Ag/AgCl/MIL-101(Fe) Catalyzed Degradation of Methylene Blue under Visible Light Irradation
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Catalyst Preparation
2.2.1. The preparation of MIL-101 (Fe)
2.2.2. The preparation of Ag/AgCl/MIL-101 (Fe)
2.3. Characterization
2.4. Photo-Fenton Catalytic Experiments and Analytical Methods
3. Results and Discussion
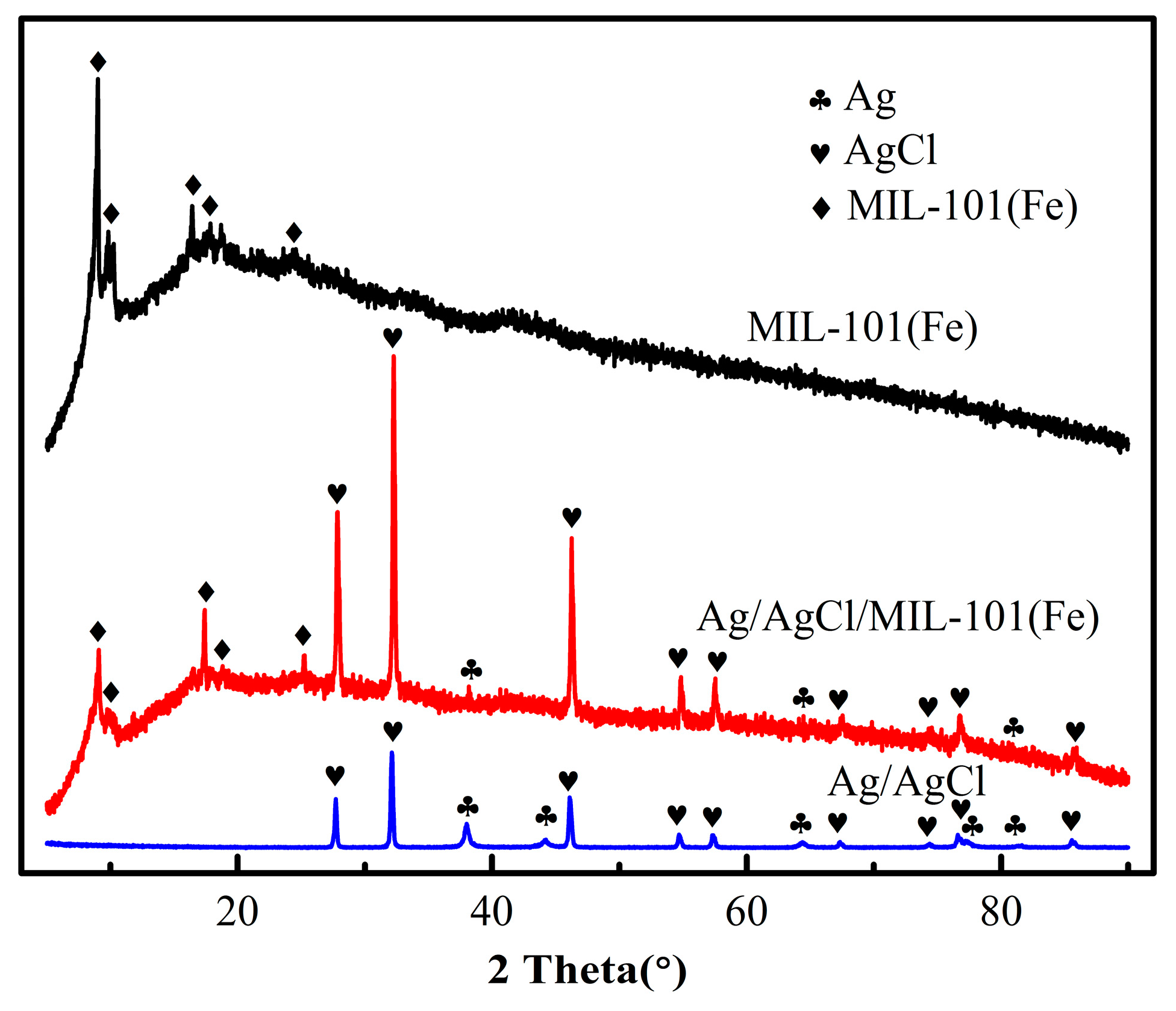
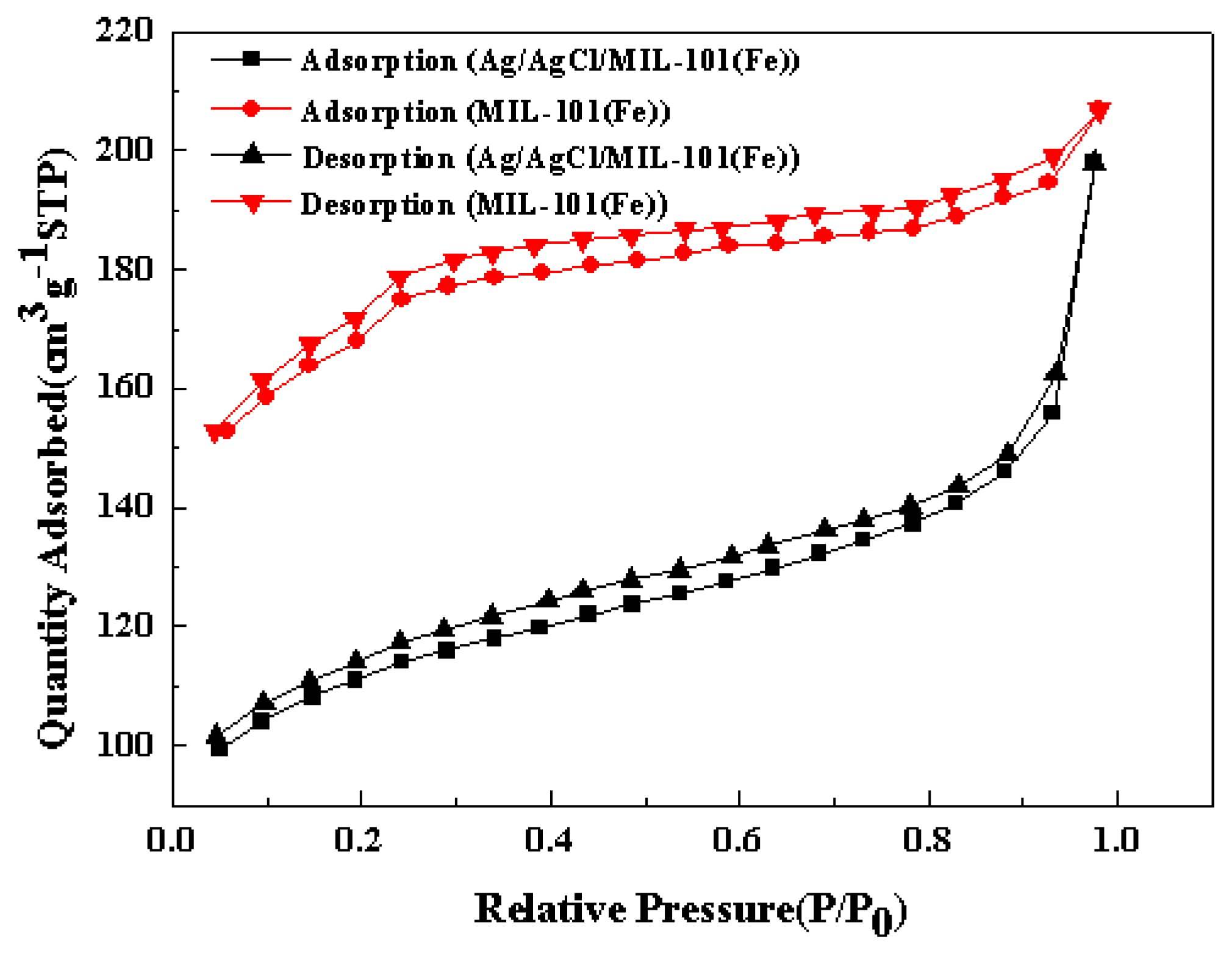
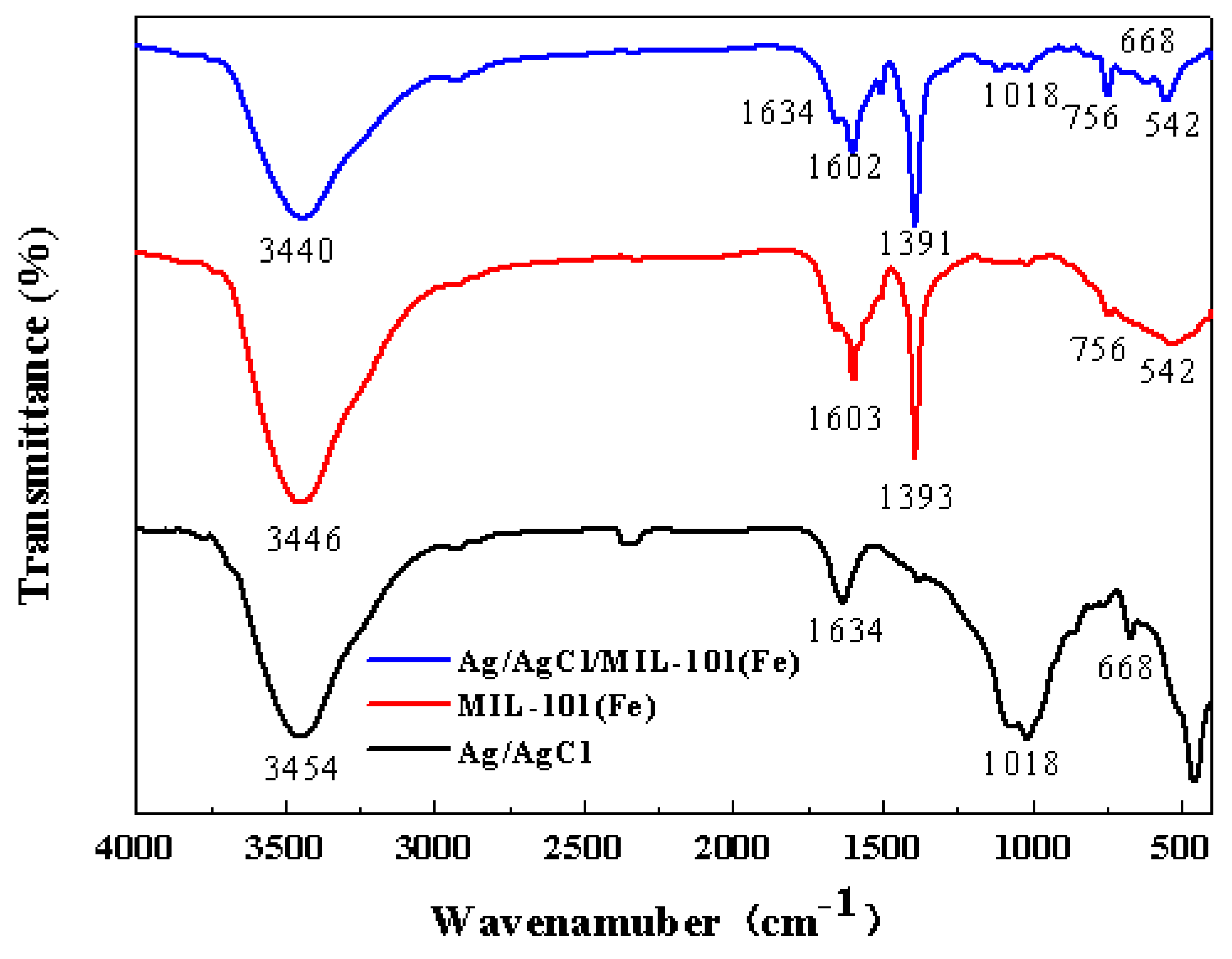
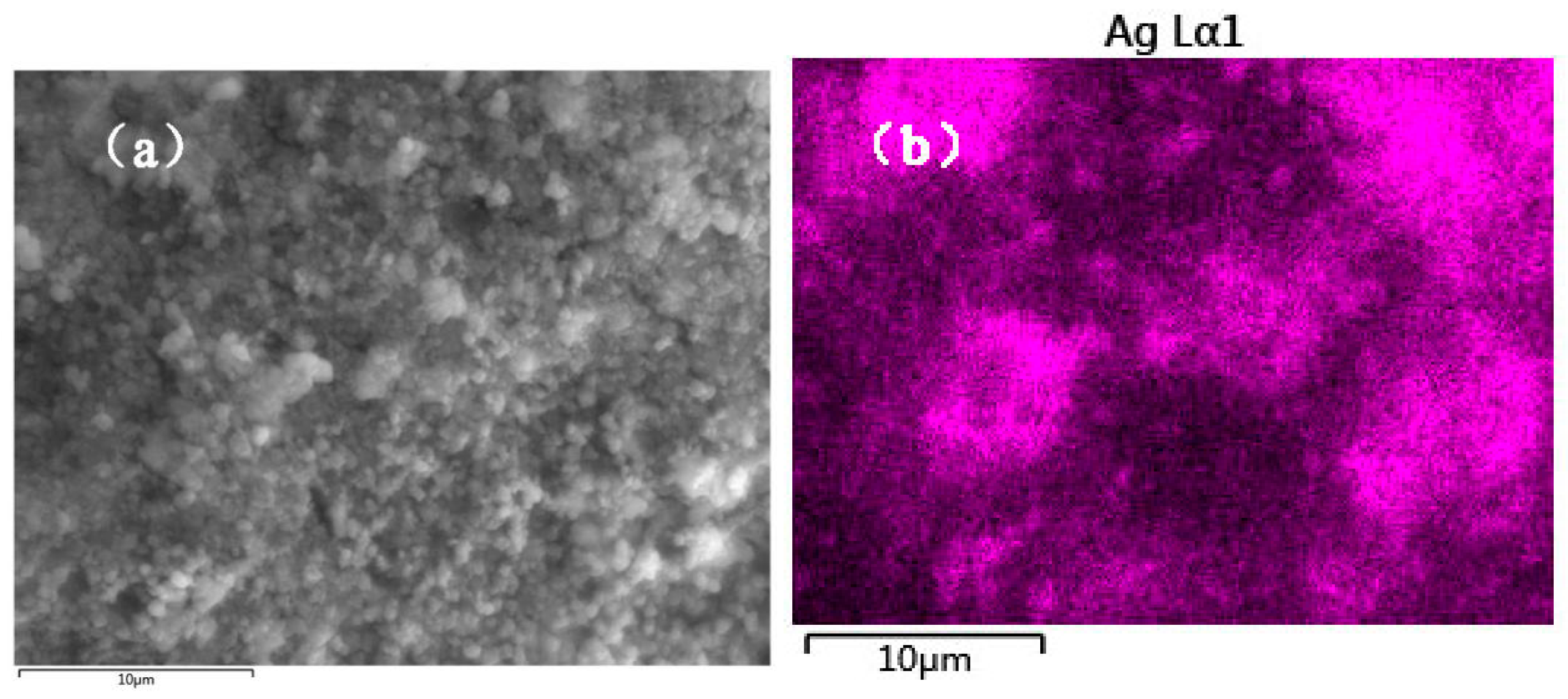
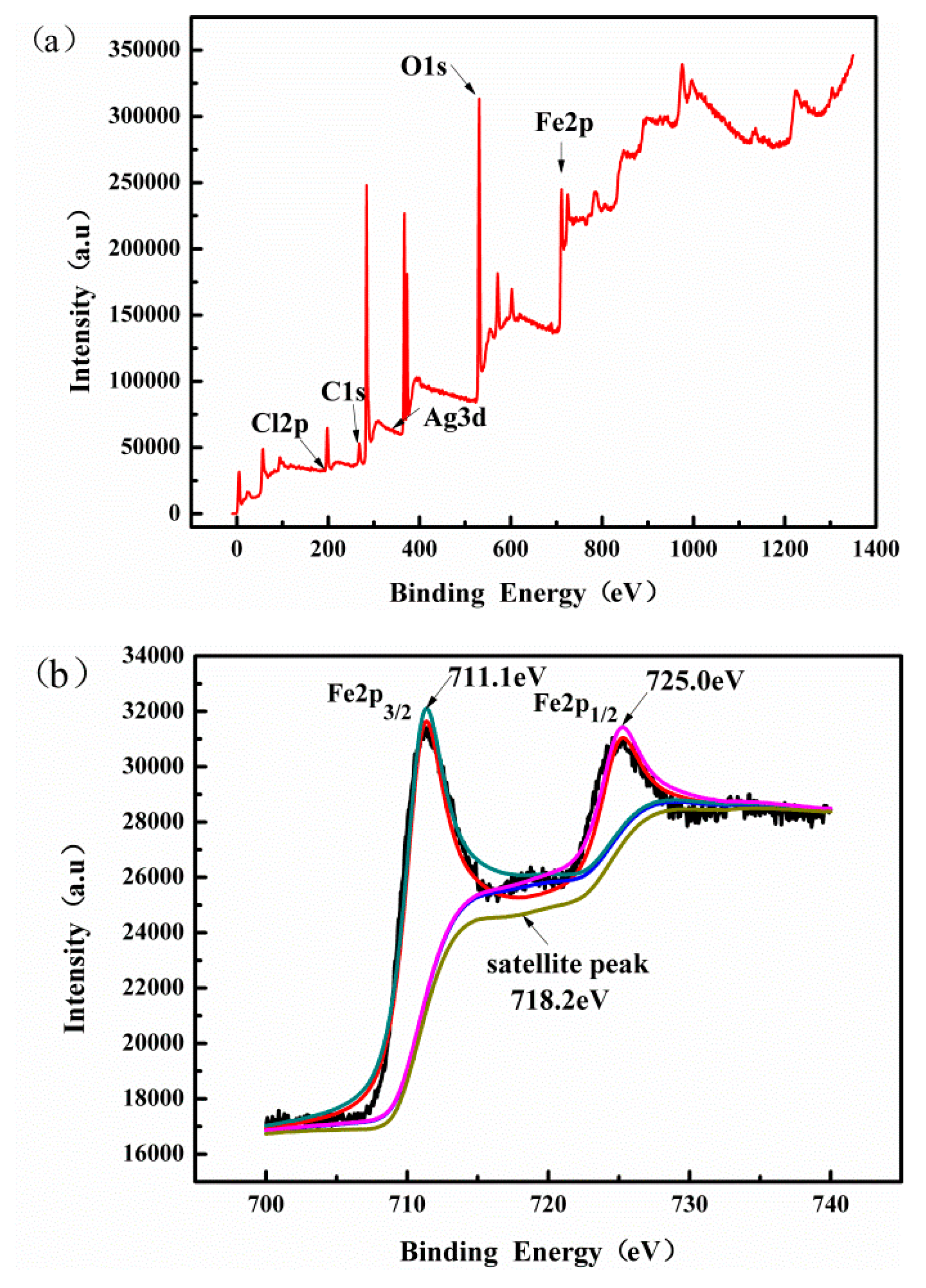
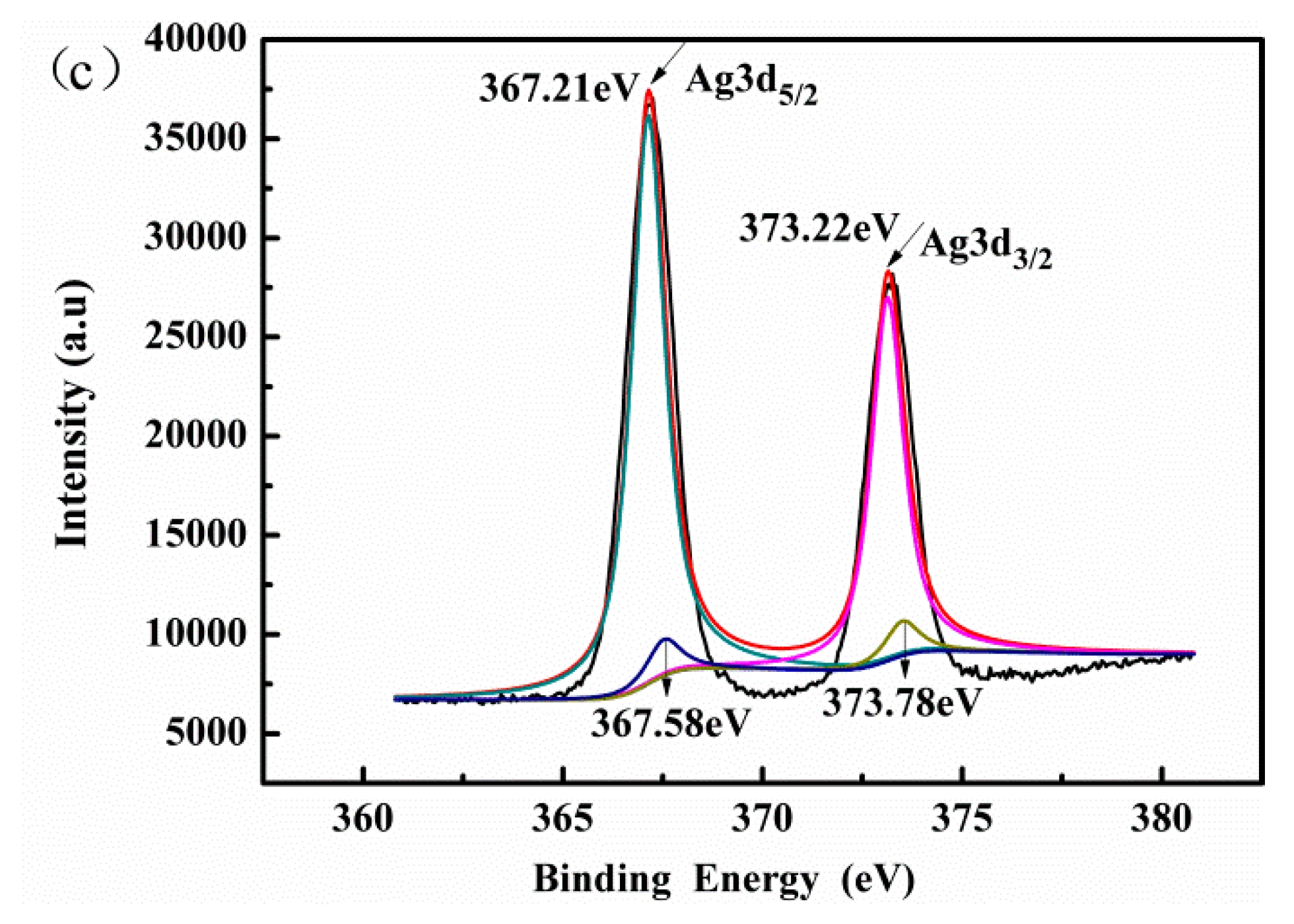
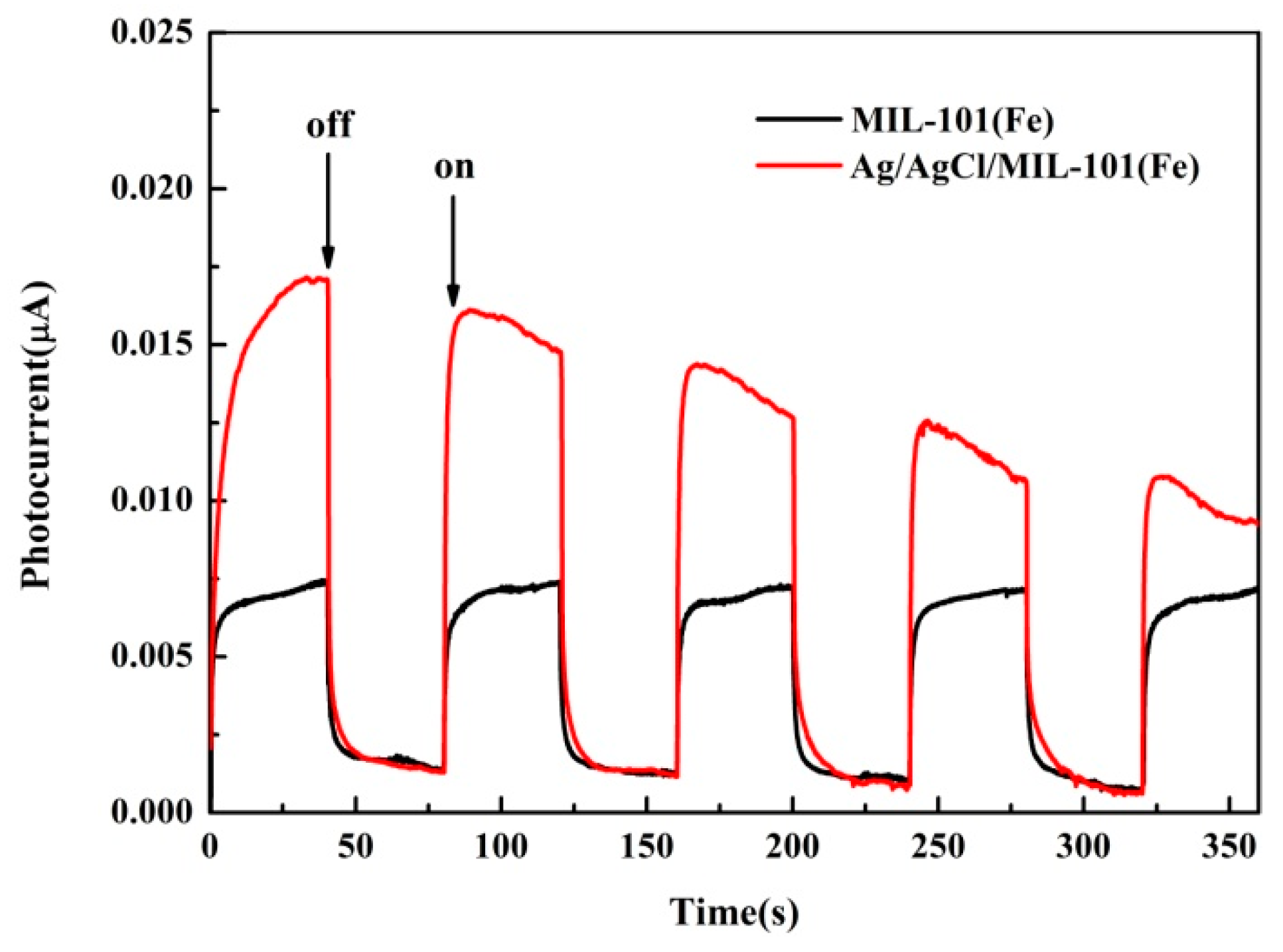
3.1. Characterization of the Catalysts
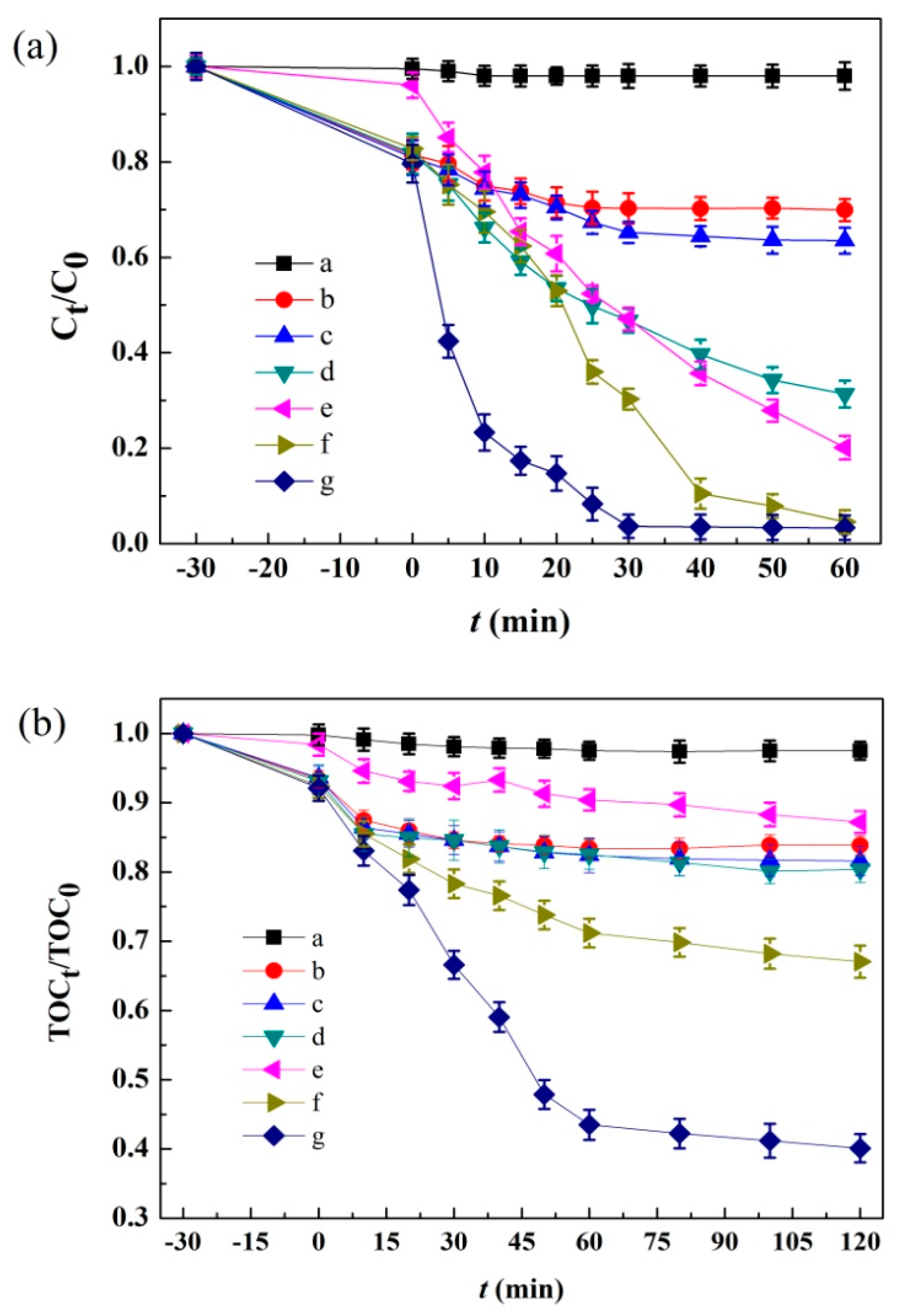
3.2. Photo-Fenton Catalytic Activity
3.3. Fitting Model and Analysis of Variance (ANOVA)
3.4. Response Surface Analysis
3.5. Model Validation and Experimental Confirmation
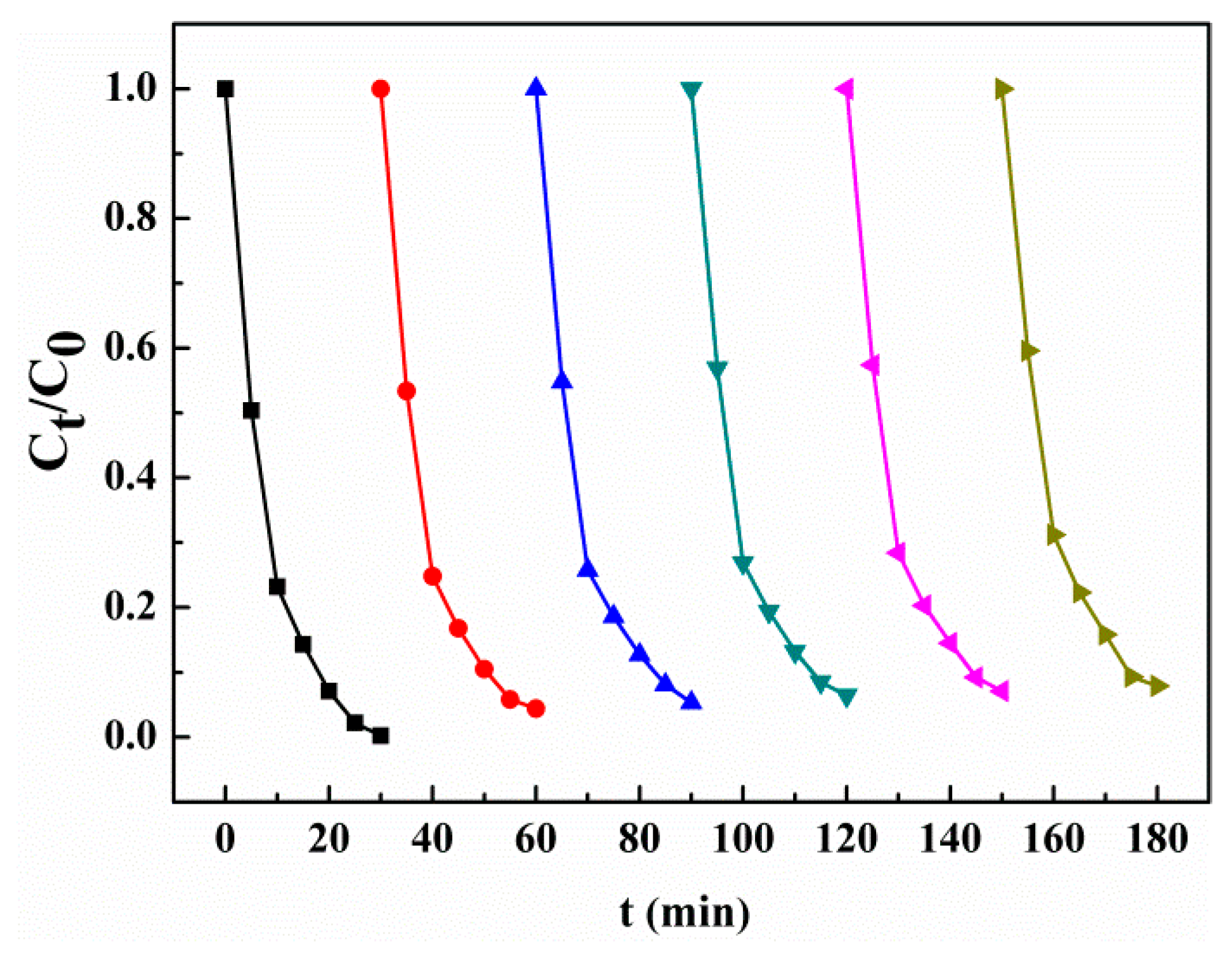
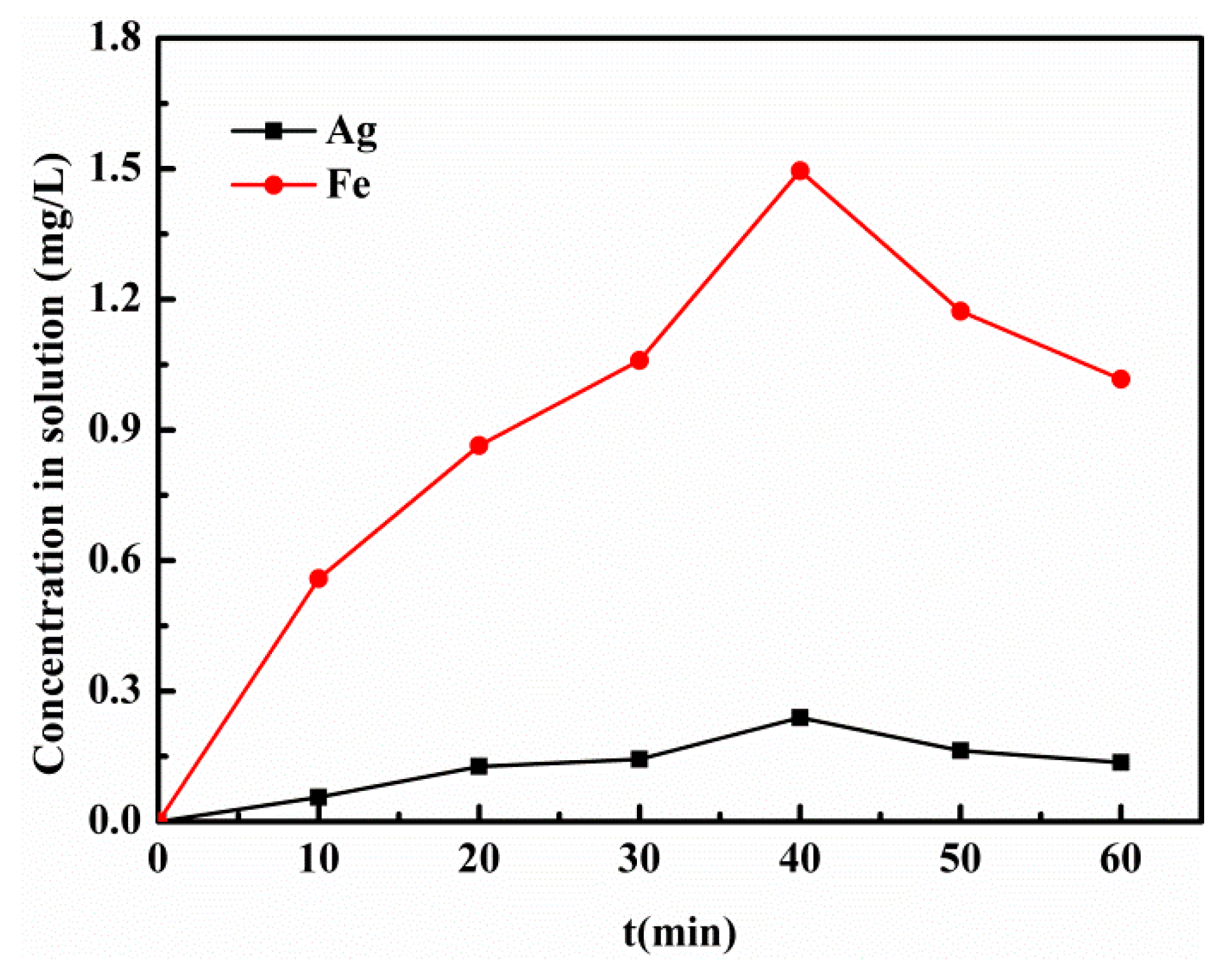
3.6. Recyclability
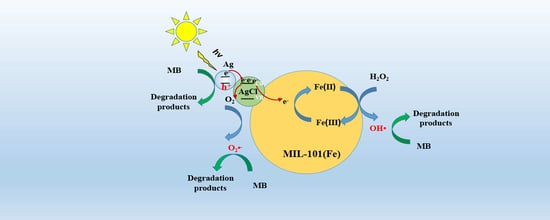
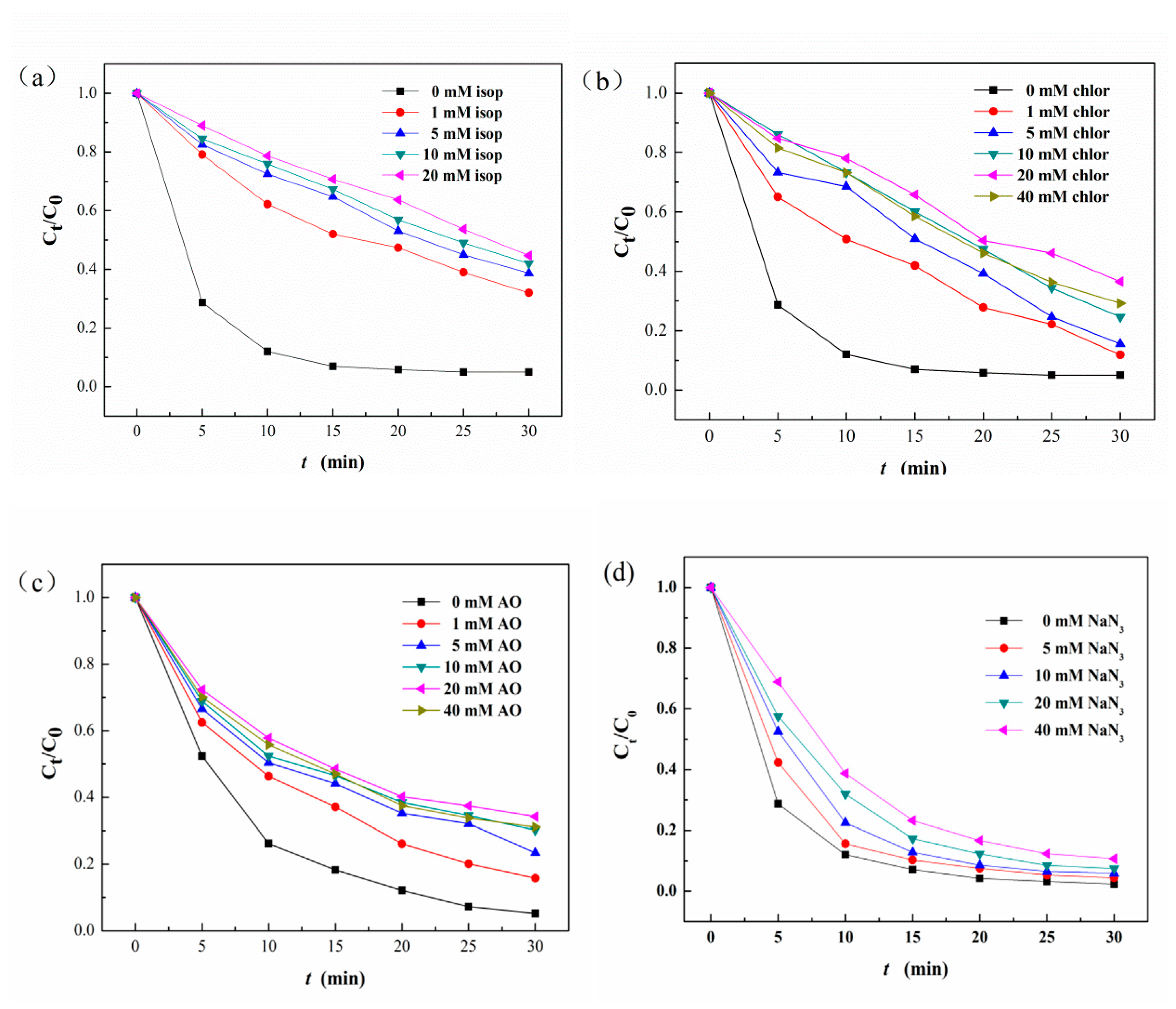
3.7. Proposed Photo-Fenton Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mathur, N.; Bhatnagar, P. Mutagenicity assessment of textile dyes from Sanganer (Rajasthan). J. Environ. Biol. 2007, 28, 123–126. [Google Scholar] [PubMed]
- Rai, H.S.; Bhattacharyya, M.S.; Singh, J.; Bansal, T.K.; Vats, P.; Banerjee, U.C. Removal of dyes from the effluent of textile and dyestuff manufacturing industry: A review of emerging techniques with reference to biological treatment. Crit. Rev. Environ. Sci. Technol. 2005, 35, 219–238. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Sánchezpérez, J.A. Combination of advanced oxidation processes and biological treatments for wastewater decontamination—A review. Energy Environ. Prot. 2012, 409, 4141–4166. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, J.; Jin, W. Transformation of oxidation products and reduction of estrogenic activity of 17β-estradiol by a heterogeneous photo-fenton reaction. Environ. Sci. Technol. 2008, 42, 5277–5284. [Google Scholar] [CrossRef]
