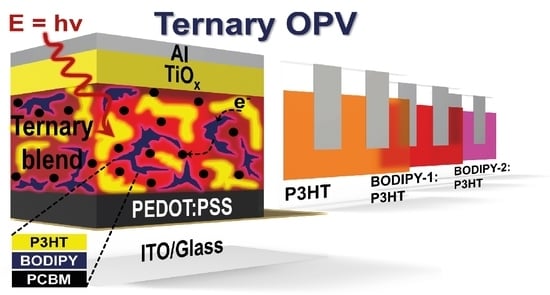
Improved Performance of Ternary Solar Cells by Using BODIPY Triads
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.2.1. Synthesis of 2,6-diiodo-BODIPY
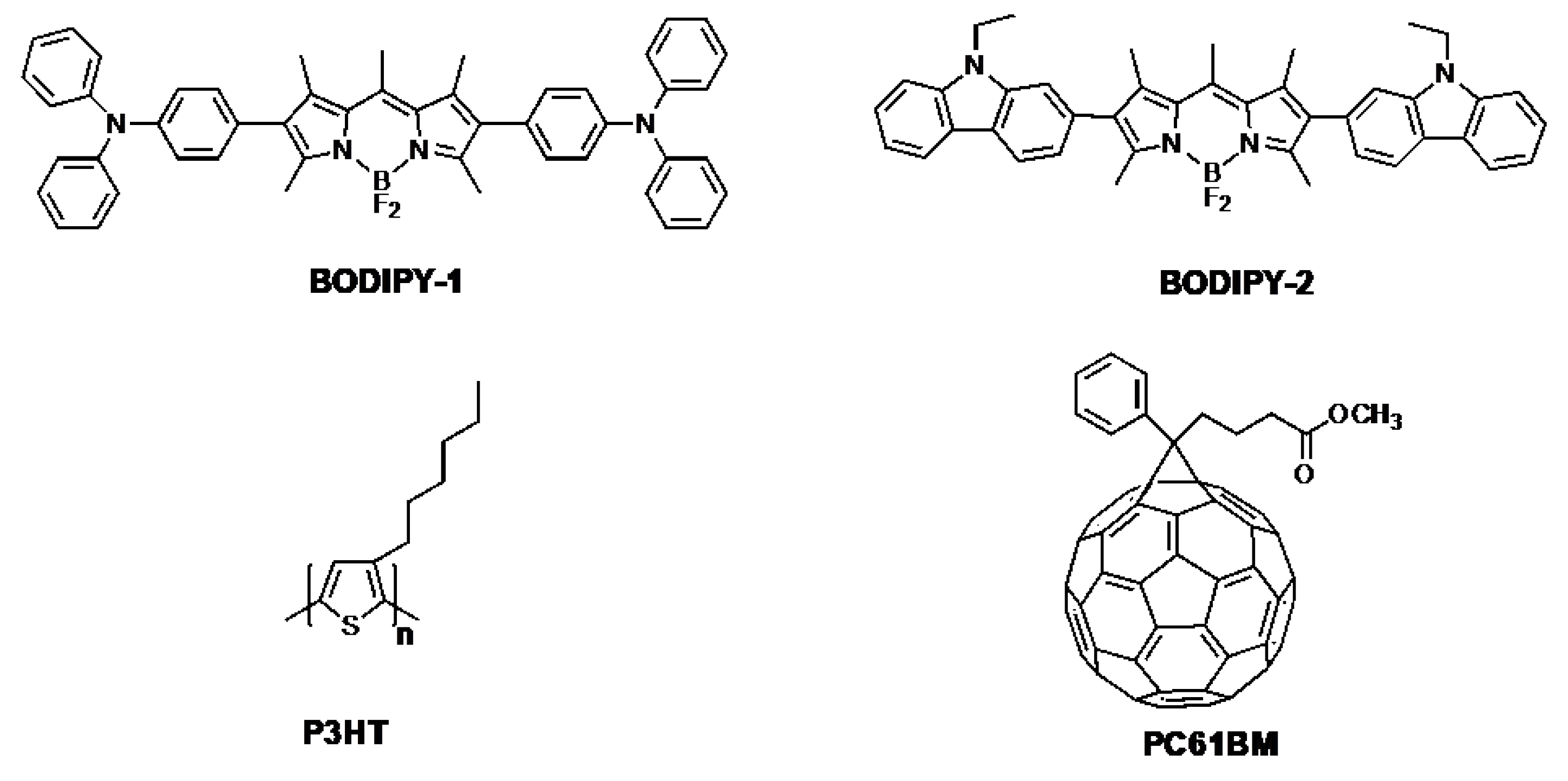
2.2.2. Synthesis of BODIPY-1 (TPA-BODIPY-TPA)
2.2.3. BODIPY-2 (CBZ-BODIPY-CBZ)
2.3. Solar Cell Fabrication
2.4. Instrumentations
3. Results and Discussion
3.1. Synthesis
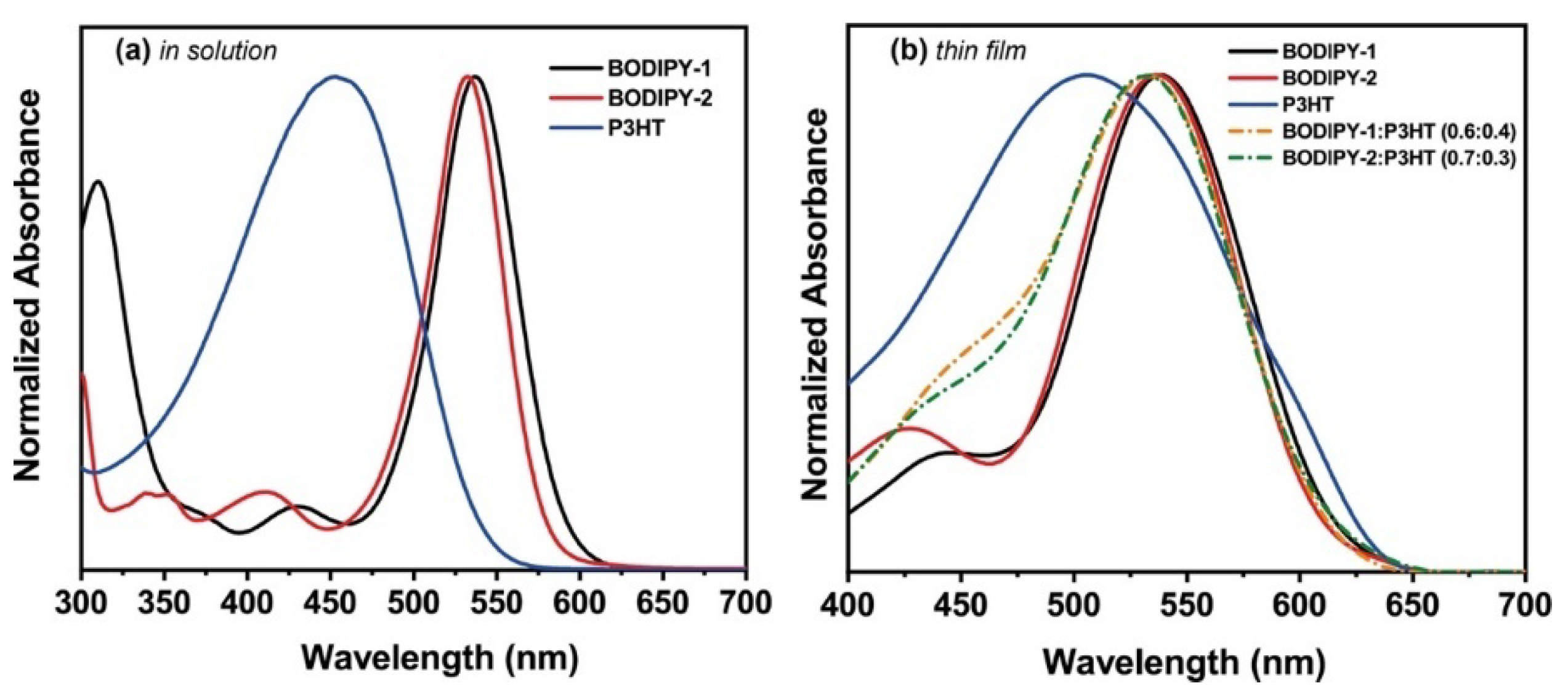
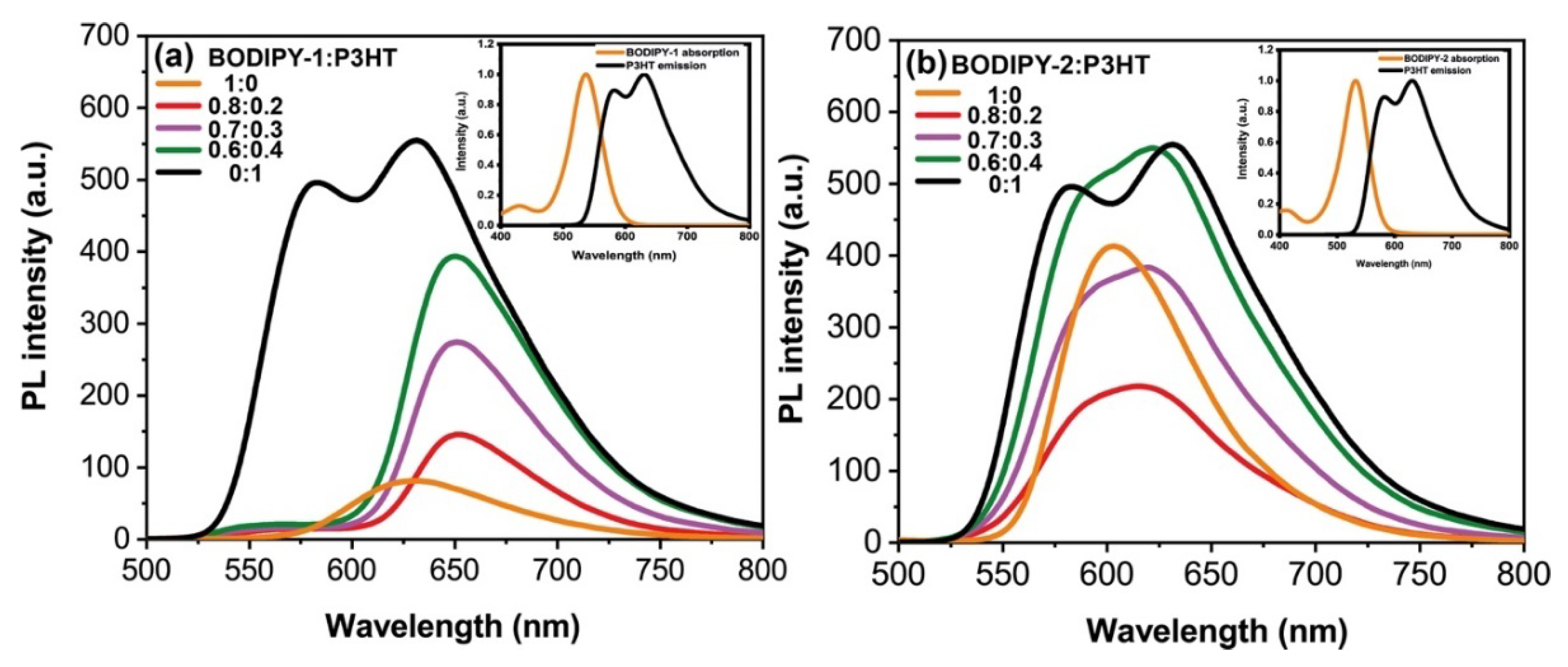
3.2. Optical Characterizations
3.3. Photovoltaic Performance
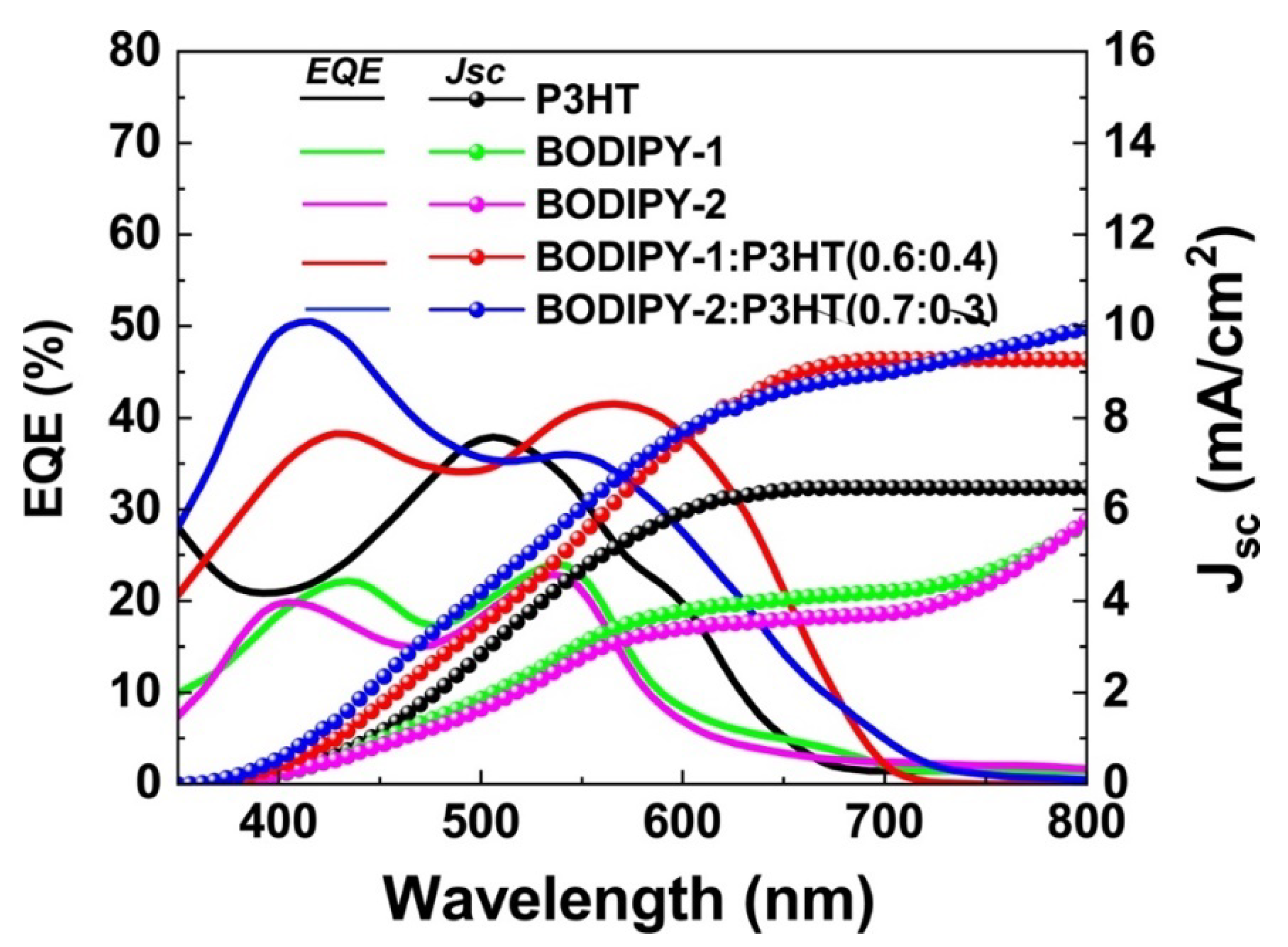
3.4. External Quantum Efficiency (EQE)
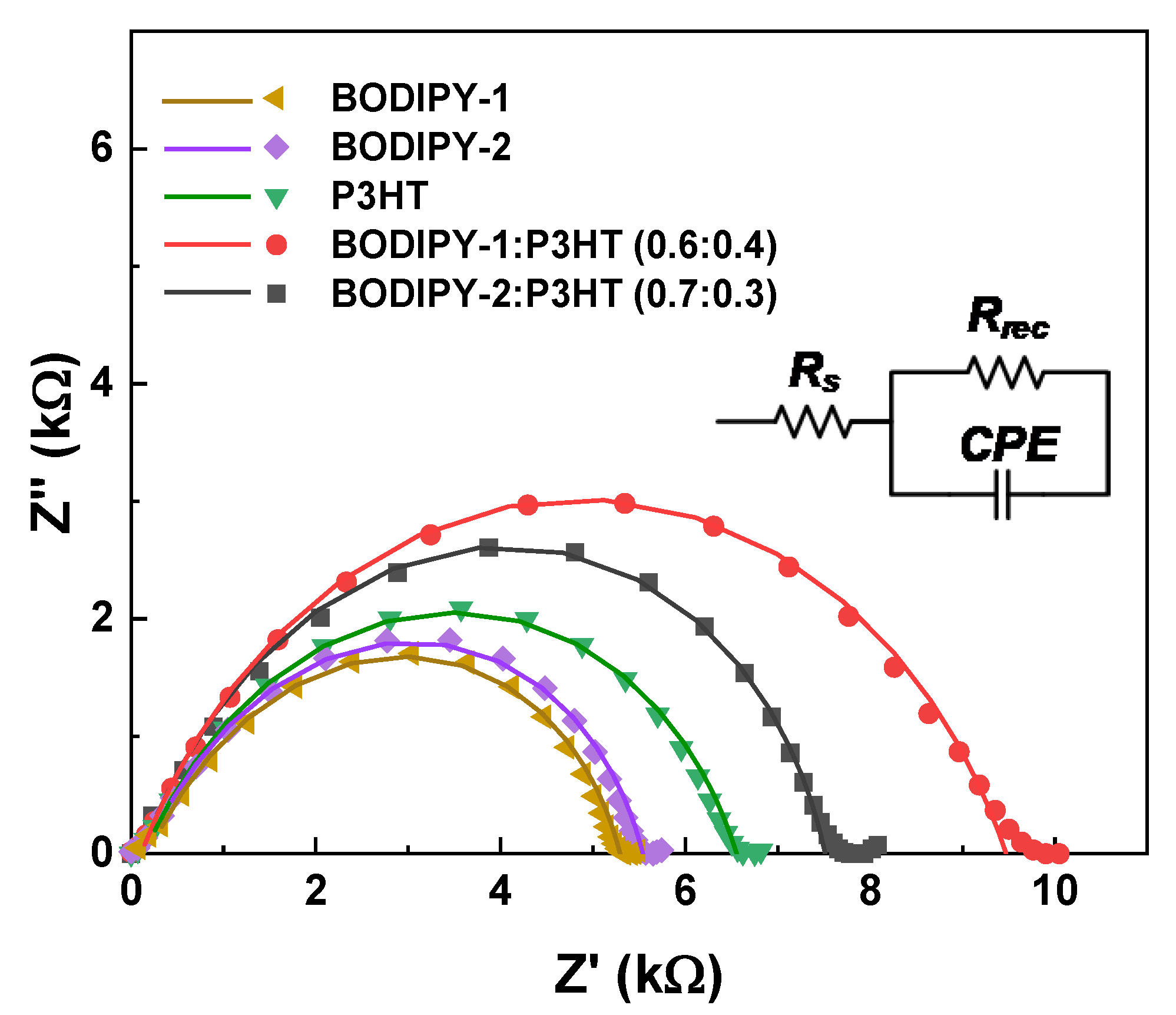
3.5. Impedance Analysis
3.6. Morphology Study
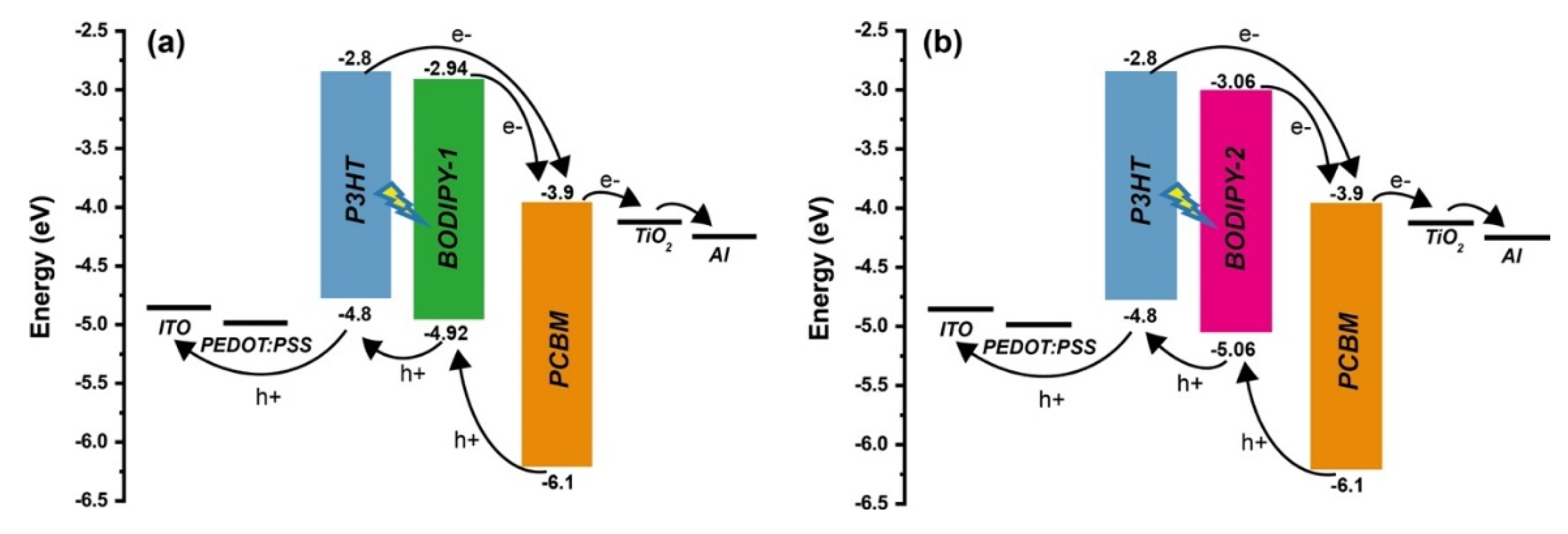
3.7. Proposed Operating Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dou, L.; Liu, Y.; Hong, Z.; Li, G.; Yang, Y. Low-Bandgap Near-IR Conjugated Polymers/Molecules for Organic Electronics. Chem. Rev. 2015, 115, 12633–12665. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhong, C.; Su, S.; Xu, M.; Wu, H.; Cao, Y. Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure. Nat. Photonics 2012, 6, 591–595. [Google Scholar] [CrossRef]
- Green, M.A.; Emery, K.; Hishikawa, Y.; Warta, W.; Dunlop, E.D. Solar cell efficiency tables (version 39). Prog. Photovolt. Res. Appl. 2011, 20, 12–20. [Google Scholar] [CrossRef]
- Liu, Q.; Toudert, J.; Li, T.; Kramarenko, M.; Martínez-Denegri, G.; Ciammaruchi, L.; Zhan, X.; Martorell, J. Inverse Optical Cavity Design for Ultrabroadband Light Absorption Beyond the Conventional Limit in Low-Bandgap Nonfullerene Acceptor–Based Solar Cells. Adv. Energy Mater. 2019, 9. [Google Scholar] [CrossRef]
- Sun, J.; Ma, X.; Zhang, Z.; Yu, J.; Zhou, J.; Yin, X.; Yang, L.; Geng, R.; Zhu, R.; Zhang, F.; et al. Dithieno (3,2-b:2′,3′-d)pyrrol Fused Nonfullerene Acceptors Enabling Over 13% Efficiency for Organic Solar Cells. Adv. Mater. 2018, 30, 1707150. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Zhu, Q.; Xu, Z.; Su, W.; Chen, J.; Wu, J.; Guo, X.; Ma, W.; Zhang, M.; Li, Y. Chlorine substituted 2D-conjugated polymer for high-performance polymer solar cells with 13.1% efficiency via toluene processing. Nano Energy 2018, 48, 413–420. [Google Scholar] [CrossRef]
- Sun, L.; Xu, X.; Song, S.; Zhang, Y.; Miao, C.; Liu, X.; Xing, G.; Zhang, S. Medium-Bandgap Conjugated Polymer Donors for Organic Photovoltaics. Macromol. Rapid Commun. 2019, 40, e1900074. [Google Scholar] [CrossRef]
