Accuracy Evaluation of 14 Maxillary Full Arch Implant Treatments Performed with Da Vinci Bridge: A Case Series
Abstract
1. Introduction
- Minimally invasive (no bone grafting) approach;
- Avoid posterior prosthetic cantilevers;
- Predictability;
- Shorter period of treatment;
- Stable during the time;
- Immediate loading is possible;
- Decreasing of costs (patient and dentist).
2. Material and Methods
2.1. Study Design
2.2. Study Population/Demographics
2.3. Inclusion and Exclusion Criteria
2.3.1. Inclusion Criteria
- Any totally or partially edentulous patient that needs a full arch implant prosthetic rehabilitation.
- Bone crest width and height in the area between right and left premolars enough to place two to four implants with at least 1 mm of bone around the implant.
- Bone width and height in the pterygoid area enough to place one implant at least 13 mm long per side.
2.3.2. Exclusion Criteria
- Patients with general contraindications to implant surgery.
- Patients with systemic diseases that could influence the outcome of the therapy (i.e., diabetes with HbA1c ≥ 6.5%, osteoporosis, or use of bisphosphonate medications).
- Patients with a history of radiation to the head and neck region.
- Patients who are pregnant or nursing.
- Patients with no available bone to plan a pterygoid implant.
- Patients with residual bone in the molar area higher and wider than 6 mm.
- Patients with insufficient residual bone crest in the frontal area to place two to four implants.
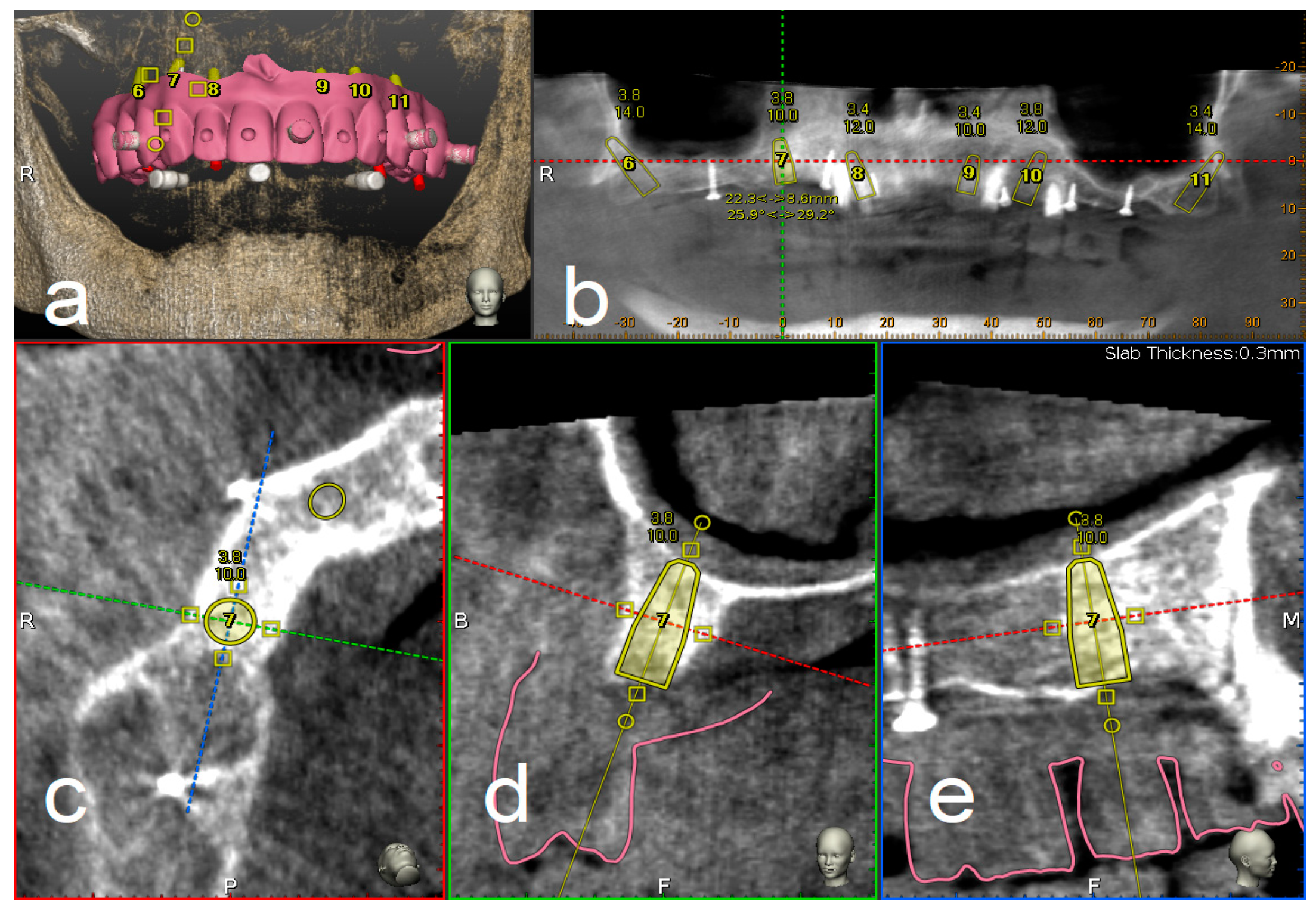
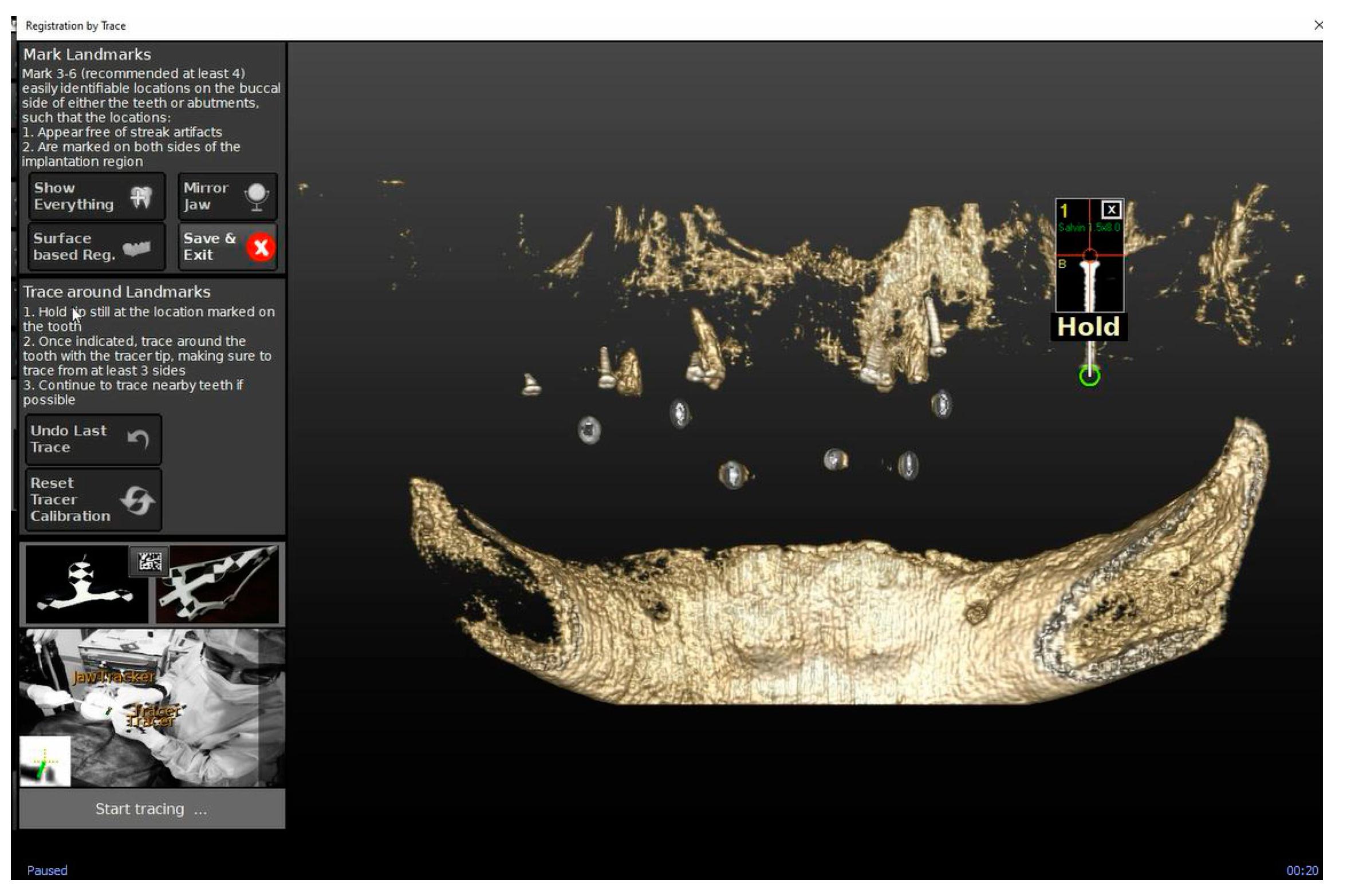
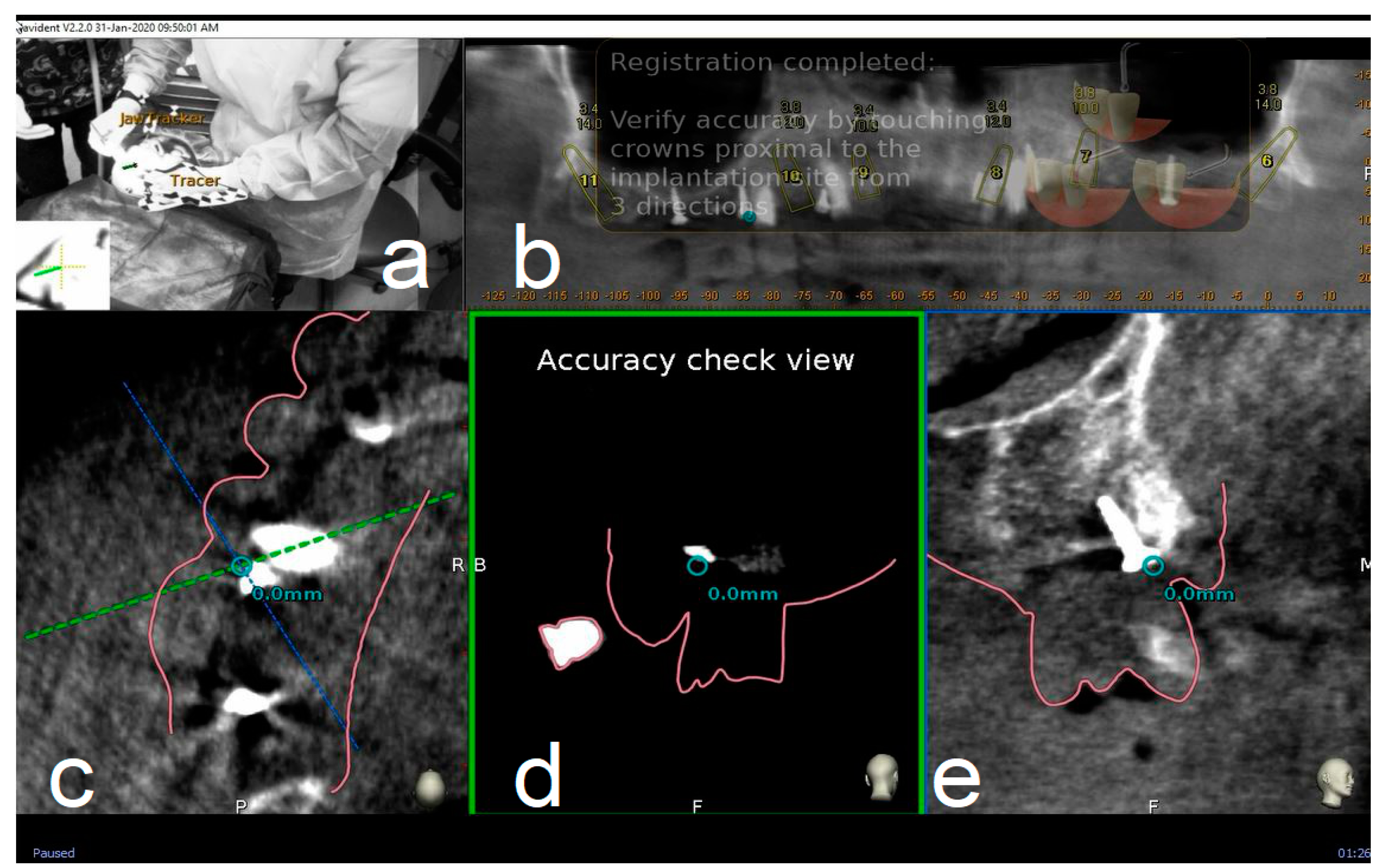
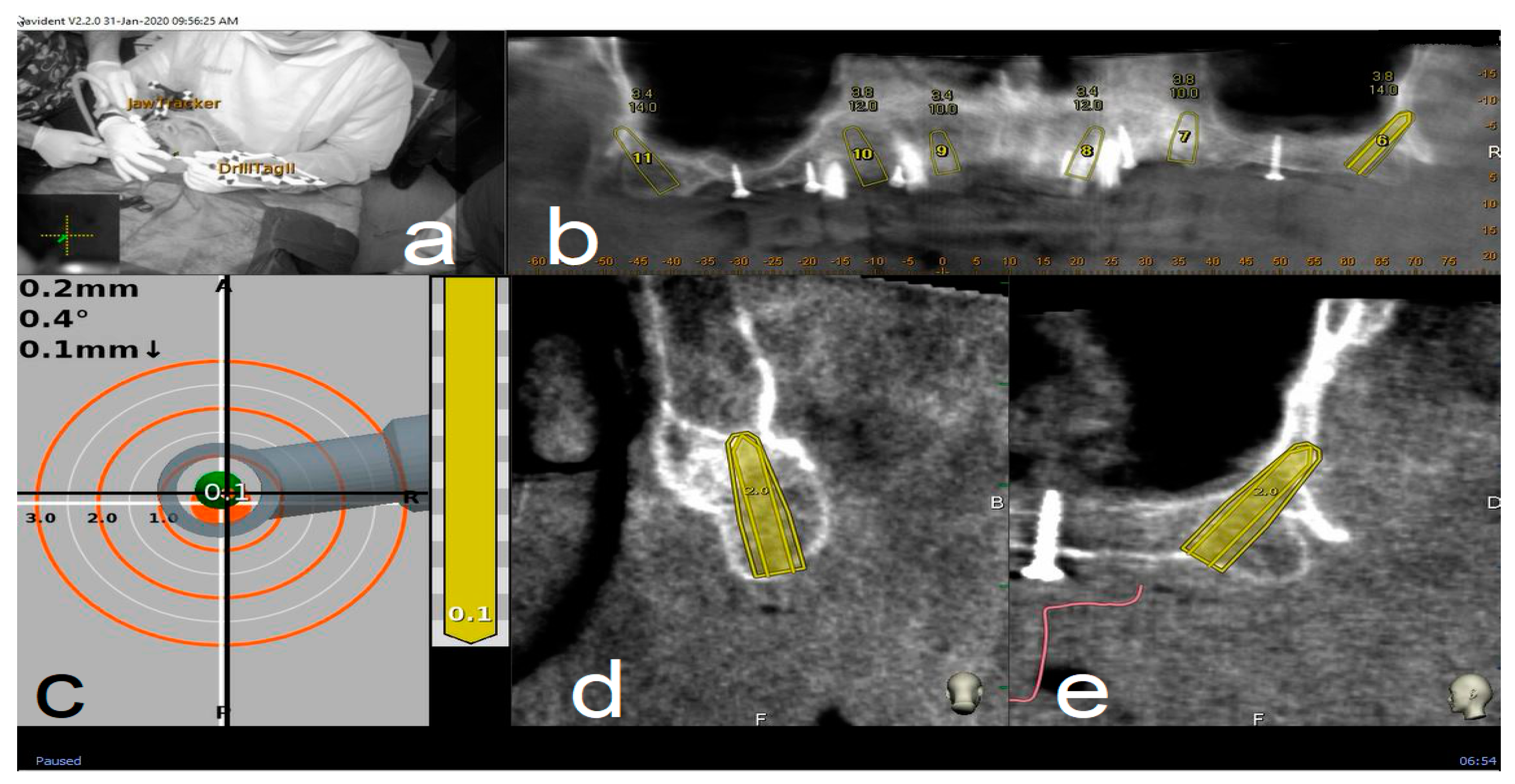
2.4. Trace and Place (TaP™) Protocol
2.4.1. Plan
2.4.2. Trace
2.4.3. Place
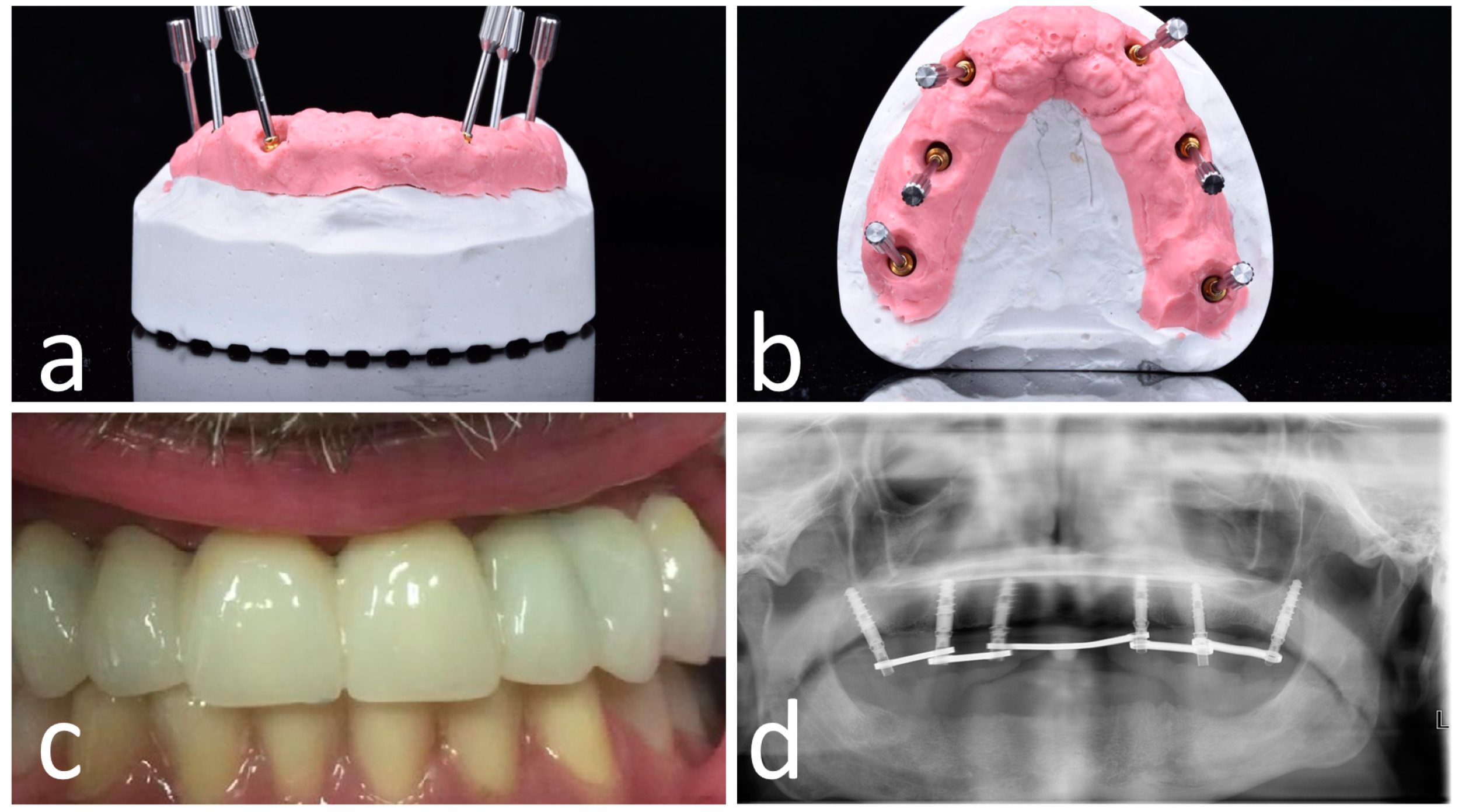
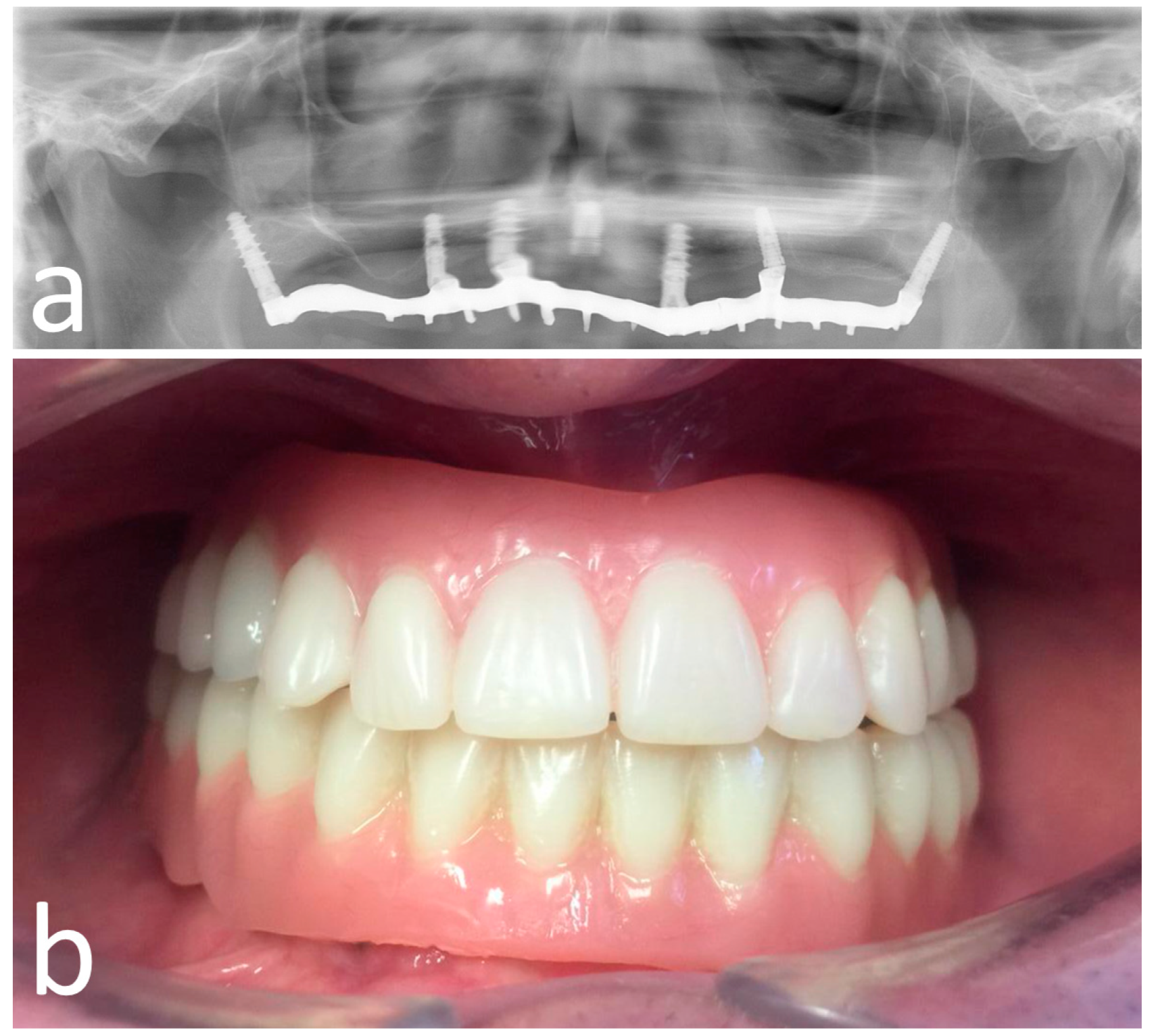
2.5. Surgical Treatment
2.6. Post-Surgical Protocol
2.7. Outcome Measures
2.7.1. Post-Surgery Complications