- Lan, H.; Wang, A.; Liu, R.; Liu, H.; Qu, J. Heterogeneous photo-Fenton degradation of acid red B over Fe2O3 supported on activated carbon fiber. J. Hazard. Mater. 2015, 285, 167–172. [Google Scholar] [PubMed]
- Feng, J.; Hu, X.; Yue, P.L. Effect of initial solution pH on the degradation of orange II using clay-based Fe nanocomposites as heterogeneous photo-fenton catalyst. Water Res. 2006, 40, 641–646. [Google Scholar] [CrossRef]
- Kasiri, M.B.; Aleboyeh, H.; Aleboyeh, A. Degradation of acid blue 74 using Fe-ZSM5 zeolite as a heterogeneous photo-fenton catalyst. Appl. Catal. B Environ. 2008, 84, 9–15. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced oxidation processes for wastewater treatment: Formation of hydroxyl Radical and application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Xamena, F.L.I.; Casanova, O.; Tailleur, R.G.; Garcia, H.; Corma, A. Metal organic frameworks (MOFs) as catalysts: A combination of Cu2+ and Co2+ MOFs as an efficient catalyst for tetralin oxidation. J. Catal. 2008, 255, 220–227. [Google Scholar]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Henninger, S.K.; Habib, H.A.; Janiak, C. MOFs as adsorbents for low temperature heating and cooling applications. J. Am. Chem. Soc. 2009, 131, 2776–2777. [Google Scholar] [CrossRef]
- Car, A.; Stropnik, C.; Peinemann, K.V. Hybrid membrane materials with different metal–organic frameworks (MOFs) for gas separation. Desalination 2006, 200, 424–426. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Zhong, R.; Van den Bosch, S.; Coman, S.M.; Parvulescu, V.I.; Sels, B.F. Functionalised heterogeneous catalysts for sustainable biomass valorisation. Chem. Soc. Rev. 2018, 47, 8349–8402. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhao, H.; Cao, T.; Lin, Q.; Wang, Y.; Zhao, G. Efficient degradation of high concentration azo-dye wastewater by heterogeneous Fenton process with iron-based metal-organic framework. J. Mol. Catal. A Chem. 2015, 400, 81–89. [Google Scholar] [CrossRef]
- Gao, C.; Chen, S.; Quan, X.; Yu, H.; Zhang, Y. Enhanced fenton-like catalysis by iron-based metal organic frameworks for degradation of organic pollutants. J. Catal. 2017, 356, 125–132. [Google Scholar] [CrossRef]
- Bazaga-Garcia, M.; Cabeza, A.; Olivera-Pastor, P.; Santacruz, I.; Colodrero, R.M.; Aranda, M.A. Photodegradation of phenol over a hybrid organo-inorganic material: Iron(II) hydroxyphosphonoacetate. J. Phys. Chem. C 2012, 116, 14526–14533. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Cyclodextrin-functionalized Ag/AgCl foam with enhanced photocatalytic performance for water purification. J. Colloid Interface Sci. 2018, 531, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Naik, G.V.; Shalaev, V.M.; Boltasseva, A. Alternative plasmonic materials: Beyond gold and silver. Adv. Mater. 2013, 25, 3264–3294. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Chen, J.; Li, G.; Nie, X.; Zhao, H.; Wong, P.K.; An, T. Synthesis and characterization of novel plasmonic Ag/AgX-CNTs (X = Cl, Br, I) nanocomposite photocatalysts and synergetic degradation of organic pollutant under visible light. ACS Appl. Mater. Interfaces 2013, 5, 6959–6967. [Google Scholar] [CrossRef]
- Li, W.; Hua, F.; Yue, J.; Li, J. Ag@AgCl plasmon-induced sensitized ZnO particle for high-efficiency photocatalytic property under visible light. Appl. Surf. Sci. 2013, 285, 490–497. [Google Scholar] [CrossRef]
