Photoluminescence Characteristics of Sn2+ and Ce3+-Doped Cs2SnCl6 Double-Perovskite Crystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of SnCl2 Solution
2.3. Synthesis of Cs2SnCl6 Crystals
2.4. Synthesis of Ce3+-Doped Cs2SnCl6 Crystals
2.5. Measurements
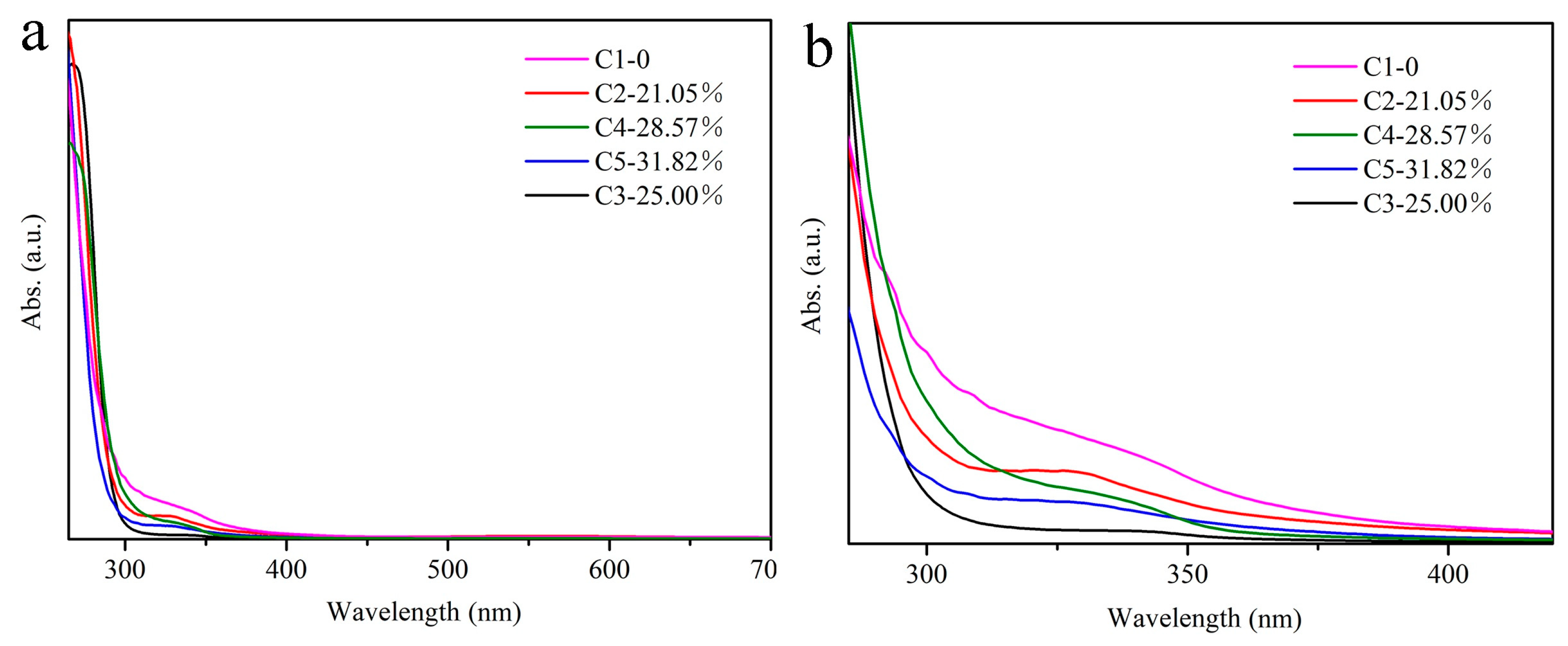
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, A.; Yan, X.; Zhang, M.; Sun, S.; Yang, M.; Shen, W.; Pan, X.; Wang, P.; Deng, Z. Controlled Synthesis of Lead-Free and Stable Perovskite Derivative Cs2SnI6 Nanocrystals via a Facile Hot-Injection Process. Chem. Mater. 2016, 28, 8132–8140. [Google Scholar] [CrossRef]
- Kagan, C.R.; Mitzi, D.B.; Dimitrakopoulos, C.D. Organic-inorganic Hybrid Materials as Semiconducting Channels in Thin-film Field-effect Transistors. Science 1999, 286, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Talapin, D.V.; Lee, J.S.; Kovalenko, M.V. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef] [PubMed]
- Beal, R.E.; Slotcavage, D.J.; Leijtens, T. Cesium Lead Halide Perovskites with Improved Stability for Tandem Solar Cells. J. Phys. Chem. Lett. 2016, 7, 746–751. [Google Scholar] [CrossRef]
