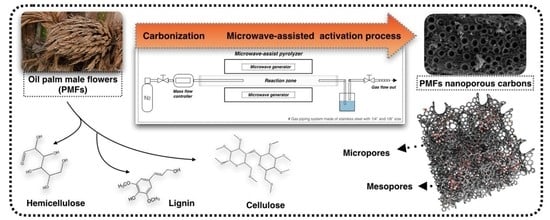
Parametric Study on Microwave-Assisted Pyrolysis Combined KOH Activation of Oil Palm Male Flowers Derived Nanoporous Carbons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Oil Palm Male Flowers Derived Nanoporous Carbon (PMFC)
2.3. Characterization
2.3.1. Proximate and Ultimate Analysis
2.3.2. Surface Characteristics
2.3.3. Surface Functional, Crystallinity, and Morphology Analysis
3. Results and Discussion
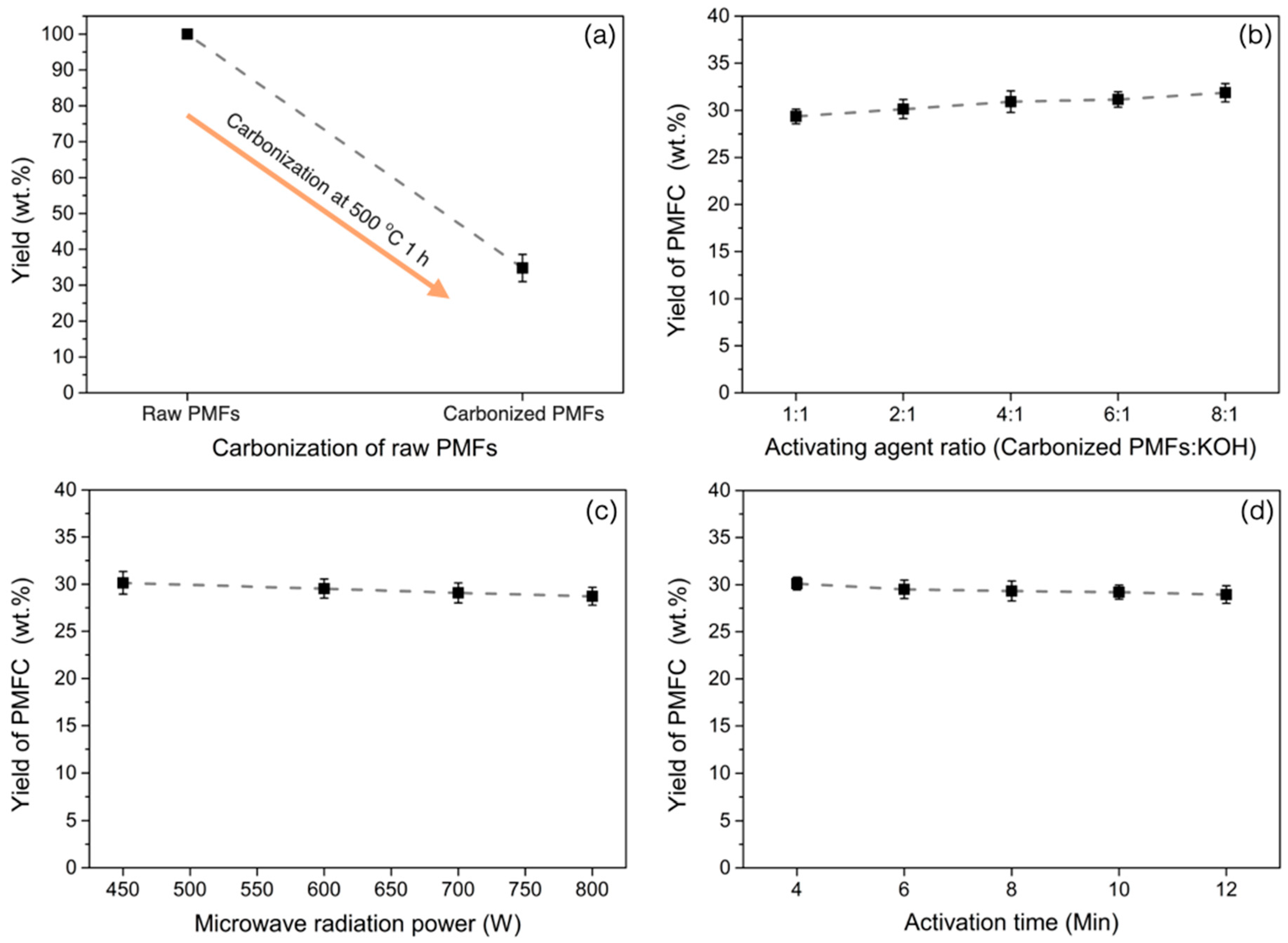
3.1. The Effects of Preparation Variables on Production Yield of PMFCs
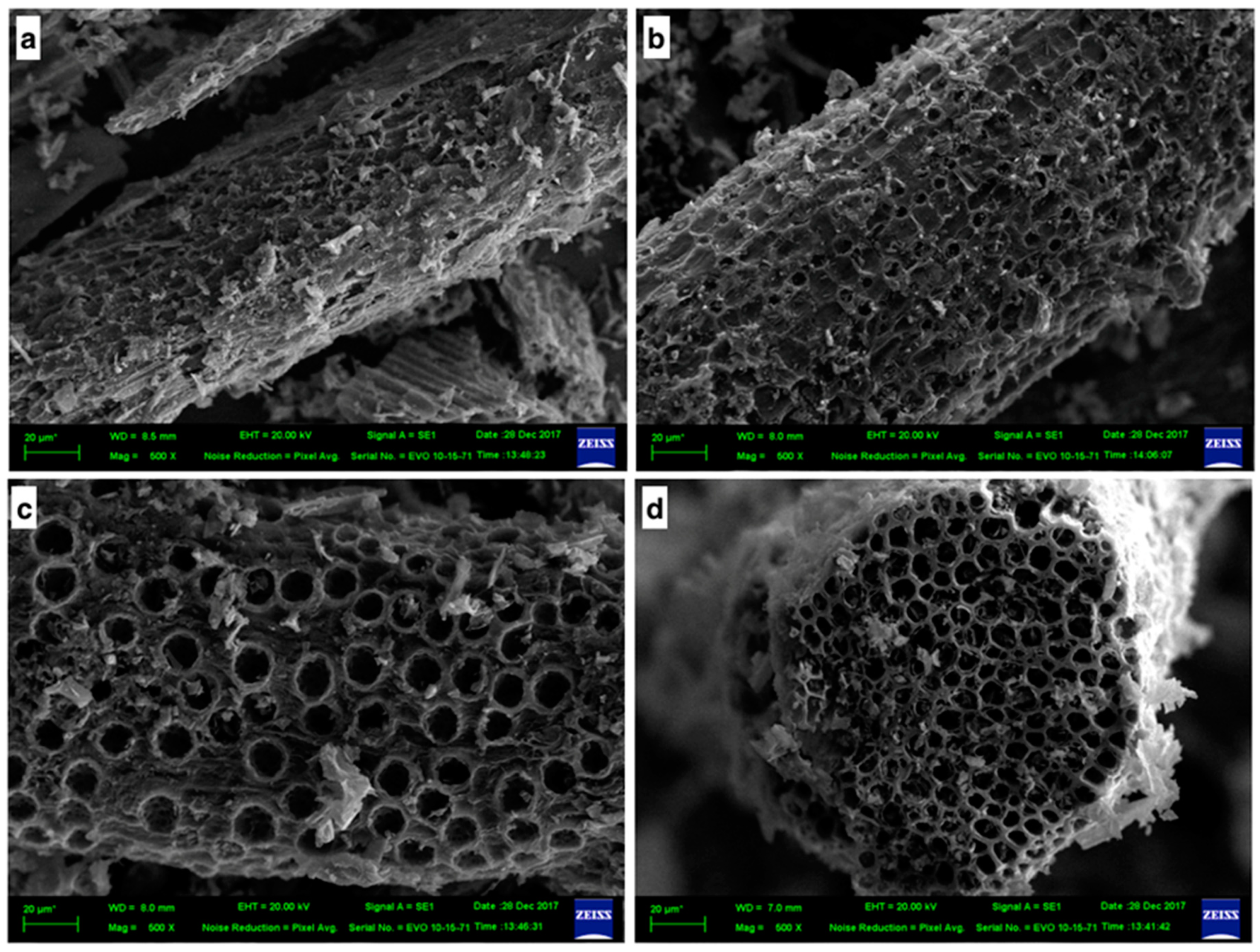
3.2. Surface Morphology
3.3. The Effects of Experimental Variables on Surface Area
3.3.1. Effect of Activating Agent Ratios on BET Surface Area
3.3.2. Effect of Microwave Radiation Powers on BET Surface Area
3.3.3. Effect of Activation Holding Times on BET Surface Area
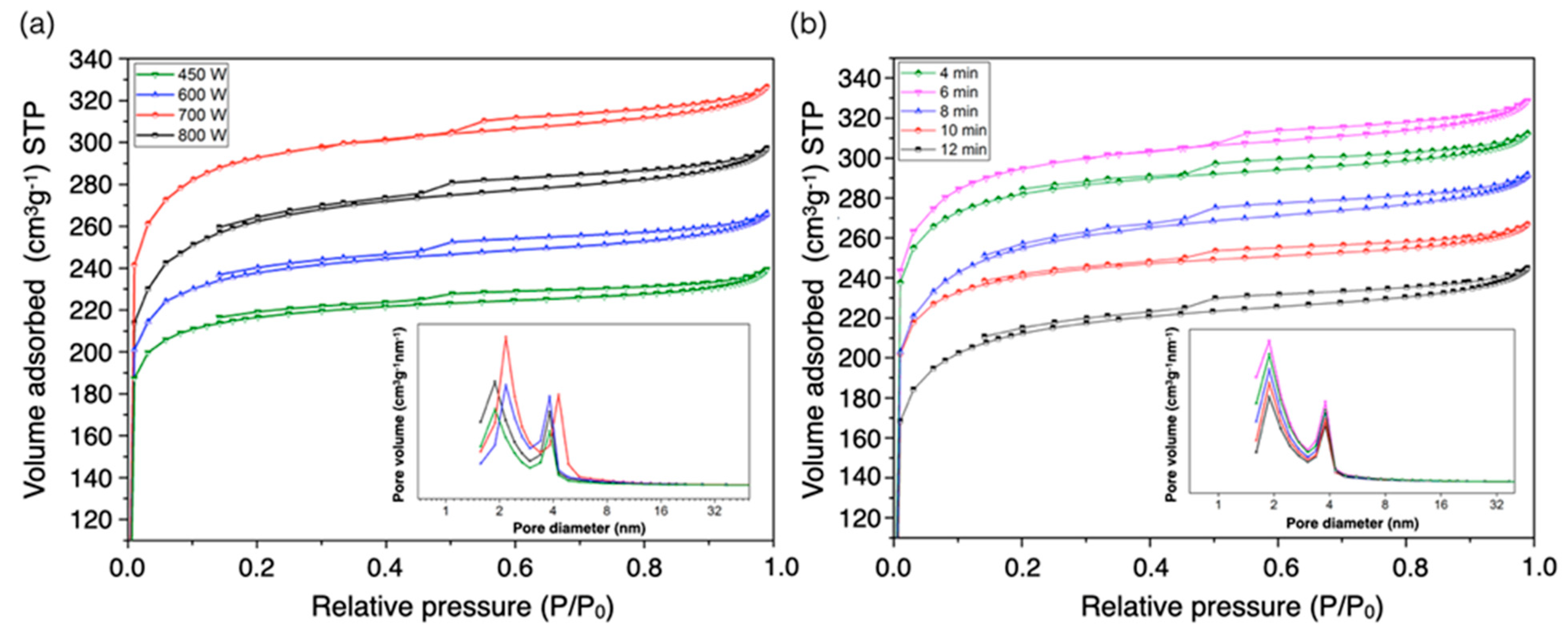
3.4. Nitrogen Adsorption/Desorption Isotherm and Pore Size Distribution
3.5. Proximate and Ultimate Analysis of PMFCs
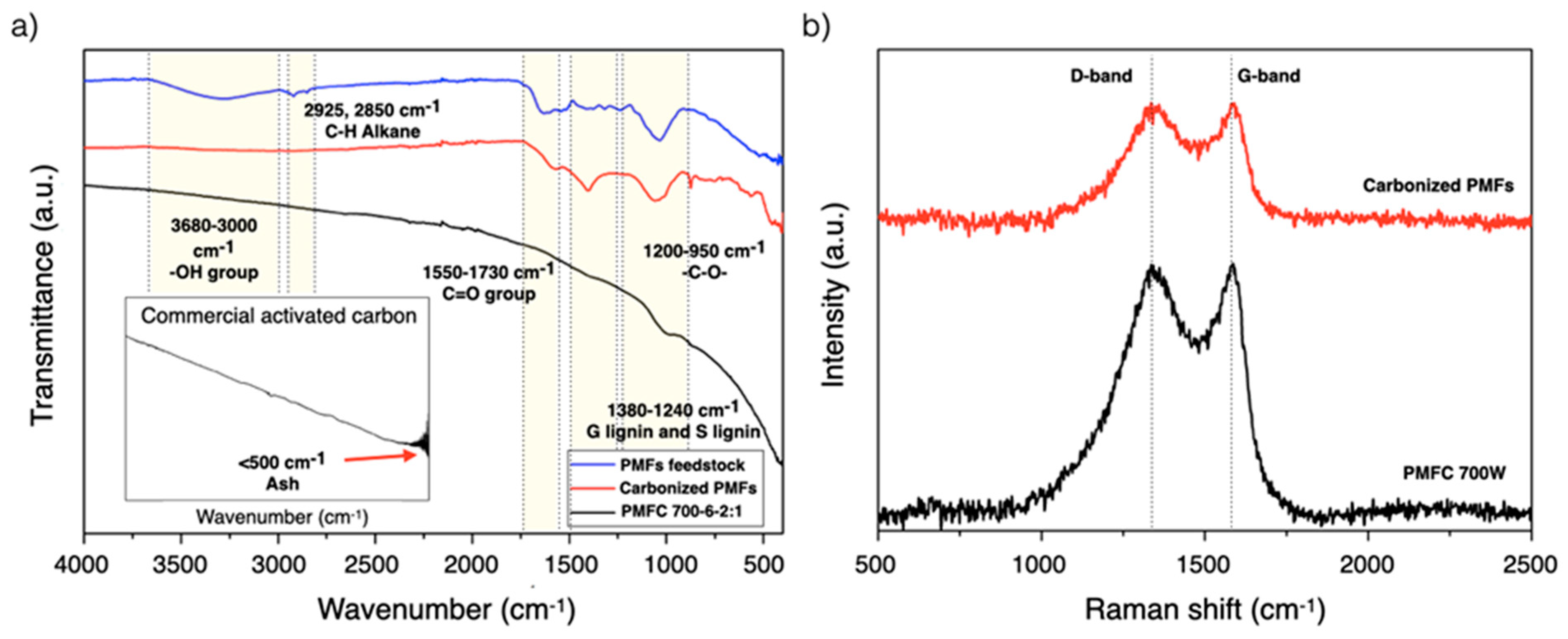
3.6. Fourier Transform Infrared Spectroscopy (FTIR) and Raman Spectroscopy Analyses
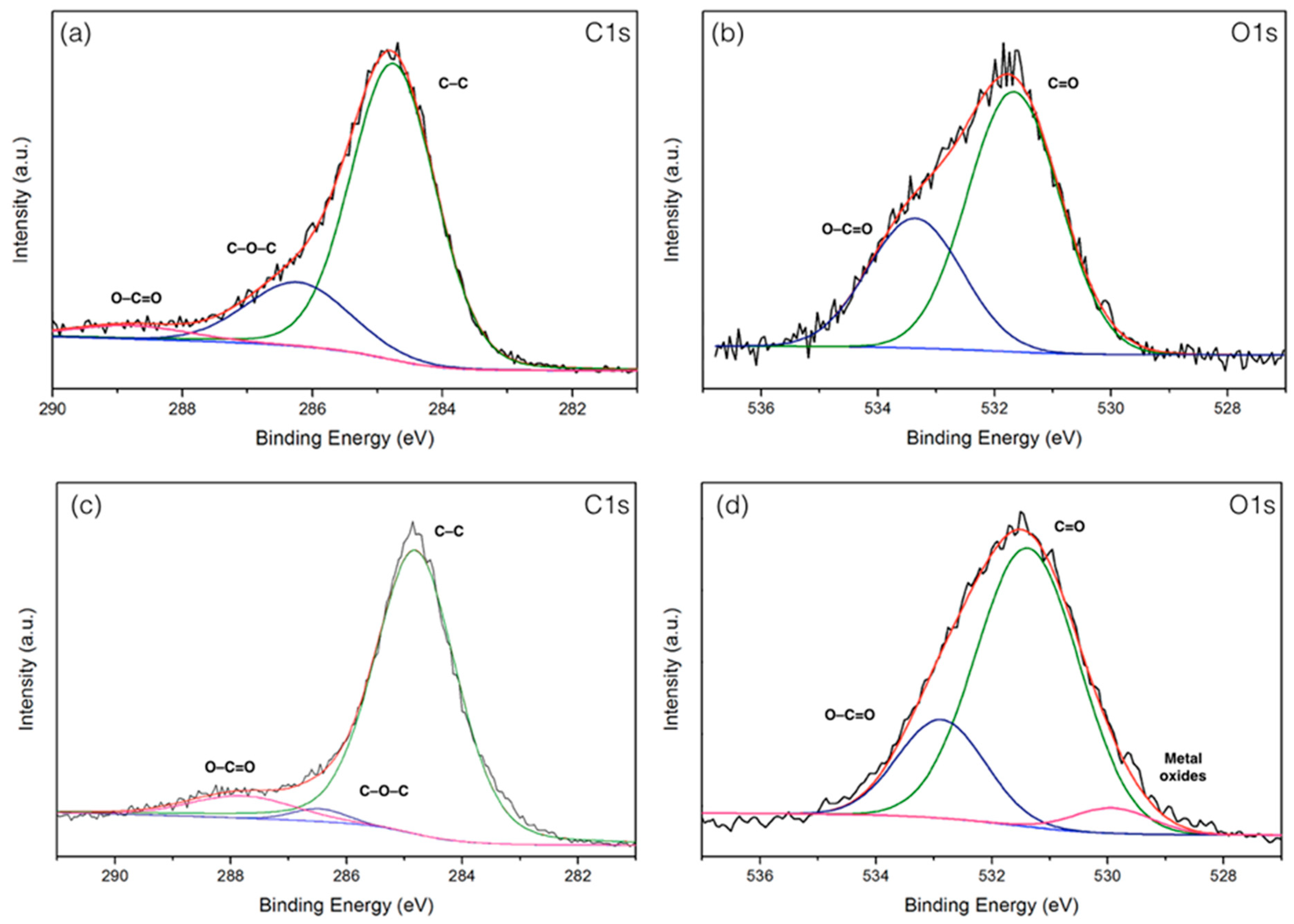
3.7. X-ray Photoelectron Spectroscopy (XPS) Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sathishkumar, P.; Arulkumar, M.; Thayumanavan, P. Utilization of agro-industrial waste Jatropha curcas pods as an activated carbon for the adsorption of reactive dye Remazol Brilliant Blue R (RBBR). J. Clean. Prod. 2012, 22, 67–75. [Google Scholar] [CrossRef]
- Olcese, R.; Bettahar, M.; Malaman, B.; Ghanbaja, J.; Tibavizco, L.; Petitjean, D.; Dufour, A. Gas-phase hydrodeoxygenation of guaiacol over iron-based catalysts. Effect of gases composition, iron load and supports (silica and activated carbon). Appl. Catal. B Environ. 2013, 129, 528–538. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, L.; Sun, L.; Zhu, S.; Huang, Z.; Mao, Y.; Lu, W.; Chen, W. Efficient removal of dyes using heterogeneous Fenton catalysts based on activated carbon fibers with enhanced activity. Chem. Eng. Sci. 2013, 101, 424–431. [Google Scholar] [CrossRef]
- Yeh, C.-L.; Hsi, H.-C.; Li, K.-C.; Hou, C.-H. Improved performance in capacitive deionization of activated carbon electrodes with a tunable mesopore and micropore ratio. Desalination 2015, 367, 60–68. [Google Scholar] [CrossRef]
