Methods of Gold and Silver Nanoparticles Preparation
Abstract
1. Introduction
2. Historical Overview
3. Basic Properties of Colloidal Solutions
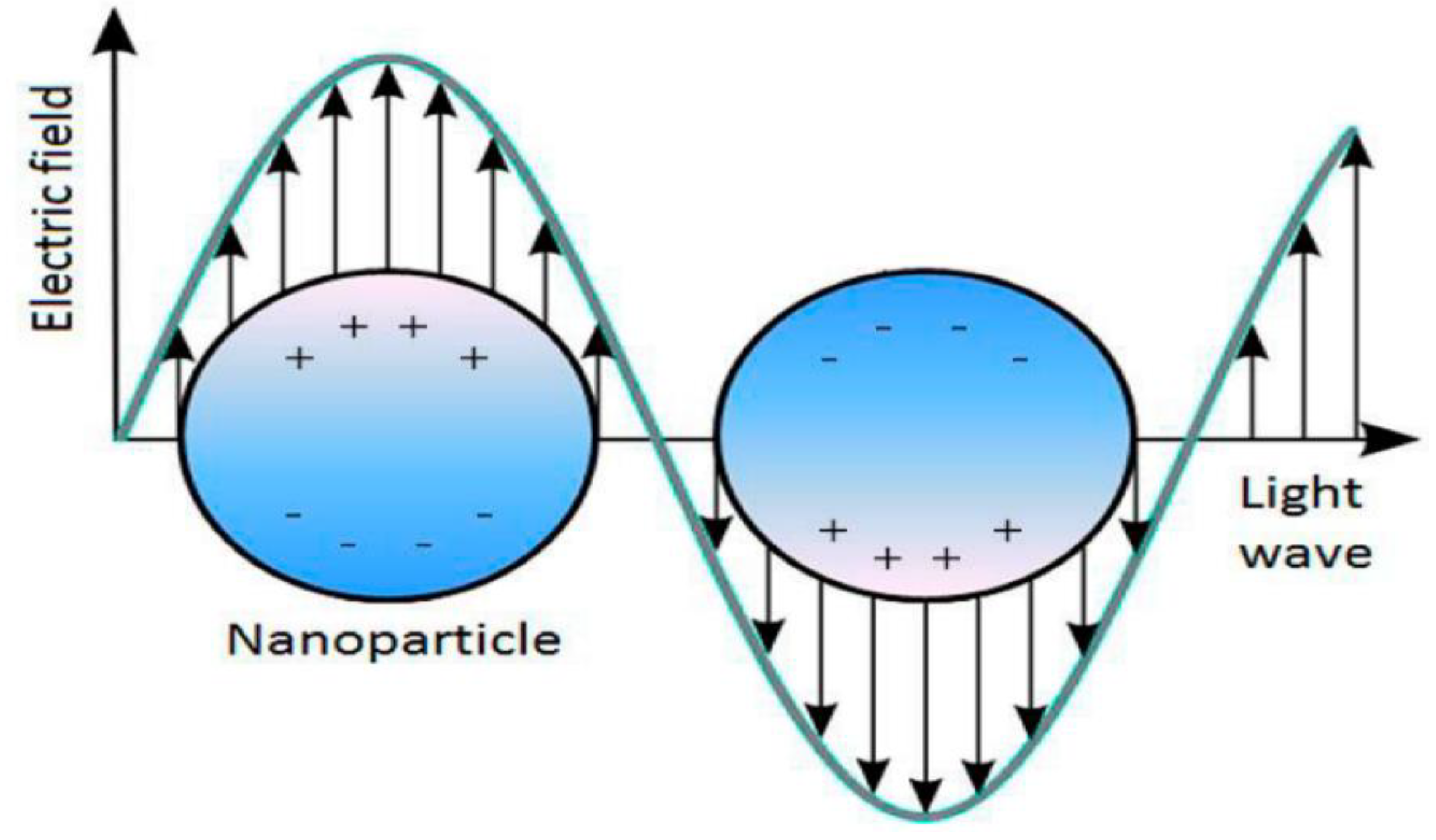
3.1. Optical Properties
3.2. Stability of Solutions
3.3. Antibacterial Effects of Silver and Gold
4. Methods of Nanoparticles Preparation
4.1. Dispergation Methods
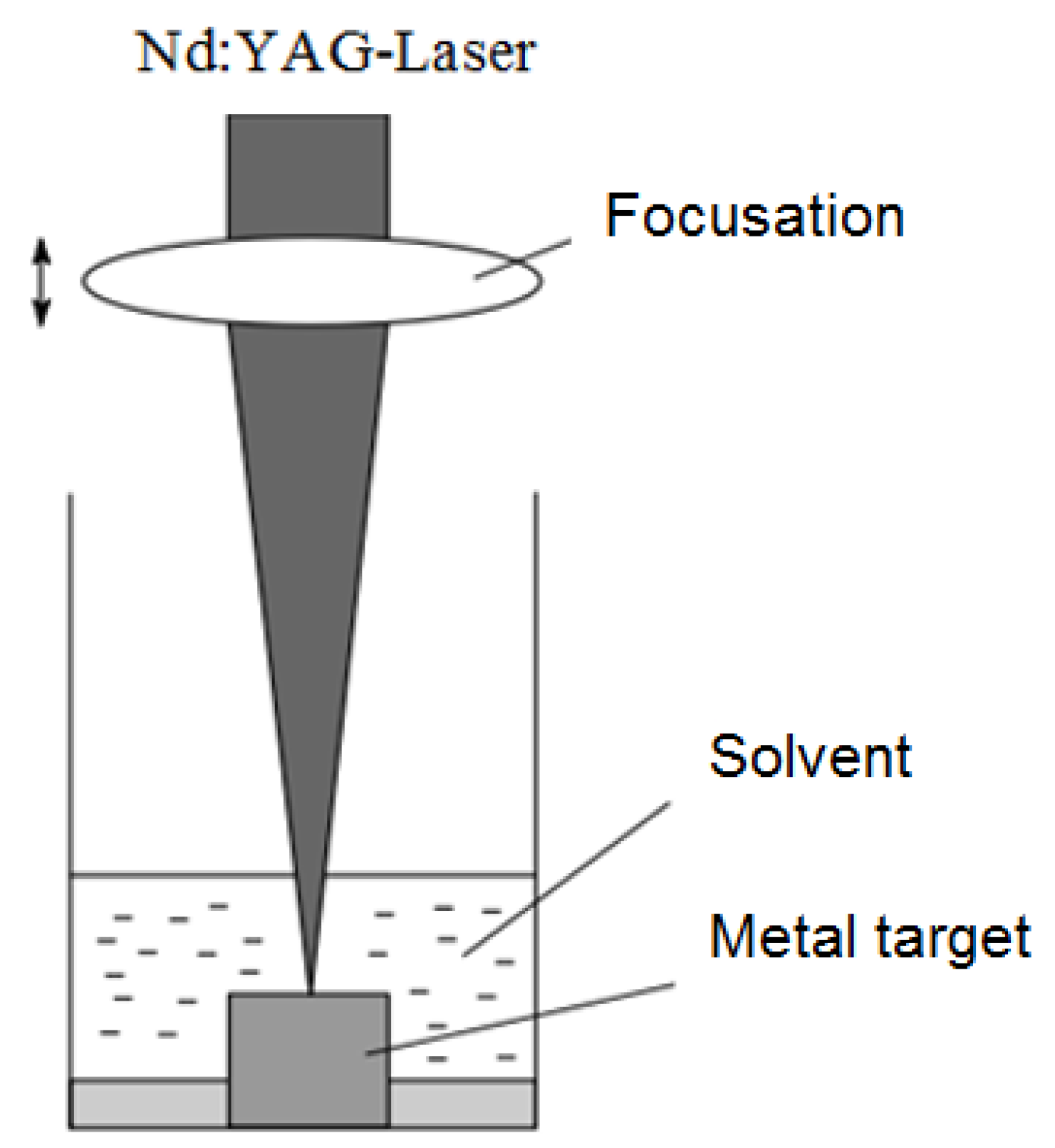
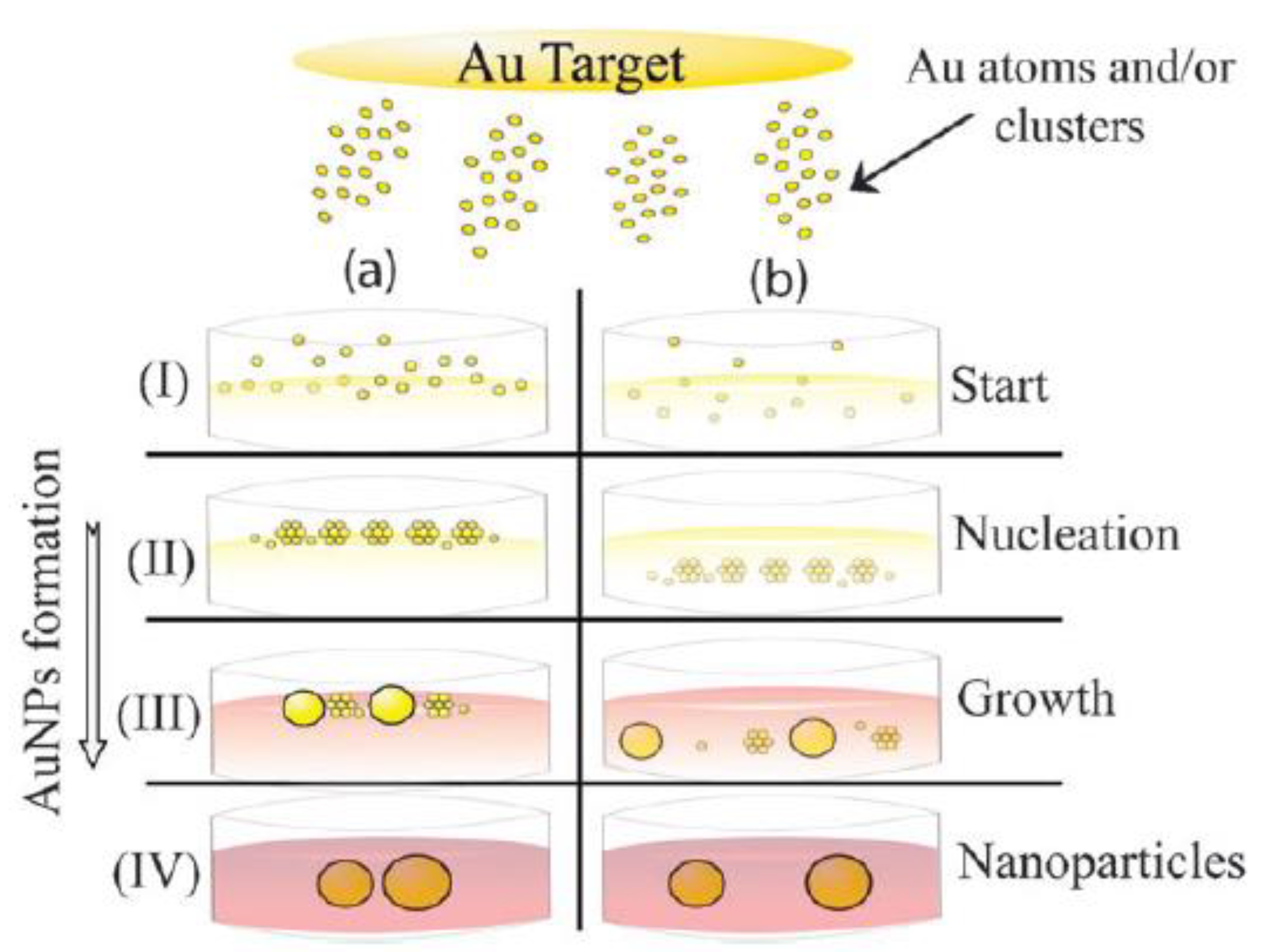
4.1.1. Laser Ablation
4.1.2. Vacuum Sputtering
4.2. Condensation Methods
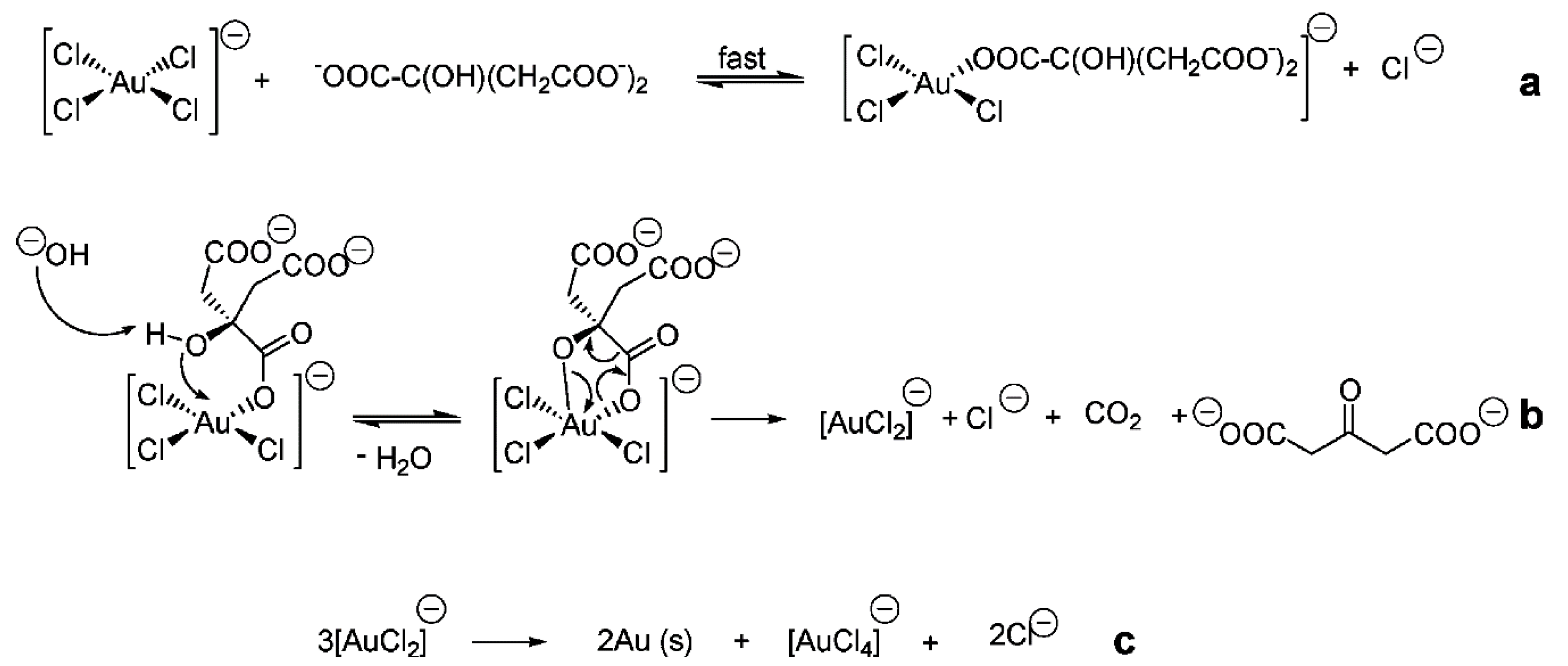
4.2.1. Reduction in Solution
4.2.2. Brust–Schiffrin Method
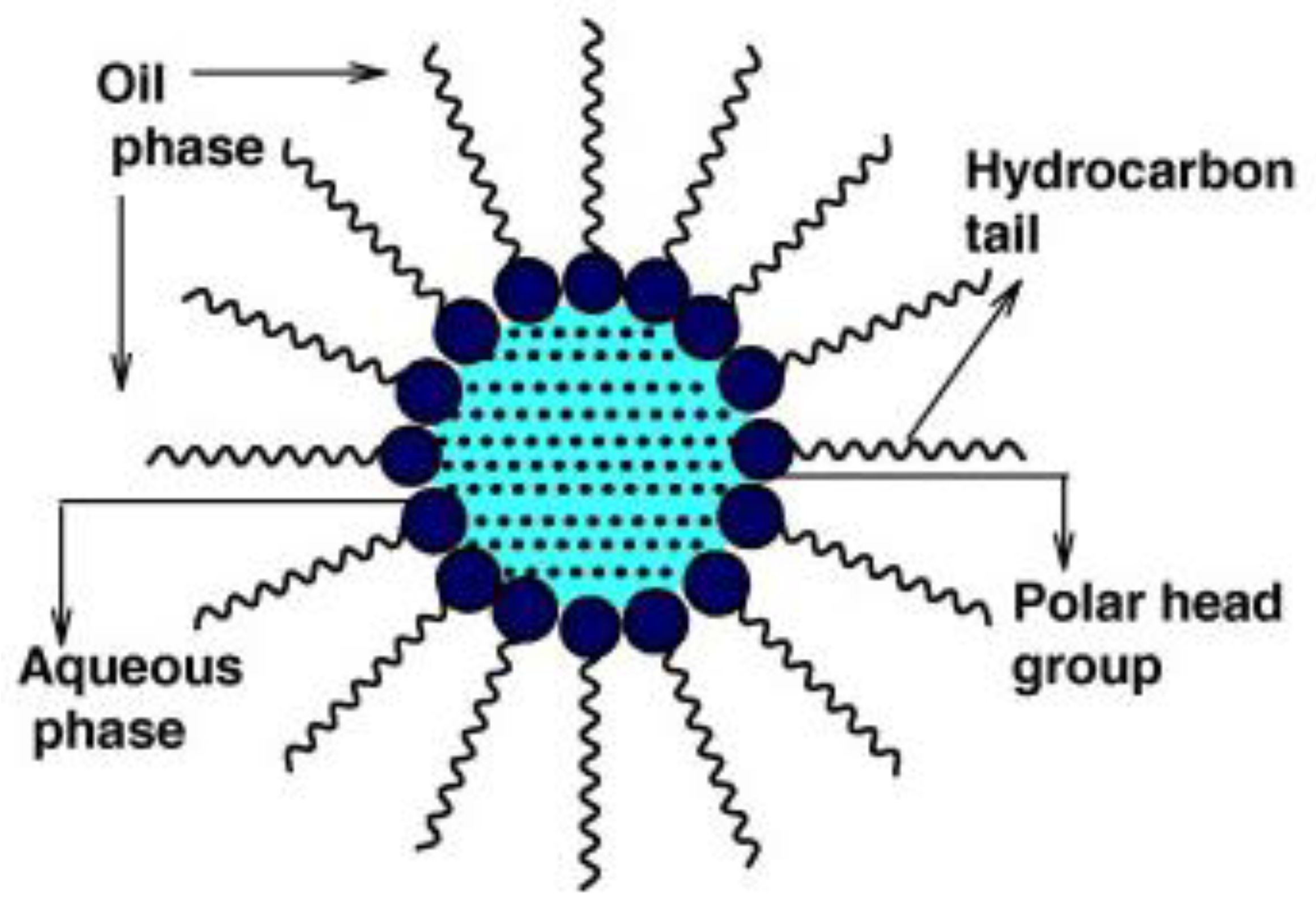
4.2.3. Synthesis in Reverse Micelles
4.2.4. Method Based on Ultraviolet Light
4.2.5. Biosynthesis of Silver and Gold Nanoparticles
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, J.; Ralston, J.; Sedef, R.; Beattie, D.A. Functionalized gold nanoparticles: Synthesis, structure and colloid stability. J. Colloid Interface Sci. 2009, 331, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, E. Metal nanoclusters: New fluorescent probes for sensors and bioimaging. Nano Today 2014, 9, 132–157. [Google Scholar] [CrossRef]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.V. Photophysical, Photochemical and Photocatalytic Aspects of Metal Nanoparticles. J. Phys. Chem. B 2002, 106, 7729–7744. [Google Scholar] [CrossRef]
- Mingos, D.M.P. Historical Introduction to Gold Colloids, Clusters and Nanoparticles; Springer Nature Switzerland AG: Basel, Switzerland, 2014. [Google Scholar]
- Sahoo, G.P.; Basu, S.; Samanta, S.; Misra, A. Microwave-assisted synthesis of anisotropic gold nanocrystals in polymer matrix and their catalytic activities. J. Exp. Nanosci. 2014, 10, 690–702. [Google Scholar] [CrossRef]
- Fajstavr, D.; Slepička, P.; Švorčík, V. LIPSS with gold nanoclusters prepared by combination of heat treatment and KrF exposure. Appl. Surf. Sci. 2019, 465, 919–928. [Google Scholar] [CrossRef]
- Scholl, J.A.; Koh, A.L.; Dionne, J.A. Quantum plasmon resonances of individual metallic nanoparticles. Nature 2012, 483, 421–427. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Shape-Controlled Synthesis of Gold and Silver Nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef]
- Naidu, K.B.; Govender, P.; Adam, J.K. Biomedical applications and toxicity of nanosilver: A review. Med. Technol. SA 2015, 29, 13–19. [Google Scholar]
- Yu, S.J.; Yin, Y.G.; Liu, J.F. Silver nanoparticles in the environment. Environ. Sci. Proc. Impacts 2013, 15, 78–92. [Google Scholar] [CrossRef]
- Edwards-Jones, V. The benefits of silver in hygiene, personal care and healthcare. Lett. Appl. Microbiol. 2009, 49, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. Engl. 2013, 52, 1636–1653. [Google Scholar] [CrossRef] [PubMed]
- Simon-Deckers, A.; Gouget, B.; Mayne-L’hermite, M.; Herlin-Boime, N.; Reynaud, C.; Carriere, M. In vitro investigation of oxide nanoparticle and carbon nanotube toxicity and intracellular accumulation in A549 human pneumocytes. Toxicology 2008, 253, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-G.; Kim, K.-T.; Ryu, T.-K.; Lee, J.-W.; Kim, J.-E.; Kim, J. Stepwise embryonic toxicity of silver nanoparticles on Oryzias latipes. BioMed. Res. Int. 2013, 2013, 1–7. [Google Scholar]
- Gaillet, S.; Rouanet, J.M. Silver nanoparticles: Their potential toxic effects after oral exposureand underlying mechanisms–A review. Food Chem. Toxicol. 2015, 77, 58–63. [Google Scholar] [CrossRef]
- Hornyak, G.L.; Tibbals, H.; Dutta, J. Introduction to Nanoscience; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Barber, D.; Freestone, I. An investigation of the origin of the colour of the Lycurgus Cup by analytical transmission electron microscopy. Archaeometry 1990, 32, 33–45. [Google Scholar] [CrossRef]