- Ma, Q.; Huang, S.; Chen, S. The Effect of Stoichiometry on the Stability of Inorganic Cesium Lead Mixed-Halide Perovskites Solar Cells. J. Phys. Chem. C 2017, 121, 19642–19649. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; D’Innocenzo, V.; Accornero, S. Tuningthe Optical Properties of Cesium Lead HalidePerovskite Nanocrystals by Anion Exchange Reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. [Google Scholar] [CrossRef]
- Huang, S.; Li, Z.; Wang, B. Morphology Evolution and Degradation of CsPbBr3 Nanocrystals under Blue Light Emitting Diode Illumination. ACS Appl. Mater. Interfaces 2017, 9, 7249–7258. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhao, F.; Liu, L. Emulsion Synthesis of Size-Tunable CH3NH3PbBr3 Nanocrystal: An Alternative Route toward Efficient Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2015, 7, 28128–28133. [Google Scholar] [CrossRef]
- Dutta, A.; Behera, R.; Pal, P.; Baitalik, S.; Pradhan, N. Near-Unity Photoluminescence Quantum Efficiency for All CsPbX3 (X = Cl, Br and I) Perovskite Nanocrystals: A Generic Synthesis Approach. Angew. Chem. Int. Ed. 2019, 58, 5552–5556. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Swarnkar, A.; Chulliyil, R.; Ravi, V.K. Colloidal CsPbBr3 Perovskite Nanocrystals: Luminescence beyond Traditional Nanocrystal. Angew. Chem. Int. Ed. 2015, 54, 15424–15428. [Google Scholar] [CrossRef]
- Lin, C.C.; Meijerink, A.; Liu, R.S. Critical Red Components for Next-Generation White LEDs. J. Phys. Chem. Lett. 2016, 7, 495–503. [Google Scholar] [CrossRef]