- Taylor-pashow, K.M.; Rocca, J.D.; Xie, Z.; Tran, S.; Lin, W. Post-Synthetic modifications of iron-carboxylate nanoscale metal-organic frameworks for imaging and drug delivery. J. Am. Chem. Soc. 2009, 131, 14261–14263. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, H.; Chen, Y.; Chen, Y.; Zhuang, L.; Ou, H. Enhanced photocatalysis degradation of organophosphorus flame retardant using MIL-101(Fe)/persulfate: Effect of irradiation wavelength and real water matrixes. Chem. Eng. J. 2019, 368, 273–284. [Google Scholar] [CrossRef]
- Liu, N.; Huang, W.; Zhang, X.; Tang, L.; Wang, L.; Wang, Y.; Wu, M. Ultrathin graphene oxide encapsulated in uniform MIL-88A (Fe) for enhanced visible light-driven photodegradation of RhB. Appl. Catal. B Environ. 2018, 221, 119–128. [Google Scholar] [CrossRef]
- Guo, J.F.; Ma, B.; Yin, A.; Fan, K.; Dai, W.L. Highly stable and efficient Ag/AgCl@TiO2 photocatalyst: Preparation, characterization, and application in the treatment of aqueous hazardous pollutants. J. Hazard. Mater. 2012, 211, 77–82. [Google Scholar] [CrossRef]
- Kalil, S.J.; Maugeri, F.; Rodrigues, M.I. Response surface analysis and simulation as a tool for bioprocess design and optimization. Process. Biochem. 2000, 35, 539–550. [Google Scholar] [CrossRef]
- Tang, X.; Yun, L. Heterogeneous photo-fenton degradation of methylene blue under visible irradiation by iron tetrasulphophthalocyanine immobilized layered double hydroxide at circumneutral pH. Dyes Pigments 2016, 134, 397–408. [Google Scholar] [CrossRef]
- Duan, M.J.; Guan, Z.Y.; Ma, Y.W.; Wan, J.Q.; Wang, Y.; Qu, Y.F. A novel catalyst of MIL-101(Fe) doped with Co and Cu as persulfate activator: Synthesis, characterization, and catalytic performance. Chem. Pap. 2018, 72, 235–250. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Lou, Z.; Zhang, X.; Qin, X.; Dai, Y.; Zheng, Z.; Wang, X. Synthesis of highly efficient Ag@AgCl plasmonic photocatalysts with various structures. Chem. A Eur. J. 2010, 16, 538–544. [Google Scholar] [CrossRef]
