Structure Dependence of Poisson’s Ratio in Cesium Silicate and Borate Glasses
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
3. Results
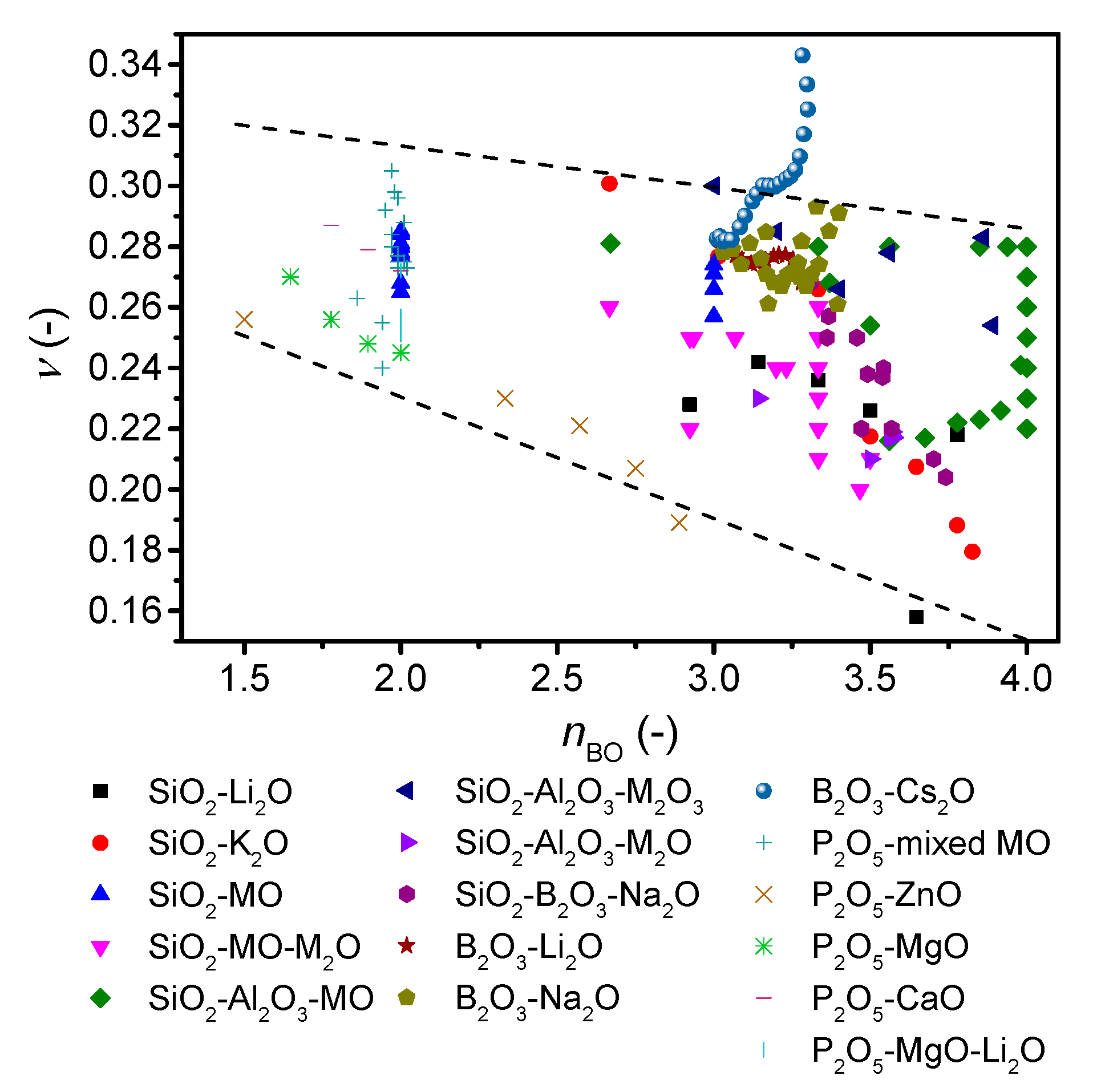
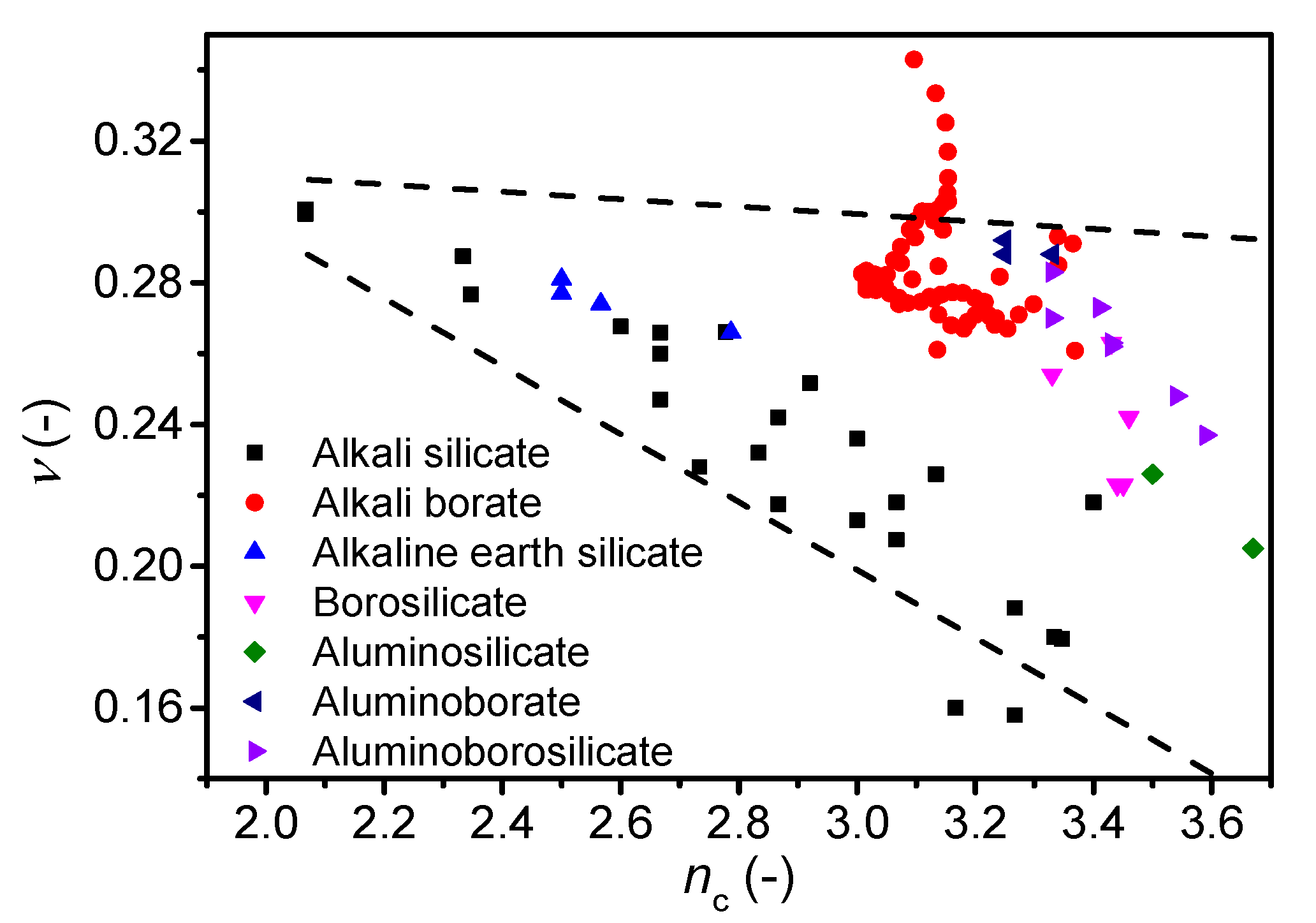
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sehgal, J.; Ito, S. Brittleness of glass. J. Non Cryst. Solids 1999, 253, 126–132. [Google Scholar] [CrossRef]
- Wondraczek, L.; Mauro, J.C.; Eckert, J.; Kühn, U.; Horbach, J.; Deubener, J.; Rouxel, T. Towards ultrastrong glasses. Adv. Mater. 2011, 23, 4578–4586. [Google Scholar] [CrossRef] [PubMed]
- Rouxel, T.; Yoshida, S. The fracture toughness of inorganic glasses. J. Am. Ceram. Soc. 2017, 100, 4374–4396. [Google Scholar] [CrossRef]
- Bertoldi, M.; Sglavo, V.M. Soda–borosilicate glass: Normal or anomalous behavior under Vickers indentation? J. Non Cryst. Solids 2004, 344, 51–59. [Google Scholar] [CrossRef]
- Rouxel, T.; Ji, H.; Hammouda, T.; Moréac, A. Poisson’s ratio and the densification of glass under high pressure. Phys. Rev. Lett. 2008, 100, 1–4. [Google Scholar] [CrossRef]
- Grima-Cornish, J.N.; Vella-Zarb, L.; Grima, J.N. Negative linear compressibility and auxeticity in boron arsenate. Annalen der Physik 2020, 532, 1900550. [Google Scholar] [CrossRef]
- Rouxel, T. Elastic properties and short-to medium-range order in glasses. J. Am. Ceram. Soc. 2007, 90, 3019–3039. [Google Scholar] [CrossRef]
- Deschamps, T.; Margueritat, J.; Martinet, C.; Mermet, A.; Champagnon, B. Elastic moduli of permanently densified silica glasses. Sci. Rep. 2014, 4, 7193. [Google Scholar] [CrossRef]
- Lewandowski, J.J.; Wang, W.H.; Greer, A.L. Intrinsic plasticity or brittleness of metallic glasses. Philos. Mag. Lett. 2005, 85, 77–87. [Google Scholar] [CrossRef]
- Greaves, G.N.; Greer, A.L.; Lakes, R.S.; Rouxel, T. Poisson’s ratio and modern materials. Nat. Mater. 2011, 10, 823–838. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, M.B.; Hansen, S.R.; Januchta, K.; To, T.; Rzoska, S.J.; Bockowski, M.; Bauchy, M.; Smedskjaer, M.M. Revisiting the dependence of poisson’s ratio on liquid fragility and atomic packing density in oxide glasses. Materials (Basel) 2019, 12, 2439. [Google Scholar] [CrossRef] [PubMed]
