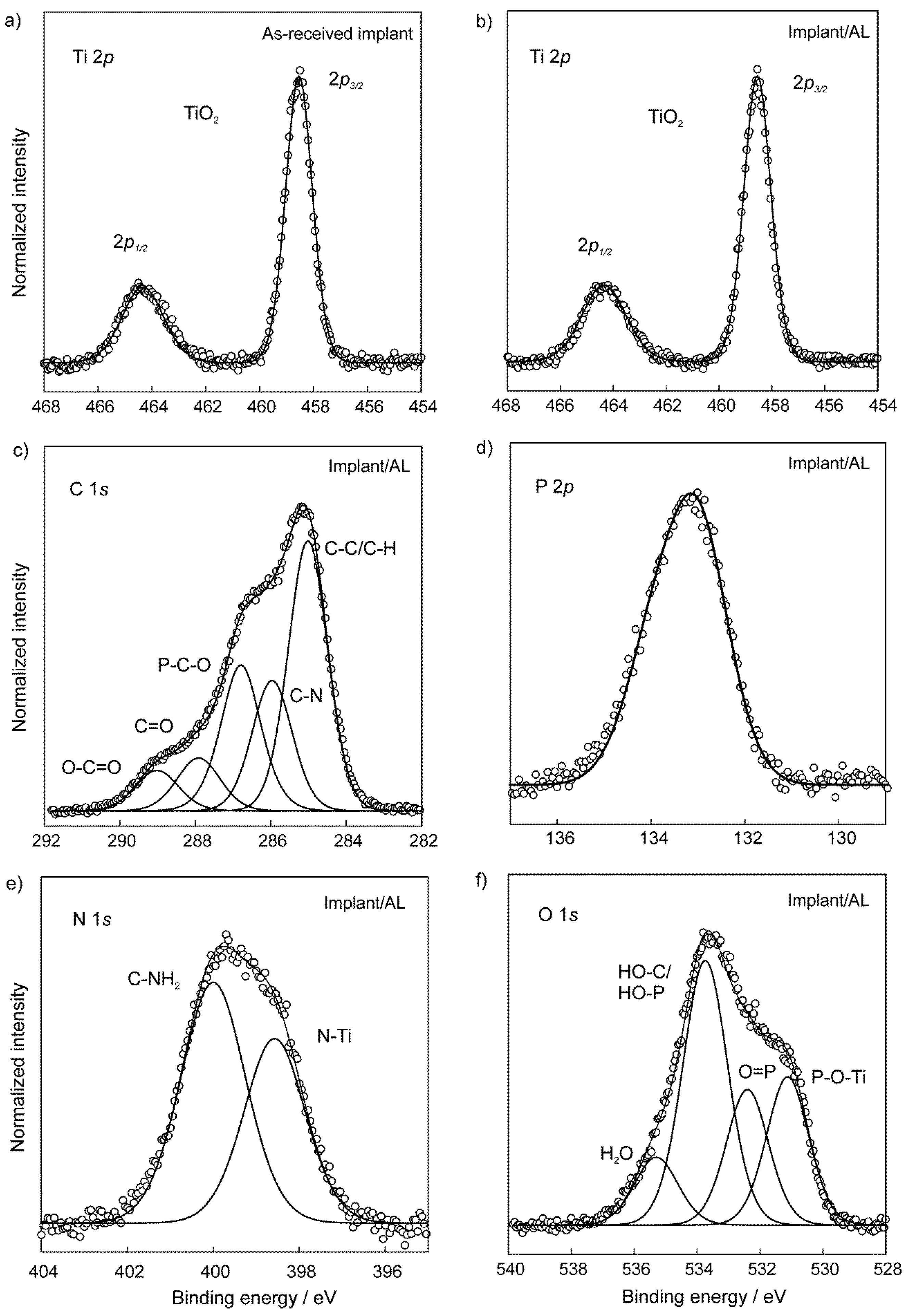
3.2. The Chemical Characterization of Implant Samples
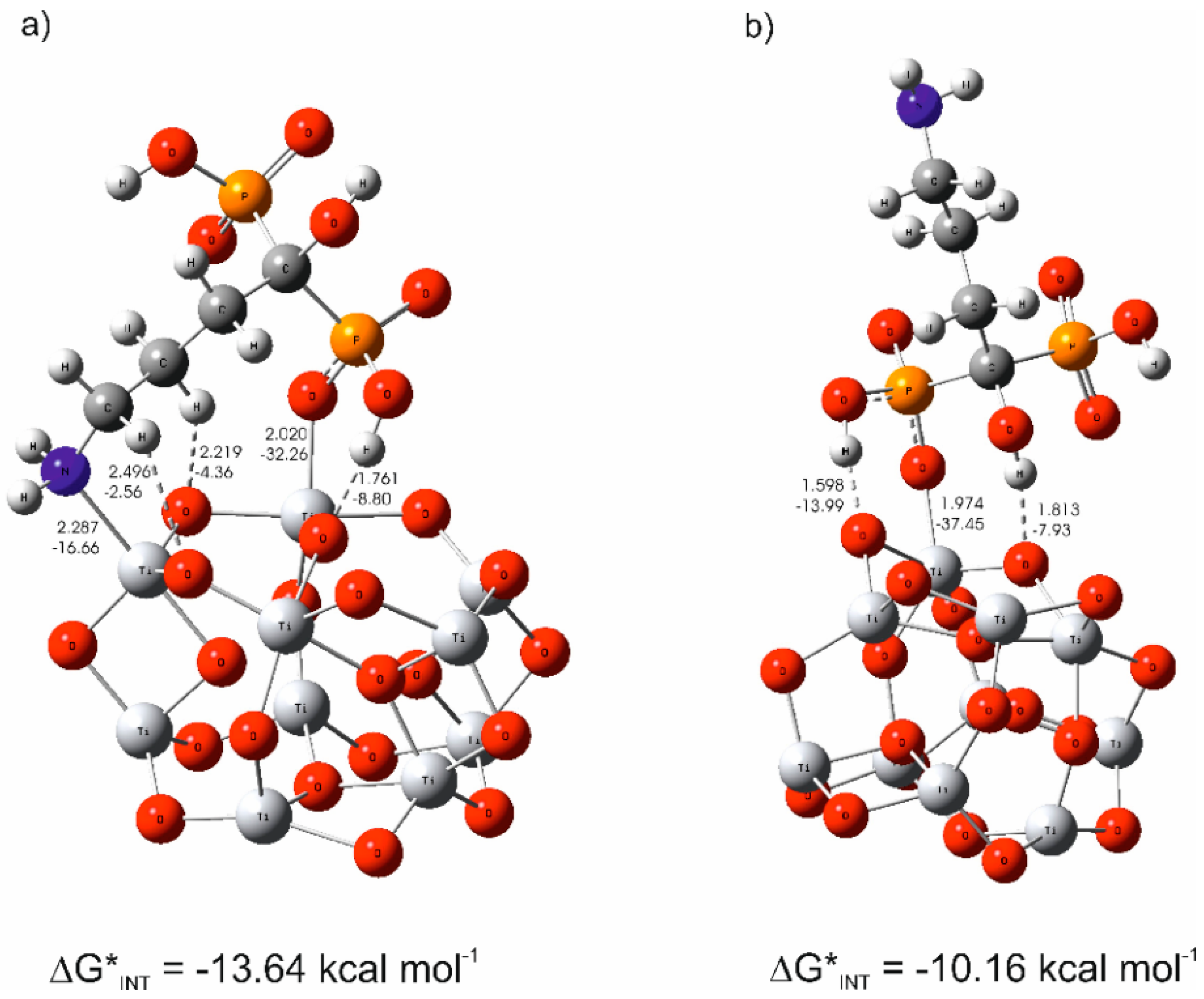
3.3. The Mechanism of the Alendronate Coating’s Formation on the Implant Surface
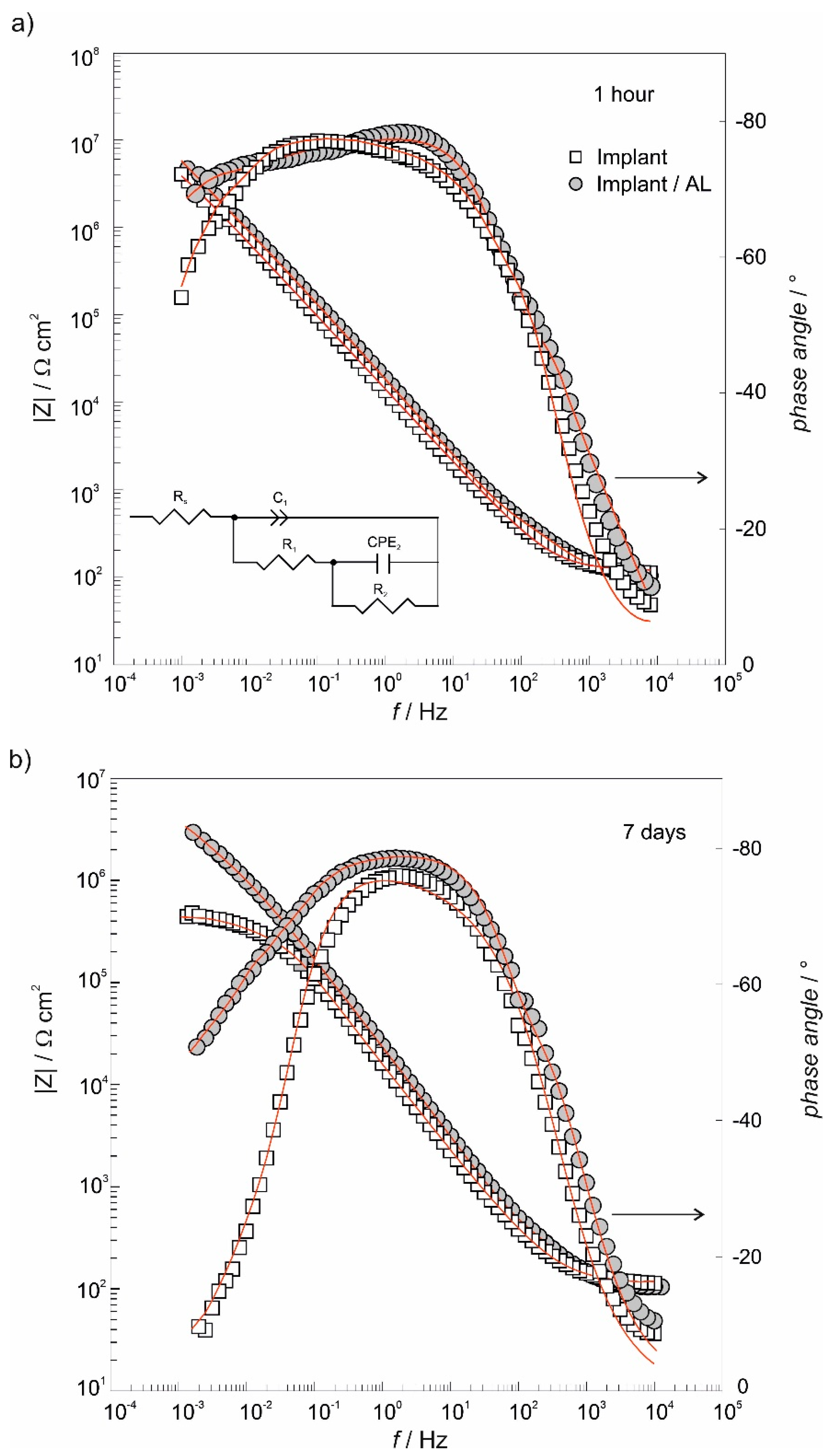
3.4. The Electrochemical Chracterization of Implant Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, C.; Ashok, D.; Nisbet, D.R.; Gautam, V. Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterials 2019, 219, 119366. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.B.; Yao, Z.; Keeney, M.; Yang, F. The future of biologic coatings for orthopaedic implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef] [PubMed]
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current trends in dental implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50–60. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, e6285620. [Google Scholar] [CrossRef] [PubMed]
- Brånemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindström, J.; Ohlsson, A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Brånemark, P.I.; Adell, R.; Albrektsson, T.; Lekholm, U.; Lundkvist, S.; Rockler, B. Osseointegrated titanium fixtures in the treatment of edentulousness. Biomaterials 1983, 4, 25–28. [Google Scholar] [CrossRef]
- McLeod, K.; Kumar, S.; Smart, R.S.C.; Dutta, N.; Voelcker, N.H.; Anderson, G.I.; Sekel, R. XPS and bioactivity study of the bisphosphonate pamidronate adsorbed onto plasma sprayed hydroxyapatite coatings. Appl. Surf. Sci. 2006, 253, 2644–2651. [Google Scholar] [CrossRef]
- Yuan, H.; Yang, Z.; Li, Y.; Zhang, X.; De Bruijn, J.D.; De Groot, K. Osteoinduction by calcium phosphate biomaterials. J. Mater. Sci. Mater. Med. 1998, 9, 723–726. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, L.L.; Ricci, V.P.; Prado, D.G.; Apolinario, R.C.; De, O.; Vercik, L.C.; Da, S.; Rigo, E.C.; Dos, S.; Fernandes, M.C.; et al. Titanium Coating with Hydroxyapatite and Chitosan Doped with Silver Nitrate. Mater. Res. 2017, 20, 863–868. [Google Scholar] [CrossRef][Green Version]
