Functionalized Wool as an Efficient and Sustainable Adsorbent for Removal of Zn(II) from an Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Wool Sample Preparation and Modification
Physical Modification
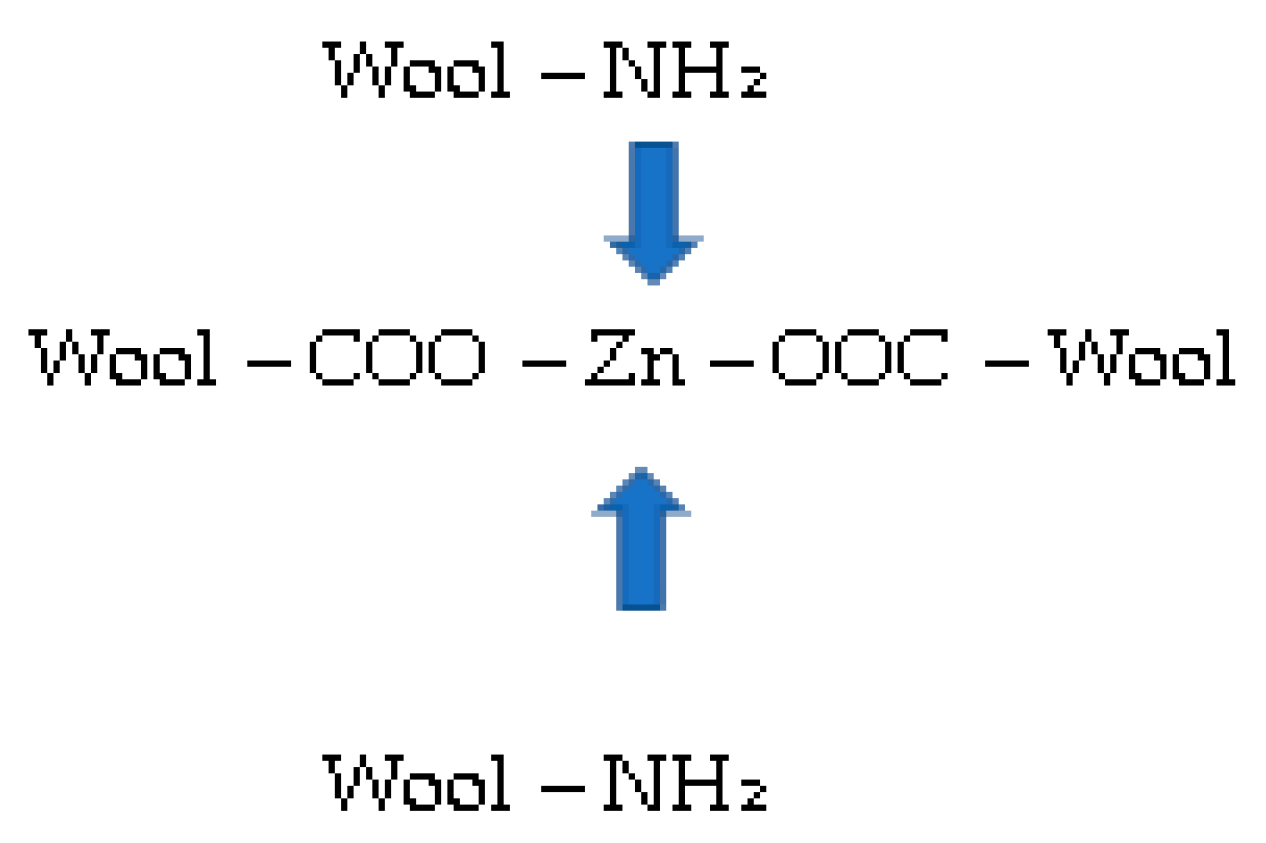
Chemical Grafting
2.2. Methods
2.2.1. Wool Characterization
Attenuated Total Reflectance—Fourier Transform Infrared Spectroscopy (ATR-FTIR)
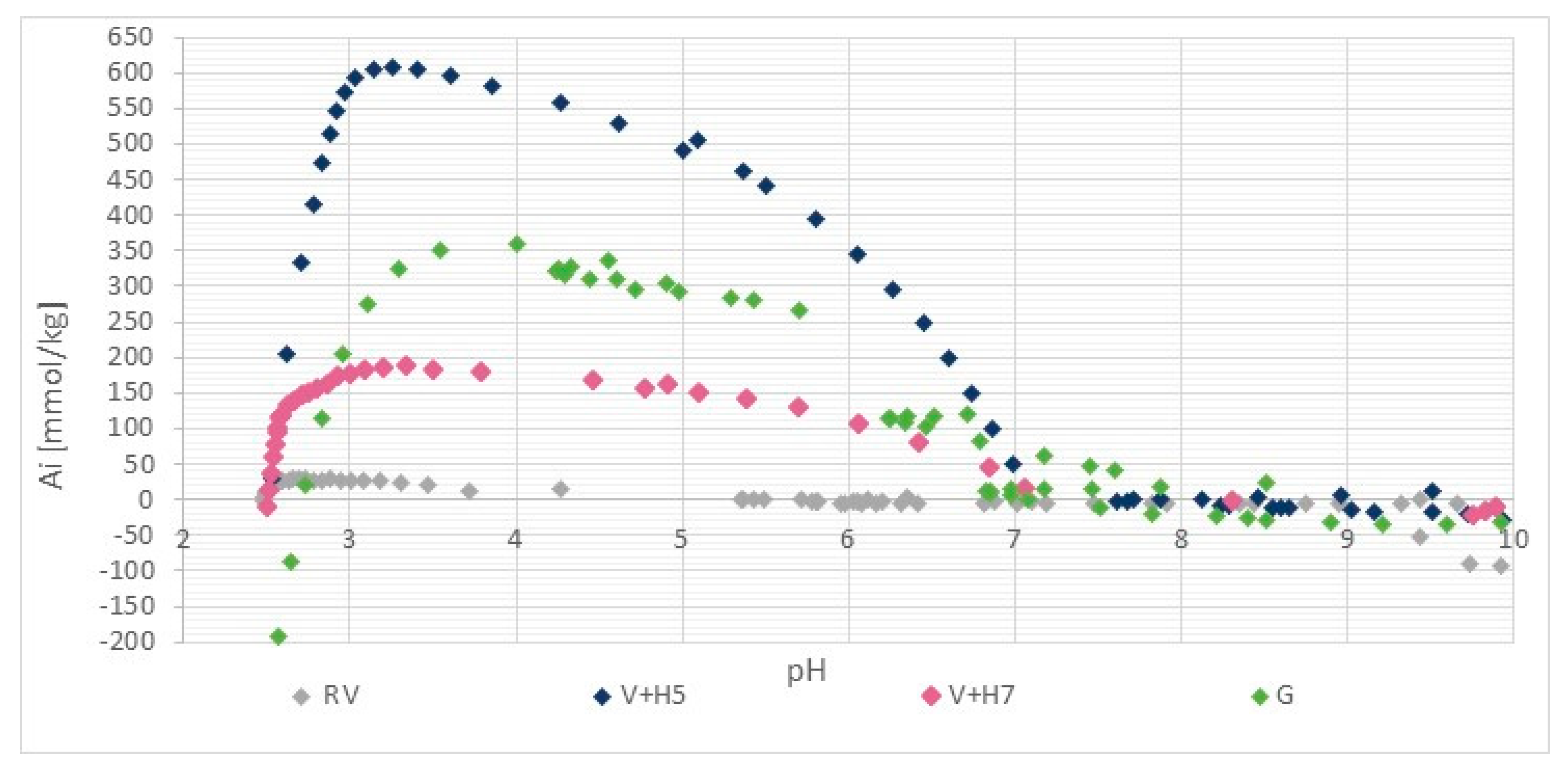
2.2.2. Potentiometric Titration
2.2.3. Polyelectrolyte Titration
2.2.4. Sorption Experiments
Atomic Absorption Spectroscopy (AAS)
- qi the concentration of adsorbed ions (mg/g) in time t (h)
- k1 Pseudo-first order constant (h−1)
3. Results and Discussion
3.1. Characterization of Wool
3.1.1. Chemical Composition of Functionalized Wool
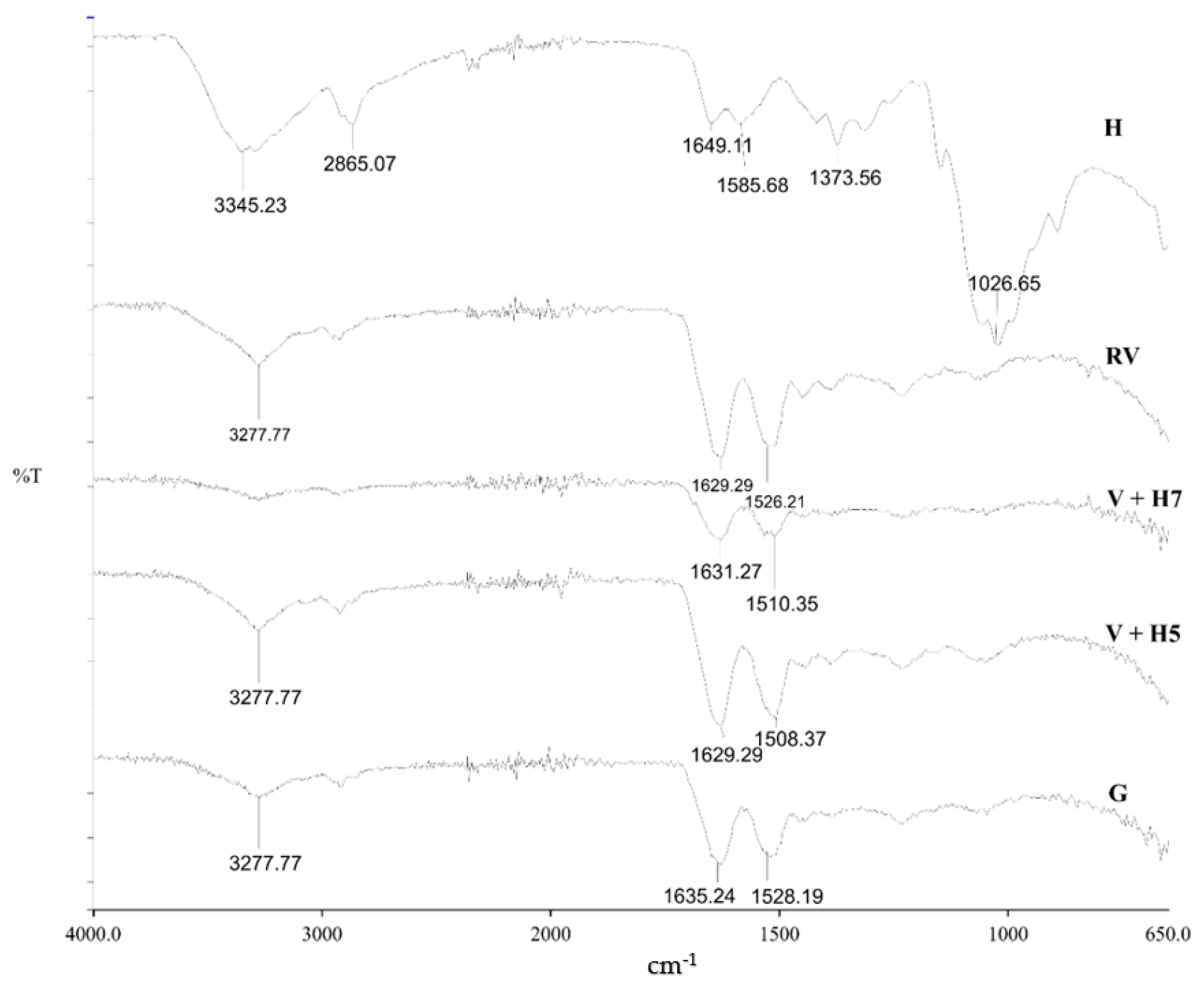
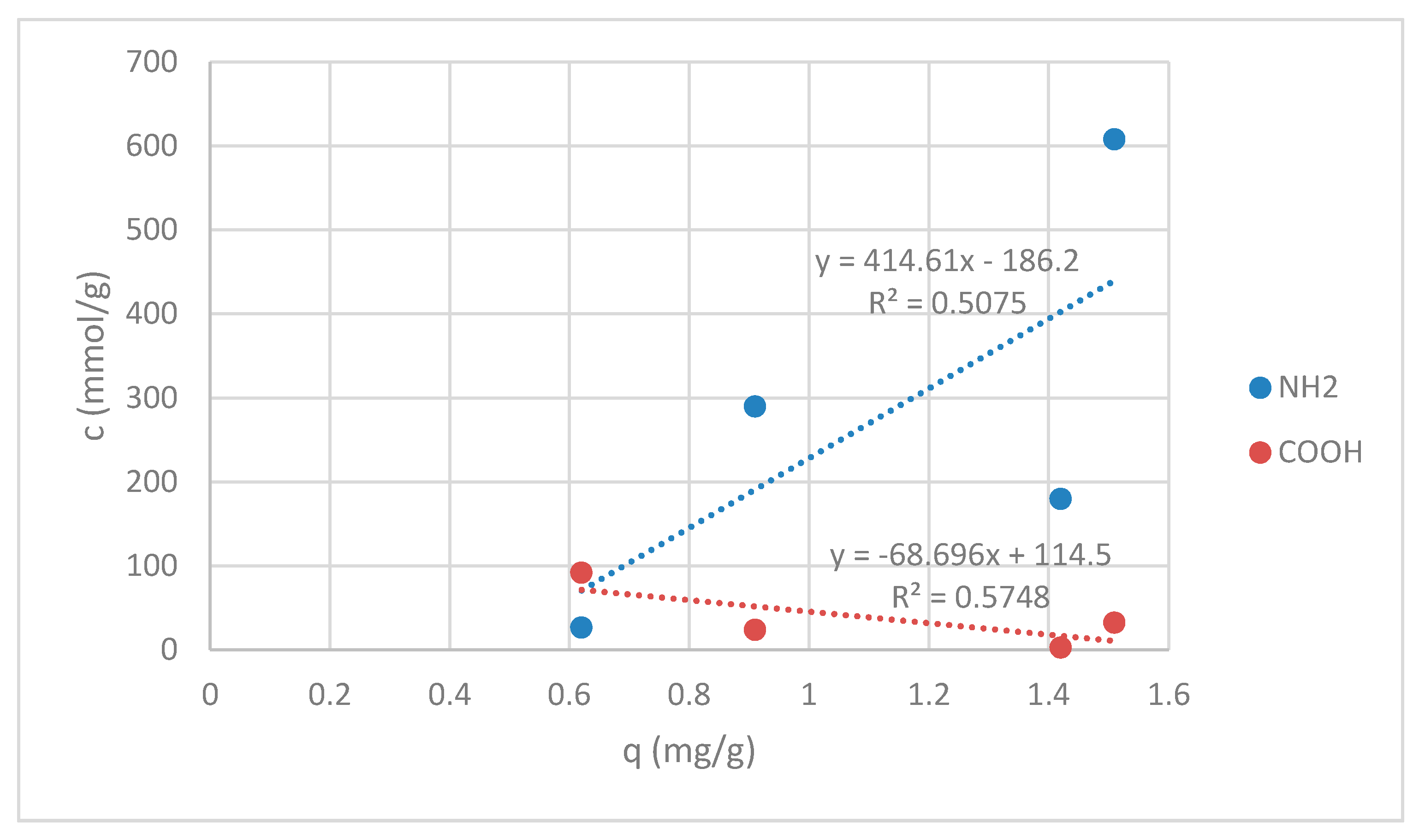
3.1.2. Functional Groups Determination
3.1.3. Stability of Chitosan Coatings
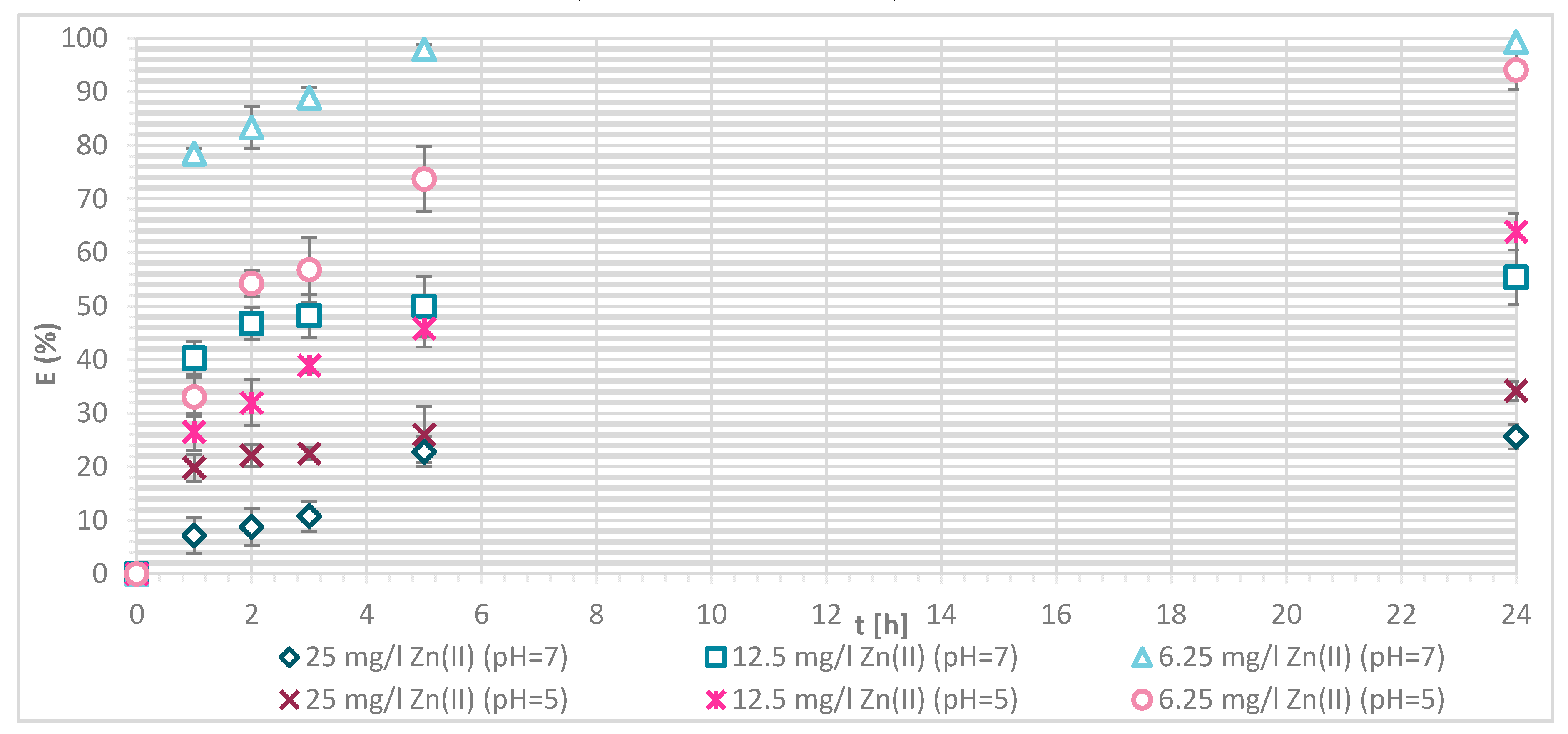
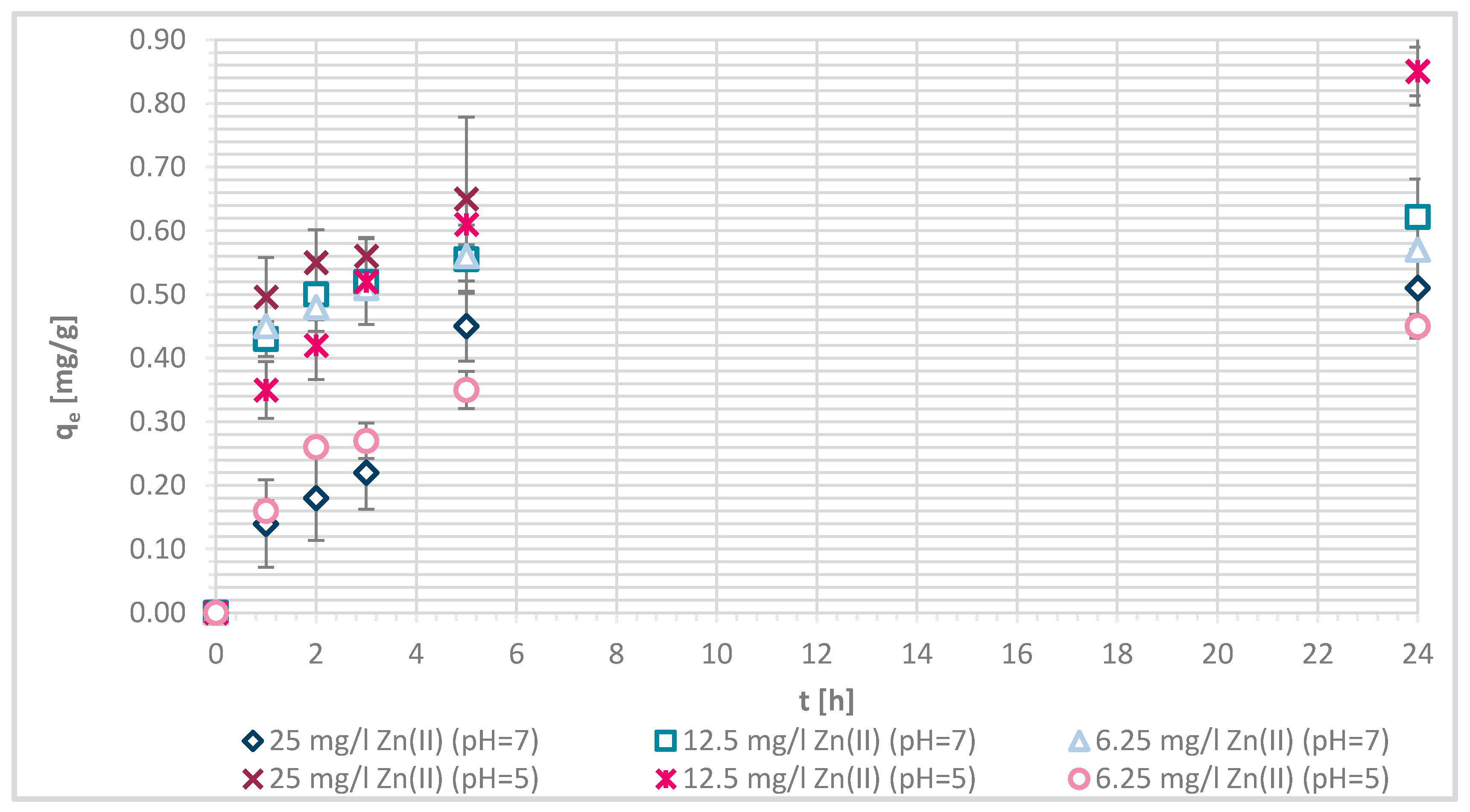
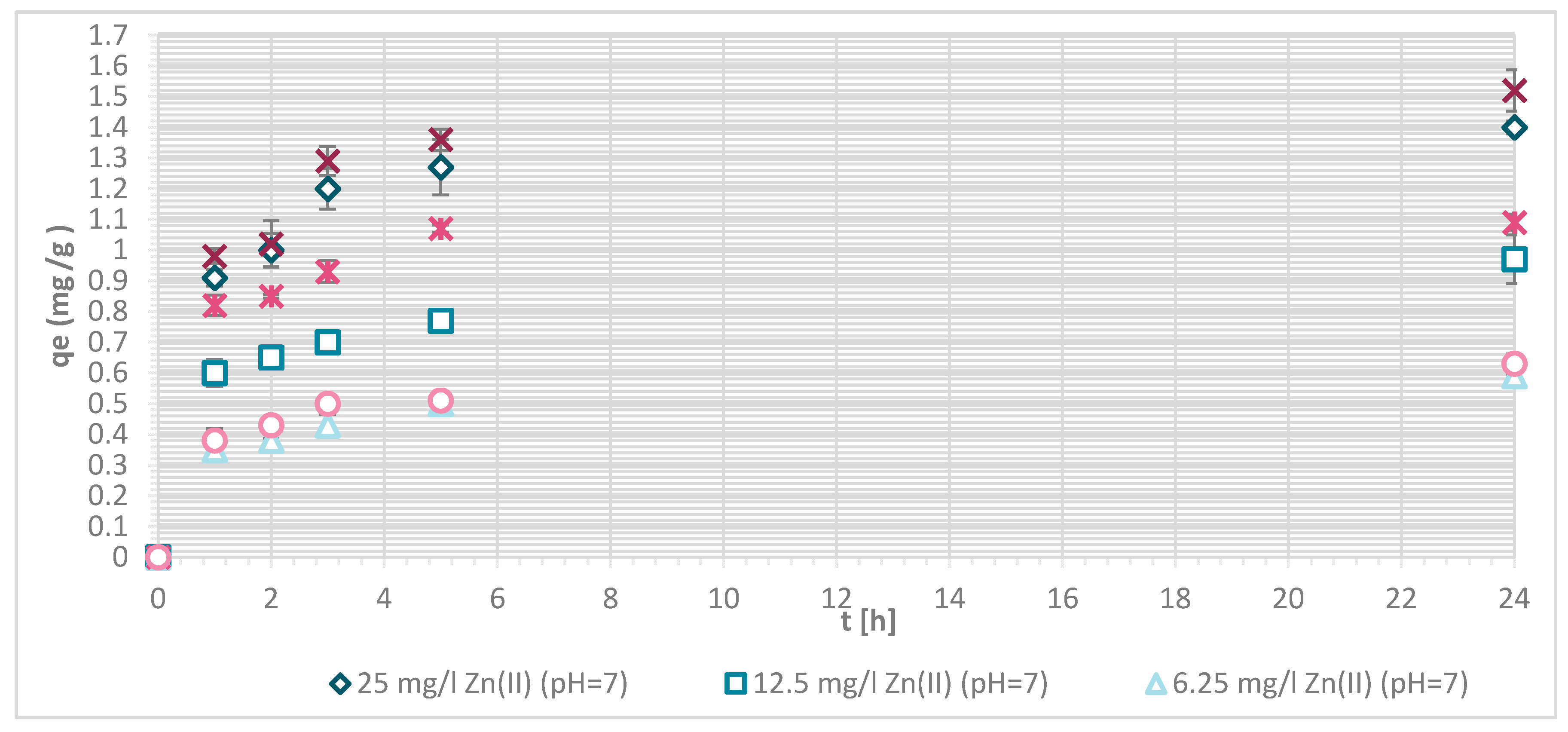
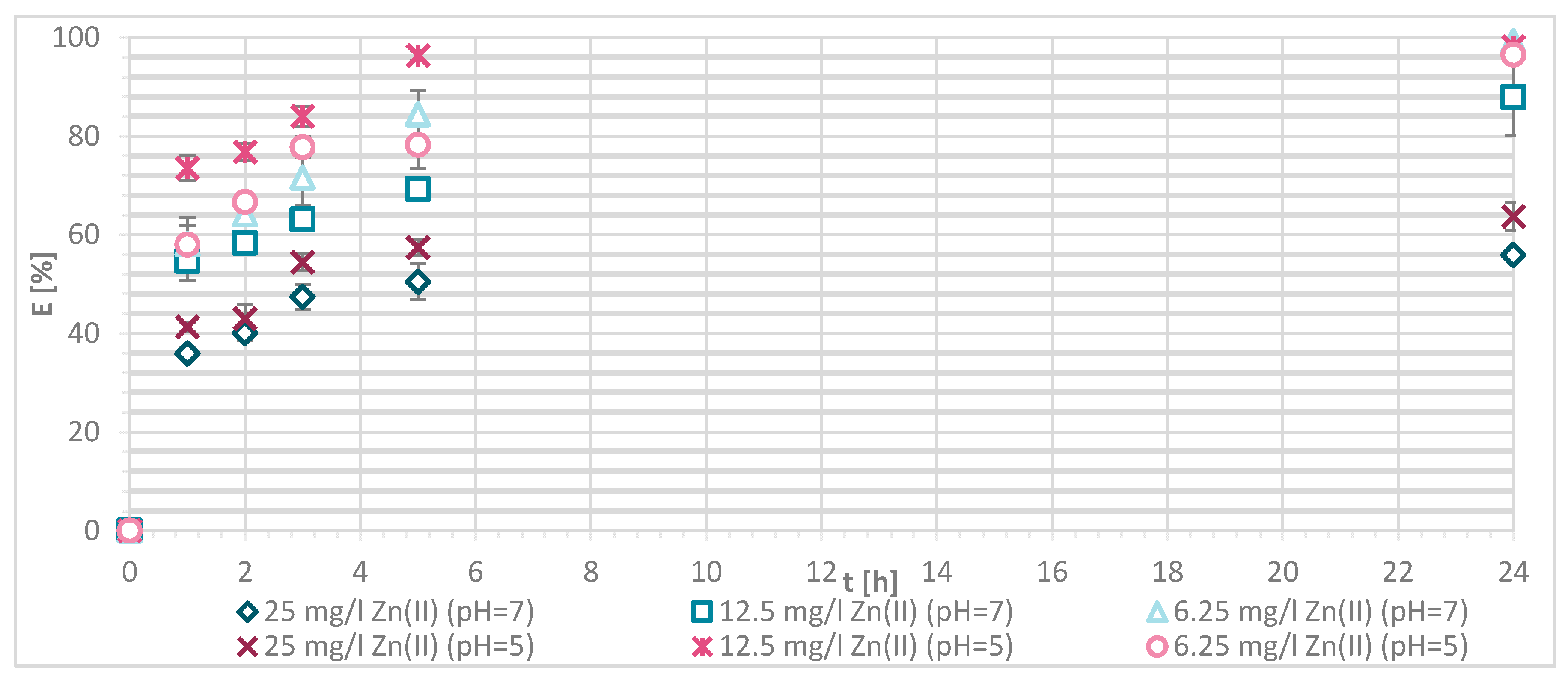
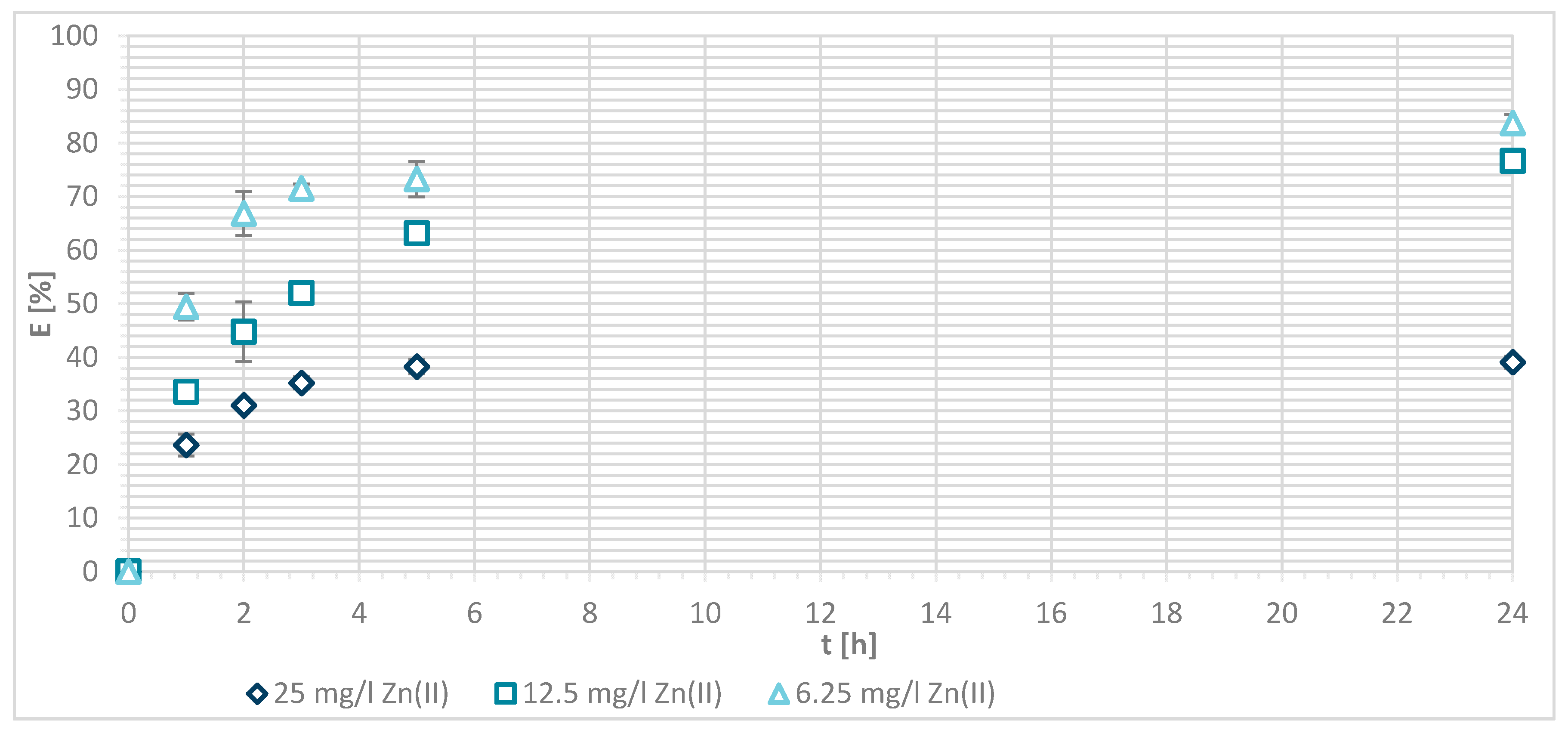
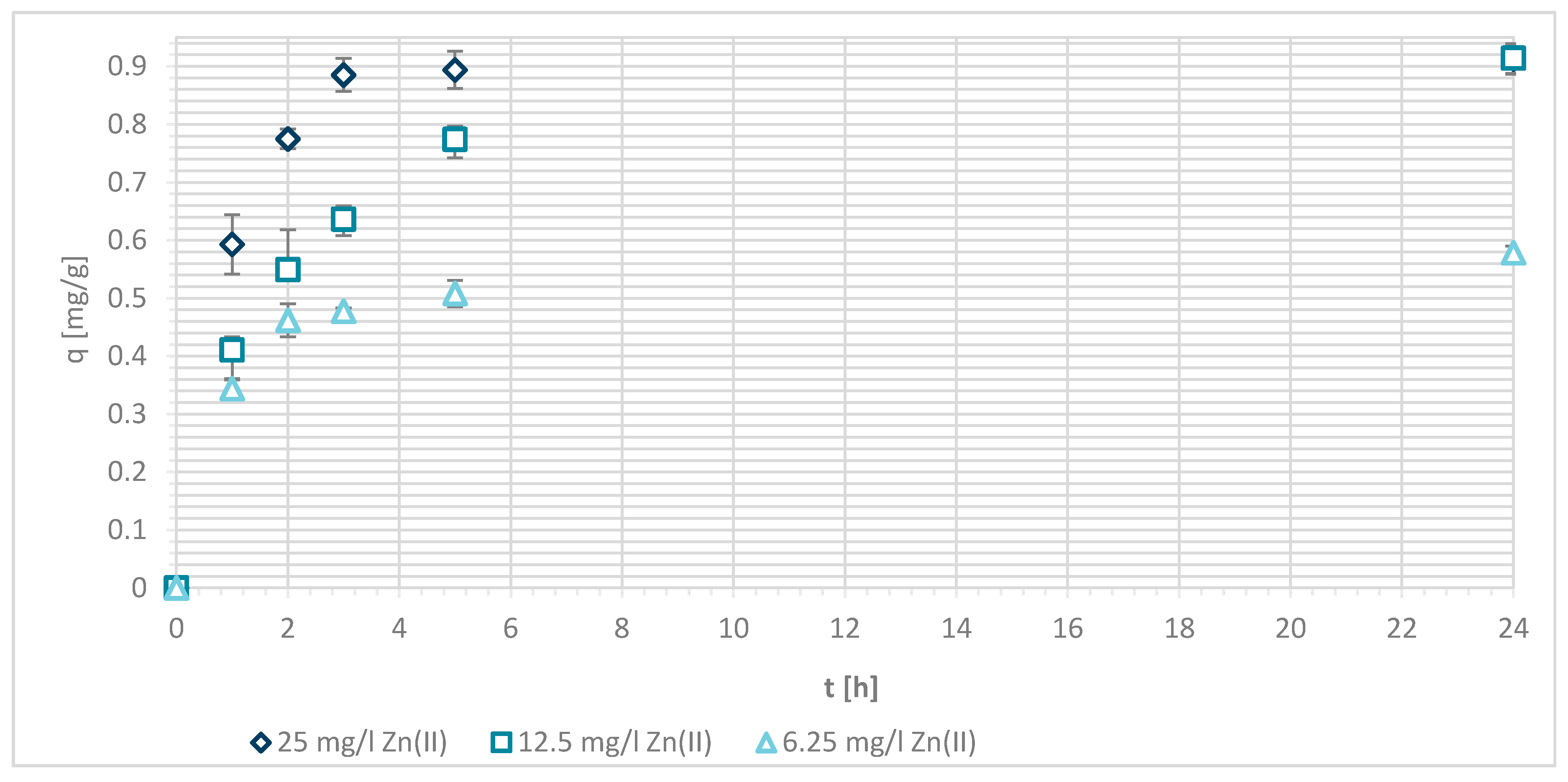
3.1.4. Sorption Experiments Result
3.1.5. Wool Physically Functionalized by Chitosan
3.1.6. Wool Chemically Functionalized by Chitosan
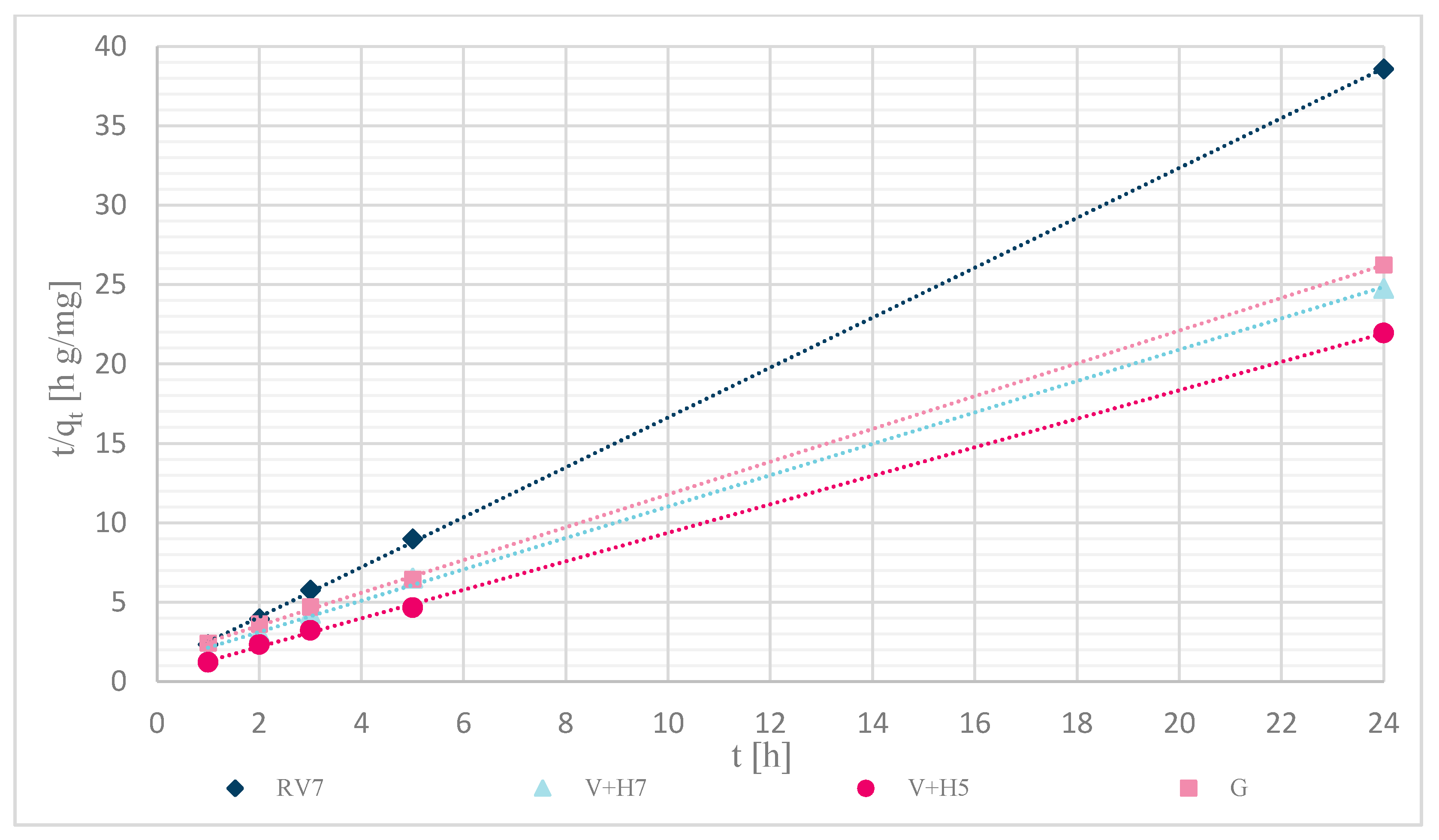
3.1.7. Sorption Kinetics
3.1.8. Sorption Isotherms
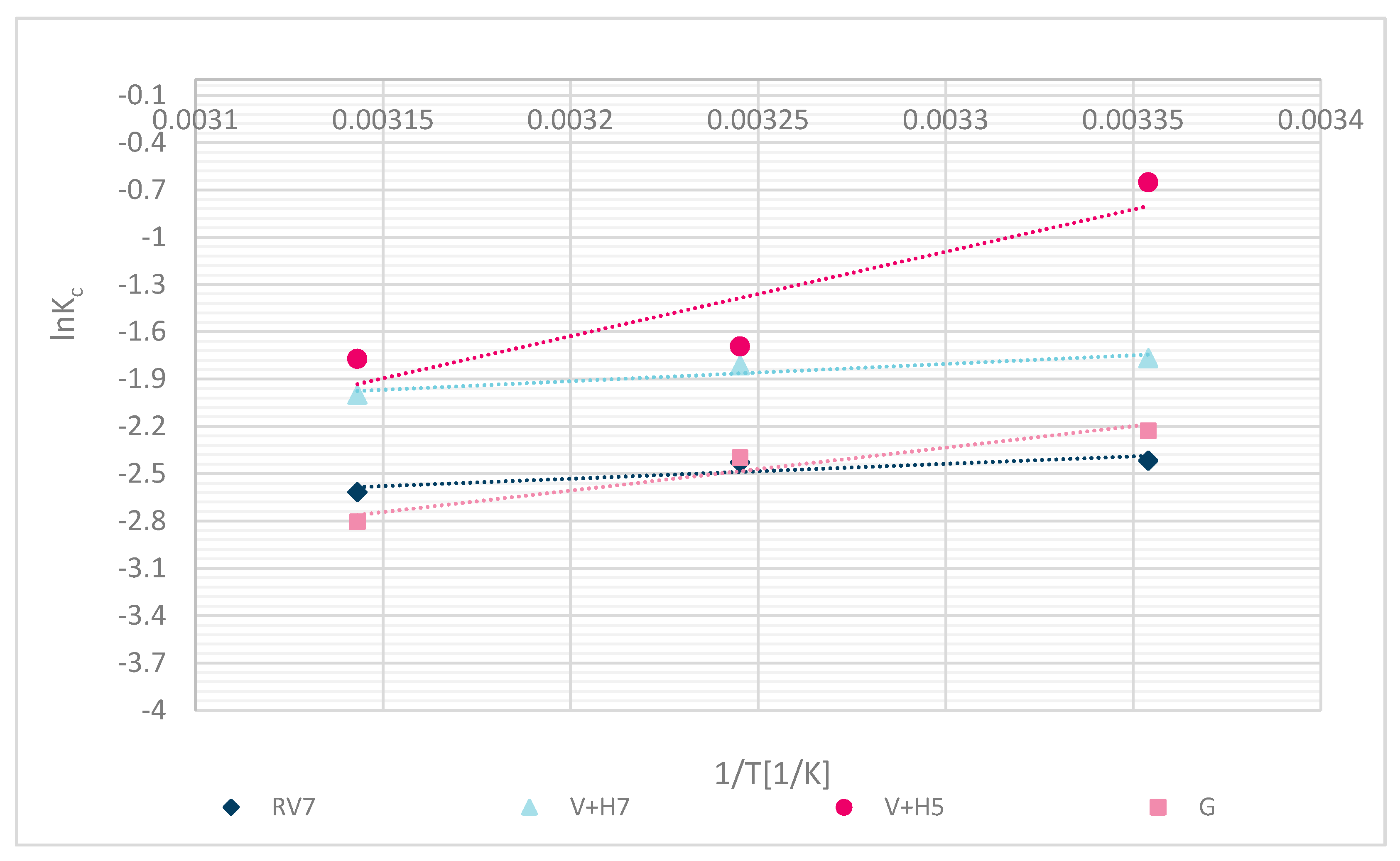