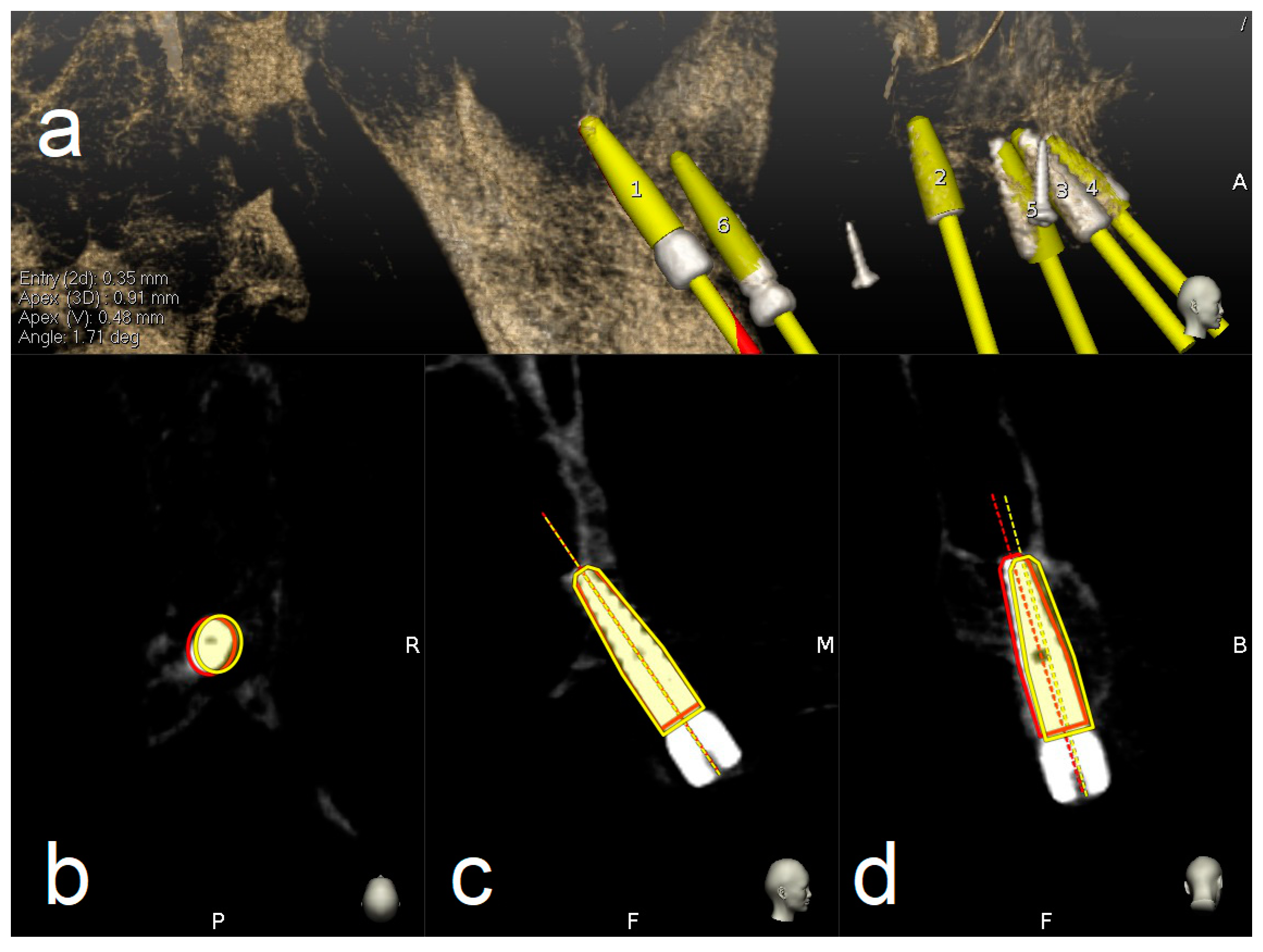
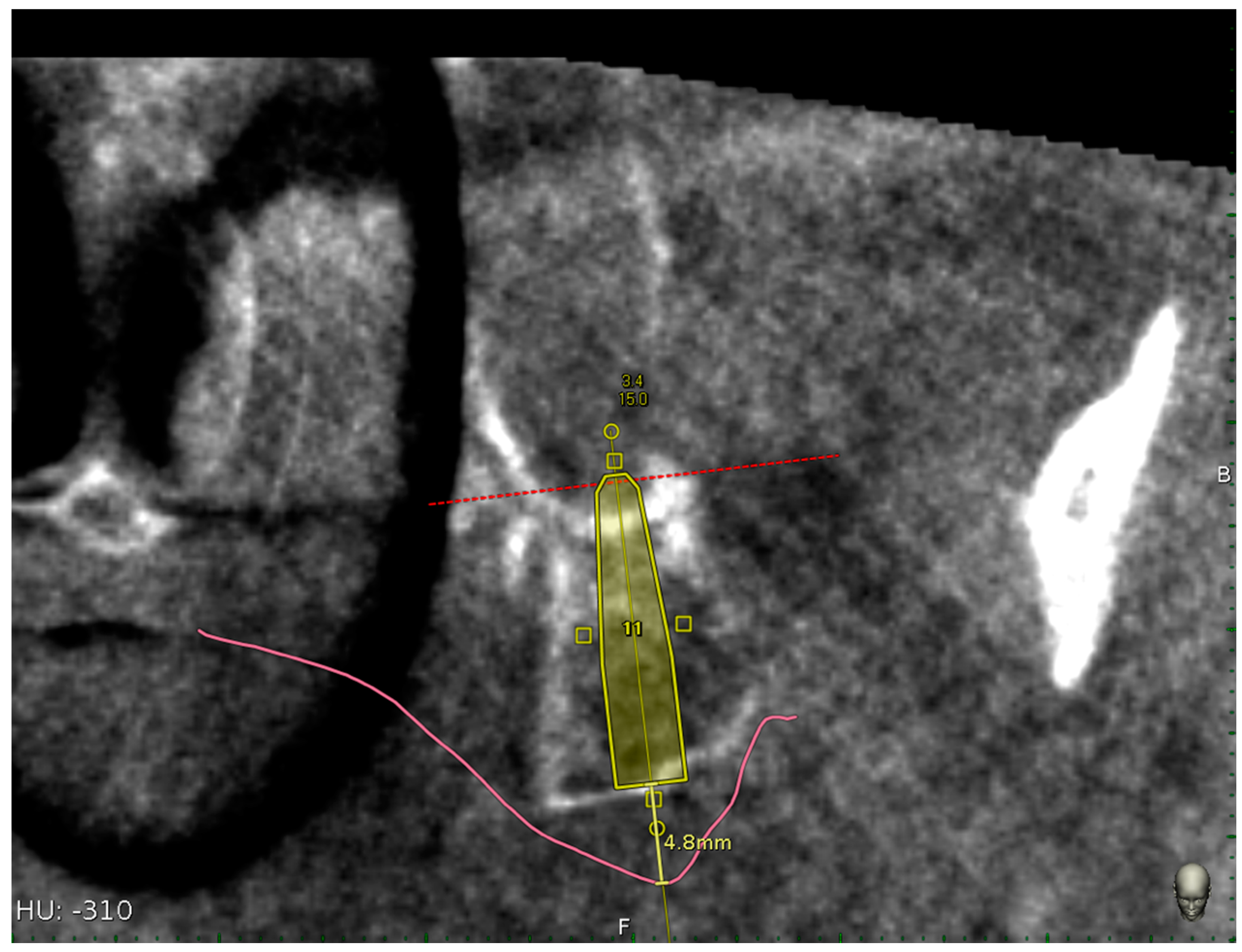
2.7.2. Accuracy Evaluation Assessment
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Adell, R.; Eriksson, B.; Lekholm, U.; Branemark, P.I.; Jemt, T. Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int. J. Oral Maxillofac. Implant. 1990, 5, 347–359. [Google Scholar]
- Guarnieri, R.; Di Nardo, D.; Gaimari, G.; Miccoli, G.; Testarelli, L. Short vs. Standard Laser-Microgrooved Implants Supporting Single and Splinted Crowns: A Prospective Study with 3 Years Follow-Up. J. Prosthodont. 2019, 28, e771–e779. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Tan, K.; Lang, N.P.; Bragger, U.; Egger, M.; Zwahlen, M.A. Systematic review of the survival and complication rates of fixed partial dentures (FPDs) after an osservation period of at least 5 years. Clin. Oral Implant. Res. 2004, 15, 625–642. [Google Scholar] [CrossRef]
- Sharan, A.; Madjiar, D. Maxillary sinus pneumatization following extractions: A radiographic study. Int. J. Oral Maxillofac. Implant. 2008, 23, 48–56. [Google Scholar]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–324. [Google Scholar]
- Ellegaard, B.; Kolsen-Petersen, J.; Baelum, V. Implant therapy involving maxillary sinus lift in periodontally compromised patients. Clin. Oral Implant. Res. 1997, 8, 305–315. [Google Scholar] [CrossRef]
- Lollobrigida, M.; Fortunato, L.; Serafini, G.; Mazzucchi, G.; Bozzuto, G.; Molinari, A.; Serra, E.; Menchini, F.; Vozza, I.; De Biase, A. The prevention of implant surface alterations in the treatment of peri-implantitis: Comparison of three different mechanical and physical treatments. Int J. Environ. Res. Public Health. 2020, 17, 2624. [Google Scholar] [CrossRef] [PubMed]