- Łukaszewska-Kuska, M.; Krawczyk, P.; Martyla, A.; Hędzelek, W.; Dorocka-Bobkowska, B. Hydroxyapatite coating on titanium endosseous implants for improved osseointegration: Physical and chemical considerations. Adv. Clin. Exp. Med. 2018, 27, 1055–1059. [Google Scholar] [CrossRef]
- Lin, K.; Zhou, Y.; Zhou, Y.; Qu, H.; Chen, F.; Zhu, Y.; Chang, J. Biomimetic hydroxyapatite porous microspheres with co-substituted essential trace elements: Surfactant-free hydrothermal synthesis, enhanced degradation and drug release. J. Mater. Chem. 2011, 21, 16558–16565. [Google Scholar] [CrossRef]
- Yagi, R.; Mochizuki, C.; Sato, M.; Toyama, T.; Hirota, M.; Hayakawa, T.; Ohkubo, C. Characterization and Bone Response of Carbonate-Containing Apatite-Coated Titanium Implants Using an Aqueous Spray Coating. Materials 2017, 10, 1416. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhang, Y.; Ma, P.; Sutrisno, L.; Luo, Z.; Hu, Y.; Yu, Y.; Tao, B.; Li, C.; Cai, K. Fabrication of magnesium/zinc-metal organic framework on titanium implants to inhibit bacterial infection and promote bone regeneration. Biomaterials 2019, 212, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Stanić, V.; Dimitrijević, S.; Antić-Stanković, J.; Mitrić, M.; Jokić, B.; Plećaš, I.B.; Raičević, S. Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders. Appl. Surf. Sci. 2010, 256, 6083–6089. [Google Scholar] [CrossRef]
- Ito, A.; Otsuka, M.; Kawamura, H.; Ikeuchi, M.; Ohgushi, H.; Sogo, Y.; Ichinose, N. Zinc-containing tricalcium phosphate and related materials for promoting bone formation. Curr. Appl. Phys. 2005, 5, 402–406. [Google Scholar] [CrossRef]