- Li, W.; Chen, M.; Cai, J.; Spooner, E.L.; Zhang, H.; Gurney, R.S.; Liu, D.; Xiao, Z.; Lidzey, D.G.; Ding, L.; et al. Molecular Order Control of Non-fullerene Acceptors for High-Efficiency Polymer Solar Cells. Joule 2019, 3, 819–833. [Google Scholar] [CrossRef]
- Zheng, B.; Huo, L.; Li, Y. Benzodithiophenedione-based polymers: Recent advances in organic photovoltaics. NPG Asia Mater. 2020, 12, 1–22. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Z.; Zhao, S.; Xu, Z.; Qiao, B.; Song, D.; Wageh, S.; Al-Ghamdi, A.A. With PBDB-T as the Donor, the PCE of Non-Fullerene Organic Solar Cells Based on Small Molecule INTIC Increased by 52.4%. Materials 2020, 13, 1324. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Ding, K.; Forrest, S. Energy Loss in Organic Photovoltaics: Nonfullerene Versus Fullerene Acceptors. Phys. Rev. Appl. 2019, 11, 024060. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef] [PubMed]
- Ameri, T.; Li, N.; Brabec, C.J. Highly efficient organic tandem solar cells: A follow up review. Energy Environ. Sci. 2013, 6, 2390. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Y.; Wan, X.; Li, C.; Zhang, X.; Wang, Y.; Ke, X.; Xiao, Z.; Ding, L.; Xia, R.; et al. Organic and solution-processed tandem solar cells with 17.3% efficiency. Science 2018, 361, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Genene, Z.; Mammo, W.; Wang, E.; Andersson, M.R. Recent Advances in n-Type Polymers for All-Polymer Solar Cells. Adv. Mater. 2019, 31, e1807275. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, Y.S.; Ahn, H.; Jo, W.H. Ternary Blend Composed of Two Organic Donors and One Acceptor for Active Layer of High-Performance Organic Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 10961–10967. [Google Scholar] [CrossRef]
- Yang, L.; Yan, L.; You, W. Organic Solar Cells beyond One Pair of Donor–Acceptor: Ternary Blends and More. J. Phys. Chem. Lett. 2013, 4, 1802–1810. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, H.; Price, S.C.; You, W. Parallel-like Bulk Heterojunction Polymer Solar Cells. J. Am. Chem. Soc. 2012, 134, 5432–5435. [Google Scholar] [CrossRef]
- Ameri, T.; Khoram, P.; Min, J.; Brabec, C.J. Organic Ternary Solar Cells: A Review. Adv. Mater. 2013, 25, 4245–4266. [Google Scholar] [CrossRef]
- Bi, P.; Hao, X.-T. Versatile Ternary Approach for Novel Organic Solar Cells: A Review (Solar RRL 1/2019). Sol. RRL 2019, 3, 3. [Google Scholar] [CrossRef]
- Gasparini, N.; Salleo, A.; McCulloch, I.; Baran, D. The role of the third component in ternary organic solar cells. Nat. Rev. Mater. 2019, 4, 229–242. [Google Scholar] [CrossRef]