- Baggi, L.; Capelloni, I.; Di Girolamo, M.; Maceri, F.; Vairo, G. The influence of implant diameter and length on stress distribution of osseointegrated implants related to crestal bone geometry: A three-dimensional finite element analysis. J. Prosthet Dent. 2008, 100, 422–431. [Google Scholar] [CrossRef]
- Jaffin, R.A.; Berman, C.L. The excessive loss of Branemark fixtures in type IV bone: A 5-year analysis. J. Periodontol. 1991, 62, 2–4. [Google Scholar] [CrossRef]
- Balshi, T.J.; Wolfinger, G.J.; Slauch, R.W.; Balshi, S.F. Brånemark system implant lengths in the pterygomaxillary region: A retrospective comparison. Implant. Dent. 2013, 22, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Bäckström, M.; Sennerby, L. Immediate and early loading of oxidized tapered implants in the partially edentulous maxilla: A 1-year prospective clinical, radiographic, and resonance frequency analysis study. Clin. Implant Dent. Relat. Res. 2009, 11, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.S.; Froum, S.J. Effect of maxillary sinus augmentation on the survival of endosseous dental implants. A systematic review. Ann. Periodontol. 2003, 8, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, M.; Testori, T.; Francetti, L.; Weinstein, R. Systematic review of survival rates for implants placed in the grafted maxillary sinus. Int. J. Periodontics Restor. Dent. 2004, 24, 565–577. [Google Scholar] [CrossRef]
- Rose, P.S.; Summers, R.B.; Mellado, J.R.; Salkin, L.M.; Shanaman, R.H.; Marks, M.H.; Fugazzotto, P.A. Bone-added osteotome sinus floor elevation technique: Multicenter retrospective report of consecutively treated patients. Int. J. Oral Maxillofac. Implant. 1999, 14, 853–858. [Google Scholar]
- Bahat, O.; Fontanessi, R.V. Efficacy of implant placement after bone grafting for three-dimensional reconstruction of the posterior jaw. Int. J. Periodontics Restor. Dent. 2001, 21, 220–231. [Google Scholar]