4. Thermodynamic Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Namdeo, M.; Bajpai, S.K. Chitosan-magnetite nanocomposites (CMNs) as magnetic carrier particles for removal of Fe(III) from aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2008, 320, 161–168. [Google Scholar] [CrossRef]
- Bendak, A.; Allam, E.E.; Allam, O.G. Chitosan Treatment of Wool to Improve its Reactive Dyeability. In Proceedings of the International Conference of Textile Research Division, Cairo, Egypt, 11–13 April 2005. [Google Scholar]
- Zhang, Q.F.; Yang, W.F.; Qiao, Y.L.; Shen, X.X. Application of Chitosan on Anti-Shrinkage of Wool Fabric. Adv. Mater. Res. 2011, 331, 283–286. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, N.; Wang, Q.; Yuan, J.; Yu, Y.; Wang, P.; Fan, X. Eco-friendly Grafting of Chitosan as a Biopolymer onto Wool Fabrics Using Horseradish Peroxidase. Fibers Polym. 2019, 20, 261–270. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Arami, M.; Bahrami, H.; Mazaheri, F.; Mahmoodi, N.M. Grafting of chitosan as a biopolymer onto wool fabric using anhydride bridge and its antibacterial property. Colloids Surf. B Biointerfaces 2010, 76, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Gawish, S.M.; Abo El-Ola, S.M.; Ramadan, A.M.; Abou El-Kheir, A.A. Citric acid used as a crosslinking agent for the grafting of chitosan onto woolen fabric. J. Appl. Polym. Sci. 2012, 123, 3345–3353. [Google Scholar] [CrossRef]