- Palazon, F.; Di Stasio, F.; Akkerman, Q.A.; Krahne, R.; Prato, M.; Manna, L. Polymer-Free Films of Inorganic Halide Perovskite Nanocrystals as UV-to-White Color-Conversion Layers in LEDs. Chem. Mater. 2016, 28, 2902–2906. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum Dot Light-Emitting Diodes Based on Inorganic Perovskite Cesium Lead Halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Song, J.; Xiao, L.; Zeng, H.; Sun, H. All-Inorganic Colloidal Perovskite Quantum Dots: A New Class of Lasing Materials with Favorable Characteristics. Adv. Mater. 2015, 27, 7101–7108. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, Q.; Zhang, C.; Wang, R.; Wu, H.; Zhang, X.; Xing, G.; Yu, W.W.; Wang, X.; Zhang, Y.; et al. Two-Photon-Pumped Perovskite Semiconductor Nanocrystal Lasers. J. Am. Chem. Soc. 2016, 138, 3761–3768. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, P.; Lim, D.-H.; Kim, B.; Lee, S.-H.; Lee, M.-S.; Lee, J.-S. All-inorganic cesium lead halide perovskite nanocrystals for photodetector applications. Chem. Commun. 2016, 52, 2067–2070. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 2019. [Google Scholar] [CrossRef]