- Felice, P.; Barausse, C.; Pistilli, R.; Ippolito, D.R.; Esposito, M. Short implants versus longer implants in vertically augmented posterior mandibles: Result at 8 years after loading from a randomized controlled trial. Eur. J. Oral Implantol. 2018, 11, 385–395. [Google Scholar]
- Felice, P.; Barausse, C.; Pistilli, V.; Piattelli, M.; Ippolito, D.R.; Esposito, M. Posterior atrophic jaws rehabilitated with prostheses supported by 6 mm long × 4 mm wide implants or by longer implants in augmented bone. 3-year post-loading results from a randomized controlled trial. Eur. J. Oral Implantol. 2018, 11, 175–187. [Google Scholar]
- Fan, T.; Li, Y.; Deng, W.W.; Wu, T.; Zhang, W. Short implants (5–8 mm) versus longer implants (>8 mm) with sinus lifting in atrophic posterior maxilla: A meta-analysis of RCTs. Clin. Implant. Dent. Relat. Res. 2017, 19, 207–215. [Google Scholar] [CrossRef]
- Anitua, E.; Flores, J.; Flores, C.; Alkhraisat, M.H. Long-term outcomes of immediate loading of short implants: A controlled retrospective cohort study. Int. J. Oral Maxillofac. Implant. 2016, 31, 1360–1366. [Google Scholar] [CrossRef]
- Bechara, S.; Kubilius, R.; Veronesi, G.; Pires, J.T.; Shibli, J.A.; Mangano, F.G. Short (6-mm) dental implants versus sinus floor elevation and placement of longer (≥10 mm) dental implants: A randomized controlled trial with a 3-year follow-up. Clin. Oral Implant. Res. 2017, 28, 1097–1107. [Google Scholar] [CrossRef]
- Chana, H.; Smith, G.; Bansal, H.; Zahra, D. A Retrospective Cohort Study of the Survival Rate of 88 Zygomatic Implants Placed Over an 18-year Period. Int. J. Oral Maxillofac. Implant. 2019, 34, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Petrungaro, P.S.; Kurtzman, G.M.; Gonzales, S.; Villegas, C. Zygomatic Implants for the Management of Severe Alveolar Atrophy in the Partial or Completely Edentulous Maxilla. Compend. Contin. Educ. Dent. 2018, 39, 636–645. [Google Scholar] [PubMed]