- Mehlman, F. Phaidon Guide to Glass; Phaidon Press LTD: London, UK, 1982; ISBN 9780714822020. [Google Scholar]
- Kumar, N.; Kumar, R. Nanotechnology and Nanomaterials in the Treatment of Life-Threatening Diseases; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–51. ISBN 978-0-323-26433-4. [Google Scholar]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Tweney, R.D. Discovering discovery: How Faraday found the first metallic colloid. Perspect. Sci. 2006, 14, 97–121. [Google Scholar] [CrossRef]
- Rayavarapu, R.G.; Petersen, W.; Ungureanu, C.; Post, J.N.; van Leeuwen, T.G.; Manohar, S. Synthesis and bioconjugation of gold nanoparticles as potential molecular probes for light-based imaging techniques. Int. J. Biomed. Imaging 2007, 2007, 29817. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications–A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef]
- Sawant, S.N. Development of Biosensors from Biopolymer Composites. In Biopolymer Composites in Electronics; Sadasivuni, K.K., Cabibihan, J.J., Ponnamma, D., AlMaadeed, M.A.A., Kim, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Liedberg, B.; Johansen, K. Affinity biosensing based on surface plasmon resonance detection. In Affinity Biosensors; Rogers, K.R., Mulchandani, A., Eds.; Springer: Berlin, Germany, 1998; pp. 31–53. [Google Scholar]
- Slepickova Kasalkova, N.; Žáková, P.; Stibor, I.; Slepička, P.; Kolská, Z.; Karpíšková, J.; Švorčík, V. Carbon nanostructures grafted biopolymers for medical applications. Mater. Technol. 2019, 34, 376–385. [Google Scholar] [CrossRef]
- Žáková, P.; Slepičková Kasálková, N.; Slepička, P.; Kolská, Z.; Karpíšková, J.; Stibor, I.; Švorčík, V. Cytocompatibility of polyethylene grafted with triethylenetetramine functionalized carbon nanoparticles. Appl. Surf. Sci. 2017, 422, 809–816. [Google Scholar] [CrossRef]
- Lu, J.; Elam, J.W.; Stair, P. Atomic layer deposition-Sequential self-limiting surface reactions for advanced catalyst “bottom-up” synthesis. Surf. Sci. Rep. 2016, 71, 410–472. [Google Scholar] [CrossRef]
- Schmid, G. Large clusters and colloids. Metals in the embryonic state. Chem. Rev. 1992, 92, 1709–1727. [Google Scholar] [CrossRef]
- Hosokawa, M.; Nogi, K.; Naito, M.; Yokoyama, T. Nanoparticle Technology Handbook; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Slepička, P.; Elashnikov, R.; Ulbrich, P.; Staszek, M.; Kolská, Z.; Švorčík, V. Stabilization of sputtered gold and silver nanoparticles in PEG colloid solutions. J. Nanopart. Res. 2015, 17, 11–26. [Google Scholar] [CrossRef]
- Unser, S.; Bruzas, I.; He, J.; Sagle, L. Localized Surface Plasmon Resonance Biosensing: Current Challenges and Approaches. Sensors 2015, 15, 15684–15716. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles? The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2002, 107, 668–677. [Google Scholar] [CrossRef]
- Cao, G.; Wang, Y. Nanostructures and Nanomaterials: Synthesis, Properties, and Applications; World Scientific Series in Nanoscience and Nanotechnology; World Scientific Co. Pte Ltd.: Singapore, 2011; Volume 2. [Google Scholar]
- Amendola, V.; Meneghetti, M. Size Evaluation of Gold Nanoparticles by UV−vis Spectroscopy. J. Phys. Chem. C 2009, 113, 4277–4285. [Google Scholar] [CrossRef]
- Niskanen, I.; Forsberg, V.; Zakrisson, D.; Reza, S.; Hummelgård, M.; Andres, B.; Fedorov, I.; Suopajärvi, T.; Liimatainen, H.; Thungström, G. Determination of nanoparticle size using Rayleigh approximation and Mie theory. Chem. Eng. Sci. 2019, 201, 222–229. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Zhang, L.; Yan, Y.; Jiang, Y. Controllable plasmon-induced catalytic reaction by surface-enhanced and tip-enhanced Raman spectroscopy. Spectrochim. Acta A 2019, 219, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.N.; Ho, C.M.; Chen, R.; He, Q.Y.; Yu, W.Y.; Sun, H.; Tam, P.H.; Chiu, J.F.; Che, C.M. Silver nanoparticles: Partial oxidation and antibacterial activities. J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Krutyakov, Y.A.; Kudrinskiy, A.A.; Olenin, A.Y.; Lisichkin, G.V. Synthesis and properties of silver nanoparticles: Advances and prospects. Russ. Chem. Rev. 2008, 77, 233. [Google Scholar] [CrossRef]