- Nowicki, P.; Kazmierczak-Razna, J.; Pietrzak, R. Comparison of physicochemical and sorption properties of activated carbons prepared by physical and chemical activation of cherry stones. Powder Technol. 2015, 269, 312–319. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Xing, Z.-J.; Duan, Z.-K.; Li, M.; Wang, Y. Effects of steam activation on the pore structure and surface chemistry of activated carbon derived from bamboo waste. Appl. Surf. Sci. 2014, 315, 279–286. [Google Scholar] [CrossRef]
- Islam, A.; Tan, I.; Benhouria, A.; Asif, M.; Hameed, B.H. Mesoporous and adsorptive properties of palm date seed activated carbon prepared via sequential hydrothermal carbonization and sodium hydroxide activation. Chem. Eng. J. 2015, 270, 187–195. [Google Scholar] [CrossRef]
- Sayğili, H.; Güzel, F. High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: Process optimization, characterization and dyes adsorption. J. Clean. Prod. 2016, 113, 995–1004. [Google Scholar] [CrossRef]
- Yakout, S.M.; El-Deen, G.S. Characterization of activated carbon prepared by phosphoric acid activation of olive stones. Arab. J. Chem. 2016, 9, S1155–S1162. [Google Scholar] [CrossRef] [Green Version]
- Marrakchi, F.; Ahmed, M.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous-activated carbon prepared from chitosan flakes via single-step sodium hydroxide activation for the adsorption of methylene blue. Int. J. Biol Macromol. 2017, 98, 233–239. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Wong, Y.M.; Yek, P.N.Y.; Ma, N.L.; Lee, C.L.; Chase, H.A. Microwave-assisted pyrolysis with chemical activation, an innovative method to convert orange peel into activated carbon with improved properties as dye adsorbent. J. Clean. Prod. 2017, 162, 1376–1387. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Han, K.; Li, J.; Li, M.; Lu, C. Preparation and characterization of super activated carbon produced from gulfweed by KOH activation. Microporous Mesoporous Mater. 2017, 243, 291–300. [Google Scholar] [CrossRef]
