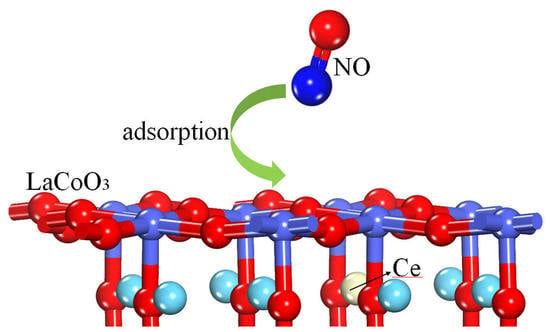
DFT Analysis of NO Adsorption on the Undoped and Ce-Doped LaCoO3 (011) Surface
Abstract
1. Introduction
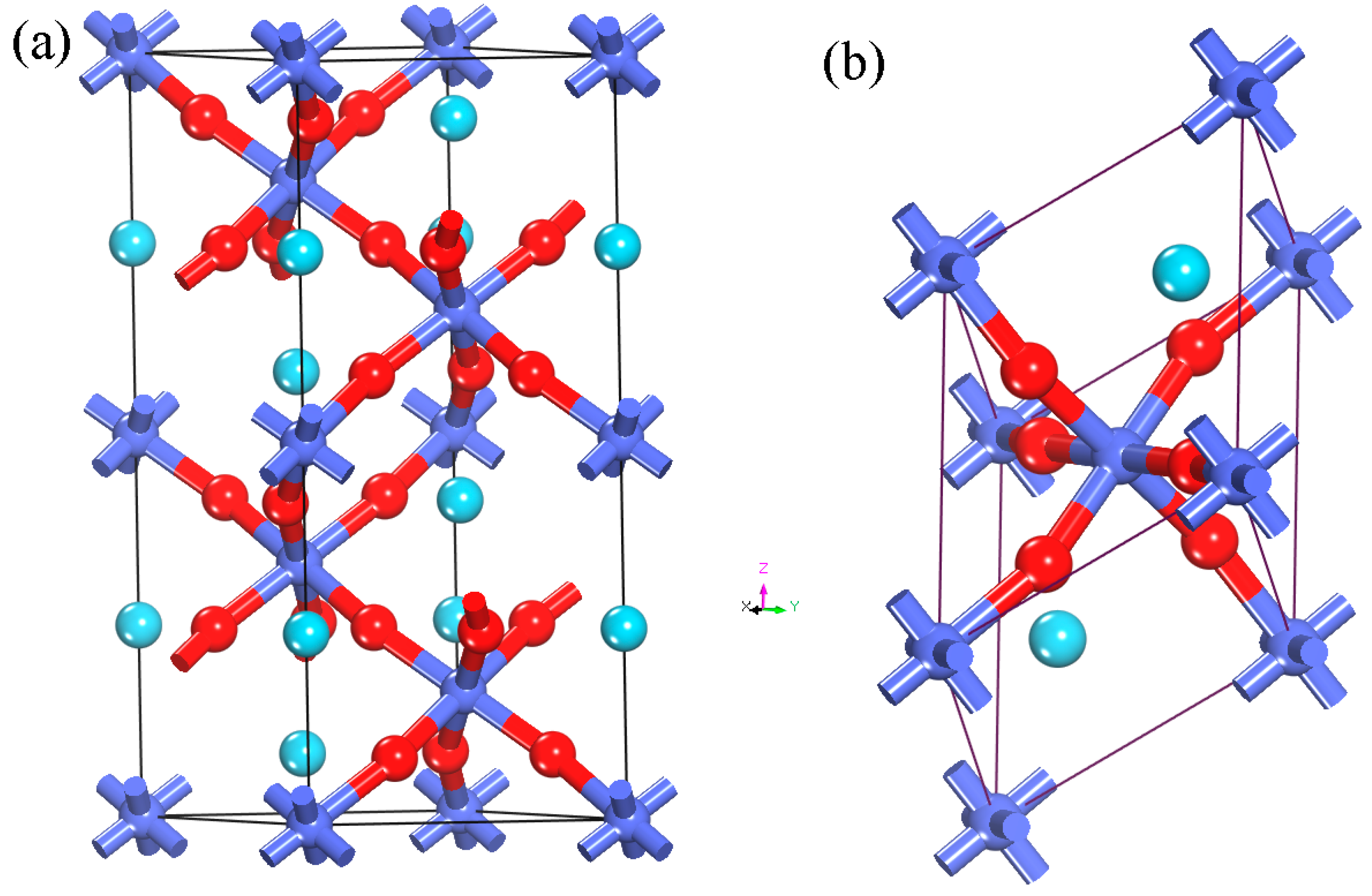
2. Computational Methods and Models