- Saparov, B.; Sun, J.-P.; Meng, W.; Xiao, Z.; Duan, H.-S.; Gunawan, O.; Shin, D.; Hill, I.G.; Yan, Y.; Mitzi, D.B. Thin-Film Deposition and Characterization of a Sn-Deficient Perovskite Derivative Cs2SnI6. Chem. Mater. 2016, 28, 2315–2322. [Google Scholar] [CrossRef]
- Giustino, F.; Snaith, H.J. Toward Lead-Free Perovskite Solar Cells. ACS Energy Lett. 2016, 1, 1233–1240. [Google Scholar] [CrossRef]
- Lee, B.; Stoumpos, C.C.; Zhou, N.; Hao, F.; Malliakas, C.; Yeh, C.Y.; Marks, T.J.; Kanatzidis, M.G.; Chang, R.P. Air-stable molecular semiconducting iodosalts for solar cell applications: Cs2SnI6 as a hole conductor. J. Am. Chem. Soc. 2014, 136, 15379–15385. [Google Scholar] [CrossRef]
- Qiu, X.; Cao, B.; Yuan, S.; Chen, X.; Qiu, Z.; Jiang, Y.; Ye, Q.; Wang, H.; Zeng, H.; Liu, J.; et al. From unstable CsSnI3 to air-stable Cs2SnI6: A lead-free perovskite solar cell light absorber with bandgap of 1.48 eV and high absorption coefficient. Sol. Energy Mater. Sol. Cells 2017, 159, 227–234. [Google Scholar] [CrossRef]
- Noel, N.K.; Stranks, S.D.; Abate, A.; Wehrenfennig, C.; Guarnera, S.; Haghighirad, A.-A.; Sadhanala, A.; Eperon, G.E.; Pathak, S.K.; Johnston, M.B.; et al. Lead-free organic–inorganic tin halide perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 3061–3068. [Google Scholar] [CrossRef]