- Schmid, G.; Corain, B. Nanoparticulated gold: Syntheses, structures, electronics, and reactivities. Eur. J. Inorg. Chem. 2003, 2003, 3081–3098. [Google Scholar] [CrossRef]
- Valueva, S.V.; Kipper, A.I.; Borovikova, L.N.; Matveeva, N.A. The influence of the nature of a nanoparticle and polymer matrix on the morphological characteristics of polymeric nanostructures. Russ. J. Phys. Chem. 2010, 84, 2110–2115. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics? Angew. Chem. Int. Ed. 2008, 48, 60–103. [Google Scholar] [CrossRef]
- Yu, D.; Yam, V.W.-W. Controlled Synthesis of Monodisperse Silver Nanocubes in Water. J. Am. Chem. Soc. 2004, 126, 13200–13201. [Google Scholar] [CrossRef]
- Reznickova, A.; Slepicka, P.; Slavikova, N.; Staszek, M.; Svorcik, V. Preparation, aging and temperature stability of PEGylated gold nanoparticles. Colloid. Surf. A 2017, 523, 91–97. [Google Scholar] [CrossRef]
- Deori, K.; Gupta, D.; Saha, B.; Deka, S. Design of 3-Dimensionally Self-Assembled CeO2 Nanocube as a Breakthrough Catalyst for Efficient Alkylarene Oxidation in Water. ACS Catal. 2014, 4, 3169–3179. [Google Scholar] [CrossRef]
- Dykman, L.; Bogatyrev, V.; Shchegolev, S.Y.; Khlebtsov, N. Gold Nanoparticles: Synthesis, Proper Ties, and Biomedical Applications; Nauka: Moscow, Russia, 2008. [Google Scholar]
- Panáček, A.; Kvítek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizúrová, N.; Sharma, V.K.; Nevěcná, T.; Zbořil, R. Silver Colloid Nanoparticles? Synthesis, Characterization, and Their Antibacterial Activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef]
- Tran, Q.H.; Nguyen, V.Q.; Le, A.T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. 2013, 4, 033001. [Google Scholar] [CrossRef]
- Dibrov, P.; Dzioba, J.; Gosink, K.K.; Häse, C.C. Chemiosmotic mechanism of antimicrobial activity of Ag+ in Vibrio cholerae. Antimicrob. Agents Chemother. 2002, 46, 2668–2670. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microb. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Singaravelu, G.; Arockiamary, J.; Kumar, V.G.; Govindaraju, K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloid Surf. B 2007, 57, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Arockiya Aarthi Rajathi, F.; Parthiban, C.; Ganesh Kumar, V.; Anantharaman, P. Biosynthesis of antibacterial gold nanoparticles using brown alga, Stoechospermum marginatum (kützing). Spectrochim. Acta A 2012, 99, 166–173. [Google Scholar] [CrossRef]
- Nguyenova, H.Y.; Vokata, B.; Zaruba, K.; Siegel, J.; Kolska, Z.; Svorcik, V.; Slepicka, P.; Reznickova, A. Silver nanoparticles grafted onto PET: Effect of preparation method on antibacterial activity. React. Funct. Polym. 2019, 145, 104376. [Google Scholar] [CrossRef]
- Neděla, O.; Slepička, P.; Slepickova Kasalkova, N.; Sajdl, P.; Kolská, Z.; Rimpelová, S.; Švorčík, V. Antibacterial properties of angle-dependent nanopatterns on polystyrene. React. Funct. Polym. 2019, 136, 173–180. [Google Scholar] [CrossRef]
- Neděla, O.; Slepička, P.; Švorčík, V. Surface Modification of Polymer Substrates for Biomedical Applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Pillai, Z.S.; Kamat, P.V. What factors control the size and shape of silver nanoparticles in the citrate ion reduction method? J. Phys. Chem. B 2004, 108, 945–951. [Google Scholar] [CrossRef]