- Saucier, C.; Adebayo, M.A.; Lima, E.C.; Cataluña, R.; Thue, P.S.; Prola, L.; Puchana-Rosero, M.; Machado, F.M.; Pavan, F.A.; Dotto, G.L. Microwave-assisted activated carbon from cocoa shell as adsorbent for removal of sodium diclofenac and nimesulide from aqueous effluents. J. Hazard. Mater. 2015, 289, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, G.S.; Wilhelm, M.; Silva, T.C.D.A.; Rezwan, K.; Sampaio, C.H.; Lima, E.C.; De Souza, S.M.A.G.U. The use of design of experiments for the evaluation of the production of surface rich activated carbon from sewage sludge via microwave and conventional pyrolysis. Appl. Therm. Eng. 2016, 93, 590–597. [Google Scholar] [CrossRef]
- Abioye, A.M.; Ani, F.N. Advancement in the production of Activated Carbon from Biomass Using Microwave Heating. J. Teknol. 2017, 79, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Köseoğlu, E.; Akmil-Başar, C. Preparation, structural evaluation and adsorptive properties of activated carbon from agricultural waste biomass. Adv. Powder Technol. 2015, 26, 811–818. [Google Scholar] [CrossRef]
- Alabadi, A.; Razzaque, S.; Yang, Y.; Chen, S.; Tan, B. Highly porous activated carbon materials from carbonized biomass with high CO2 capturing capacity. Chem. Eng. J. 2015, 281, 606–612. [Google Scholar] [CrossRef]
- Watson, V.J.; Nieto-Delgado, C.; Logan, B.E. Influence of Chemical and Physical Properties of Activated Carbon Powders on Oxygen Reduction and Microbial Fuel Cell Performance. Environ. Sci. Technol. 2013, 47, 6704–6710. [Google Scholar] [CrossRef]
- Sarswat, A.; Mohan, D. Sustainable development of coconut shell activated carbon (CSAC) & a magnetic coconut shell activated carbon (MCSAC) for phenol (2-nitrophenol) removal. RSC Adv. 2016, 6, 85390–85410. [Google Scholar] [CrossRef]
- Choi, G.-G.; Oh, S.-J.; Lee, S.-J.; Kim, J.-S. Production of bio-based phenolic resin and activated carbon from bio-oil and biochar derived from fast pyrolysis of palm kernel shells. Bioresour. Technol. 2015, 178, 99–107. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Loh, M.; Aziz, J. Preparation and characterization of activated carbon from oil palm wood and its evaluation on Methylene blue adsorption. Dye. Pigment. 2007, 75, 263–272. [Google Scholar] [CrossRef]
- Bouchelta, C.; Medjram, M.S.; Bertrand, O.; Bellat, J.-P. Preparation and characterization of activated carbon from date stones by physical activation with steam. J. Anal. Appl. Pyrolysis 2008, 82, 70–77. [Google Scholar] [CrossRef]