- Huang, H.; Yang, L.; Sharma, B. Recent advances in organic ternary solar cells. J. Mater. Chem. A 2017, 5, 11501–11517. [Google Scholar] [CrossRef]
- Lu, L.; Kelly, M.A.; You, W.; Yu, L. Status and prospects for ternary organic photovoltaics. Nat. Photonics 2015, 9, 491–500. [Google Scholar] [CrossRef]
- Lu, L.; Chen, W.; Xu, T.; Yu, L. High-performance ternary blend polymer solar cells involving both energy transfer and hole relay processes. Nat. Commun. 2015, 6, 7327. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ohkita, H.; Tamai, Y.; Benten, H.; Ito, S. Interface Engineering for Ternary Blend Polymer Solar Cells with a Heterostructured Near-IR Dye. Adv. Mater. 2015, 27, 5868–5874. [Google Scholar] [CrossRef]
- An, Q.; Zhang, J.; Zhang, J.; Tang, W.; Deng, Z.; Hu, B. Versatile ternary organic solar cells: A critical review. Energy Environ. Sci. 2016, 9, 281–322. [Google Scholar] [CrossRef]
- Yan, H.; Li, D.; Zhang, Y.; Yang, Y.; Wei, Z. Rational Design of Ternary-Phase Polymer Solar Cells by Controlling Polymer Phase Separation. J. Phys. Chem. C 2014, 118, 10552–10559. [Google Scholar] [CrossRef]
- Huang, J.-S.; Goh, T.; Li, X.; Sfeir, M.Y.; Bielinski, E.A.; Tomasulo, S.; Lee, M.L.; Hazari, N.; Taylor, A.D. Polymer bulk heterojunction solar cells employing Förster resonance energy transfer. Nat. Photonics 2013, 7, 479–485. [Google Scholar] [CrossRef]
- Hwang, H.; Sin, D.H.; Park, C.; Cho, K. Ternary Organic Solar Cells Based on a Wide-Bandgap Polymer with Enhanced Power Conversion Efficiencies. Sci. Rep. 2019, 9, 12081. [Google Scholar] [CrossRef]
- Sun, Y.; Welch, G.C.; Leong, W.L.; Takacs, C.J.; Bazan, G.C.; Heeger, A.J. Solution-processed small-molecule solar cells with 6.7% efficiency. Nat. Mater. 2011, 11, 44–48. [Google Scholar] [CrossRef]
- Qi, X.; Lo, Y.-C.; Zhao, Y.; Xuan, L.; Ting, H.-C.; Wong, K.-T.; Rahaman, M.; Chen, Z.; Xiao, L.; Qu, B. Two Novel Small Molecule Donors and the Applications in Bulk-Heterojunction Solar Cells. Front. Chem. 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Zhang, F.; Li, L.; Wang, J.; Sun, Q.; Zhang, J.; Tang, W.; Deng, Z. Simultaneous Improvement in Short Circuit Current, Open Circuit Voltage, and Fill Factor of Polymer Solar Cells through Ternary Strategy. ACS Appl. Mater. Interfaces 2015, 7, 3691–3698. [Google Scholar] [CrossRef] [PubMed]