- Yu, J.; Dai, G.; Huang, B. Fabrication and characterization of visible-light-driven plasmonic photocatalyst Ag/AgCl/TiO2 nanotube arrays. J. Phys. Chem. C 2009, 113, 16394–16401. [Google Scholar] [CrossRef]
- Denny, J.M.S.; Cohen, S.M. In situ modification of metal-organic frameworks in mixed-matrix membranes. Angew. Chem. Int. Ed. 2015, 54, 9029–9032. [Google Scholar] [CrossRef]
- Sangwichien, C.; Aranovich, G.L.; Donohue, M.D. Density functional theory predictions of adsorption isotherms with hysteresis loops. Colloids Surf. A Physicochem. Eng. Asp. 2012, 206, 313–320. [Google Scholar] [CrossRef]
- Ping, W.; Ming, T.; Wang, G.; Wang, X.; Yu, H.; Yu, J. Cocatalyst modification and nanonization of Ag/AgCl photocatalyst with enhanced photocatalytic performance. J. Mol. Catal. A Chem. 2014, 381, 114–119. [Google Scholar]
- Deng, Y.; Zhang, R.; Li, D.; Sun, P.; Su, P.; Yang, Y. Preparation of iron-based MIL-101 functionalized polydopamine@Fe3O4 magnetic composites for extracting sulfonylurea herbicides from environmental water and vegetable samples. J. Sep. Sci. 2018, 41, 2046–2055. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.X.; Dan, Z.Y.; Wei, J.Z.; Mei, W.D.; Zhi, H.C.; Fang, L.Y. Fe3O4 and metal-organic framework MIL-101(Fe) composites catalyze luminol chemiluminescence for sensitively sensing hydrogen peroxide and glucose. Talanta 2018, 179, 43–50. [Google Scholar] [CrossRef]
- Xu, H.; Li, H.; Xia, J.; Yin, S.; Luo, Z.; Liu, L.; Xu, L. One-pot synthesis of visible-light-driven plasmonic photocatalyst Ag/AgCl in ionic liquid. ACS Appl. Mater. Interfaces 2011, 3, 22–29. [Google Scholar] [CrossRef]