- Davó, R.; Felice, P.; Pistilli, R.; Barausse, C.; Marti-Pages, C.; Ferrer-Fuertes, A.; Ippolito, D.R.; Esposito, M. Immediately loaded zygomatic implants vs conventional dental implants in augmented atrophic maxillae: 1-year post-loading results from a multicentre randomised controlled trial. Eur. J. Oral Implantol. 2018, 11, 145–161. [Google Scholar] [PubMed]
- Tulasne, J.F. Implant treatment of missing posterior dentition. In The Brånemark Osseointegrated Implant; Albrektson, T., Zarb, G., Eds.; Quintessence: Chicago, IL, USA, 1989; pp. 103–158. ISBN 0-86715-208-7. [Google Scholar]
- Tulasne, J.F. Osseointegrated fixtures in the pterygoid region. In Advanced Osseointegration Surgery, Applications in the Maxillofacial Region; Worthington, P., Brånemark, P.I., Eds.; Quintessence: Chicago, IL, USA, 1992; pp. 182–188. ISBN 0-86715-242-7. [Google Scholar]
- Uchida, Y.; Yamashita, Y.; Danjo, A.; Shibata, K.; Kuraoka, A. Computed tomography and anatomical measurements of critical sites for endosseous implants in the pterygomaxillary region: A cadaveric study. Int. J. Oral Maxillofac. Surg. 2017, 46, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, X.; Lucas-Taulé, E.; Elnayef, B.; Altuna, P.; Gargallo-Albiol, J.; Peñarrocha Diago, M.; Hernandez-Alfaro, F. Anatomical and radiological approach to pterygoid implants: A cross-sectional study of 202 cone beam computed tomography examinations. Int. J. Oral Maxillofac. Surg. 2016, 45, 636–640. [Google Scholar] [CrossRef]
- Bidra, A.S.; Huynh-Ba, G. Implants in the pterygoid region: A systematic review of the literature. Int. J. Oral Maxillofac. Surg. 2011, 40, 773–781. [Google Scholar] [CrossRef]
- Candel, E.; Peñarrocha, D.; Peñarrocha, M. Rehabilitation of the atrophic posterior maxilla with pterygoid implants: A review. J. Oral Implantol. 2012, 38, 461–466. [Google Scholar] [CrossRef]