- Sun, Z.L.; Wataha, J.C.; Hanks, J.C. Effects of metal ions on osteoblast-like cell metabolism and differentiation. J. Biomed. Mater. Res. 1997, 34, 29–37. [Google Scholar] [CrossRef]
- Elsayed, H.; Brunello, G.; Gardin, C.; Ferroni, L.; Badocco, D.; Pastore, P.; Sivolella, S.; Zavan, B.; Biasetto, L. Bioactive Sphene-Based Ceramic Coatings on cpTi Substrates for Dental Implants: An In Vitro Study. Materials 2018, 11, 2234. [Google Scholar] [CrossRef]
- Biasetto, L.; Elsayed, H.; Bonollo, F.; Colombo, P. Polymer-derived sphene biocoating on cpTi substrates for orthopedic and dental implants. Surf. Coat. Technol. 2016, 301, 140–147. [Google Scholar] [CrossRef]
- Cheng, D.A.; Liu, D.P.; Tang, T.H.; Zhang, X.R.; Jia, X.L.; Cai, Q.; Yang, X.P. Effects of Ca/P molar ratios on regulating biological functions of hybridized carbon nanofibers containing bioactive glass nanoparticles. Biomed. Mater. 2017, 12, 025019. [Google Scholar] [CrossRef]
- Yang, H.; Zhu, Q.; Qi, H.; Liu, H.; Ma, M.; Chen, Q. A Facile Flow-Casting Production of Bioactive Glass Coatings on Porous Titanium for Bone Tissue Engineering. Materials 2018, 11, 1540. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; González-García, C.; Torstrick, B.; Guldberg, R.E.; Salmerón-Sánchez, M.; García, A.J. Simple Coating with Fibronectin Fragment Enhances Stainless Steel Screw Osseointegration in Healthy and Osteoporotic Rats. Biomaterials 2015, 63, 137–145. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Huang, T.; Gan, L.; Zhou, Z.; Jiang, B.; Wu, Y.; Wu, F.; Gu, Z. Collagen-infiltrated porous hydroxyapatite coating and its osteogenic properties: In vitro and in vivo study. J. Biomed. Mater. Res. Part A 