- Liu, Q.; Zeng, C.; Ai, L.; Hao, Z.; Jiang, J. Boosting visible light photoreactivity of photoactive metal-organic framework: Designed plasmonic Z-scheme Ag/AgCl@MIL-53-Fe. Appl. Catal. B Environ. 2018, 224, 38–45. [Google Scholar] [CrossRef]
- Warang, T.; Patel, N.; Santini, A.; Bazzanella, N.; Kale, A.; Miotello, A. Pulsed laser deposition of Co3O4 nanoparticles assembled coating: Role of substrate temperature to tailor disordered to crystalline phase and related photocatalytic activity in degradation of methylene blue. Appl. Catal. A Gen. 2012, 423, 21–27. [Google Scholar] [CrossRef]
- Galindo, C.; Jacques, P.; Kalt, A. Photodegradation of the aminoazobenzene acid orange 52 by three advanced oxidation processes: UV/H2O2, UV/TiO2 and VIS/TiO2: Comparative mechanistic and kinetic investigations. J. Photochem. Photobiol. A Chem. 2000, 130, 35–47. [Google Scholar] [CrossRef]
- Pujari, V.; Chandra, T.S. Statistical optimization of medium components for enhanced riboflavin production by a UV-mutant of Eremothecium ashbyii. Process. Biochem. 2000, 36, 31–37. [Google Scholar] [CrossRef]
- Maran, J.P.; Manikandan, S.; Thirugnanasambandham, K.; Nivetha, C.V.; Dinesh, R. Box–Behnken design based statistical modeling for ultrasound-assisted extraction of corn silk polysaccharide. Carbohydr. Polym. 2013, 92, 604–611. [Google Scholar] [CrossRef]
- Tripathi, P.; Srivastava, V.C.; Kumar, A. Optimization of an azo dye batch adsorption parameters using Box-Behnken design. Desalination 2009, 249, 1273–1279. [Google Scholar] [CrossRef]
- Bali, U.; Catalkaya, E.; Sengül, F. Photodegradation of reactive black 5, direct red 28 and direct yellow 12 using UV, UV/H2O2 and UV/H2O2/Fe2+: A comparative study. J. Hazard. Mater. 2004, 114, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.S.; Peres, J.A. Decolorization of the azo dye reactive black 5 by fenton and photo-fenton oxidation. Dyes Pigments 2006, 71, 236–244. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, Z.; Jin, L.; Ni, S.; Hui, Z.; Dionysiou, D.D. Visible light-assisted heterogeneous fenton with ZnFe2O4 for the degradation of orange II in water. Appl. Catal. B Environ. 2016, 182, 456–468. [Google Scholar] [CrossRef]