- Niksiar, A.; Nasernejad, B. Activated carbon preparation from pistachio shell pyrolysis and gasification in a spouted bed reactor. Biomass Bioenergy 2017, 106, 43–50. [Google Scholar] [CrossRef]
- Ma, X.; Ouyang, F. Adsorption properties of biomass-based activated carbon prepared with spent coffee grounds and pomelo skin by phosphoric acid activation. Appl. Surf. Sci. 2013, 268, 566–570. [Google Scholar] [CrossRef]
- Liu, H.; Jiaqiang, E.; Deng, Y.; Xie, C.; Zhu, H. Experimental study on pyrolysis characteristics of the tobacco stem based on microwave heating method. Appl. Therm. Eng. 2016, 106, 473–479. [Google Scholar] [CrossRef]
- Li, W.-H.; Yue, Q.; Gao, B.; Wang, X.-J.; Qi, Y.-F.; Zhao, Y.-Q.; Li, Y.-J. Preparation of sludge-based activated carbon made from paper mill sewage sludge by steam activation for dye wastewater treatment. Desalination 2011, 278, 179–185. [Google Scholar] [CrossRef]
- Karim, M.R.; Hashim, H.; Razak, H.A.; Yusoff, S. Characterization of palm oil clinker powder for utilization in cement-based applications. Constr. Build. Mater. 2017, 135, 21–29. [Google Scholar] [CrossRef]
- Tonks, A.J.; Aplin, P.; Beriro, D.J.; Cooper, H.; Evers, S.; Vane, C.H.; Sjögersten, S. Impacts of conversion of tropical peat swamp forest to oil palm plantation on peat organic chemistry, physical properties and carbon stocks. Geoderma 2017, 289, 36–45. [Google Scholar] [CrossRef]
- Ocampo-Peñuela, N.; Garcia-Ulloa, J.; Ghazoul, J.; Etter, A. Quantifying impacts of oil palm expansion on Colombia’s threatened biodiversity. Biol. Conserv. 2018, 224, 117–121. [Google Scholar] [CrossRef]
- Thushari, I.; Babel, S. Sustainable utilization of waste palm oil and sulfonated carbon catalyst derived from coconut meal residue for biodiesel production. Bioresour. Technol. 2018, 248, 199–203. [Google Scholar] [CrossRef]
- Said, A.; Tekasakul, S.; Phoungthong, K. Investigation of Hydrochar Derived from Male Oil Palm Flower: Characteristics and Application for Dye Removal. Pol. J. Environ. Stud. 2019, 29, 807–815. [Google Scholar] [CrossRef]
- Dizbay-Onat, M.; Vaidya, U.K.; Lungu, C.T. Preparation of industrial sisal fiber waste derived activated carbon by chemical activation and effects of carbonization parameters on surface characteristics. Ind. Crop. Prod. 2017, 95, 583–590. [Google Scholar] [CrossRef]