- Bi, P.-Q.; Wu, B.; Zheng, F.; Xu, W.-L.; Yang, X.; Feng, L.; Zhu, F.; Hao, X.-T. An Obvious Improvement in the Performance of Ternary Organic Solar Cells with “Guest” Donor Present at the “Host” Donor/Acceptor Interface. ACS Appl. Mater. Interfaces 2016, 8, 23212–23221. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Nogami, T.; Ohkita, H.; Benten, H.; Ito, S. Improvement of the Light-Harvesting Efficiency in Polymer/Fullerene Bulk Heterojunction Solar Cells by Interfacial Dye Modification. ACS Appl. Mater. Interfaces 2009, 1, 804–810. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Chen, J.; Kim, H.D.; Gao, P.; Wang, B.; Iriguchi, R.; Ohkita, H. Quadrupolar D–A–D diketopyrrolopyrrole-based small molecule for ternaryblend polymer solar cells. Dye. Pigment. 2018, 158, 213–218. [Google Scholar] [CrossRef]
- Baran, D.; Ashraf, R.S.; Hanifi, D.A.; Abdelsamie, M.; Gasparini, N.; Rohr, J.A.; Holliday, S.; Wadsworth, A.; Lockett, S.; Neophytou, M.; et al. Reducing the efficiency–stability–cost gap of organic photovoltaics with highly efficient and stable small molecule acceptor ternary solar cells. Nat. Mater. 2016, 16, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Ozdemir, R.; Kim, H.; Earmme, T.; Usta, H.; Kim, C. BODIPY-Based Semiconducting Materials for Organic Bulk Heterojunction Photovoltaics and Thin-Film Transistors. ChemPlusChem 2018, 84, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Conron, S.M.; Erwin, P.; Dimitriou, M.; McAlahney, K.; Thompson, M.E. High-Efficiency BODIPY-Based Organic Photovoltaics. ACS Appl. Mater. Interfaces 2014, 7, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Gayathri, T. Evolution of BODIPY Dyes as Potential Sensitizers for Dye-Sensitized Solar Cells. Eur. J. Org. Chem. 2014, 2014, 4689–4707. [Google Scholar] [CrossRef]
- Wanwong, S.; Sangkhun, W.; Wootthikanokkhan, J. The effect of co-sensitization methods between N719 and boron dipyrromethene triads on dye-sensitized solar cell performance. RSC Adv. 2018, 8, 9202–9210. [Google Scholar] [CrossRef]
- Gkini, K.; Verykios, A.; Balis, N.; Kaltzoglou, A.; Papadakis, M.; Adamis, K.S.; Armadorou, K.-K.; Soultati, A.; Drivas, C.; Gardelis, S.; et al. Enhanced Organic and Perovskite Solar Cell Performance through Modification of the Electron-Selective Contact with a Bodipy–Porphyrin Dyad. ACS Appl. Mater. Interfaces 2019, 12, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.; Dhokale, B.; Mobin, S.M.; Misra, R. Ferrocenyl BODIPYs: Synthesis, structure and properties. RSC Adv. 2012, 2, 12105. [Google Scholar] [CrossRef]