2012, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Yun, Y.P.; Lee, J.Y.; Shim, J.S.; Park, K.; Huh, J.B. Co-delivery of platelet-derived growth factor (PDGF-BB) and bone morphogenic protein (BMP-2) coated onto heparinized titanium for improving osteoblast function and osteointegration. J. Tissue Eng. Regen. Med. 2015, 9, E219–E228. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Bae, E.-B.; Kim, S.-E.; Yun, Y.-P.; Kim, H.-J.; Choi, J.-W.; Lee, J.-J.; Huh, J.-B. Effects of Immobilization of rhBMP-2 and/or rhPDGF-BB on Titanium Implant Surfaces on Osseointegration and Bone Regeneration. Coatings 2018, 8, 17. [Google Scholar] [CrossRef]
- Giger, E.V.; Castagner, B.; Leroux, J.C. Biomedical applications of bisphosphonates. J. Control. Release 2013, 167, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Vohra, F.; Al-Rifaiy, M.Q.; Almas, K.; Jared, F. Efficacy of systemic bisphosphonate delivery on osseointegration of implants under osteoporotic conditions: Lessons from animal studies. Arch. Oral Biol. 2014, 59, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Mendes, V.; Dos Santos, G.O.; Calasans-Maia, M.D.; Granjeiro, J.M.; Moraschini, V. Impact of bisphosphonate therapy on dental implant outcomes: An overview of systematic review evidence. Int. J. Oral Maxillofac. Surg. 2019, 48, 373–381. [Google Scholar] [CrossRef]
- Hotieba, A.A.; Sharara, A.A.; Osman, S.M. The Effect of Sodium Alendronate Gel on Osseointegration of Submerged Dental Implants. Alex. Dent. J. 2020, 45, 1–6. [Google Scholar] [CrossRef]
- Kellesarian, S.V.; Abduljabbar, T.; Vohra, F.; Malignaggi, V.R.; Malmstrom, H.; Romanos, G.H.; Javed, F. Role of local alendronate delivery on the osseointegration of implants: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2017, 46, 912–921. [Google Scholar] [CrossRef]