- Feng, J.; Hu, X.; Yue, P.L. Novel bentonite clay-based Fe−nanocomposite as a heterogeneous catalyst for photo-fenton discoloration and mineralization of orange II. Environ. Sci. Technol. 2004, 38, 269–275. [Google Scholar] [CrossRef]
- Gao, S.; Feng, T.; Feng, C.; Shang, N.; Wang, C. Novel visible-light-responsive Ag/AgCl@MIL-101 hybrid materials with synergistic photocatalytic activity. J. Colloid Interface Sci. 2016, 466, 284–290. [Google Scholar] [CrossRef]



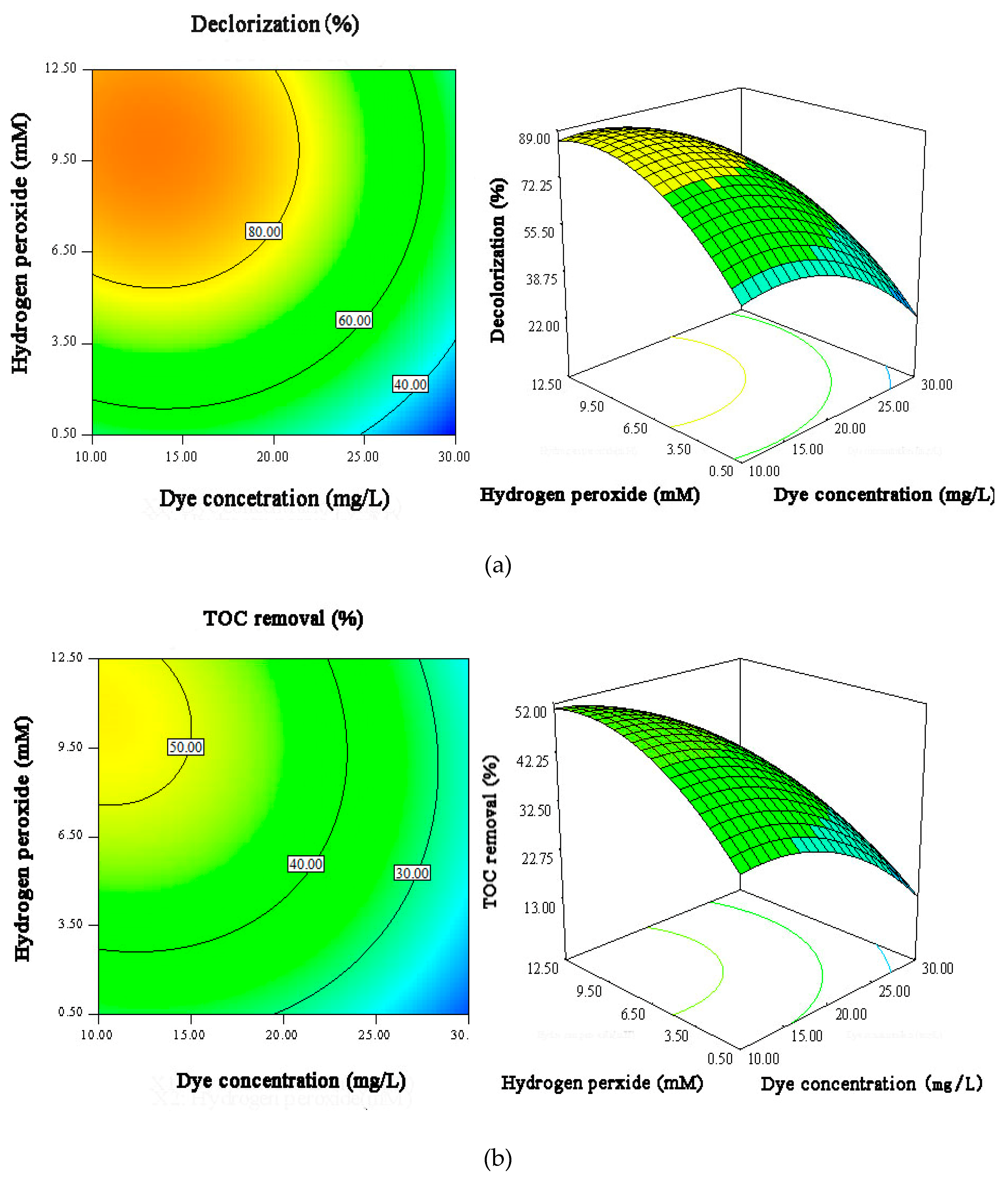
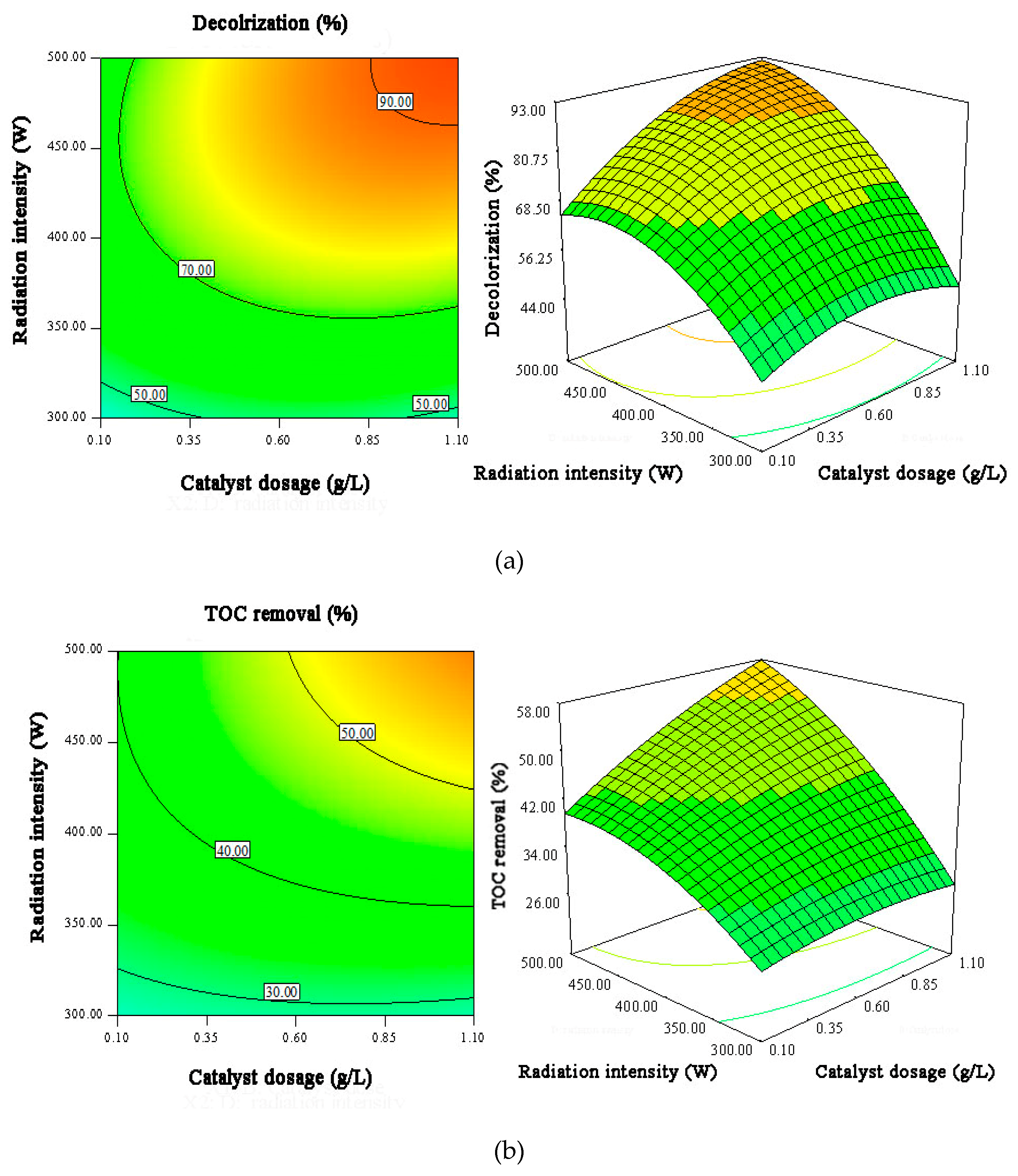



| Influence Factors | Code | Levels Low (−) | Levels Middle (0) | Levels High (+) |
|---|---|---|---|---|
| Dye concentration(mg/L) | A | 10 | 20 | 30 |
| Hydrogen peroxide(mM) | B | 0.5 | 6.5 | 12.5 |
| Catalyst dosage(g/L) | C | 0.2 | 0.7 | 1.2 |
| Radiation intensity (W) | D | 300 | 400 | 500 |
| Source | Decolorization | TOC Removal | |||||||
|---|---|---|---|---|---|---|---|---|---|
| D | Sum of | Mean | F Value | Prob > F | Sum of | Mean | F Value | Prob > F | |
| F | Square | Square | Square | Square | |||||
| Model | 14 | 12376.46 | 12376.46 | 54.76 | <0.0001 | 4873.04 | 4873.04 | 13.23 | <0.0001 |
| A | 1 | 3178.48 | 3178.48 | 133.59 | <0.0001 | 1658.0 | 1658.0 | 63.06 | <0.0001 |
| B | 1 | 665.88 | 665.88 | 128.72 | 0.0017 | 274.85 | 274.85 | 10.45 | 0.006 |
| C | 1 | 2761.85 | 2761.85 | 164.48 | <0.0001 | 601.83 | 601.83 | 22.88 | 0.0003 |
| D | 1 | 3274.59 | 3274.59 | 211.95 | <0.0001 | 1418.32 | 1418.32 | 53.92 | <0.0001 |
| AB | 1 | 63.04 | 63.04 | 12.21 | 0.2547 | 144.36 | 144.36 | 5.49 | 0.0344 |