- Purc, A.; Sobczyk, K.; Sakagami, Y.; Ando, A.; Kamada, K.; Gryko, D. Strategy towards large two-photon absorption cross-sections for diketopyrrolopyrroles. J. Mater. Chem. C 2015, 3, 742–749. [Google Scholar] [CrossRef]
- Wanwong, S.; Surawatanawong, P.; Khumsubdee, S.; Kanchanakungwankul, S.; Wootthikanokkhan, J. Synthesis, optical, and electrochemical properties, and theoretical calculations of BODIPY containing triphenylamine. Heteroat. Chem. 2016, 27, 306–315. [Google Scholar] [CrossRef]
- Sengupta, S.; Athresh, E.U.; Pandey, U.K. Regioisomeric donor–acceptor–donor triads based on benzodithiophene and BODIPY with distinct optical properties and mobilities. RSC Adv. 2016, 6, 73645–73649. [Google Scholar] [CrossRef]
- Bonnier, C.; Machin, D.D.; Abdi, O.; Koivisto, B. Manipulating non-innocent ?-spacers: The challenges of using 2,6-disubstituted BODIPY cores within donor–acceptor light-harvesting motifs. Org. Biomol. Chem. 2013, 11, 3756. [Google Scholar] [CrossRef]
- Yang, J.; Devillers, C.H.; Fleurat-Lessard, P.; Jiang, H.; Wang, S.; Gros, C.P.; Gupta, G.; Sharma, G.D.; Xu, H.-J. Carbazole-based green and blue-BODIPY dyads and triads as donors for bulk heterojunction organic solar cells. Dalton Trans. 2020, 49, 5606–5617. [Google Scholar] [CrossRef]
- Min, J.; Ameri, T.; Gresser, R.; Lorenz-Rothe, M.; Baran, D.; Troeger, A.; Sgobba, V.; Leo, K.; Riede, M.K.; Guldi, D.M.; et al. Two Similar Near-Infrared (IR) Absorbing Benzannulated Aza-BODIPY Dyes as Near-IR Sensitizers for Ternary Solar Cells. ACS Appl. Mater. Interfaces 2013, 5, 5609–5616. [Google Scholar] [CrossRef]
- Wanwong, S.; Khomein, P.; Thayumanavan, S. BODIPY dyads and triads: Synthesis, optical, electrochemical and transistor properties. Chem. Central J. 2018, 12, 60. [Google Scholar] [CrossRef]
- Doumon, N.Y.; Dryzhov, M.V.; Houard, F.; Le Corre, V.M.; Chatri, A.R.; Christodoulis, P.; Koster, L.J.A. Photostability of Fullerene and Non-Fullerene Polymer Solar Cells: The Role of the Acceptor. ACS Appl. Mater. Interfaces 2019, 11, 8310–8318. [Google Scholar] [CrossRef]
- Gregory, D.G.; Lu, L.; Kiely, C.J.; Snyder, M.A. Interfacial Stabilization of Metastable TiO2 Films. J. Phys. Chem. C 2017, 121, 4434–4442. [Google Scholar] [CrossRef]