- Xing, G.; Kumar, M.H.; Chong, W.K.; Liu, X.; Cai, Y.; Ding, H.; Asta, M.; Gratzel, M.; Mhaisalkar, S.; Mathews, N.; et al. Solution-Processed Tin-Based Perovskite for Near-Infrared Lasing. Adv. Mater. 2016, 28, 8191–8196. [Google Scholar] [CrossRef]
- Lai, M.L.; Tay, T.Y.; Sadhanala, A.; Dutton, S.E.; Li, G.; Friend, R.H.; Tan, Z.K. Tunable Near-Infrared Luminescence in Tin Halide Perovskite Devices. J. Phys. Chem. Lett. 2016, 7, 2653–2658. [Google Scholar] [CrossRef]
- Qiu, X.; Jiang, Y.; Zhang, H.; Qiu, Z.; Yuan, S.; Wang, P.; Cao, B. Lead-free mesoscopic Cs2SnI6 perovskite solar cells using different nanostructured ZnO nanorods as electron transport layers. Phys. Status Solidi Rapid Res. Lett. 2016, 10, 587–591. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, Y.; Hosono, H.; Kamiya, T. Intrinsic defects in a photovoltaic perovskite variant Cs2SnI6. Phys. Chem. Chem. Phys. 2015, 17, 18900–18903. [Google Scholar] [CrossRef] [PubMed]
- Kaltzoglou, A.; Antoniadou, M.; Kontos, A.G.; Stoumpos, C.C.; Perganti, D.; Siranidi, E.; Raptis, V.; Trohidou, K.; Psycharis, V.; Kanatzidis, M.G.; et al. Optical-Vibrational Properties of the Cs2SnX6(X = Cl, Br, I) Defect Perovskites and Hole-Transport Efficiency in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2016, 120, 11777–11785. [Google Scholar] [CrossRef]