- Januchta, K.; Bauchy, M.; Youngman, R.E.; Rzoska, S.J.; Bockowski, M.; Smedskjaer, M.M. Modifier field strength effects on densification behavior and mechanical properties of alkali aluminoborate glasses. Phys. Rev. Mater. 2017, 1, 063603. [Google Scholar] [CrossRef]
- Wilkinson, C.J.; Zheng, Q.; Huang, L.; Mauro, J.C. Topological constraint model for the elasticity of glass-forming systems. J. Non-Cryst. Solids X 2019, 2, 100019. [Google Scholar] [CrossRef]
- Smedskjaer, M.M.; Mauro, J.C.; Yue, Y. Prediction of glass hardness using temperature-dependent constraint theory. Phys. Rev. Lett. 2010, 105, 115503. [Google Scholar] [CrossRef] [PubMed]
- Smedskjaer, M.M.; Mauro, J.C.; Sen, S.; Yue, Y. Quantitative design of glassy materials using temperature-dependent constraint theory. Chem. Mater. 2010, 22, 5358–5365. [Google Scholar] [CrossRef]
- Makishima, A.; Mackenzie, J.D. Calculation of bulk modulus, shear modulus, and Poisson’s ratio of glass. J. Non Cryst. Solids 1975, 17, 147–157. [Google Scholar] [CrossRef]
- Pedone, A.; Malavasi, G.; Cormack, A.N.; Segre, U.; Menziani, M.C. Insight into elastic properties of binary alkali silicate glasses; Prediction and interpretation through atomistic simulation techniques. Chem. Mater. 2007, 19, 3144–3154. [Google Scholar] [CrossRef]
- Novikov, V.N.; Sokolov, A.P. Poisson’s ratio and the fragility of glass-forming liquids. Nature 2004, 431, 961–963. [Google Scholar] [CrossRef]
- Sidebottom, D.L. Fragility of network-forming glasses: A universal dependence on the topological connectivity. Phys. Rev. E 2015, 92, 1–9. [Google Scholar] [CrossRef]
- Yannopoulos, S.N.; Johari, G.P. Glass behaviour: Poisson’s ratio and liquid’s fragility. Nature 2006, 442, E7–E8. [Google Scholar] [CrossRef]
- Kodama, M.; Kojima, S. Velocity of sound in and elastic properties of alkali metal borate glasses. Phys. Chem. Glas. Eur. J. Glas. Sci. Technol. B 2014, 55, 1–12. [Google Scholar] [CrossRef]
- Dupree, R.; Holland, D.; Williams, D.S. The structure of binary alkali silicate glasses. J. Non Cryst. Solids 1986, 81, 185–200. [Google Scholar] [CrossRef]