- Moon, H.-J.; Yun, Y.-P.; Han, C.-W.; Kim, M.S.; Kim, S.E.; Bae, M.S.; Kim, G.-T.; Choi, Y.-S.; Hwang, E.-H.; Lee, J.W.; et al. Effect of heparin and alendronate coating on titanium surfaces on inhibition of osteoclast and enhancement of osteoblast function. Biochem. Biophys. Res. Commun. 2011, 413, 194–200. [Google Scholar] [CrossRef]
- Bronze-Uhle, E.S.; Dias, L.F.G.; Trino, L.D.; Matos, A.A.; De Oliveira, R.C.; Lisboa-Filho, P.N. Physicochemical bisphosphonate immobilization on titanium dioxide thin films surface by UV radiation for bio-application. Surf. Coat. Technol. 2019, 357, 36–47. [Google Scholar] [CrossRef]
- Corrado, A.; Colia, R.; Cantatore, F.P. Neridronate: From Experimental Data to Clinical Use. Clin. Med. Insights Ther. 2017, 9, 1–8. [Google Scholar] [CrossRef]
- Rojo, L.; Gharibi, B.; McLister, R.; Meenan, B.J.; Deb, S. Self-assembled monolayers of alendronate on Ti6Al4V alloy surfaces enhance osteogenesis in mesenchymal stem cells. Sci. Rep. 2016, 6, 30548. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Harmankaya, N.; Allard, S.; Palmquist, A.; Halvarsson, M.; Tengvall, P.; Andersson, M. Ex vivo alendronate localization at the mesoporous titania implant/bone interface. J. Mater. Sci. Mater. Med. 2015, 26, 11. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Neoh, K.G.; Kang, E.-T. Immobilization of alendronate on titanium via its different functional groups and the subsequent effects on cell functions. J. Colloid Interface Sci. 2017, 487, 1–11. [Google Scholar] [CrossRef]
- Aggarwal, R.; Babaji, P.; Nathan, S.S.; Attokaran, G.; Santosh Kumar, S.M.; Sathnoorkar, S. Comparative clinicoradiographical evaluation of effect of aminobisphosphonate (sodium alendronate) on peri-implant bone status: Controlled clinical trial. J. Int. Soc. Prev. Commun. Dent. 2016, 6, 285–290. [Google Scholar] [CrossRef]