- Reese, M.O.; A Gevorgyan, S.; Jørgensen, M.; Bundgaard, E.; Kurtz, S.R.; Ginley, D.S.; Olson, D.C.; Lloyd, M.T.; Morvillo, P.; Katz, E.A.; et al. Consensus stability testing protocols for organic photovoltaic materials and devices. Sol. Energy Mater. Sol. Cells 2011, 95, 1253–1267. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Qian, D.; Gautam, B.; Yang, G.; Zhao, J.; Bergqvist, J.; Zhang, F.; Ma, W.; Ade, H.; et al. Fast charge separation in a non-fullerene organic solar cell with a small driving force. Nat. Energy 2016, 1, 16089. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, J.; Deng, W.; Luo, M.; Xie, Y.; Liang, Q.; Zou, Y.; He, Z.; Wu, H.; Cao, Y. High-efficiency organic solar cells with low non-radiative recombination loss and low energetic disorder. Nat. Photonics 2020, 14, 300–305. [Google Scholar] [CrossRef]
- Yang, D.; Wang, Y.; Sano, T.; Gao, F.; Sasabe, H.; Kido, J. A minimal non-radiative recombination loss for efficient non-fullerene all-small-molecule organic solar cells with a low energy loss of 0.54 eV and high open-circuit voltage of 1.15 V. J. Mater. Chem. A 2018, 6, 13918–13924. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.; Liang, N.; Yao, H.; Wei, Z.; He, C.; Yuan, X.; Hou, J. Effects of energy-level offset between a donor and acceptor on the photovoltaic performance of non-fullerene organic solar cells. J. Mater. Chem. A 2019, 7, 18889–18897. [Google Scholar] [CrossRef]
- Liu, W.; Yao, J.; Zhan, C. A Novel BODIPY-Based Low-Band-Gap Small-Molecule Acceptor for Efficient Non-fullerene Polymer Solar Cells. Chin. J. Chem. 2017, 35, 1813–1823. [Google Scholar] [CrossRef]
- Elumalai, N.K.; Uddin, A. Open circuit voltage of organic solar cells: An in-depth review. Energy Environ. Sci. 2016, 9, 391–410. [Google Scholar] [CrossRef]
- Zheng, Y.; Goh, T.; Fan, P.; Shi, W.; Yu, J.; Taylor, A.D. Toward Efficient Thick Active PTB7 Photovoltaic Layers Using Diphenyl Ether as a Solvent Additive. ACS Appl. Mater. Interfaces 2016, 8, 15724–15731. [Google Scholar] [CrossRef]
- Luck, K.A.; Sangwan, V.K.; Hartnett, P.E.; Arnold, H.N.; Wasielewski, M.R.; Marks, T.J.; Hersam, M.C. Correlated In Situ Low-Frequency Noise and Impedance Spectroscopy Reveal Recombination Dynamics in Organic Solar Cells Using Fullerene and Non-Fullerene Acceptors. Adv. Funct. Mater. 2017, 27, 1703805. [Google Scholar] [CrossRef]
- Li, J.; Liang, Z.; Peng, Y.; Lv, J.; Ma, X.; Wang, Y.; Xia, Y. 36% Enhanced Efficiency of Ternary Organic Solar Cells by Doping a NT-Based Polymer as an Electron-Cascade Donor. Polymers 2018, 10, 703. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yang, F.; Li, Y.; Uddin, M.A.; Bi, P.; Fan, B.; Cai, Y.; Hao, X.-T.; Woo, H.Y.; Li, W.; et al. Morphology Control Enables Efficient Ternary Organic Solar Cells. Adv. Mater. 2018, 30, 30. [Google Scholar] [CrossRef] [PubMed]