- Maekawa, H.; Maekawa, T.; Kawamura, K.; Yokokawa, T. The structural groups of alkali silicate glasses determined from 29Si MAS-NMR. J. Non Cryst. Solids 1991, 127, 53–64. [Google Scholar] [CrossRef]
- Du, J.; Corrales, L.R. First sharp diffraction peak in silicate glasses: Structure and scattering length dependence. Phys. Rev. B 2005, 72, 092201. [Google Scholar] [CrossRef]
- Feller, S.A.; Dell, W.J.; Bray, P.J. 10B NMR studies of lithium borate glasses. J. Non Cryst. Solids 1982, 51, 21–30. [Google Scholar] [CrossRef]
- Bray, P.J.; Feller, S.A.; Jellison, G.E.; Yun, Y.H. B10 NMR studies of the structure of borate glasses. J. Non Cryst. Solids 1980, 38, 93–98. [Google Scholar] [CrossRef]
- Konijnendijk, W.L.; Stevels, J.M. The structure of borate glasses studied by raman scattering. J. Non Cryst. Solids 1975, 18, 307–331. [Google Scholar] [CrossRef]
- Dietzel, A. The cation field strengths and their relation to devitrifying processes, to compound formation and to the melting points of silicates. Z. Elektrochem. Angew. Phys. Chemie 1942, 48, 9–23. [Google Scholar]
- Frederiksen, K.F.; Januchta, K.; Mascaraque, N.; Youngman, R.E.; Bauchy, M.; Rzoska, S.J.; Bockowski, M.; Smedskjaer, M.M. structural compromise between high hardness and crack resistance in aluminoborate Glasses. J. Phys. Chem. B 2018, 122, 6287–6295. [Google Scholar] [CrossRef]
- Tiegel, M.; Hosseinabadi, R.; Kuhn, S.; Herrmann, A.; Rüssel, C. Young’s modulus, Vickers hardness and indentation fracture toughness of alumino silicate glasses. Ceram. Int. 2015, 41, 7267–7275. [Google Scholar] [CrossRef]
- Burkhard, D.J.M. Elastic properties of alkali silicate glasses with iron oxide: Relation to glass structure. Solid State Commun. 1997, 101, 903–907. [Google Scholar] [CrossRef]
- Yiannopoulos, Y.D.; Chryssikos, G.D.; Kamitsos, E.I. Structure and properties of alkaline earth borate glasses. Phys. Chem. Glas. 2001, 42, 164–172. [Google Scholar]
- Berkemeier, F.; Voss, S. Molar volume, glass-transition temperature, and ionic conductivity of Na- and Rb-borate glasses in comparison with mixed Na-Rb borate glasses. J. Non Cryst. Solids 2005, 351, 3816–3825. [Google Scholar] [CrossRef]