| AC | 1 | 9.33 | 9.33 | 0.074 | 0.6542 | 18.53 | 18.53 | 0.70 | 0.4154 |
| AD | 1 | 190.16 | 190.16 | 54.13 | 0.0528 | 104.96 | 104.96 | 3.99 | 0.0656 |
| BC | 1 | 22.71 | 22.71 | 36.71 | 0.1017 | 4.96 | 4.96 | 0.19 | 0.6224 |
| BD | 1 | 137.1 | 137.1 | 36.94 | 0.0001 | 69.81 | 69.81 | 2.65 | 0.1256 |
| CD | 1 | 0.71 | 0.71 | 0.0052 | 0.0054 | 77.44 | 77.44 | 2.94 | 0.1082 |
| A2 | 1 | 996.37 | 996.37 | 22.29 | 0.0003 | 274.14 | 274.14 | 10.42 | 0.0061 |
| B2 | 1 | 225.13 | 225.13 | 5.04 | 0.0415 | 13.80 | 13.80 | 0.52 | 0.4808 |
| C2 | 1 | 1162.34 | 1162.34 | 26.01 | 0.0002 | 290.51 | 290.51 | 11.04 | 0.0050 |
| D2 | 1 | 661.86 | 661.86 | 14.81 | 0.0018 | 91.69 | 91.69 | 3.49 | 0.0830 |
| Residual | 14 | 625.67 | 44.69 | 368.28 | 26.31 | ||||
| Pure Error | 4 | 0 | 8.330 × 10−3 | 0 | 1.770 × 10−3 | ||||
| R2 = 0.9821; R2adj = 0.9641 | R2 = 0.9737; R2adj = 0.9423 | ||||||||
| R2pred = 0.9021; adeq precision = 16.920 | R2pred = 0.8577; adeq precision = 13.62 | ||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xie, Y.; Dai, M.; Gong, Q.; Dang, Z. Ag/AgCl/MIL-101(Fe) Catalyzed Degradation of Methylene Blue under Visible Light Irradation. Materials 2019, 12, 1453. https://doi.org/10.3390/ma12091453
Liu Y, Xie Y, Dai M, Gong Q, Dang Z. Ag/AgCl/MIL-101(Fe) Catalyzed Degradation of Methylene Blue under Visible Light Irradation. Materials. 2019; 12(9):1453. https://doi.org/10.3390/ma12091453
Chicago/Turabian StyleLiu, Yun, Yuanhong Xie, Mingjin Dai, Qingjiao Gong, and Zhi Dang. 2019. "Ag/AgCl/MIL-101(Fe) Catalyzed Degradation of Methylene Blue under Visible Light Irradation" Materials 12, no. 9: 1453. https://doi.org/10.3390/ma12091453
APA StyleLiu, Y., Xie, Y., Dai, M., Gong, Q., & Dang, Z. (2019). Ag/AgCl/MIL-101(Fe) Catalyzed Degradation of Methylene Blue under Visible Light Irradation. Materials, 12(9), 1453. https://doi.org/10.3390/ma12091453