- Panigrahi, S.; Kundu, S.; Ghosh, S.; Nath, S.; Pal, T. General method of synthesis for metal nanoparticles. J. Nanopart. Res. 2004, 6, 411–414. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Karacharov, A.; Likhatski, M.; Podlipskaya, T.; Zubavichus, Y.; Veligzhanin, A.; Zaikovski, V. Submicrometer intermediates in the citrate synthesis of gold nanoparticles: New insights into the nucleation and crystal growth mechanisms. J. Colloid Interface Sci. 2011, 362, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Kohler, J.M. Continuous synthesis of gold nanoparticles in a microreactor. Nano Lett. 2005, 5, 685591. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.; Kvítek, O.; Ulbrich, P.; Kolská, Z.; Slepička, P.; Švorčík, V. Progressive approach for metal nanoparticle synthesis. Mater. Lett. 2012, 89, 47–50. [Google Scholar] [CrossRef]
- Staszek, M.; Siegel, J.; Kolarova, K.; Rimpelova, S.; Svorcik, V. Formation and antibacterial action of Pt and Pd nanoparticles sputtered into liquid. Micro Nano Lett. 2014, 9, 778–781. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, M.; Liu, M.; Xiao, Y.; Li, Z.; Chen, J.; Sun, Y.; Zhao, J.; Fang, S.; Jia, D.; et al. Homogeneous Pd nanoparticles produced in direct reactions: Green synthesis, formation mechanism and catalysis properties. J. Mater. Chem. A 2014, 2, 1369–1374. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatized gold nanoparticles in a 1994, 2-phase liquid-liquid system. J. Chem. Soc. Chem. Commun. 1994, 801–802. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Yuan, M.; Guo, R. Preparation and characterization of casein-stabilized gold nanoparticles for catalytic applications. Colloid Surf. A 2013, 417, 18–25. [Google Scholar] [CrossRef]
- Doyen, M.; Bartik, K.; Bruylants, G. UV-Vis and NMR study of the formation of gold nanoparticles by citrate reduction: Observation of gold-citrate aggregates. J. Colloid Interface Sci. 2013, 399, 1–5. [Google Scholar] [CrossRef]
- De, S.; Kundu, R.; Biswas, A. Synthesis of gold nanoparticles in niosomes. J. Colloid Interface Sci. 2012, 386, 9–15. [Google Scholar] [CrossRef]
- Wangoo, N.; Bhasin, K.K.; Mehta, S.K.; Suri, C.R. Synthesis and capping of water-dispersed gold nanoparticles by an amino acid: Bioconjugation and binding studies. J. Colloid Interface Sci. 2008, 323, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Nagata, K.; Iwase, A.; Hori, F. Synthesis of Cu nanoparticles using gamma-ray irradiation reduction method. Jpn. J. Appl. Phys. 2014, 53, 05FC05. [Google Scholar] [CrossRef]
- Yokoyama, S.; Takahashi, H.; Itoh, T.; Motomiya, K.; Tohji, K. Synthesis of metallic Cu nanoparticles by controlling Cu complexes in aqueous solution. Adv. Powder Technol. 2014, 25, 999–1006. [Google Scholar] [CrossRef]
- Bankar, A.; Joshi, B.; Kumar, A.R.; Zinjarde, S. Banana peel extract mediated synthesis of gold nanoparticles. Colloids Surf. B 2010, 80, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Bankar, A.; Joshi, B.; Kumar, A.R.; Zinjarde, S. Banana peel extract mediated novel route for the synthesis of palladium nanoparticles. Mater. Lett. 2010, 64, 1951–1953. [Google Scholar] [CrossRef]
- Bankar, A.; Joshi, B.; Kumar, A.R.; Zinjarde, S. Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Colloid Surf. A 2010, 368, 58–63. [Google Scholar] [CrossRef]
- Das, R.K.; Sharma, P.; Nahar, P.; Bora, U. Synthesis of gold nanoparticles using aqueous extract of Calotropis procera latex. Mater. Lett. 2011, 65, 610–613. [Google Scholar] [CrossRef]
- Ren, F.; He, X.; Wang, K.; Yin, J. Biosynthesis of gold nanoparticles using catclaw buttercup (Radix Ranunculi Ternati) and evaluation of its colloidal stability. J. Biomed. Nanotechnol. 2012, 8, 586–593. [Google Scholar] [CrossRef]
- Im, H.-J.; Lee, B.C.; Yeon, J.-W. Preparation and characterization of Ag nanoparticle-embedded blank and ligand-anchored silica gels. J. Nanosci. Nanotechnol. 2013, 13, 7643–7647. [Google Scholar] [CrossRef]