- Rouse, G.B.; Kamitsos, E.I.; Risen, W.M. Brillouin spectra of mixed alkali glasses: xCs2O(1-x)Na2O5SiO2. J. Non Cryst. Solids 1981, 45, 257–269. [Google Scholar] [CrossRef]
- Royle, M.; MacKenzie, J.; Taylor, J.; Sharma, M.; Feller, S. Densities, glass transition temperatures, and structural models resulting from extremely modified caesium and rubidium borate glasses. J. Non Cryst. Solids 1994, 177, 242–248. [Google Scholar] [CrossRef]
- Yue, Y. Characteristic temperatures of enthalpy relaxation in glass. J. Non Cryst. Solids 2008, 354, 1112–1118. [Google Scholar] [CrossRef]
- Zheng, Q.; Mauro, J.C.; Yue, Y. Reconciling calorimetric and kinetic fragilities of glass-forming liquids. J. Non Cryst. Solids 2017, 456, 95–100. [Google Scholar] [CrossRef]
- Bødker, M.S.; Sørensen, S.S.; Mauro, J.C.; Smedskjaer, M.M. Predicting composition-structure relations in alkali borosilicate glasses using statistical mechanics. Front. Mater. 2019, 6, 1–11. [Google Scholar] [CrossRef]
- Zhong, J.; Bray, P.J. Change in boron coordination in alkali borate glasses, and mixed alkali effects, as elucidated by NMR. J. Non Cryst. Solids 1989, 111, 67–76. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chaleogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Nascimento, M.L.F.; Do Nascimento, E.; Watanabe, S. Test of Anderson-Stuart model and the “universal” conductivity in rubidium and cesium silicate glasses. Braz. J. Phys. 2005, 35, 626–631. [Google Scholar] [CrossRef]
- Kodama, M.; Nakashima, N.; Matsushita, T. Velocity of Sound in and Elastic Properties of Cs2O-B2O3 glasses. Jpn. J. Appl. Phys. 1993, 32, 2227. [Google Scholar] [CrossRef]
- Sidebottom, D.L.; Tran, T.D.; Schnell, S.E. Building up a weaker network: The effect of intermediate range glass structure on liquid fragility. J. Non Cryst. Solids 2014, 402, 16–20. [Google Scholar] [CrossRef]
- Ferreira Nascimento, M.L.; Aparicio, C. Viscosity of strong and fragile glass-forming liquids investigated by means of principal component analysis. J. Phys. Chem. Solids 2007, 68, 104–110. [Google Scholar] [CrossRef]