- Araujo, R.Z.; Santiago, Júnior, J.F.; Cardoso, C.L.; Benites Condezo, A.F.; Moreira Júnior, R.; Curi, M.M. Clinical outcomes of pterygoid implants: Systematic review and meta-analysis. J. Craniomaxillofac. Surg. 2019, 47, 651–660. [Google Scholar] [CrossRef]
- Block, M.S.; Emery, R.W.; Cullum, D.R.; Sheikh, A. Implant placement is more accurate using dynamic Navigation. J. Oral Maxillofac. Surg. 2017, 75, 1377–1386. [Google Scholar] [CrossRef]
- Mandelaris, G.A.; Stefanelli, L.V.; DeGroot, B.S. Dynamic Navigation for Surgical Implant Placement: Overview of Technology, Key Concepts, and a Case Report. Compend. Contin. Educ. Dent. 2018, 39, 614–621. [Google Scholar]
- Stefanelli, L.V.; DeGroot, B.S.; Lipton, D.I.; Mandelaris, G.A. Accuracy of a Dynamic Dental Implant Navigation System in a Private Practice. Int. J. Oral Maxillofac. Implant. 2019, 34, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Stefanelli, L.V.; Mandelaris, G.A.; DeGroot, B.S.; Gambarini, G.; De Angelis, F.; Di Carlo, S. Accuracy of a novel trace registration method for dynamic navigation surgery. Int. J. Periodontics Restor. Dent. 2020, 40, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.; Veronesi, G. Digital versus Analog procedures for the prosthetic restoration of single implants: A randomized controlled trial with 1 year of follow-up. Biomed. Res. Int. 2018, 2018, 5325032. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.G.; Hauschild, U.; Admakin, O. Full in-Office guided surgery with open selective tooth-supported templates: A prospective clinical study on 20 patients. Int. J. Environ. Res. Public Health 2018, 25, 2361. [Google Scholar] [CrossRef]
- Pellegrino, G.; Taraschi, V.; Andrea, Z.; Ferri, A.; Marchetti, C. Dynamic navigation: A prospective clinical trial to evaluate the accuracy of implant placement. Int. J. Comput. Dent. 2019, 22, 139–147. [Google Scholar]