- Sarkar, P.; Bhui, D.K.; Bar, H.; Sahoo, G.P.; De, S.P.; Misra, A. Synthesis and photophysical study of silver nanoparticles stabilized by unsaturated dicarboxylates. J. Lumin. 2009, 129, 704–709. [Google Scholar] [CrossRef]
- Mafune, F.; Kohno, J.-Y.; Takeda, Y.; Kondow, T.; Sawabe, H. Formation and size control of silver nanoparticles by laser ablation in aqueous solution. J. Phys. Chem. B 2000, 104, 9111–9117. [Google Scholar] [CrossRef]
- Hatakeyama, Y.; Morita, T.; Takahashi, S.; Onishi, K.; Nishikawa, K. Synthesis of Gold Nanoparticles in Liquid Polyethylene Glycol by Sputter Deposition and Temperature Effects on their Size and Shape. J. Phys. Chem. C 2011, 115, 3279–3285. [Google Scholar] [CrossRef]
- Gracia, R.; Vijayakrishna, K.; Mecerreyes, D. Poly (ionic liquid) s with redox active counter-anions: All-in-one reactants and stabilizers for the synthesis of functional colloids. React. Funct. Polym. 2014, 79, 54–58. [Google Scholar] [CrossRef]
- Torimoto, T.; Okazaki, K.-I.; Kiyama, T.; Hirahara, K.; Tanaka, N.; Kuwabata, S. Sputter deposition onto ionic liquids: Simple and clean synthesis of highly dispersed ultrafine metal nanoparticles. Appl. Phys. Lett. 2006, 89, 243117. [Google Scholar] [CrossRef]
- Wender, H.; de Oliveira, L.F.; Feil, A.F.; Lissner, E.; Migowski, P.; Meneghetti, M.R.; Teixeira, S.R.; Dupont, J. Synthesis of gold nanoparticles in a biocompatible fluid from sputtering deposition onto castor oil. Chem. Commun. 2010, 46, 7019–7021. [Google Scholar] [CrossRef] [PubMed]
- Vanecht, E.; Binnemans, K.; Patskovsky, S.; Meunier, M.; Seo, J.W.; Stappers, L.; Fransaer, J. Stability of sputter-deposited gold nanoparticles in imidazolium ionic liquids. Phys. Chem. Chem. Phys. 2012, 14, 5662–5671. [Google Scholar] [CrossRef]
- Hatakeyama, Y.; Onishi, K.; Nishikawa, K. Effects of sputtering conditions on formation of gold nanoparticles in sputter deposition technique. RSC Adv. 2011, 1, 1815–1821. [Google Scholar] [CrossRef]
- Hatakeyama, Y.; Takahashi, S.; Nishikawa, K. Can Temperature Control the Size of Au Nanoparticles Prepared in Ionic Liquids by the Sputter Deposition Technique? J. Phys. Chem. C 2010, 114, 11098–11102. [Google Scholar] [CrossRef]
- Ye, G.X.; Zhang, Q.R.; Feng, C.M.; Ge, H.L.; Jiao, Z.K. Structural and electrical properties of a metallic rough-thin-film system deposited on liquid substrates. Phys. Rev. B 1996, 54, 14754–14757. [Google Scholar] [CrossRef]
- Ye, G.X.; Geng, C.M.; Zhang, Z.R.; Ge, H.L.; Zhang, X.J. Structural and critical behaviors of ag rough films deposited on liquid substrates. Chin. Phys. Lett. 1996, 13, 772–774. [Google Scholar] [CrossRef]
- Da Silva, E.C.; da Silva MG, A.; Meneghetti, S.M.P.; Machado, G.; Alencar, M.A.R.C.; Hickmann, J.M.; Meneghetti, M.R. Synthesis of colloids based on gold nanoparticles dispersed in castor oil. J. Nanopart. Res. 2008, 10, 201–208. [Google Scholar] [CrossRef]
- Wender, H.; Goncalves, R.V.; Feil, A.F.; Migowski, P.; Poletto, F.S.; Pohlmann, A.R.; Dupont, J.; Teixeira, S.R. Sputtering onto liquids: From thin films to nanoparticles. J. Phys. Chem. C 2011, 115, 16362–16367. [Google Scholar] [CrossRef]
- Dupont, J.; de Souza, R.F.; Suarez, P.A.Z. Ionic liquid (molten salt) phase organometallic catalysis. Chem. Rev. 2002, 102, 3667–3691. [Google Scholar] [CrossRef] [PubMed]
- Wender, H.; Migowski, P.; Feil, A.F.; Teixeira, S.R.; Dupont, J. Sputtering deposition of nanoparticles onto liquid substrates: Recent advances and future trends. Coord. Chem. Rev. 2013, 257, 2468–2483. [Google Scholar] [CrossRef]
- Antonietti, M.; Kuang, D.B.; Smarsly, B.; Yong, Z. Ionic liquids for the convenient synthesis of functional nanoparticles and other inorganic nanostructures. Angew. Chem. Int. Ed. 2004, 43, 4988–4992. [Google Scholar] [CrossRef] [PubMed]