- Tengvall, P.; Skoglund, B.; Askendal, A.; Aspenberg, P. Surface immobilized bisphosphonate improves stainless-steel screw fixation in rats. Biomaterials 2004, 25, 2133–2138. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zou, S.; Liu, X.; Bao, C.; Hu, J. The effect of surface immobilized bisphosphonates on the fixation of hydroxyapatite-coated titanium implants in ovariectomized rats. Biomaterials 2009, 30, 1790–1796. [Google Scholar] [CrossRef]
- Agholme, F.; Andersson, T.; Tengvall, P.; Aspenberg, P. Local bisphosphonate release versus hydroxyapatite coating for stainless steel screw fixation in rat tibiae. J. Mater. Sci. Mater. Med. 2012, 23, 743–752. [Google Scholar] [CrossRef]
- Dentsply Sirona. Croatia, (n.d.). Available online: https://www.dentsplysirona.com/content/dentsply-sirona/hr-hr.html (accessed on 9 May 2020).
- CP Grade 2 Titanium Sheet, Coil, Bar, Plate—AMS 4902, (n.d.). Available online: https://www.upmet.com/products/titanium/cp-grade-2 (accessed on 9 May 2020).
- Quiñones, R.; Gawalt, E.S. Study of the Formation of Self-Assembled Monolayers on Nitinol. Langmuir 2007, 23, 10123–10130. [Google Scholar] [CrossRef]
- Petrović, Ž.; Katić, J.; Metikoš-Huković, M.; Dadafarin, H.; Omanovic, S. Modification of a Nitinol Surface by Phosphonate Self-Assembled Monolayers. J. Electrochem. Soc. 2011, 158, F159. [Google Scholar] [CrossRef]
- Petrović, Ž.; Katić, J.; Šarić, A.; Despotović, I.; Matijaković, N.; Kralj, D.; Petković, M. Influence of Biocompatible Coating on Titanium Surface Characteristics. Innov. Corros. Mater. Sci. 2020, 10. [Google Scholar] [CrossRef]
- Peak Shape Analysis of Core Level Photoelectron Spectra Using Unifit for Windows. SpringerLink, (n.d.). Available online: https://link.springer.com/article/10.1007/s002160051443 (accessed on 15 May 2020).