- Vercruyssen, M.; Cox, C.; Coucke, W.; Naert, I.; Jacobs, R.; Quirynen, M. A randomized clinical trial comparing guided implant surgery (bone- or mucosa-supported) with mental navigation or the use of a pilot-drill template. J. Clin. Periodontol. 2014, 41, 717–723. [Google Scholar] [CrossRef]
- Van Assche, N.; Quirynen, M. Tolerance within a surgical guide. Clin. Oral Implant. Res. 2010, 21, 455–458. [Google Scholar] [CrossRef]
- Park, Y.J.; Cho, S.A. Retrospective chart analysis on survival rate of fixtures installed at the tuberosity bone for cases with missing unilateral upper molars: A study of 7 cases. J. Oral Maxillofac. Surg. 2010, 68, 1338–1344. [Google Scholar] [CrossRef]
- Graves, S.L. The pterygoid plate implant: A solution for restoring the posterior maxilla. Int. J. Periodontics Restor. Dent. 1994, 14, 512–523. [Google Scholar]
- Salinas-Goodier, C.; Rojo, R.; Murillo-González, J.; Prados-Frutos, J.C. Three-dimensional descriptive study of the pterygomaxillary region related to pterygoid implants: A retrospective study. Sci. Rep. 2019, 9, 16179. [Google Scholar] [CrossRef]


| Deviation of the Total Implant Inserted (78) | Mean | Minimum | Maximum | Standard Deviation |
|---|---|---|---|---|
| Coronal (mm) | 0.66 | 0.04 | 2.19 | 0.35 |
| Apical 3D (mm) | 1.01 | 0.23 | 2.52 | 0.46 |
| Apical (depth) (mm) | 0.52 | 0.02 | 1.27 | 0.30 |
| Angular degree (°) | 2.61 | 0.39 | 7.86 | 1.29 |
| Deviation of the Pterygoid Implants Inserted (28) | Mean | Minimum | Maximum | Standard Deviation |
|---|---|---|---|---|
| Coronal (mm) | 0.72 | 0.25 | 1.42 | 0.28 |
| Apical 3D (mm) | 1.25 | 0.56 | 2.52 | 0.46 |
| Apical (depth) (mm) | 0.69 | 0.06 | 1.27 | 0.33 |
| Angular degree (°) | 2.86 | 0.57 | 7.86 | 1.56 |
| Deviation of the Frontal Implant Inserted (56) | Mean | Minimum | Maximum | Standard Deviation |
|---|---|---|---|---|
| Coronal (mm) | 0.64 | 0.04 | 2.19 | 0.37 |
| Apical 3D (mm) | 0.89 | 0.23 | 2.01 | 0.42 |
| Apical (depth) (mm) | 0.46 | 0.02 | 1.22 | 0.26 |
| Angular degree (°) | 2.49 | 0.39 | 5.21 | 1.14 |