- Borchard, N.; Wolf, A.; Laabs, V.; Aeckersberg, R.; Scherer, H.W.; Moeller, A.; Amelung, W. Physical activation of biochar and its meaning for soil fertility and nutrient leaching—A greenhouse experiment. Soil Use Manag. 2012, 28, 177–184. [Google Scholar] [CrossRef]
- Njoku, V.O.; Islam, A.; Asif, M.; Hameed, B.H. Utilization of sky fruit husk agricultural waste to produce high quality activated carbon for the herbicide bentazon adsorption. Chem. Eng. J. 2014, 251, 183–191. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Preparation and characterization of activated carbon from sunflower seed oil residue via microwave assisted K2CO3 activation. Bioresour. Technol. 2011, 102, 9794–9799. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Boateng, A.A.; Qi, P.X.; Lima, I.M.; Chang, J. Heavy metal and phenol adsorptive properties of biochars from pyrolyzed switchgrass and woody biomass in correlation with surface properties. J. Environ. Manag. 2013, 118, 196–204. [Google Scholar] [CrossRef]
- Abioye, A.M.; Ani, F.N. The Characteristics of Oil Palm Shell Biochar and Activated Carbon Produced via Microwave Heating. Appl. Mech. Mater. 2014, 695, 12–15. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Microwave-assisted preparation and adsorption performance of activated carbon from biodiesel industry solid reside: Influence of operational parameters. Bioresour. Technol. 2012, 103, 398–404. [Google Scholar] [CrossRef]
- Rajagopal, R.R.; Aravinda, L.; Rajarao, R.; Bhat, B.R.; Sahajwalla, V. Activated carbon derived from non-metallic printed circuit board waste for supercapacitor application. Electrochim. Acta 2016, 211, 488–498. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Coconut husk derived activated carbon via microwave induced activation: Effects of activation agents, preparation parameters and adsorption performance. Chem. Eng. J. 2012, 184, 57–65. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Preparation of activated carbon by microwave heating of langsat (Lansium domesticum) empty fruit bunch waste. Bioresour. Technol. 2012, 116, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Li, G.; Yang, H.; Tang, J.; Tang, J. Preparation of activated carbons from cotton stalk by microwave assisted KOH and K2CO3 activation. Chem. Eng. J. 2010, 163, 373–381. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, G.; Xu, X.; Tao, G.; Dai, J. Optimization of preparation of activated carbon from cotton stalk by microwave assisted phosphoric acid-chemical activation. J. Hazard. Mater. 2010, 182, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Peng, H.; Deng, S.; Yang, X.; Zhang, Y.; Li, Y. Preparation of activated carbon from edible fungi residue by microwave assisted K2CO3 activation—Application in reactive black 5 adsorption from aqueous solution. Bioresour. Technol. 2012, 111, 127–133. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Liou, T.-H. Development of mesoporous structure and high adsorption capacity of biomass-based activated carbon by phosphoric acid and zinc chloride activation. Chem. Eng. J. 2010, 158, 129–142. [Google Scholar] [CrossRef]
- Maldhure, A.V.; Ekhe, J. Preparation and characterizations of microwave assisted activated carbons from industrial waste lignin for Cu(II) sorption. Chem. Eng. J. 2011, 168, 1103–1111. [Google Scholar] [CrossRef]
- Tseng, R.-L.; Tseng, S.-K.; Wu, F.-C.; Hu, C.-C.; Wang, C.-C. Effects of micropore development on the physicochemical properties of KOH-activated carbons. J. Chin. Inst. Chem. Eng. 2008, 39, 37–47. [Google Scholar] [CrossRef]
- Duan, X.-H.; Srinivasakannan, C.; Peng, J.-H.; Zhang, L.-B.; Zhang, Z.-Y. Comparison of activated carbon prepared from Jatropha hull by conventional heating and microwave heating. Biomass Bioenergy 2011, 35, 3920–3926. [Google Scholar] [CrossRef]
- Tay, T.; Uçar, S.; Karagöz, S. Preparation and characterization of activated carbon from waste biomass. J. Hazard. Mater. 2009, 165, 481–485. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, H.; Dai, P.; Shen, X.; Zhang, J.; Zhao, T.; Zhu, Z. Microwave-assisted preparation and characterization of mesoporous activated carbon from mushroom roots by phytic acid (C6H18O24P6) activation. J. Taiwan Inst. Chem. Eng. 2016, 67, 532–537. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Li, H.; Li, G.; Gao, B.; Yue, Q.; Li, X. Characterization and ciprofloxacin adsorption properties of activated carbons prepared from biomass wastes by H3PO4 activation. Bioresour. Technol. 2016, 217, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Nabais, J.V.; Nunes, P.; Carrott, P.J.M.; Carrott, M.R.; Garcia, A.M.; Diaz-Diez, M. Production of activated carbons from coffee endocarp by CO2 and steam activation. Fuel Process. Technol. 2008, 89, 262–268. [Google Scholar] [CrossRef]