- Mai, J.; Lu, H.; Lau, T.-K.; Peng, S.-H.; Hsu, C.S.; Hua, W.; Zhao, N.; Xiao, X.; Lu, X. High efficiency ternary organic solar cell with morphology-compatible polymers. J. Mater. Chem. A 2017, 5, 11739–11745. [Google Scholar] [CrossRef]
- Mai, J.Q.; Lau, T.K.; Xiao, T.; Su, C.J.; Jeng, U.S.; Zhao, N.; Xiao, X.D.; Lu, X.H. A ternary morphology facilitated thick-film organic solar cell. Rsc Adv. 2015, 5, 88500–88507. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, F.; Xu, W.; Bi, P.; Feng, L.; Liu, J.; Hao, X.-T. Improving the Compatibility of Donor Polymers in Efficient Ternary Organic Solar Cells via Post-Additive Soaking Treatment. ACS Appl. Mater. Interfaces 2016, 9, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Bonasera, A.; Giuliano, G.; Arrabito, G.; Pignataro, B. Tackling Performance Challenges in Organic Photovoltaics: An Overview about Compatibilizers. Molecules 2020, 25, 2200. [Google Scholar] [CrossRef]
- Du, X.; Lin, H.; Chen, X.; Tao, S.; Zheng, C.-J.; Zhang, X. Ternary organic solar cells with a phase-modulated surface distribution via the addition of a small molecular luminescent dye to obtain a high efficiency over 10.5%. Nanoscale 2018, 10, 16455–16467. [Google Scholar] [CrossRef]
- Sun, Y.; Chien, S.-C.; Yip, H.-L.; Chen, K.-S.; Zhang, Y.; Davies, J.A.; Chen, F.-C.; Lin, B.; Jen, A.K.-Y. Improved thin film morphology and bulk-heterojunction solar cell performance through systematic tuning of the surface energy of conjugated polymers. J. Mater. Chem. 2012, 22, 5587. [Google Scholar] [CrossRef]
- An, Q.; Zhang, F.; Sun, Q.; Zhang, M.; Zhang, J.; Tang, W.; Yin, X.; Deng, Z. Efficient organic ternary solar cells with the third component as energy acceptor. Nano Energy 2016, 26, 180–191. [Google Scholar] [CrossRef]
- Neumann, A.; Good, R.; Hope, C.; Sejpal, M. An equation-of-state approach to determine surface tensions of low-energy solids from contact angles. J. Colloid Interface Sci. 1974, 49, 291–304. [Google Scholar] [CrossRef]
- Li, D.; Neumann, A. A reformulation of the equation of state for interfacial tensions. J. Colloid Interface Sci. 1990, 137, 304–307. [Google Scholar] [CrossRef]
- Li, D.; Neumann, A. Contact angles on hydrophobic solid surfaces and their interpretation. J. Colloid Interface Sci. 1992, 148, 190–200. [Google Scholar] [CrossRef]
- Sumita, M.; Sakata, K.; Asai, S.; Miyasaka, K.; Nakagawa, H. Dispersion of fillers and the electrical conductivity of polymer blends filled with carbon black. Polym. Bull. 1991, 25, 265–271. [Google Scholar] [CrossRef]