- Tan, Z.; Li, J.; Zhang, C.; Li, Z.; Hu, Q.; Xiao, Z.; Kamiya, T.; Hosono, H.; Niu, G.; Lifshitz, E.; et al. Highly Efficient Blue-Emitting Bi-Doped Cs2SnCl6 Perovskite Variant: Photoluminescence Induced by Impurity Doping. Adv. Funct. Mater. 2018, 28, 1801131. [Google Scholar] [CrossRef]
- Kaltzoglou, A.; Antoniadou, M.; Perganti, D.; Siranidi, E.; Raptis, V.; Trohidou, K.; Psycharis, V.; Kontos, A.G.; Falaras, P. Mixed-halide Cs2SnI3Br3 perovskite as low resistance hole-transporting material in dye-sensitized solar cells. Electrochim. Acta 2015, 184, 466–474. [Google Scholar] [CrossRef]
- Huang, H.M.; Jiang, Z.Y.; Luo, S.J. First-principles investigations on the mechanical, thermal, electronic, and optical properties of the defect perovskites Cs2SnX6 (X = Cl, Br, I). Chin. Phys. B 2017, 26, 096301. [Google Scholar] [CrossRef]
- Giuseppe, A.; Nuccioo, B.; John, R.F.; Antta, F.; Giovanna, I.; Giovanni, P.; Maria, V.R. Mössbauer, Far-Infrared, and XPS Investigations of SnCl2 and SnCl4 Introduced in Polyconjugated Monosubstituted Acetylene Matrices. Appl. Spectrosc. 1995, 49, 237–240. [Google Scholar]
- Larachi, F.; Pierre, J.; Adnot, A.; Bernis, A. Ce 3d XPS study of composite CexMn1−xO2−y wet oxidation catalysts. Appl. Surf. Sci. 2002, 195, 236–250. [Google Scholar] [CrossRef]