- Periolatto, M.; Ferrero, F.; Vineis, C.; Rombaldoni, F. Multifunctional finishing of wool fabrics by chitosan UV-grafting: An approach. Carbohydr. Polym. 2013, 98, 624–629. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Kessler, R. Handbook of Composites from Renewable Materials; Functionalization, John Wiley & Sons, Scriver Publishing LLC: Hoboken, NJ, USA, 2017. [Google Scholar]
- Davidson, R.S.; Xue, Y. Improving the dyeability of wool by treatment with chitosan. J. Soc. Dye. Colour. 2008, 110, 24–29. [Google Scholar] [CrossRef]
- Braniša, J.; Jomová, K.; Porubská, M. Scouring Test of Sheep Wool Intended for Sorption. Fibres Text. East. Eur. 2019, 27, 24–29. [Google Scholar] [CrossRef]
- Al-Karawi, A.J.M.; Al-Qaisi, Z.H.J.; Abdullah, H.I.; Al-Mokaram, A.M.A.; Al-Heetimi, D.T.A. Synthesis, characterization of acrylamide grafted chitosan and its use in removal of copper(II) ions from water. Carbohydr. Polym. 2011, 83, 495–500. [Google Scholar] [CrossRef]
- Dakiky, M.; Khamis, M.; Manassra, A.; Mer’eb, M. Selective adsorption of chromium(VI) in industrial wastewater using low-cost abundantly available adsorbents. Adv. Environ. Res. 2002, 6, 533–540. [Google Scholar] [CrossRef]
- Ahmed, A.W.; Shamran Jassim, S.; Jassim, A.H. Removal of Zinc ions from industrial wastewater with wool fibers. Baghdad Sci. J. 2010, 7. [Google Scholar] [CrossRef]