- Zhu, K.; Tang, D.; Zhang, K.; Wang, Z.; Ding, L.; Liu, Y.; Yuan, L.; Fan, J.; Song, B.; Zhou, Y.; et al. A two-dimension-conjugated small molecule for efficient ternary organic solar cells. Org. Electron. 2017, 48, 179–187. [Google Scholar] [CrossRef]
- Mohapatra, A.A.; Kim, V.; Puttaraju, B.; Sadhanala, A.; Jiao, X.; McNeill, C.R.; Friend, R.H.; Patil, S. Förster Resonance Energy Transfer Drives Higher Efficiency in Ternary Blend Organic Solar Cells. ACS Appl. Energy Mater. 2018, 1, 4874–4882. [Google Scholar] [CrossRef]
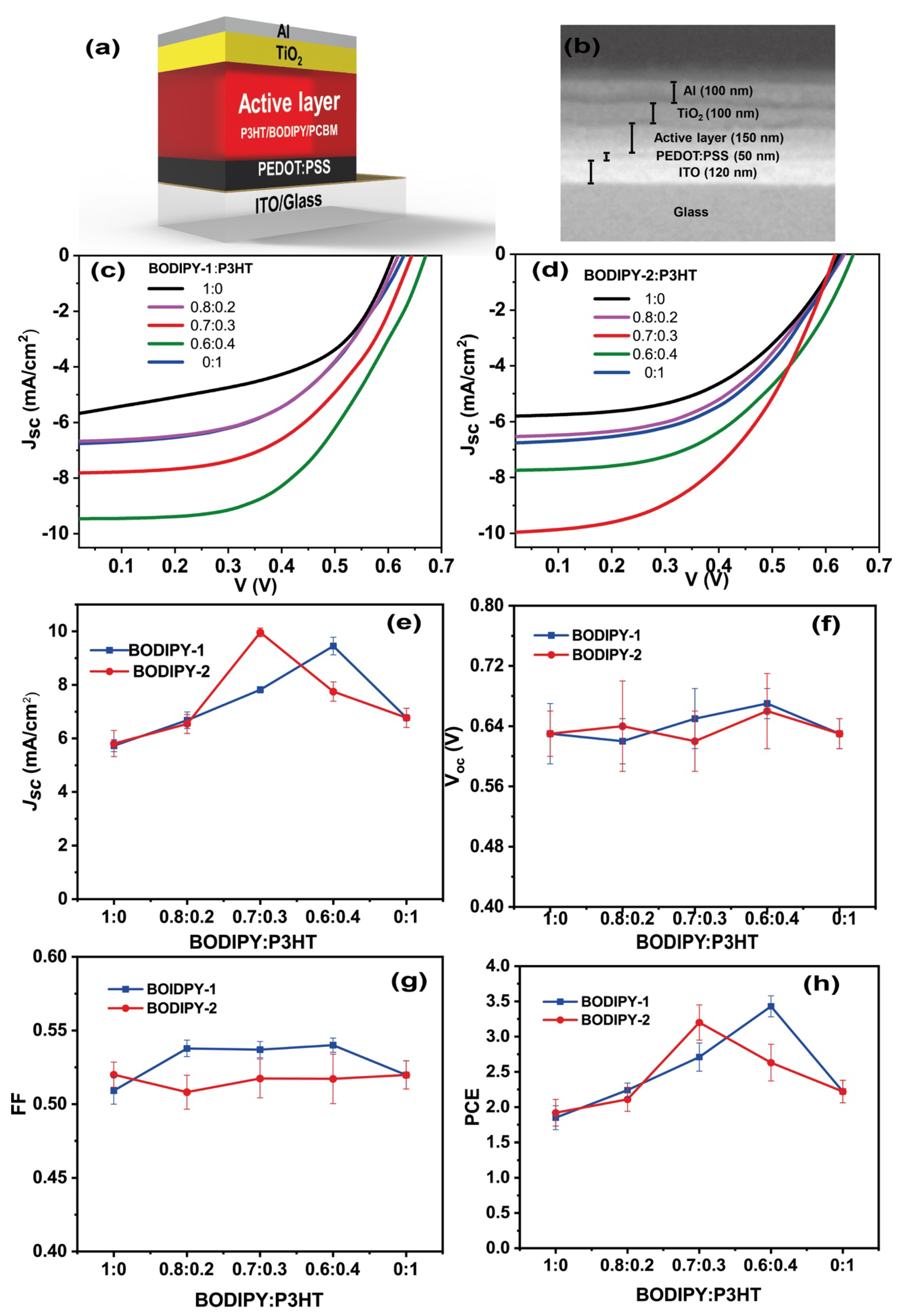

| Devices | Donor Concentration a (by wt.) | Jsc (mA/cm2) | Voc (V) | FF | PCE (%) (Best) (Average) |
|---|---|---|---|---|---|
| 1 | P3HT | 6.77 ± 0.36 | 0.63 ± 0.02 | 51.98 ± 0.96 | 2.48, 2.22 ± 0.16 |
| 2 | BODIPY-1 | 5.73 ± 0.22 | 0.63 ± 0.04 | 50.91 ± 0.92 | 2.00, 1.85 ± 0.17 |
| 3 | BODIPY-2 | 5.81 ± 0.49 | 0.63 ± 0.03 | 52.01 ± 0.85 | 2.29, 1.92 ± 0.19 |
| 4 | BODIPY-1:P3HT (0.8:0.2) | 6.68 ± 0.31 | 0.62 ± 0.01 | 53.78 ± 0.56 | 2.33, 2.24 ± 0.10 |
| 5 | BODIPY-1:P3HT (0.7:0.3) | 7.82 ± 0.12 | 0.65 ± 0.04 | 53.70 ± 0.55 | 2.90, 2.71 ± 0.20 |
| 6 | BODIPY-1:P3HT (0.6:0.4) | 9.45 ± 0.33 | 0.67 ± 0.02 | 54.00 ± 0.48 | 3.71, 3.43 ± 0.15 |
| 7 | BODIPY-2:P3HT (0.8:0.2) | 6.54 ± 0.35 | 0.64 ± 0.06 | 50.81 ± 1.15 | 2.23, 2.11 ± 0.17 |
| 8 | BODIPY-2:P3HT (0.7:0.3) | 9.96 ± 0.16 | 0.62 ± 0.04 | 51.74 ± 1.32 | 3.38, 3.20 ± 0.25 |
| 9 | BODIPY-2:P3HT (0.6:0.4) | 7.75 ± 0.36 | 0.66 ± 0.05 | 51.71 ± 1.68 | 2.99, 2.63 ± 0.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanwong, S.; Sangkhun, W.; Kumnorkaew, P.; Wootthikanokkhan, J. Improved Performance of Ternary Solar Cells by Using BODIPY Triads. Materials 2020, 13, 2723. https://doi.org/10.3390/ma13122723
Wanwong S, Sangkhun W, Kumnorkaew P, Wootthikanokkhan J. Improved Performance of Ternary Solar Cells by Using BODIPY Triads. Materials. 2020; 13(12):2723. https://doi.org/10.3390/ma13122723
Chicago/Turabian StyleWanwong, Sompit, Weradesh Sangkhun, Pisist Kumnorkaew, and Jatuphorn Wootthikanokkhan. 2020. "Improved Performance of Ternary Solar Cells by Using BODIPY Triads" Materials 13, no. 12: 2723. https://doi.org/10.3390/ma13122723
APA StyleWanwong, S., Sangkhun, W., Kumnorkaew, P., & Wootthikanokkhan, J. (2020). Improved Performance of Ternary Solar Cells by Using BODIPY Triads. Materials, 13(12), 2723. https://doi.org/10.3390/ma13122723