- Schroeder, J.; Mohr, R. Rayleigh and Brillouin Scattering in K2O-SiO2 Glasses. J. Am. Ceram. Soc. 1973, 56, 510–514. [Google Scholar] [CrossRef]
- Martin, S.W.; Angell, C.A. Glass formation and transition temperatures in sodium and lithium borate and aluminoborate melts up to 72 mol.% alkali. J. Non Cryst. Solids 1984, 66, 429–442. [Google Scholar] [CrossRef]
- Shartsis, L.; Capps, W.; Spinner, S. Density and expansivity of Alkali Borates and Density. J. Am. Ceram. Soc. 1953, 36, 35–43. [Google Scholar] [CrossRef]
- Carini, G.; Carini, G.; D’Angelo, G.; Tripodo, G.; Bartolotta, A.; Salvato, G. Ultrasonic relaxations, anharmonicity, and fragility in lithium borate glasses. Phys. Rev. B Condens. Matter 2005, 72, 1–10. [Google Scholar] [CrossRef]
- Shaw, R.R.; Uhlmann, D.R. Effect of phase separation on the properties of simple glasses II. Elastic properties. J. Non Cryst. Solids 1971, 5, 237–263. [Google Scholar] [CrossRef]
- Januchta, K.; To, T.; Bødker, M.S.; Rouxel, T.; Smedskjaer, M.M. Elasticity, hardness, and fracture toughness of sodium aluminoborosilicate glasses. J. Am. Ceram. Soc. 2019, 102, 4520–4537. [Google Scholar] [CrossRef]
- Soga, N.; Yamanaka, H.; Hisamoto, C.; Kunugi, M. Elastic properties and structure of alkaline-earth silicate glasses. J. Non Cryst. Solids 1976, 22, 67–76. [Google Scholar] [CrossRef]
- Hermansen, C.; Matsuoka, J.; Yoshida, S.; Yamazaki, H.; Kato, Y.; Yue, Y. Densification and plastic deformation under microindentation in silicate glasses and the relation to hardness and crack resistance. J. Non Cryst. Solids 2013, 364, 40–43. [Google Scholar] [CrossRef]
- Yoshida, S.; Sanglebœuf, J.C.; Rouxel, T. Quantitative evaluation of indentation-induced densification in glass. J. Mater. Res. 2005, 20, 3404–3412. [Google Scholar] [CrossRef]
- Limbach, R.; Rodrigues, B.P.; Möncke, D.; Wondraczek, L. Elasticity, deformation and fracture of mixed fluoride-phosphate glasses. J. Non Cryst. Solids 2015, 430, 99–107. [Google Scholar] [CrossRef]