- Migowski, P.; Dupont, J. Catalytic applications of metal nanoparticles in imidazolium ionic liquids. Chem. Eur. J. 2007, 13, 32–39. [Google Scholar] [CrossRef]
- Wender, H.; de Oliveira, L.F.; Migowski, P.; Feil, A.F.; Lissner, E.; Prechtl, M.H.G.; Teixeira, S.R.; Dupont, J. Ionic liquid surface composition controls the size of gold nanoparticles prepared by sputtering deposition. J. Phys. Chem. C 2010, 114, 11764–11768. [Google Scholar] [CrossRef]
- Migowski, P.; Zanchet, D.; Machado, G.; Gelesky, M.A.; Teixeira, S.R.; Dupont, J. Nanostructures in ionic liquids: Correlation of iridium nanoparticles’ size and shape with imidazolium salts’ structural organization and catalytic properties. Phys. Chem. Chem. Phys. 2010, 12, 6826–6833. [Google Scholar] [CrossRef]
- Redel, E.; Thomann, R.; Janiak, C. Use of ionic liquids (ILs) for the IL-anion size-dependent formation of Cr, Mo and W nanoparticles from metal carbonyl M(CO)6 precursors. Chem. Commun. 2008, 15, 1789–1791. [Google Scholar] [CrossRef]
- Redel, E.; Thomann, R.; Janiak, C. First correlation of nanoparticle size-dependent formation with the ionic liquid anion molecular volume. Inorg. Chem. 2008, 47, 14–16. [Google Scholar] [CrossRef]
- Švorčík, V.; Kolská, Z.; Siegel, J.; Slepička, P. “Short” Dithiol and Au Nanoparticles Grafting on Plasma Treated Polyethyleneterephthalate. J. Nano Res. 2013, 25, 40–48. [Google Scholar] [CrossRef]
- Kalachyova, Y.; Lyutakov, O.; Solovyev, A.; Slepička, P.; Švorčík, V. Surface morphology and optical properties of porphyrin/Au and Au/porphyrin/Au systems. Nanoscale Res. Lett. 2013, 8, 547. [Google Scholar]
- Slepičková Kasálková, N.; Stýblová, Š.; Slepička, P.; Rimpelová, S.; Švorčík, V. Surface changes of polymer modified by gold nanoparticles. Int. J. Nanotechnol. 2017, 14, 120–132. [Google Scholar] [CrossRef]
- Slepička, P.; Přibyl, M.; Fajstavr, D.; Ulbrich, P.; Siegel, J.; Řezníčková, A.; Švorčík, V. Grafting of platinum nanostructures on biopolymer at elevated temperature. Colloids Surf. A 2018, 546, 316–325. [Google Scholar]
- Slepička, P.; Michaljaničová, I.; Slepičková Kasálková, N.; Kolská, Z.; Rimpelová, S.; Ruml, T.; Švorčík, V. Poly-l -lactic acid modified by etching and grafting with gold nanoparticles. J. Mater. Sci. 2013, 48, 5871–5879. [Google Scholar] [CrossRef]
- Reznickova, A.; Slepicka, P.; Nguyenova, H.Y.; Kolska, Z.; Dendisova, M.; Svorcik, V. Copper-gold sandwich structures on PE and PET and their SERS enhancement effect. RSC Adv. 2017, 7, 23055–23064. [Google Scholar] [CrossRef]
- Slepicka, P.; Slepickova Kasalkova, N.; Siegel, J.; Kolska, Z.; Bacakova, Z.; Svorcik, V. Nano-structured and functionalized surfaces for cytocompatibility improvement and bactericidal action. Biotechnol. Adv. 2015, 33, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Slepicka, P.; Siegel, J.; Lyutakov, O.; Slepickova Kasalkova, N.; Kolska, Z.; Bacakova, L.; Svorcik, V. Polymer nanostructures for bioapplications induced by laser treatment. Biotechnol. Adv. 2018, 36, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Slepička, P.; Malá, Z.; Rimpelová, S.; Švorčík, V. Antibacterial properties of modified biodegradable PHB non-woven fabric. Mater. Sci. Eng. C 2016, 65, 364–368. [Google Scholar] [CrossRef]
- Hayat, M.A. Colloidal Gold: Principles, Methods, and Applications; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Yi, Z.; Xu, X.; Luo, J.; Li, X.; Yi, Y.; Jiang, X.; Yi, Y.; Tang, Y. Size controllable synthesis of ultrafine spherical gold particles and their simulation of plasmonic and SERS behaviors. Phys. B 2014, 438, 22–28. [Google Scholar] [CrossRef]
- Reetz, M.T.; Helbig, W. Size-Selective Synthesis of Nanostructured Transition Metal Clusters. J. Am. Chem. Soc. 1994, 116, 7401–7402. [Google Scholar] [CrossRef]