- Atef El-Sayed, A.; Salama, M.; Kantouch, A.A.M. Wool micro powder as a metal ion exchanger for the removal of copper and zinc. Desalin. Water Treat. 2015, 56, 1010–1019. [Google Scholar] [CrossRef]
- Hanzlíková, Z.; Braniša, J.; Jomová, K.; Fülöp, M.; Hybler, P.; Porubská, M. Electron beam irradiated sheep wool—Prospective sorbent for heavy metals in wastewater. Sep. Purif. Technol. 2018, 193, 345–350. [Google Scholar] [CrossRef]
- Enkhzaya, S.; Shiomori, K.; Oyuntsetse, B. Removal of Heavy Metals from Aqueous Solution by Adsorption using Livestock Biomass of Mongolia. J. Environ. Sci. Technol. 2017, 10, 107–119. [Google Scholar] [CrossRef]
- Čakara, D.; Fras, L.Z.; Bračič, M.; Kleinschek, K.S. Protonation behavior of coton fabric with irreversibly adsorbed chitosan: A potentionmetric titration study. Carbohydr. Polym. 2009, 78, 36–40. [Google Scholar] [CrossRef]
- Ghafar, H.H.A.; Salem, T.; Radwan, E.K.; El-Sayed, A.A.; Embaby, M.A.; Salama, M. Modification of waste wool fiber as low cost adsorbent for the removal of methylene blue from aqueous solution. Egypt. J. Chem. 2017, 60, 395–406. [Google Scholar] [CrossRef]
- Mishra, V.; Balomajumder, C.; Agarwal, V.K. Zn(II) Ion Biosorption onto Surface of Eucalyptus Leaf Biomass: Isotherm, Kinetic, and Mechanistic Modeling. Clean Soil Air Water 2010, 38, 1062–1073. [Google Scholar] [CrossRef]