- Chang, J.; Gao, Z.; Wang, X.; Wu, D.; Xu, F.; Wang, X.; Guo, Y.; Jiang, K. Activated porous carbon prepared from paulownia flower for high performance supercapacitor electrodes. Electrochim. Acta 2015, 157, 290–298. [Google Scholar] [CrossRef]

| Properties | Proximate Analysis ** | Ultimate Analysis ** | ||||||
|---|---|---|---|---|---|---|---|---|
| M | VM | FC * | A | C | H | N | O * | |
| Raw PMFs | 7.61 | 61.06 | 24.01 | 7.32 | 43.73 | 2.42 | 1.24 | 52.61 |
| Conditions | Pore Characteristics | |||
|---|---|---|---|---|
| SBET (m2/g) | VT (cm3/g) | Vmic (%) | Vmes (%) | |
| PMFC450-6-1:1 | 369 | 0.21 | 57.14 | 42.86 |
| PMFC450-6-2:1 | 757 | 0.37 | 72.97 | 27.03 |
| PMFC450-6-4:1 | 447 | 0.22 | 77.27 | 22.73 |
| PMFC450-6-6:1 | 390 | 0.19 | 73.68 | 26.32 |
| PMFC450-6-8:1 | 355 | 0.18 | 61.11 | 38.89 |
| PMFC600-6-2:1 | 784 | 0.39 | 69.23 | 30.77 |
| PMFC700-6-2:1 | 991 | 0.49 | 71.12 | 28.88 |
| PMFC800-6-2:1 | 920 | 0.46 | 63.04 | 36.96 |
| PMFC700-4-2:1 | 911 | 0.45 | 73.81 | 26.19 |
| PMFC700-8-2:1 | 895 | 0.44 | 69.38 | 30.62 |
| PMFC700-10-2:1 | 866 | 0.42 | 64.10 | 36.90 |
| PMFC700-12-2:1 | 777 | 0.39 | 63.64 | 36.36 |
| Conditions | Proximate Analysis ** | Ultimate Analysis ** | ||||||
|---|---|---|---|---|---|---|---|---|
| M | VM | FC * | A | C | H | N | O * | |
| Carbonized PMFs | 2.13 | 26.86 | 64.37 | 6.64 | 68.71 | 1.27 | 0.95 | 29.07 |
| PMFC450-6-1:1 | 2.94 | 15.96 | 72.63 | 8.47 | 75.27 | 1.18 | 1.02 | 22.53 |
| PMFC450-6-2:1 | 3.12 | 15.79 | 73.47 | 7.62 | 76.59 | 0.95 | 1.08 | 21.38 |
| PMFC450-6-4:1 | 3.79 | 16.22 | 72.81 | 7.18 | 77.17 | 0.94 | 0.97 | 20.29 |
| PMFC450-6-6:1 | 3.57 | 16.03 | 73.34 | 7.06 | 78.66 | 0.91 | 0.96 | 19.47 |
| PMFC450-6-8:1 | 2.91 | 16.18 | 73.79 | 7.12 | 79.12 | 0.92 | 0.99 | 18.97 |
| PMFC600-6-2:1 | 3.16 | 15.19 | 73.14 | 8.51 | 77.63 | 0.89 | 0.97 | 20.51 |
| PMFC700-6-2:1 | 2.96 | 13.61 | 74.56 | 8.87 | 79.09 | 0.93 | 0.95 | 19.03 |
| PMFC800-6-2:1 | 2.88 | 11.45 | 76.62 | 9.05 | 80.13 | 0.84 | 0.94 | 18.09 |
| PMFC700-4-2:1 | 3.67 | 14.32 | 73.56 | 8.45 | 78.59 | 0.81 | 0.86 | 19.74 |
| PMFC700-8-2:1 | 3.29 | 12.07 | 75.83 | 8.81 | 79.15 | 0.95 | 0.79 | 19.11 |
| PMFC700-10-2:1 | 2.85 | 12.49 | 75.53 | 9.13 | 79.27 | 0.79 | 0.93 | 19.01 |