- Winterstein-Beckmann, A.; Möncke, D.; Palles, D.; Kamitsos, E.I.; Wondraczek, L. A Raman-spectroscopic study of indentation-induced structural changes in technical alkali-borosilicate glasses with varying silicate network connectivity. J. Non Cryst. Solids 2014, 405, 196–206. [Google Scholar] [CrossRef]
- Pönitzsch, A.; Nofz, M.; Wondraczek, L.; Deubener, J. Bulk elastic properties, hardness and fatigue of calcium aluminosilicate glasses in the intermediate-silica range. J. Non Cryst. Solids 2016, 434, 1–12. [Google Scholar] [CrossRef]
- Kjeldsen, J.; Smedskjaer, M.M.; Mauro, J.C.; Yue, Y. On the origin of the mixed alkali effect on indentation in silicate glasses. J. Non Cryst. Solids 2014, 406, 22–26. [Google Scholar] [CrossRef]
- Kodama, M.; Matsushita, T.; Kojima, S. Velocity of sound in and elastic properties of Li2O–B2O3 glasses. Jpn. J. Appl. Phys. 1995, 34, 2570–2574. [Google Scholar] [CrossRef]
- Sanditov, D.S.; Mashanov, A.A.; Sanditov, B.D.; Mantatov, V.V. Fragility and anharmonicity of lattice vibrations of glass-forming systems. Glas. Phys. Chem. 2008, 34, 389–393. [Google Scholar] [CrossRef]
- Hassan, A.K.; Börjesson, L.; Torell, L.M. Relaxations in Complex Systems the boson peak in glass formers of increasing fragility. J. Non Cryst. Solids 1994, 172, 154–160. [Google Scholar] [CrossRef]
- Griebenow, K.; Bragatto, C.B.; Kamitsos, E.I.; Wondraczek, L. Mixed-modifier effect in alkaline earth metaphosphate glasses. J. Non Cryst. Solids 2018, 481, 447–456. [Google Scholar] [CrossRef]
- Matori, K.A.; Zaid, M.H.M.; Quah, H.J.; Aziz, S.H.A.; Wahab, Z.A.; Ghazali, M.S.M. Studying the Effect of ZnO on Physical and Elastic Properties of (ZnO)x(P2O5)1−x Glasses Using Nondestructive Ultrasonic Method. Adv. Mater. Sci. Eng. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Striepe, S.; Deubener, J. Effect of lithium-to-magnesium ratio in metaphosphate glasses on crack-tip condensation and sub-critical crack growth. J. Non Cryst. Solids 2013, 375, 47–54. [Google Scholar] [CrossRef]
- Ashizuka, M.; Ishida, E.; Uto, S.; Bradt, R.C. Fracture toughness and surface energies of binary CaO- and MgO-phosphate glasses. J. Non Cryst. Solids 1988, 104, 316–322. [Google Scholar] [CrossRef]