| Implant Length (mm) | Implants Used (%) |
|---|---|
| 13 | 2 (7%) |
| 15 | 9 (32%) |
| 16 | 10 (36%) |
| 18 | 3 (11%) |
| 20 | 4 (14%) |
| Distances (mm) | Mean | Minimum | Maximum | Standard Deviation |
|---|---|---|---|---|
| Mucosa thickness | 5.08 | 2.6 | 8.2 | 1.77 |
| t-Test (Frontal Implants vs. Navident) | Difference between Means | Sig. (p) |
|---|---|---|
| Coronal deviation (mm) | 0.07 | p = 0.29 |
| Apical 3D (mm) | 0.36 | p = 0.001 |
| Apical (depth) (mm) | 0.20 | p = 0.01 |
| Angular deviation (°) | 0.37 | p = 0.27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanelli, L.V.; Mandelaris, G.A.; Franchina, A.; Di Nardo, D.; Galli, M.; Pagliarulo, M.; Testarelli, L.; Di Carlo, S.; Gambarini, G. Accuracy Evaluation of 14 Maxillary Full Arch Implant Treatments Performed with Da Vinci Bridge: A Case Series. Materials 2020, 13, 2806. https://doi.org/10.3390/ma13122806
Stefanelli LV, Mandelaris GA, Franchina A, Di Nardo D, Galli M, Pagliarulo M, Testarelli L, Di Carlo S, Gambarini G. Accuracy Evaluation of 14 Maxillary Full Arch Implant Treatments Performed with Da Vinci Bridge: A Case Series. Materials. 2020; 13(12):2806. https://doi.org/10.3390/ma13122806
Chicago/Turabian StyleStefanelli, Luigi V., George A. Mandelaris, Alessio Franchina, Dario Di Nardo, Massimo Galli, Michele Pagliarulo, Luca Testarelli, Stefano Di Carlo, and Gianluca Gambarini. 2020. "Accuracy Evaluation of 14 Maxillary Full Arch Implant Treatments Performed with Da Vinci Bridge: A Case Series" Materials 13, no. 12: 2806. https://doi.org/10.3390/ma13122806
APA StyleStefanelli, L. V., Mandelaris, G. A., Franchina, A., Di Nardo, D., Galli, M., Pagliarulo, M., Testarelli, L., Di Carlo, S., & Gambarini, G. (2020). Accuracy Evaluation of 14 Maxillary Full Arch Implant Treatments Performed with Da Vinci Bridge: A Case Series. Materials, 13(12), 2806. https://doi.org/10.3390/ma13122806