| PMFC700-12-2:1 | 3.78 | 13.16 | 73.54 | 9.52 | 79.93 | 0.82 | 0.97 | 18.28 |
| Raw Biomass | Reactor | Condition | SBET (m2/g) | Ref. |
|---|---|---|---|---|
| Cherry stones | Horizontal tube furnace | CO2 (0.25 L/min, and KOH (KOH/char weight ratio of 2:1), 500–800 °C, 1 h | 361–1173 | [5] |
| Bamboo waste | Vertical tube furnace | Steam, 550–850 °C, 1–2.5 h | 459–1210 | [6] |
| Date seed | Hydrothermal reactor and tube furnace | Hydrothermal at 200 °C 5 h, NaOH (1:3 HTC char/NaOH w/w), activation at 600 °C 1 h | 1282 | [7] |
| Tomato solid waste | Horizontal tube furnace | ZnCl2 (6:1 ZnCl2/TW w/w), activation at 600 °C 0.5–4 h | 522–1093 | [8] |
| Palm wood | Pilot kiln | 519–806 °C, 1–3.5 h CO2 and steam from limestone and liquefied petroleum gas (LPG) combustion | 194–1084 | [21] |
| Oil palm male flowers | Hydrothermal reactor | Hydrothermal at 180 °C 8 h, No activating agent | 5 | [31] |
| Langsat (Lansium domesticum) empty fruit bunch | Microwave-assisted pyrolyzer | Pre-carbonization at 700 °C, 1 h Activation at 600 W, 6 min 1.25 NaOH/char ratio (w/w) | 839 | [41] |
| Oil palm male flowers | Microwave-assisted pyrolyzer | Pre-carbonization at 500 °C, 1 h Activation at 450–800 W, 4–12 min 1:1, 2:1, 4:1, 6:1, 8:1 char/KOH ratio (w/w) | 355–991 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaewtrakulchai, N.; Faungnawakij, K.; Eiad-Ua, A. Parametric Study on Microwave-Assisted Pyrolysis Combined KOH Activation of Oil Palm Male Flowers Derived Nanoporous Carbons. Materials 2020, 13, 2876. https://doi.org/10.3390/ma13122876
Kaewtrakulchai N, Faungnawakij K, Eiad-Ua A. Parametric Study on Microwave-Assisted Pyrolysis Combined KOH Activation of Oil Palm Male Flowers Derived Nanoporous Carbons. Materials. 2020; 13(12):2876. https://doi.org/10.3390/ma13122876
Chicago/Turabian StyleKaewtrakulchai, Napat, Kajornsak Faungnawakij, and Apiluck Eiad-Ua. 2020. "Parametric Study on Microwave-Assisted Pyrolysis Combined KOH Activation of Oil Palm Male Flowers Derived Nanoporous Carbons" Materials 13, no. 12: 2876. https://doi.org/10.3390/ma13122876
APA StyleKaewtrakulchai, N., Faungnawakij, K., & Eiad-Ua, A. (2020). Parametric Study on Microwave-Assisted Pyrolysis Combined KOH Activation of Oil Palm Male Flowers Derived Nanoporous Carbons. Materials, 13(12), 2876. https://doi.org/10.3390/ma13122876