- Bødker, M.S.; Mauro, J.C.; Youngman, R.E.; Smedskjaer, M.M. Statistical mechanical modeling of borate glass structure and topology: Prediction of superstructural units and glass transition temperature. J. Phys. Chem. B 2019, 123, 1206–1213. [Google Scholar] [CrossRef]
- Toplis, M.J.; Kohn, S.C.; Smith, M.E.; Poplett, I.J.F. Fivefold-coordinated aluminum in tectosilicate glasses observed by triple quantum MAS NMR. Am. Mineral. 2000, 85, 1556–1560. [Google Scholar] [CrossRef]
- Stebbins, J.F.; Xu, Z. NMR evidence for excess non-bridging oxygen in an aluminosilicate glass. Nature 1997, 390, 60–62. [Google Scholar] [CrossRef]
- Konijnendijk, W.L.; Stevels, J.M. The structure of borosilicate glasses studied by Raman scattering. J. Non Cryst. Solids 1976, 20, 193–224. [Google Scholar] [CrossRef]
- Mascaraque, N.; Bauchy, M.; Smedskjaer, M.M. Correlating the network topology of oxide glasses with their chemical durability. J. Phys. Chem. B 2017, 121, 1139–1147. [Google Scholar] [CrossRef]
- Gupta, P.K.; Mauro, J.C. Composition dependence of glass transition temperature and fragility. I. A topological model incorporating temperature- dependent constraints. J. Chem. Phys. 2009, 130, 094503. [Google Scholar] [CrossRef]
- Smedskjaer, M.M.; Mauro, J.C.; Youngman, R.E.; Hogue, C.L.; Potuzak, M.; Yue, Y. Topological principles of borosilicate glass chemistry. J. Phys. Chem. B 2011, 115, 12930–12946. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.C. Topology of covalent non-crystalline solids I: Short-range order in chalcogenide alloys. J. Non Cryst. Solids 1979, 34, 153–181. [Google Scholar] [CrossRef]
- Phillips, J.C.; Thorpe, M.F. Constraint theory, vector percolation and glass formation. Solid State Commun. 1985, 53, 699–702. [Google Scholar] [CrossRef]
- Mauro, J.C. Topological constraint theory of glass. Am. Ceram. Soc. Bull. 2011, 90, 31–37. [Google Scholar]
- Micoulaut, M. Constrained interactions, rigidity, adaptative networks, and their role for the description of silicates. Am. Mineral. 2008, 93, 1732–1748. [Google Scholar] [CrossRef]
- Smedskjaer, M.M. Topological model for boroaluminosilicate glass hardness. Front. Mater. 2014, 1, 1–6. [Google Scholar] [CrossRef]