- Guang, Q.; Ning, D.; Hai, G.; Min, Y. Synthesis and spectroscopic properties of MBPO5Ce (M = Ca, Sr, Ba). Chin. J. Lumin. 2005, 26, 199–205. [Google Scholar]
- Xiao, J.; Ya, Z.; Shuai, H.; Gong, W.; Dan, C.; Qiu, Y. Radioluminescence properties of Sn2+-doped borosilicate glass with high Gd2O3. Acta Opt. Sin. 2018, 38, 1–8. [Google Scholar]
- Jian, C.; Huan, L.; Lei, S.; Jin, Y. Preparation and optical properties of Sn2+ doped copper sulfide. J. Mater. Sci. Eng. 2018, 30, 11–18. [Google Scholar]
- Karmakar, A.; Dodd, M.S.; Agnihotri, S.; Ravera, E.; Michaelis, V.K. Cu(II)-Doped Cs2SbAgCl6 Double Perovskite A Lead-Free, Low-Bandgap Material. Chem. Mater. 2018, 30, 8280–8290. [Google Scholar] [CrossRef]
- Dian, Y. Anew set of electronegativities of elements in valence states. Chin. J. Inorg. Chem. 1982, 21, 955–959. [Google Scholar]
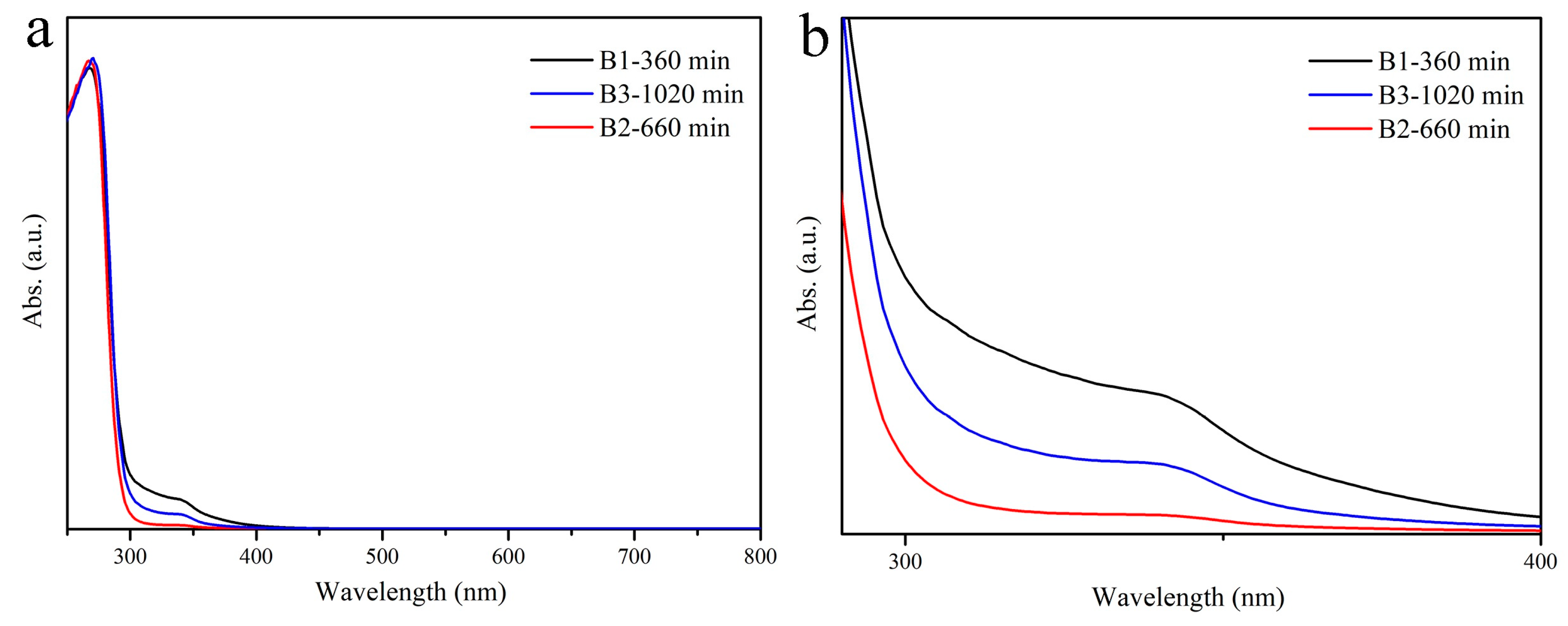
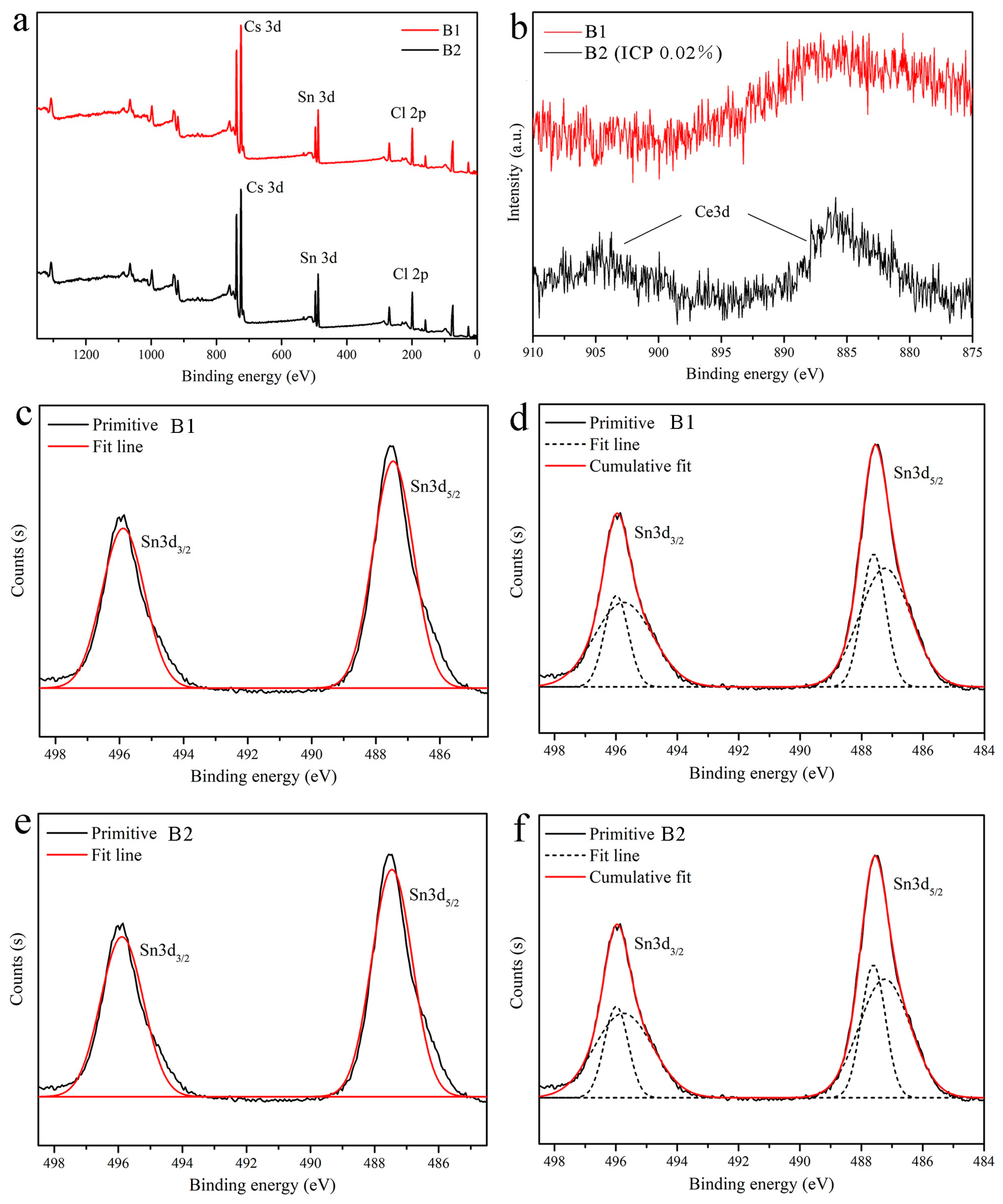
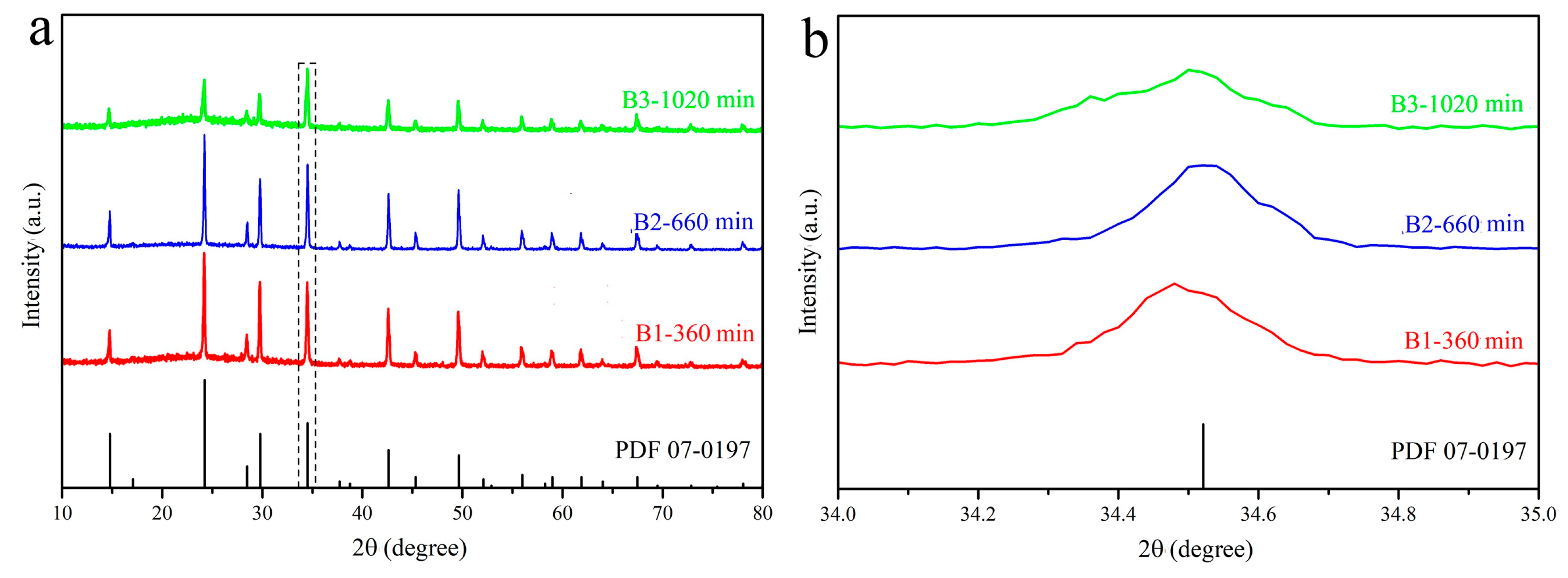
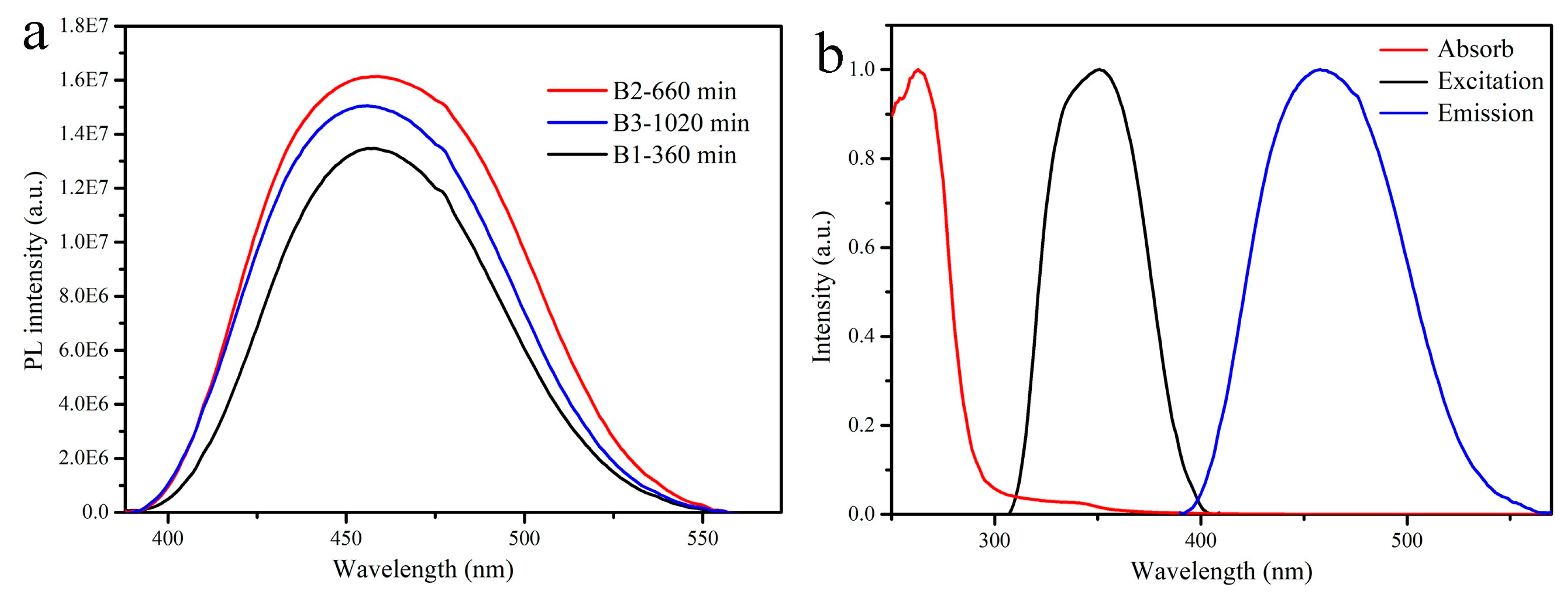

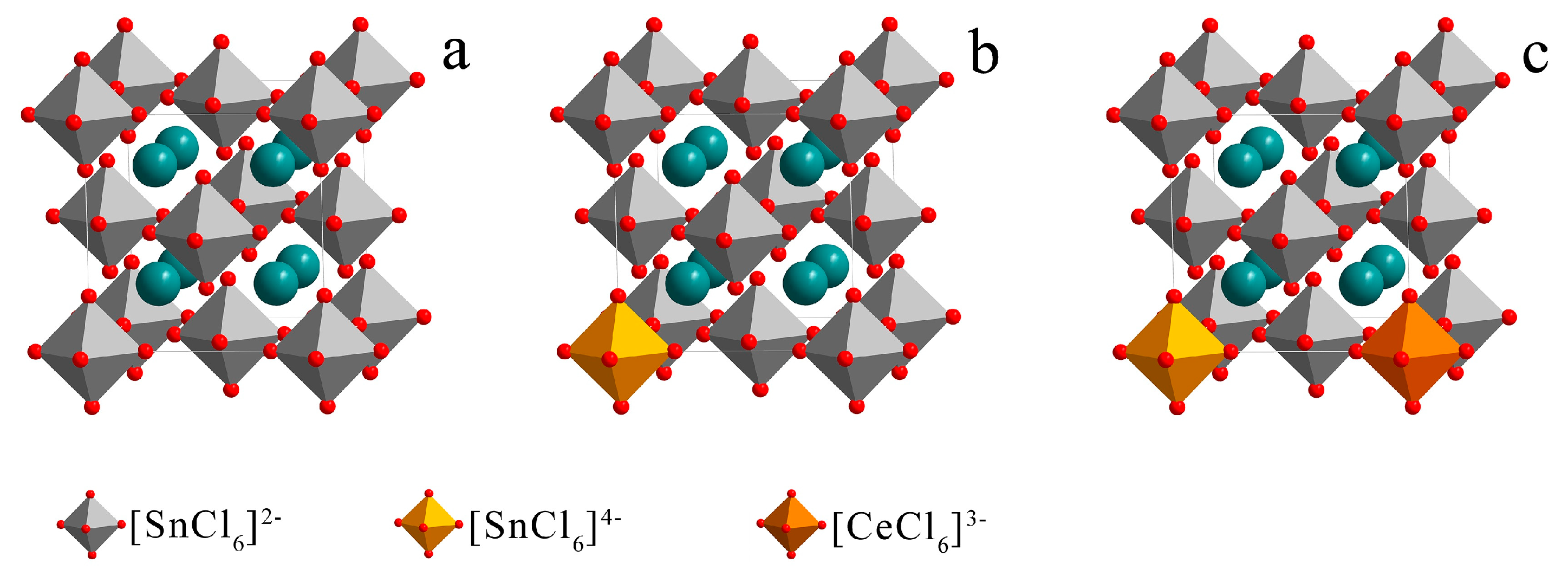
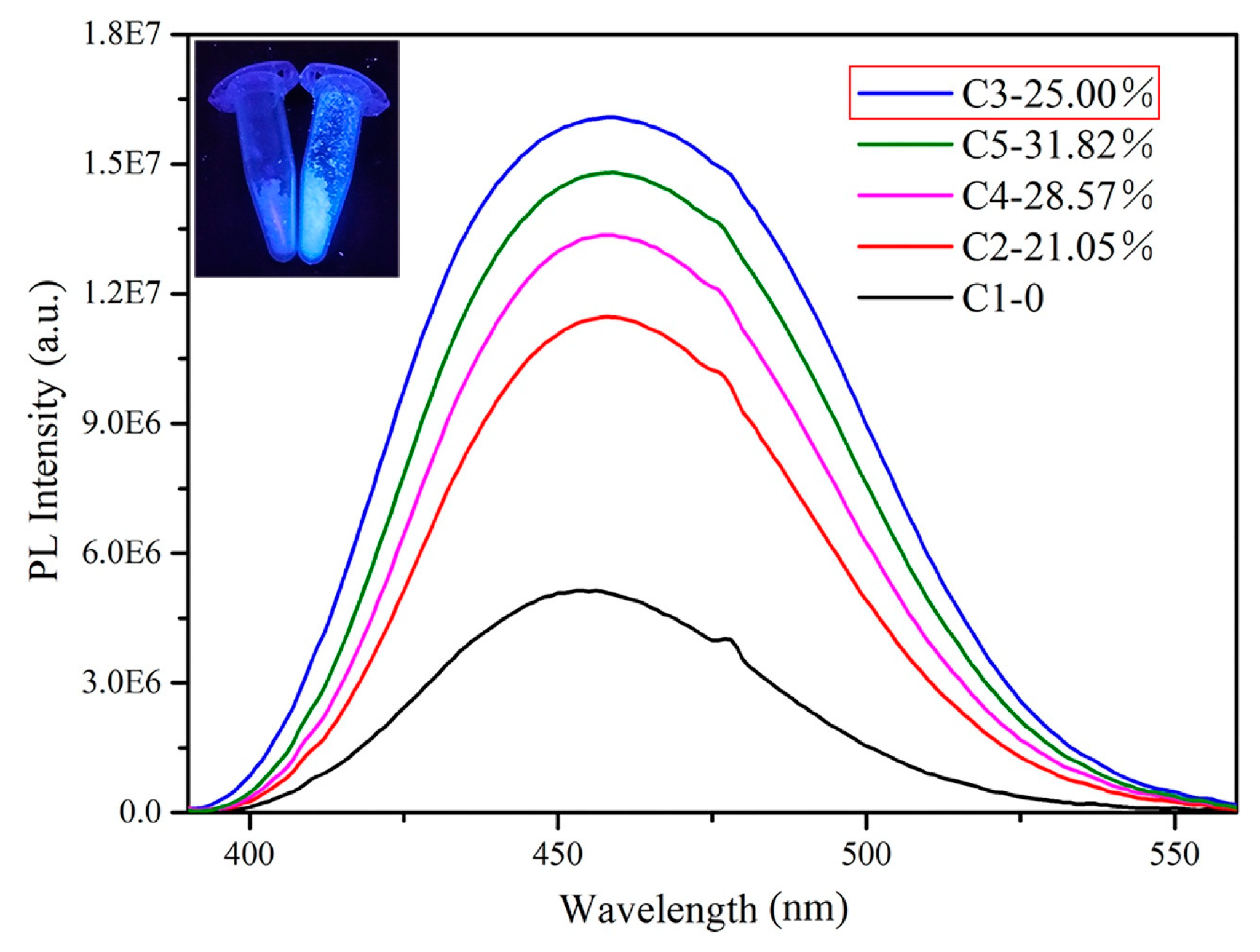
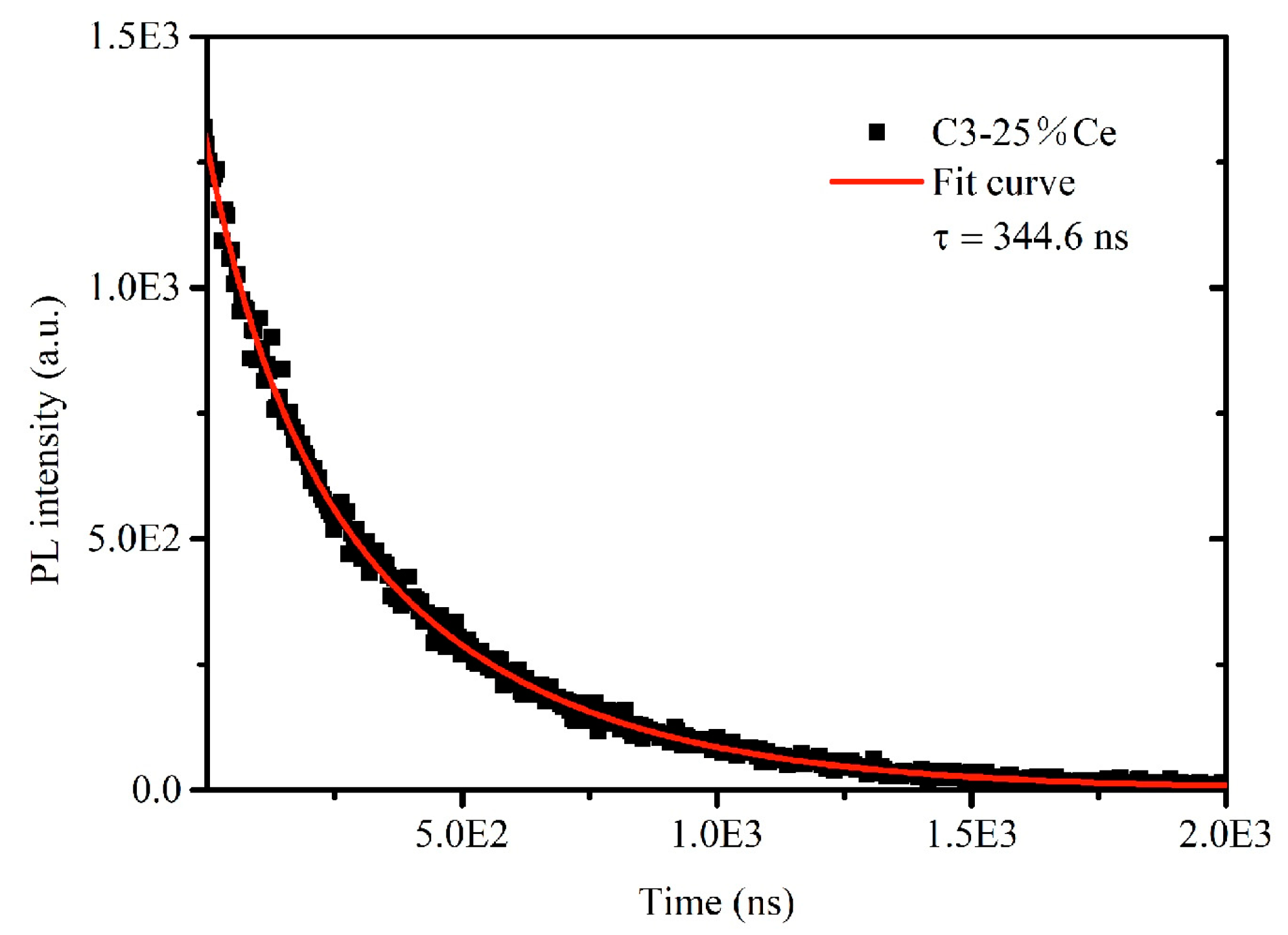
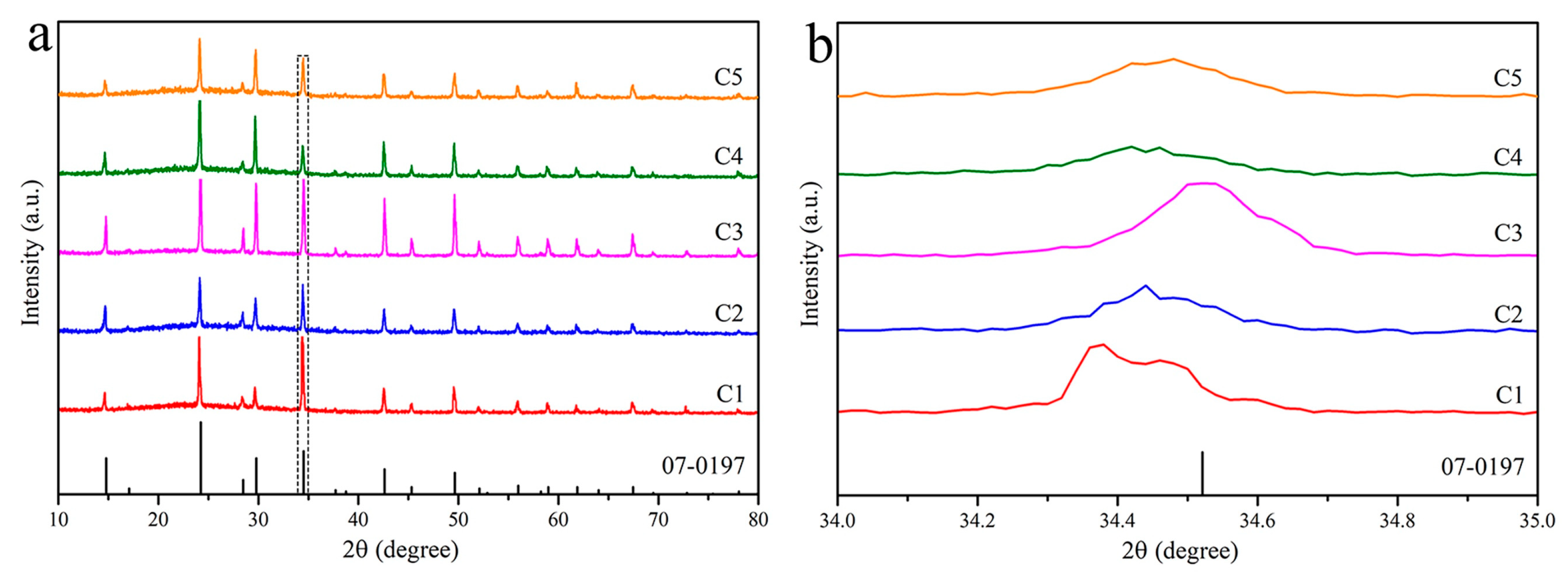
| Sample No. | Sn (mol%) | Ce (mol%) | Time (min) | Content (Ce/(Ce + Sn)) |
|---|---|---|---|---|
| A1 | 100 | 0 | 0 | – |
| A2 | 100 | 0 | 10 | – |
| A3 | 100 | 0 | 60 | – |