- Malkoc, E.; Nuhoglu, Y. Potential of tea factory waste for chromium(VI) removal from aqueous solutions: Thermodynamic and kinetic studies. Sep. Purif. Technol. 2007, 54, 291–298. [Google Scholar] [CrossRef]
- Andersson, K.I.; Eriksson, M.; Norgren, M. Removal of Lignin from Wastewater Generated by Mechanical Pulping Using Activated Charcoal and Fly Ash: Adsorption Isotherms and Thermodynamics. Ind. Eng. Chem. Res. 2011, 50, 7722–7732. [Google Scholar] [CrossRef]
- Ibrahim, M.; Osman, O.; Mahmoud, A.A. Spectroscopic Analyses of Cellulose and Chitosan: FTIR and Modeling Approach. J. Comput. Theor. Nanosci. 2011, 8, 117–123. [Google Scholar] [CrossRef]
- Paulino, A.T.; Simionato, J.I.; Garcia, J.C.; Nozaki, J. Characterization of chitosan and chitin produced from silkworm crysalides. Carbohydr. Polym. 2006, 64, 98–103. [Google Scholar] [CrossRef]
- Zargarkazemi, A.; Sadeghi-Kiakhani, M.; Arami, M.; Bahrami, S.H. Modification of wool fabric using prepared chitosan-cyanuric chloride hybrid. J. Text. Inst. 2015, 106, 80–89. [Google Scholar] [CrossRef]
- Burkinshaw, S.M. Physico-chemical Aspects of Textile Coloration; Wiley: Chichester, UK, 2016; p. 504. [Google Scholar]
- Lewis, D.M.; Rippon, J.A. The Chemical and Physical Basis for Wool Dyeing. In Coloration Wool Other Keratin Fibres; Willey, NYSE: Hoboken, NJ, USA, 2013; pp. 43–74. [Google Scholar] [CrossRef]
- Jintakosol, T.; Nitayaphat, W. Adsorption of Silver (I) From Aqueous Solution Using Chitosan/Montmorillonite Composite Beads. Mater. Res. 2016, 19, 1114–1121. [Google Scholar] [CrossRef]