- Khaydarov, R.A.; Khaydarov, R.R.; Gapurova, O.; Estrin, Y.; Scheper, T. Electrochemical method for the synthesis of silver nanoparticles. J. Nanopart. Res. 2008, 11, 1193–1200. [Google Scholar] [CrossRef]
- Turkevich, J.; Cooperá Stevenson, P.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Ojea-Jiménez, I.; Romero, F.M.; Bastús, N.G.; Puntes, V. Small gold nanoparticles synthesized with sodium citrate and heavy water: Insights into the reaction mechanism. J. Phys. Chem. C 2010, 114, 1800–1804. [Google Scholar] [CrossRef]
- Dykman, L.A.; Bogatyrev, V.A. Gold nanoparticles: Preparation, functionalisation and applications in biochemistry and immunochemistry. Russ. Chem. Rev. 2007, 76, 181. [Google Scholar] [CrossRef]
- Weiser, H.B.; Reyerson, L. Inorganic colloid chemistry. J. Phys. Chem. 1935, 39, 305–306. [Google Scholar] [CrossRef]
- Henglein, A.; Giersig, M. Formation of colloidal silver nanoparticles: Capping action of citrate. J. Phys. Chem. B 1999, 103, 9533–9539. [Google Scholar] [CrossRef]
- Nishimoto, M.; Abe, S.; Yonezawa, T. Preparation of Ag nanoparticles using hydrogen peroxide as a reducing agent. New J. Chem. 2018, 42, 14493–14501. [Google Scholar] [CrossRef]
- Rashid, M.U.; Bhuiyan, K.H.; Quayum, M.E. Synthesis of silver nano particles(Ag-NPs) and their uses for quantitative analysis of vitamin C tablets. Dhaka Univ. J. Pharm. Sci. 2013, 12, 2933. [Google Scholar] [CrossRef]
- Ghorbani, R.H.; Safekordi, A.A.; Attar, H.; Sorkhabadi, S.M.R. Biological and nonbiological methods for silver nanoparticles synthesis. Chem. Biochem. Eng. 2011, 25, 317–326. [Google Scholar]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Krutyakov, Y.A.; Olenin, A.Y.; Kudrinskii, A.; Dzhurik, P.; Lisichkin, G. Aggregative stability and polydispersity of silver nanoparticles prepared using two-phase aqueous organic systems. Nanotechnol. Russ. 2008, 3, 303–310. [Google Scholar] [CrossRef]
- El Roustom, B.; Foti, G.; Comninellis, C. Preparation of gold nanoparticles by heat treatment of sputter deposited gold on boron-doped diamond film electrode. Electrochem. Commun. 2005, 7, 398–405. [Google Scholar] [CrossRef]
- Kanninen, P.; Johans, C.; Merta, J.; Konttur, K. Influence of ligand structure on the stability and oxidation of coppernanoparticles. J. Colloid Interface Sci. 2008, 318, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Felici, S.; Lavecchia, T.; Angjellari, M.; Micheli, L.; Orlanducci, S.; Terranova, M.T.; Palleschi, G. Towards a model of electrochemical immunosensor using silver nanoparticles. Procedia Technol. 2017, 27, 155–156. [Google Scholar] [CrossRef]
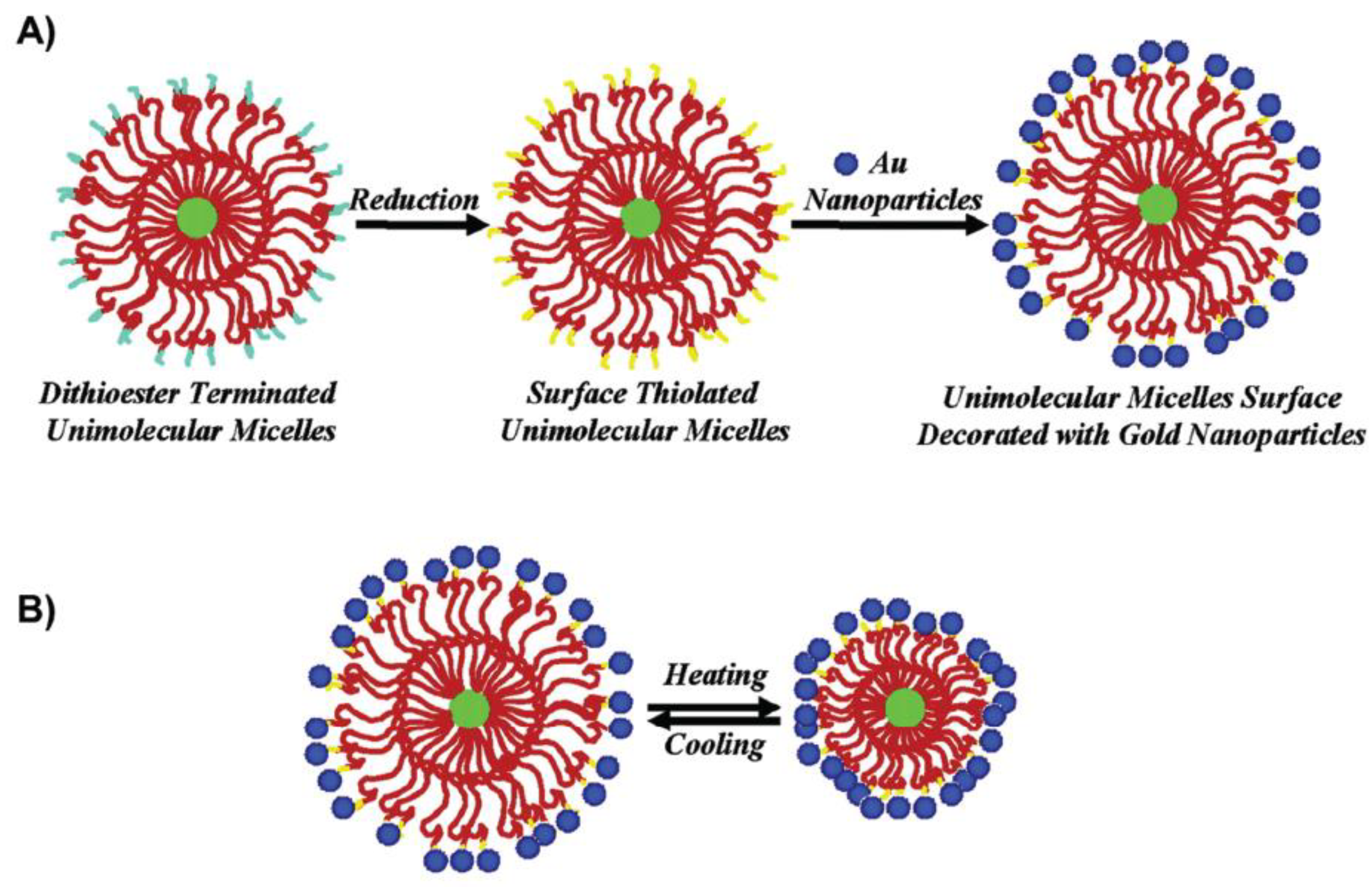
- Xu, H.; Xu, J.; Jiang, X.; Zhu, Z.; Rao, J.; Yin, J.; Wu, T.; Liu, H.; Liu, S. Thermosensitive Unimolecular Micelles Surface-Decorated with Gold Nanoparticles of Tunable Spatial Distribution. Chem. Mater. 2007, 19, 2489–2494. [Google Scholar] [CrossRef]
- Farrusseng, D.; Tuel, A. Perspectives on zeolite-encapsulated metal nanoparticles and their applications in catalysis. New J. Chem. 2016, 40, 3933–3949. [Google Scholar] [CrossRef]
- Choi, M.; Wu, Z.; Iglesia, E. Mercaptosilane-Assisted Synthesis of Metal Clusters within Zeolites and Catalytic Consequences of Encapsulation. J. Am. Chem. Soc. 2010, 132, 9129–9137. [Google Scholar] [CrossRef]
- Herrera, A.P.; Resto, O.; Briano, J.G.; Rinaldi, C. Synthesis and agglomeration of gold nanoparticles in reverse micelles. Nanotechnology 2005, 16, S618. [Google Scholar] [CrossRef]
- de Oliveira, R.; Zhao, P.; Li, N.; de Santa Maria, L.C.; Vergnaud, J.; Ruiz, J.; Astruc, D.; Barratt, G. Synthesis and in vitro studies of gold nanoparticles loaded with docetaxel. Int. J. Pharm. 2013, 454, 703–711. [Google Scholar] [CrossRef]