- Mellado-Valero, A.; Muñoz, A.I.; Pina, V.G.; Sola-Ruiz, M.F. Electrochemical Behaviour and Galvanic Effects of Titanium Implants Coupled to Metallic Suprastructures in Artificial Saliva. Materials 2018, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, B.A. A Nonlinear Least Squares Fit procedure for analysis of immittance data of electrochemical systems. Solid State Ion. 1986, 20, 31–44. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Account. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density Functional Theory for Reaction Energies: Test of Meta and Hybrid Meta Functionals, Range-Separated Functionals, and Other High-Performance Functionals. J. Chem. Theory Comput. 2011, 7, 669–676. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Bader, R.R.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, NY, USA, 1994. [Google Scholar]
- Keith, T.A. AIMAll (Version 17.01.25); TK Gristmill Software: Overland Park, KS, USA, 2017; Available online: aim.tkgristmill.com.
- Allard, M.M.; Merlos, S.N.; Springer, B.N.; Cooper, J.; Zhang, G.; Boskovic, D.S.; Kwon, S.R.; Nick, K.E.; Perry, C.C. Role of TiO2 Anatase Surface Morphology on Organophosphorus Interfacial Chemistry. J. Phys. Chem. C 2018, 122, 29237–29248. [Google Scholar] [CrossRef]
- Qu, Z.; Kroes, G.-J. Theoretical Study of Stable, Defect-Free (TiO2)n Nanoparticles with n = 10−16. J. Phys. Chem. C 2007, 111, 16808–16817. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.; Nygren, H.; Ohlson, K. Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: Cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials 2004, 25, 4759–4766. [Google Scholar] [CrossRef]
- Wall, I.; Donos, N.; Carlqvist, K.; Jones, F.; Brett, P. Modified titanium surfaces promote accelerated osteogenic differentiation of mesenchymal stromal cells in vitro. Bone 2009, 45, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Petravić, M.; Varašanec, M.; Peter, R.; Kavre, I.; Metikoš-Huković, M.; Yang, Y.-W. Electronic structure of nitinol surfaces oxidized by low-energy ion bombardment. J. Appl. Phys. 2014, 115, 243703. [Google Scholar] [CrossRef]
- Viornery, C.; Chevolot, Y.; Léonard, D.; Aronsson, B.-O.; Péchy, P.; Mathieu, H.-J.; Descouts, P.; Grätzel, M. Surface Modification of Titanium with Phosphonic Acid To Improve Bone Bonding: Characterization by XPS and ToF-SIMS. Langmuir 2002, 18, 2582–2589. [Google Scholar] [CrossRef]
- Lee, K.K.; Lee, J.-G.; Park, C.S.; Lee, S.H.; Raja, N.; Yun, H.; Lee, J.-S.; Lee, C.-S. Bone-targeting carbon dots: Effect of nitrogen-doping on binding affinity. RSC Adv. 2019, 9, 2708–2717. [Google Scholar] [CrossRef]
- Spori, D.M.; Venkataraman, N.V.; Tosatti, S.G.P.; Durmaz, F.; Spencer, N.D.; Zürcher, S. Influence of Alkyl Chain Length on Phosphate Self-Assembled Monolayers. Langmuir 2007, 23, 8053–8060. [Google Scholar] [CrossRef]
- Adden, N.; Gamble, L.J.; Castner, D.G.; Hoffmann, A.; Gross, G.; Menzel, H. Phosphonic Acid Monolayers for Binding of Bioactive Molecules to Titanium Surfaces. Langmuir 2006, 22, 8197–8204. [Google Scholar] [CrossRef]
- Mani, G.; Johnson, D.M.; Marton, D.; Dougherty, V.L.; Feldman, M.D.; Patel, D.; Ayon, A.A.; Agrawal, C.M. Stability of Self-Assembled Monolayers on Titanium and Gold. Langmuir 2008, 24, 6774–6784. [Google Scholar] [CrossRef]