| A4 | 100 | 0 | 360 | – |
| A5 | 100 | 0 | 660 | – |
| A6 | 100 | 0 | 1020 | – |
| B1 | 75.00 | 25.00 | 360 | – |
| B2 | 75.00 | 25.00 | 660 | 0.02% |
| B3 | 75.00 | 25.00 | 1020 | – |
| C1 | 100 | 0 | 660 | – |
| C2 | 78.95 | 21.05 | 660 | – |
| C3(B2) | 75.00 | 25.00 | 660 | 0.02% |
| C4 | 71.43 | 28.57 | 660 | – |
| C5 | 68.18 | 31.82 | 660 | – |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhu, L.; Cheng, J.; Chen, L.; Liu, C.; Yuan, S. Photoluminescence Characteristics of Sn2+ and Ce3+-Doped Cs2SnCl6 Double-Perovskite Crystals. Materials 2019, 12, 1501. https://doi.org/10.3390/ma12091501
Zhang H, Zhu L, Cheng J, Chen L, Liu C, Yuan S. Photoluminescence Characteristics of Sn2+ and Ce3+-Doped Cs2SnCl6 Double-Perovskite Crystals. Materials. 2019; 12(9):1501. https://doi.org/10.3390/ma12091501
Chicago/Turabian StyleZhang, Hongdan, Ludan Zhu, Jun Cheng, Long Chen, Chuanqi Liu, and Shuanglong Yuan. 2019. "Photoluminescence Characteristics of Sn2+ and Ce3+-Doped Cs2SnCl6 Double-Perovskite Crystals" Materials 12, no. 9: 1501. https://doi.org/10.3390/ma12091501
APA StyleZhang, H., Zhu, L., Cheng, J., Chen, L., Liu, C., & Yuan, S. (2019). Photoluminescence Characteristics of Sn2+ and Ce3+-Doped Cs2SnCl6 Double-Perovskite Crystals. Materials, 12(9), 1501. https://doi.org/10.3390/ma12091501