- Dee, K.C.; Puleo, D.A.; Bizios, R. Protein-Surface Interactions, An Introduction To Tissue-Biomaterial Interactions; Wiley-Liss, Inc.: New York, NY, USA, 2002; pp. 37–52. [Google Scholar] [CrossRef]
- Boguta, P.; Sokolowska, Z. Interactions of Zn(II) ions with Humic Acid isolated Type of Soils. Effect of pH, Zn concentrations and Humic Acids Chemical Properties. PLoS ONE 2016, 11(4). [Google Scholar] [CrossRef] [PubMed]
- Ristić, T.; Šauperl, O.; Fras, Z.L. Water retention value and mechanical properties of viscose fibres functionalised by chitosan and its wastersoluble derivative, N,N,N-trimethyl chitosan. Cellul. Chem. Technol. 2017, 51, 507–512. [Google Scholar]
- Vaishnav, V.; Chandra, S.; Daga, K. Adsorption Studies of Zn(II) Ions from wastewater using Calotropis procera as an adsorbent. Int. J. Sci. Eng. Res. 2012, 1, 160–165. [Google Scholar]
- Pranata Putra, W.; Kamari, A. Biosorption of Cu(II), Pb(II) and Zn(II) Ions from Aqueous Solutions Using Selected Waste Materials: Adsorpt Characterisation Studies. J. Encapsulation Adsorpt. Sci. 2014, 4, 25–35. [Google Scholar] [CrossRef]
- Nasernejad, B.; Zadeh, T.E.; Pour, B.B.; Bygi, M.E.; Zamani, A. Camparison for biosorption modeling of heavy metals (Cr (III), Cu (II), Zn(II)) adsorption from wastewater by carrot residues. Process Biochem. 2005, 40, 1319–1322. [Google Scholar] [CrossRef]