- Wagstaffe, M.; Hussain, H.; Acres, M.J.; Jones, R.; Syres, K.L.; Thomas, A.G. Structure and Reactivity of a Model Oxide Supported Silver Nanocluster Catalyst Studied by Near Ambient Pressure X-ray Photoelectron Spectroscopy. J. Phys. Chem. C 2017, 121, 21383–21389. [Google Scholar] [CrossRef]
- Pop-Georgievski, O.; Kubies, D.; Zemek, J.; Neykova, N.; Demianchuk, R.; Chánová, E.M.; Šlouf, M.; Houska, M.; Rypáček, F. Self-assembled anchor layers/polysaccharide coatings on titanium surfaces: A study of functionalization and stability. Beilstein J. Nanotechnol. 2015, 6, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Katić, J.; Šarić, A.; Despotović, I.; Matijaković, N.; Petković, M.; Petrović, Ž. Bioactive Coating on Titanium Dental Implants for Improved Anticorrosion Protection: A Combined Experimental and Theoretical Study. Coatings 2019, 9, 612. [Google Scholar] [CrossRef]
- Pan, J.; Thierry, D.; Leygraf, C. Electrochemical impedance spectroscopy study of the passive oxide film on titanium for implant application. Electrochim. Acta 1996, 41, 1143–1153. [Google Scholar] [CrossRef]
- Boubour, E.; Lennox, R.B. Insulating Properties of Self-Assembled Monolayers Monitored by Impedance Spectroscopy. Langmuir 2000, 16, 4222–4228. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy, 2nd ed; Wiley: Hoboken, NJ, USA, 2017; Wiley.Com. (n.d.). [Google Scholar]
- Brug, G.J.; Van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interf. Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Scully, J.R. Polarization Resistance Method for Determination of Instantaneous Corrosion Rates. Corrosion 2000, 56, 199–218. [Google Scholar] [CrossRef]
| Element | N | C | O | Fe | H | Ti | Other |
|---|---|---|---|---|---|---|---|
| wt % | 0.03 | 0.10 | 0.25 | 0.30 | 0.0155 | Balance | 0.4 |
| Rs/ Ω cm2 | C1/ µF cm−2 | R1/ Ω cm2 | Q2·106/ Ω−1 cm−2 sn1 | n2 | C2/ µF cm−2 | R2/ MΩ cm2 | |
|---|---|---|---|---|---|---|---|
| Exposure time of 1 h | |||||||
| Implant | 111 | 3.02 | 760 | 5.16 | 0.850 | 1.38 | 9.90 |
| Implant/AL | 109 | 2.00 | 307 | 9.21 | 0.820 | 2.03 | 39.0 |
| Exposure time of 7 days | |||||||
| Implant | 123 | 2.71 | 307 | 9.21 | 0.810 | 1.88 | 0.44 |
| Implant/AL | 109 | 1.98 | 302 | 7.23 | 0.795 | 1.15 | 5.88 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrović, Ž.; Šarić, A.; Despotović, I.; Katić, J.; Peter, R.; Petravić, M.; Petković, M. A New Insight into Coating’s Formation Mechanism Between TiO2 and Alendronate on Titanium Dental Implant. Materials 2020, 13, 3220. https://doi.org/10.3390/ma13143220
Petrović Ž, Šarić A, Despotović I, Katić J, Peter R, Petravić M, Petković M. A New Insight into Coating’s Formation Mechanism Between TiO2 and Alendronate on Titanium Dental Implant. Materials. 2020; 13(14):3220. https://doi.org/10.3390/ma13143220
Chicago/Turabian StylePetrović, Željka, Ankica Šarić, Ines Despotović, Jozefina Katić, Robert Peter, Mladen Petravić, and Marin Petković. 2020. "A New Insight into Coating’s Formation Mechanism Between TiO2 and Alendronate on Titanium Dental Implant" Materials 13, no. 14: 3220. https://doi.org/10.3390/ma13143220
APA StylePetrović, Ž., Šarić, A., Despotović, I., Katić, J., Peter, R., Petravić, M., & Petković, M. (2020). A New Insight into Coating’s Formation Mechanism Between TiO2 and Alendronate on Titanium Dental Implant. Materials, 13(14), 3220. https://doi.org/10.3390/ma13143220