- Xu, J.; Han, X.; Liu, H.; Hu, Y. Synthesis and optical properties of silver nanoparticles stabilized by gemini surfactant. Colloid Surf. A 2006, 273, 179–183. [Google Scholar] [CrossRef]
- Zana, R. Dimeric (gemini) surfactants: Effect of the spacer group on the association behavior in aqueous solution. J. Colloid Interface Sci. 2002, 248, 203–220. [Google Scholar] [CrossRef]
- Malik, M.A.; Wani, M.Y.; Hashim, M.A. Microemulsion method: A novel route to synthesize organic and inorganic nanomaterials: 1st Nano Update. Arab. J. Chem. 2012, 5, 397–417. [Google Scholar] [CrossRef]
- Yan, J.M.; Zhang, X.B.; Akita, T.; Haruta, M.; Xu, Q. One-Step Seeding Growth of Magnetically Recyclable Au@Co Core−Shell Nanoparticles: Highly Efficient Catalyst for Hydrolytic Dehydrogenation of Ammonia Borane. J. Am. Chem. Soc. 2010, 132, 5326–5327. [Google Scholar] [CrossRef]
- Henglein, A. Radiolytic preparation of ultrafine colloidal gold particles in aqueous solution: Optical spectrum, controlled growth, and some chemical reactions. Langmuir 1999, 15, 6738–6744. [Google Scholar] [CrossRef]
- Radoń, A.; Łukowiec, D. Silver nanoparticles synthesized by UV-irradiation method using chloramine T as modifier: Structure, formation mechanism and catalytic activity. Cryst. Eng. Commun. 2018, 20, 7130. [Google Scholar] [CrossRef]
- Mittelman, A.M.; Fortner, J.D.; Pennell, K.D. Effects of ultraviolet light on silver nanoparticle mobility and dissolution. Environ. Sci. Nano 2015, 2, 683–691. [Google Scholar] [CrossRef]
- Maity, A.; Panda, S.K. Colloidal silver nanoparticles prepared by UV-light induced citrate reduction technique for the quantitative detection of uric acid. AIP Conf. Proc. 2018, 1942, 050057. [Google Scholar]
- Khan, M.J.; Kumari, S.; Shameli, K.; Selamat, J.; Sazili, A.Q. Green Synthesis and Characterization of Pullulan Mediated Silver Nanoparticles through Ultraviolet Irradiation. Materials 2019, 12, 2382. [Google Scholar] [CrossRef]
- Klaus, T.; Joerger, R.; Olsson, E.; Granqvist, C.G. Silver-based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.; Alam, M.; Kumar, R. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: A novel biological approach to nanoparticle synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloid Surf. B 2003, 28, 313–318. [Google Scholar] [CrossRef]


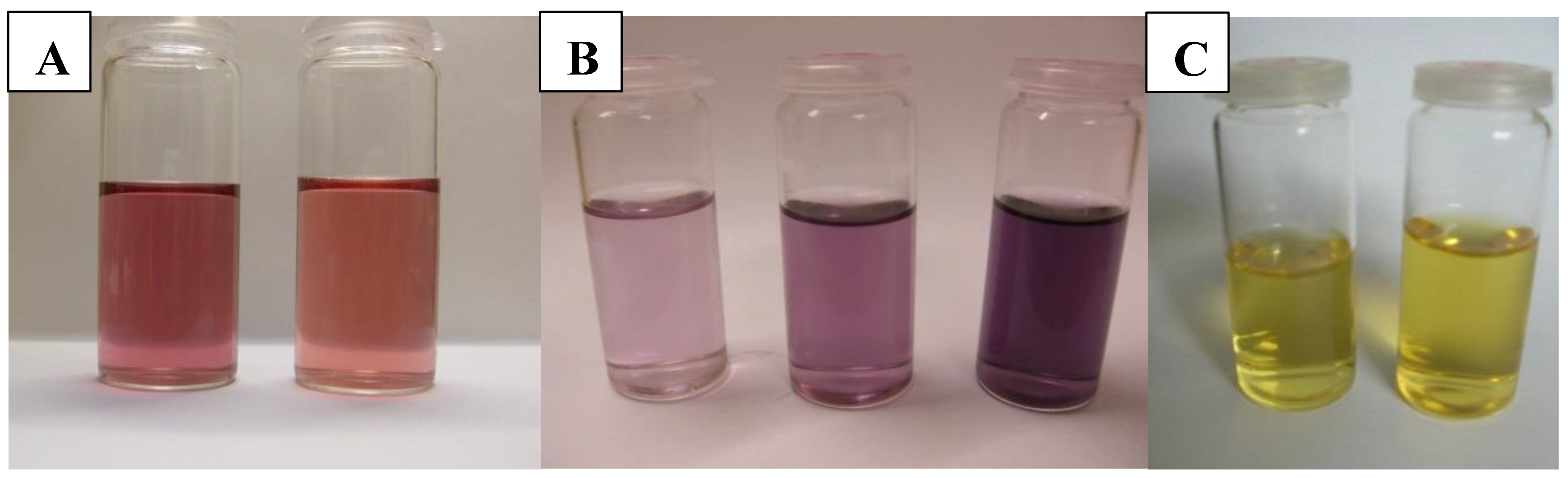
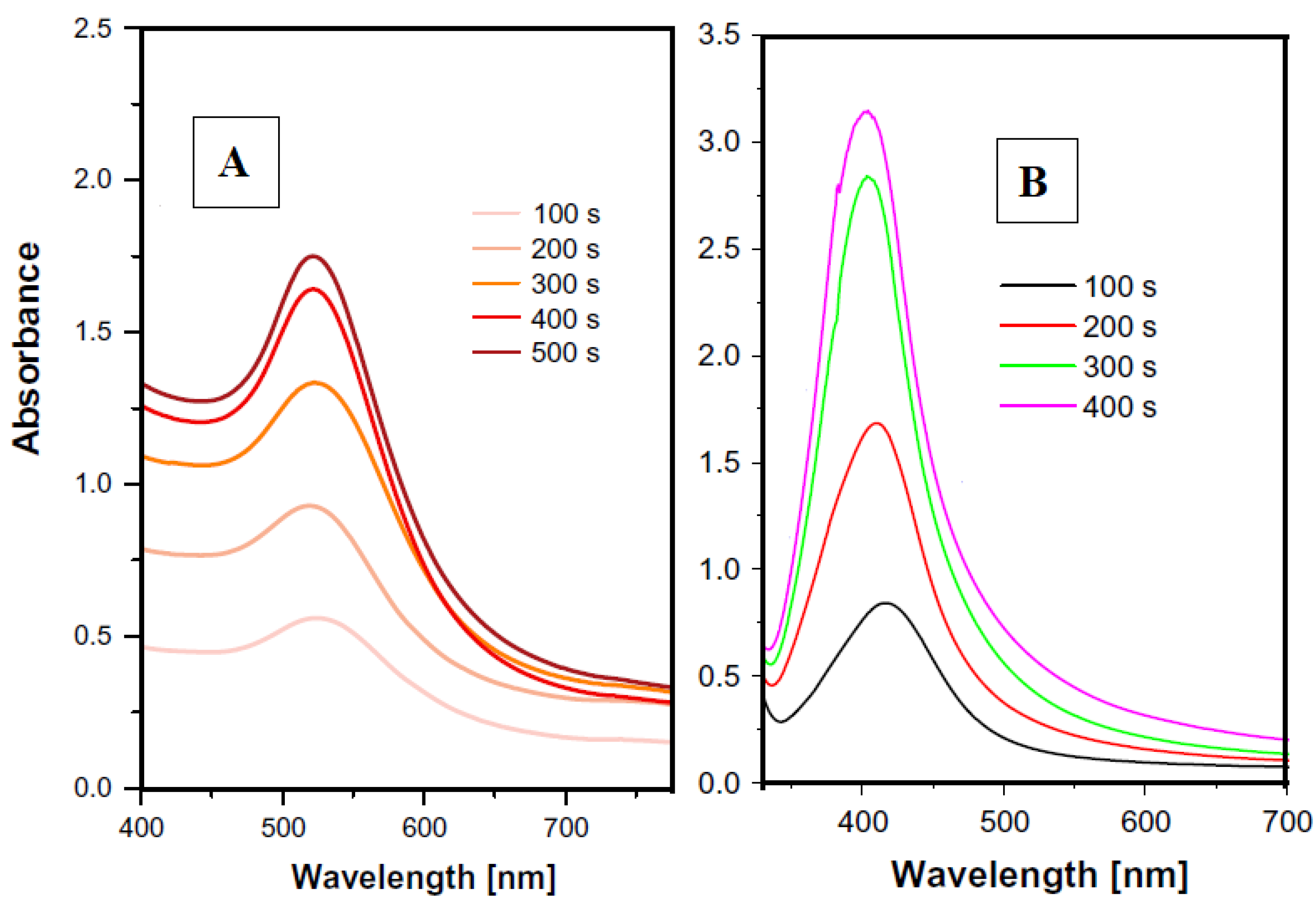


| Type of NPs | Synthetic Method | NP Size [nm] | References | ||||
|---|---|---|---|---|---|---|---|
| Au | Ag | Pd | Pt | Cu | |||
| x | Sol–gel micro reactors | 5–50 | [61,62] | ||||
| x | x | x | x | PVD into liquid substrate | 2–10 | [33,63,64] | |
| x | x | Reduction in acidic environment | 3–40 | [65] | |||
| x | Reduction process | 2–40 | [66,67,68,69,70] | ||||
| x | x | γ-Irradiation | 3–30 | [71] | |||
| x | pH control of Cu complexes | 48–150 | [72] | ||||
| x | x | x | Biosynthesis | 9–25 | [73,74,75,76,77] | ||
| x | Wet chemistry | 20–60 | [78,79] | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slepička, P.; Slepičková Kasálková, N.; Siegel, J.; Kolská, Z.; Švorčík, V. Methods of Gold and Silver Nanoparticles Preparation. Materials 2020, 13, 1. https://doi.org/10.3390/ma13010001
Slepička P, Slepičková Kasálková N, Siegel J, Kolská Z, Švorčík V. Methods of Gold and Silver Nanoparticles Preparation. Materials. 2020; 13(1):1. https://doi.org/10.3390/ma13010001
Chicago/Turabian StyleSlepička, Petr, Nikola Slepičková Kasálková, Jakub Siegel, Zdeňka Kolská, and Václav Švorčík. 2020. "Methods of Gold and Silver Nanoparticles Preparation" Materials 13, no. 1: 1. https://doi.org/10.3390/ma13010001
APA StyleSlepička, P., Slepičková Kasálková, N., Siegel, J., Kolská, Z., & Švorčík, V. (2020). Methods of Gold and Silver Nanoparticles Preparation. Materials, 13(1), 1. https://doi.org/10.3390/ma13010001