- Belhamdi, B.; Merzougui, Z.; Trari, M.; Addoun, A. A kinetic equilibrium and thermodynamic study of L-phenylalanine adsorption using activated carbon based on agricultural waste (date stones). J. Appl. Res. Technol. 2016, 14, 354–366. [Google Scholar] [CrossRef]

| Sample Notation | Description of Sample |
|---|---|
| RV | Pristine wool |
| V+H5 | Wool modified physically with chitosan macromolecular solution at pH = 5 |
| V + H7 | Wool modified physically with chitosan macromolecular solution at pH = 7 |
| G | Chemical modification of wool using the grafting procedure |
| Volume of Titrant VPES-Na (mL) | Calculated Amino Groups cc (mmol/kg) | Desorption (%) | |
|---|---|---|---|
| V + H5 | 1.1 | 220 | 36.2 |
| V + H7 | 0.3 | 60 | 33.3 |
| G | 0 | 0 | 0 |
| Groups | Protonated Amino Groups NH3+ (mmol/kg) | Deprotonated Carboxyl Groups COO− (mmol/kg) |
|---|---|---|
| RV | 26.7 | 92.2 |
| V + H5 | 607.7 | 32.6 |
| V + H7 | 180.0 | 3 |
| G | 290.0 | 24.0 |
| Sorbent | Equilibrium Concentration of Adsorbed Ions qe (mg/g) | Pseudisecond Order Constant k2 (g /(mg h)) | Determination Coefficient R2 |
|---|---|---|---|
| RV | 0.62 | 2.63 | 0.9999 |
| V + H5 | 1.09 | 1.98 | 0.9997 |
| V + H7 | 1.01 | 0.84 | 0.9987 |
| G | 0.91 | 0.72 | 0.9998 |
| Sorbent | Maximum Experimental Sorption Capacity qmax,eksp (mg/g) | Maximum Sorption Capacity qmax (mg/g) | Langmuir Constant KL (l/mg) | Determination Coefficient R2 |