- Takeda, W.; Wilkinson, C.J.; Feller, S.A.; Mauro, J.C. Journal of non-crystalline solids: X Topological constraint model of high lithium content borate glasses. J. Non-Cryst. Solids X 2019, 3, 100028. [Google Scholar] [CrossRef]
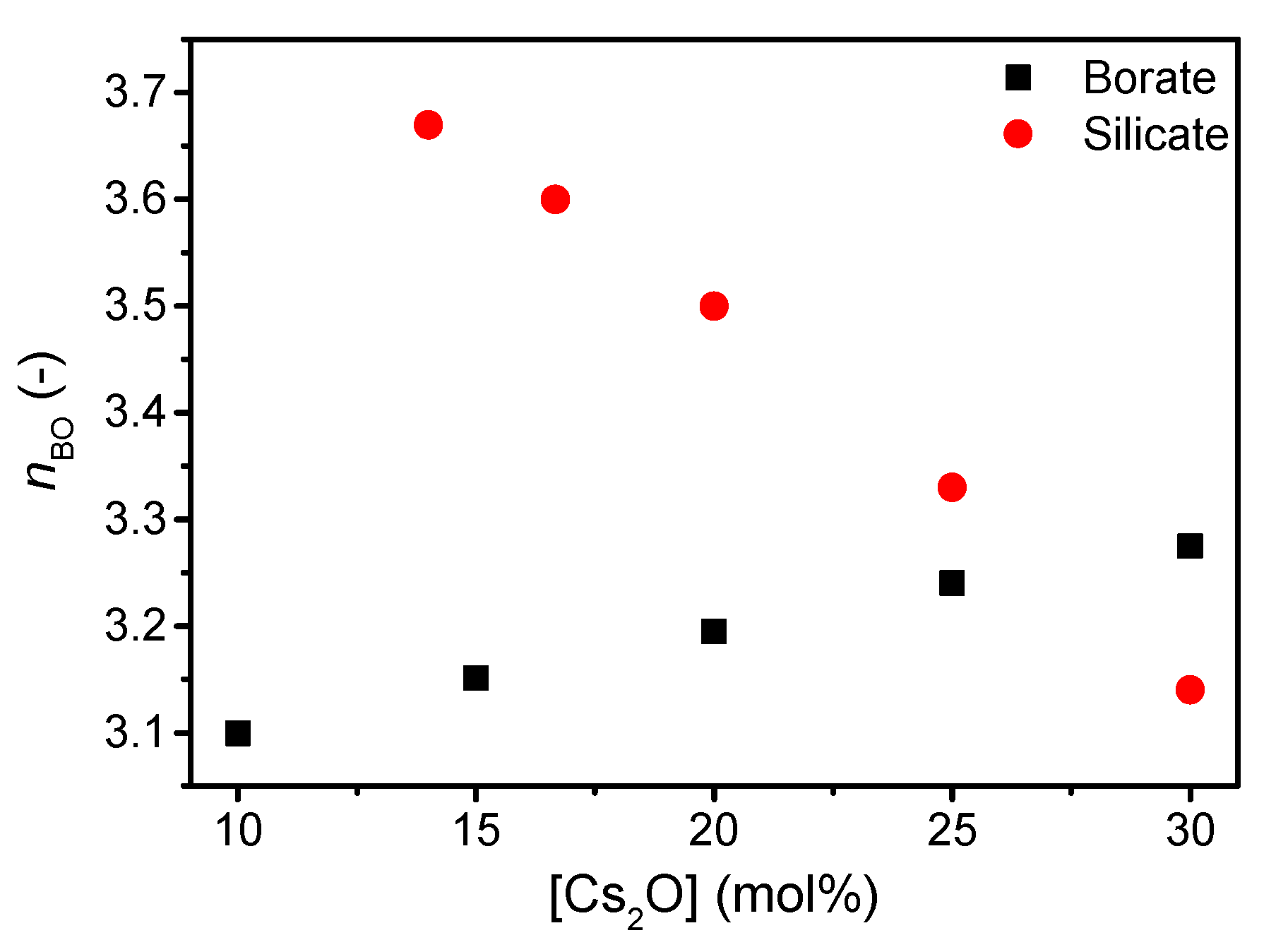

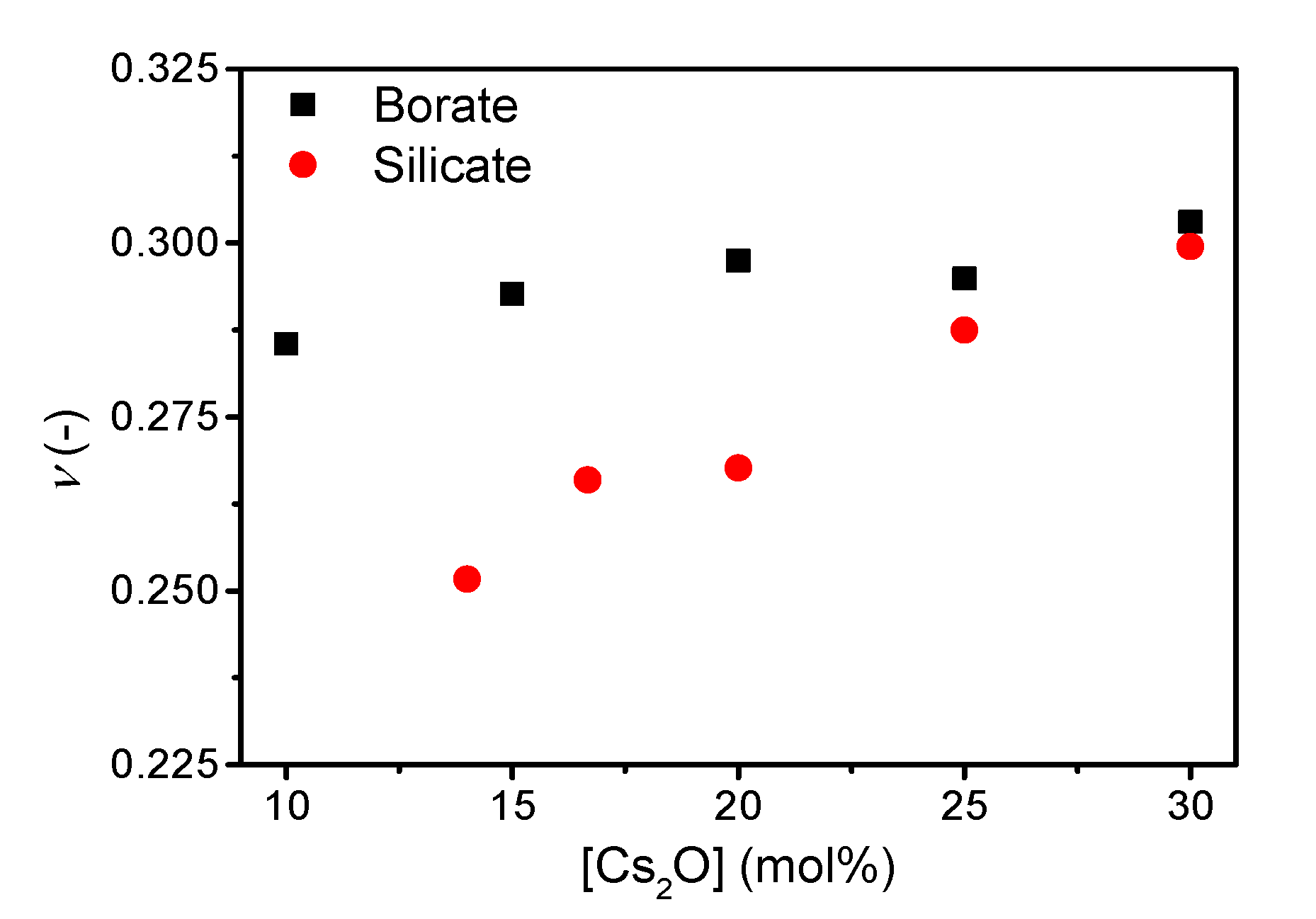
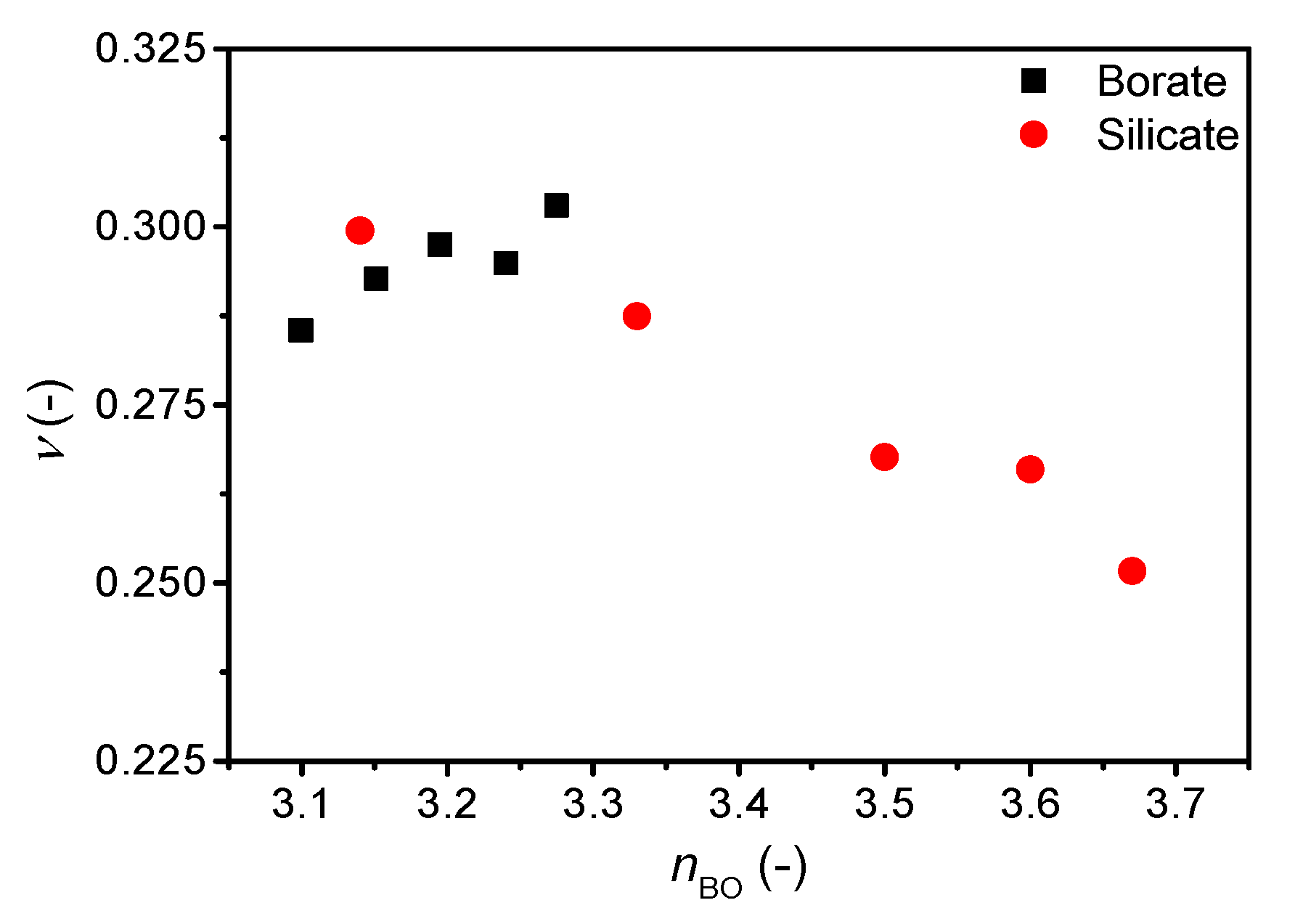
| Sample ID | Nominal Composition (mol%) | Tg (°C) | M (-) | ρ (g cm−3) | Cg (-) | E (GPa) | G (GPa) | ν (-) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | B2O3 | Cs2O | ||||||||
| Si86 | 86 | - | 14 | 555 | 20 | 2.97 | 0.484 | 44 | 18 | 0.25 |
| Si83 | 83.3 | - | 16.7 | 549 | 30 | 3.24 | 0.512 | 40 | 16 | 0.27 |
| Si80 | 80 | - | 20 | 539 | 30 | 3.25 | 0.495 | 38 | 15 | 0.27 |
| Si75 | 75 | - | 25 | 530 | 37 | 3.42 | 0.496 | 33 | 13 | 0.29 |
| Si70 | 70 | - | 30 | 490 | 47 | 3.58 | 0.499 | 31 | 12 | 0.30 |
| B90 | - | 90 | 10 | 319 | 25 | 2.41 | 0.524 | 25 | 10 | 0.29 |
| B85 | - | 85 | 15 | 343 | 30 | 2.65 | 0.530 | 26 | 10 | 0.29 |
| B80 | - | 80 | 20 | 376 | 32 | 2.85 | 0.531 | 25 | 10 | 0.30 |
| B75 | - | 75 | 25 | 416 | 47 | 3.05 | 0.532 | 30 | 11 | 0.30 |
| B70 | - | 70 | 30 | 403 | 49 | 3.33 | 0.548 | 31 | 18 | 0.30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Østergaard, M.B.; Bødker, M.S.; Smedskjaer, M.M. Structure Dependence of Poisson’s Ratio in Cesium Silicate and Borate Glasses. Materials 2020, 13, 2837. https://doi.org/10.3390/ma13122837
Østergaard MB, Bødker MS, Smedskjaer MM. Structure Dependence of Poisson’s Ratio in Cesium Silicate and Borate Glasses. Materials. 2020; 13(12):2837. https://doi.org/10.3390/ma13122837
Chicago/Turabian StyleØstergaard, Martin B., Mikkel S. Bødker, and Morten M. Smedskjaer. 2020. "Structure Dependence of Poisson’s Ratio in Cesium Silicate and Borate Glasses" Materials 13, no. 12: 2837. https://doi.org/10.3390/ma13122837
APA StyleØstergaard, M. B., Bødker, M. S., & Smedskjaer, M. M. (2020). Structure Dependence of Poisson’s Ratio in Cesium Silicate and Borate Glasses. Materials, 13(12), 2837. https://doi.org/10.3390/ma13122837