|---|---|---|---|---|
| RV | 0.62 | 0.66 | 6.14 | 0.998 |
| V + H5 | 1.52 | 1.53 | 2.84 | 0.9866 |
| V + H7 | 1.4 | 1.42 | 4.19 | 0.9971 |
| G | 0.91 | 0.94 | 2.41 | 0.9983 |
| Sorbent | Gibbs free energy ∆G⁰ (25 °C) [J/mol] | Enthalpy ∆H⁰ [J/mol] | Entropy ∆S⁰ [J/(mol K)] | Determination CoefficientR2 |
|---|---|---|---|---|
| RV | −4500.0 | −7831.7 | −46.1 | 0.7712 |
| V + H5 | −2589.4 | −44,553.9 | −156.1 | 0.8174 |
| V + H7 | −3553.8 | −9087.7 | −45.0 | 0.8786 |
| G | −2183.3 | −22,631.2 | −94.1 | 0.9378 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonič, M.; Fras Zemljič, L. Functionalized Wool as an Efficient and Sustainable Adsorbent for Removal of Zn(II) from an Aqueous Solution. Materials 2020, 13, 3208. https://doi.org/10.3390/ma13143208
Simonič M, Fras Zemljič L. Functionalized Wool as an Efficient and Sustainable Adsorbent for Removal of Zn(II) from an Aqueous Solution. Materials. 2020; 13(14):3208. https://doi.org/10.3390/ma13143208
Chicago/Turabian StyleSimonič, Marjana, and Lidija Fras Zemljič. 2020. "Functionalized Wool as an Efficient and Sustainable Adsorbent for Removal of Zn(II) from an Aqueous Solution" Materials 13, no. 14: 3208. https://doi.org/10.3390/ma13143208
APA StyleSimonič, M., & Fras Zemljič, L. (2020). Functionalized Wool as an Efficient and Sustainable Adsorbent for Removal of Zn(II) from an Aqueous Solution. Materials, 13(14), 3208. https://doi.org/10.3390/ma13143208