3. Results and Discussion
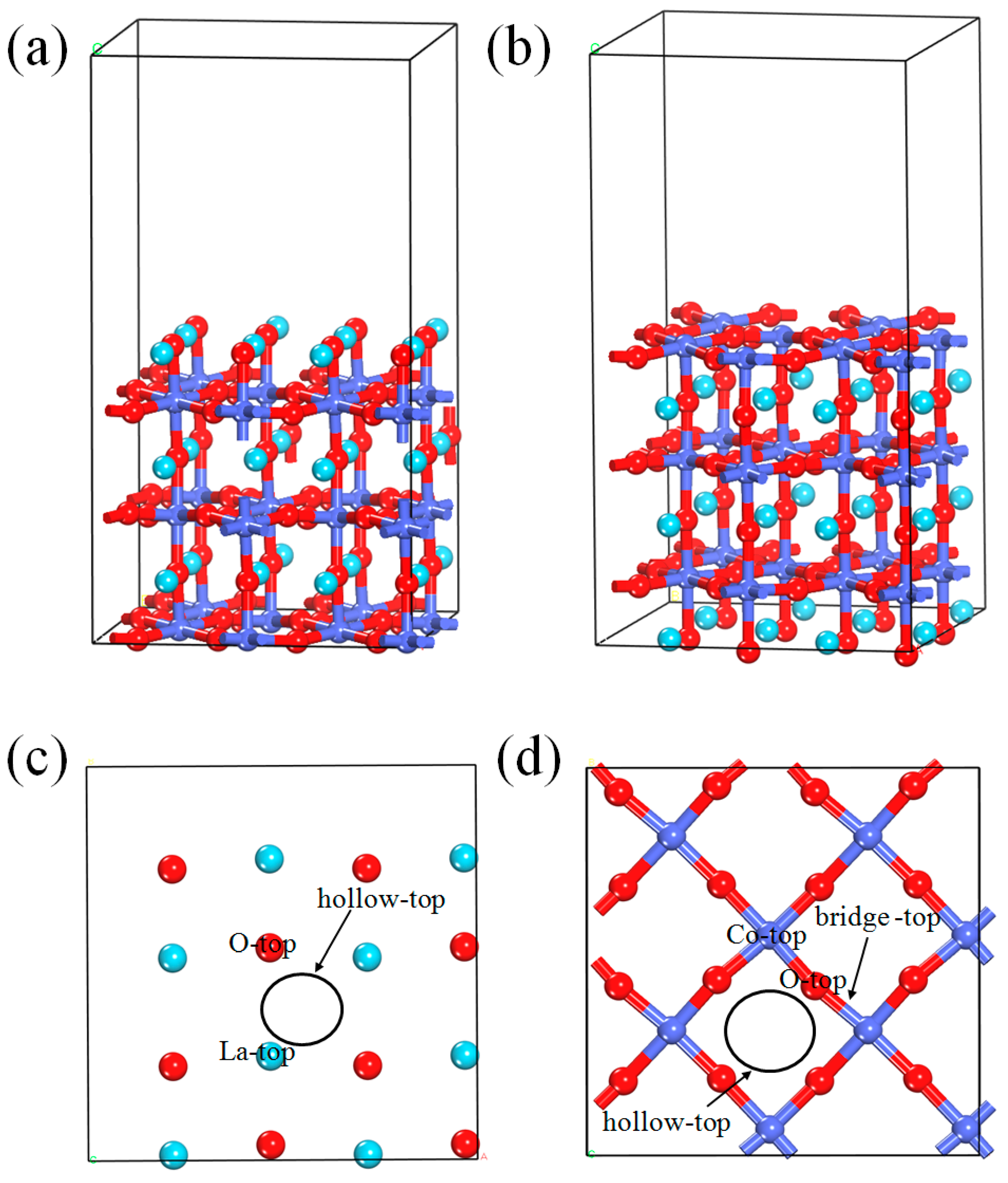
3.1. NO Adsorption on the (011) Surface of Perfect LaCoO3
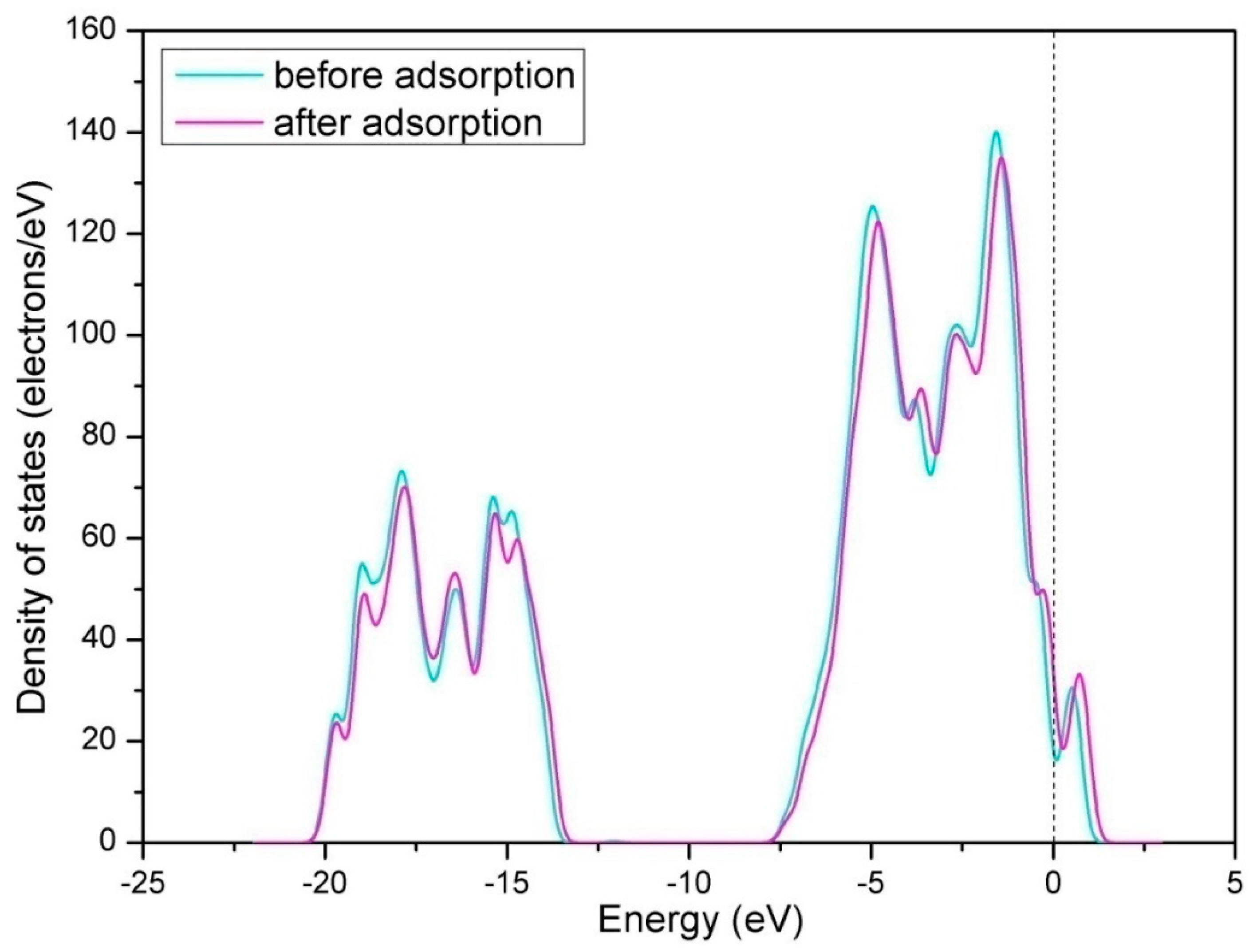
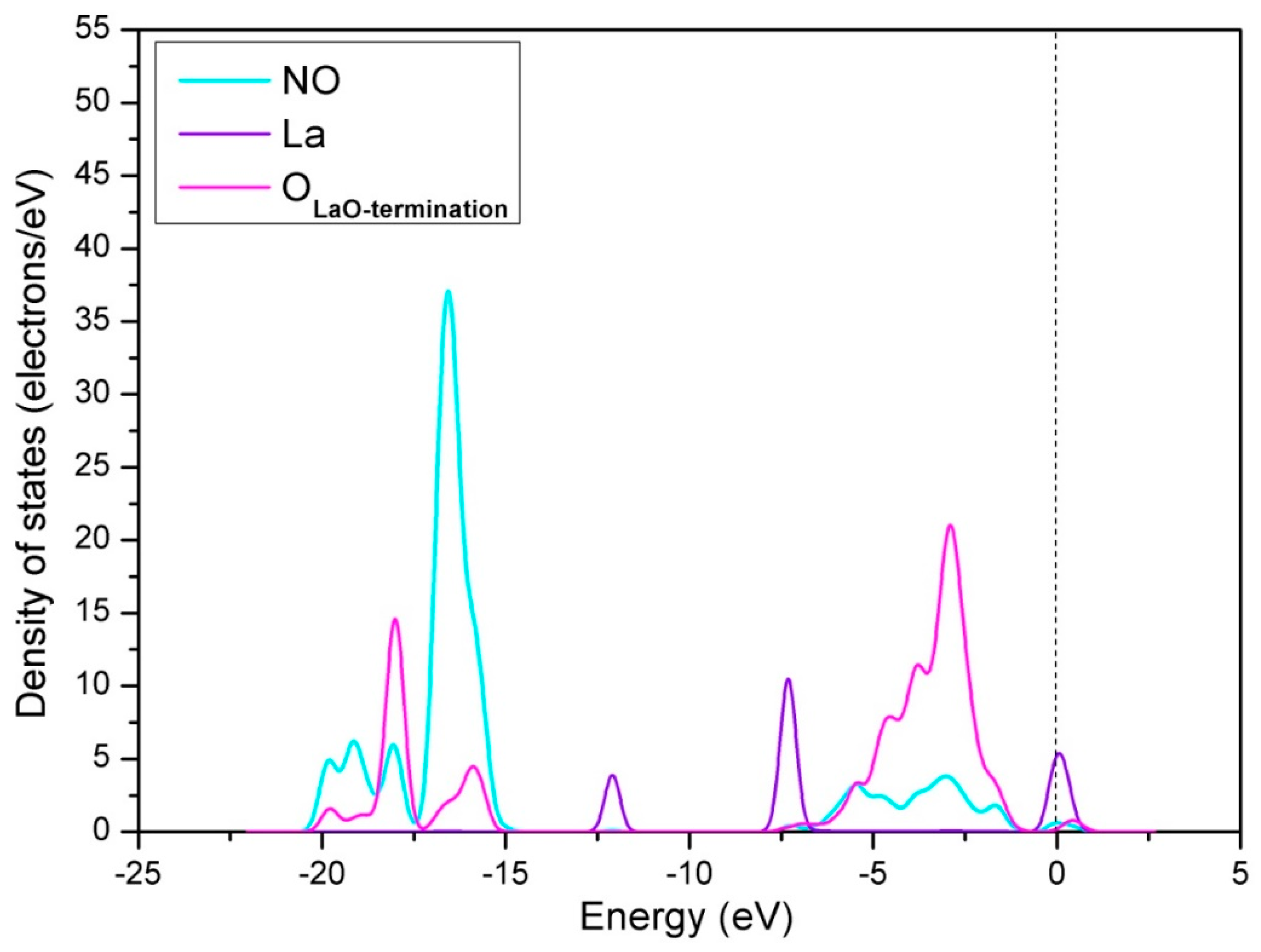
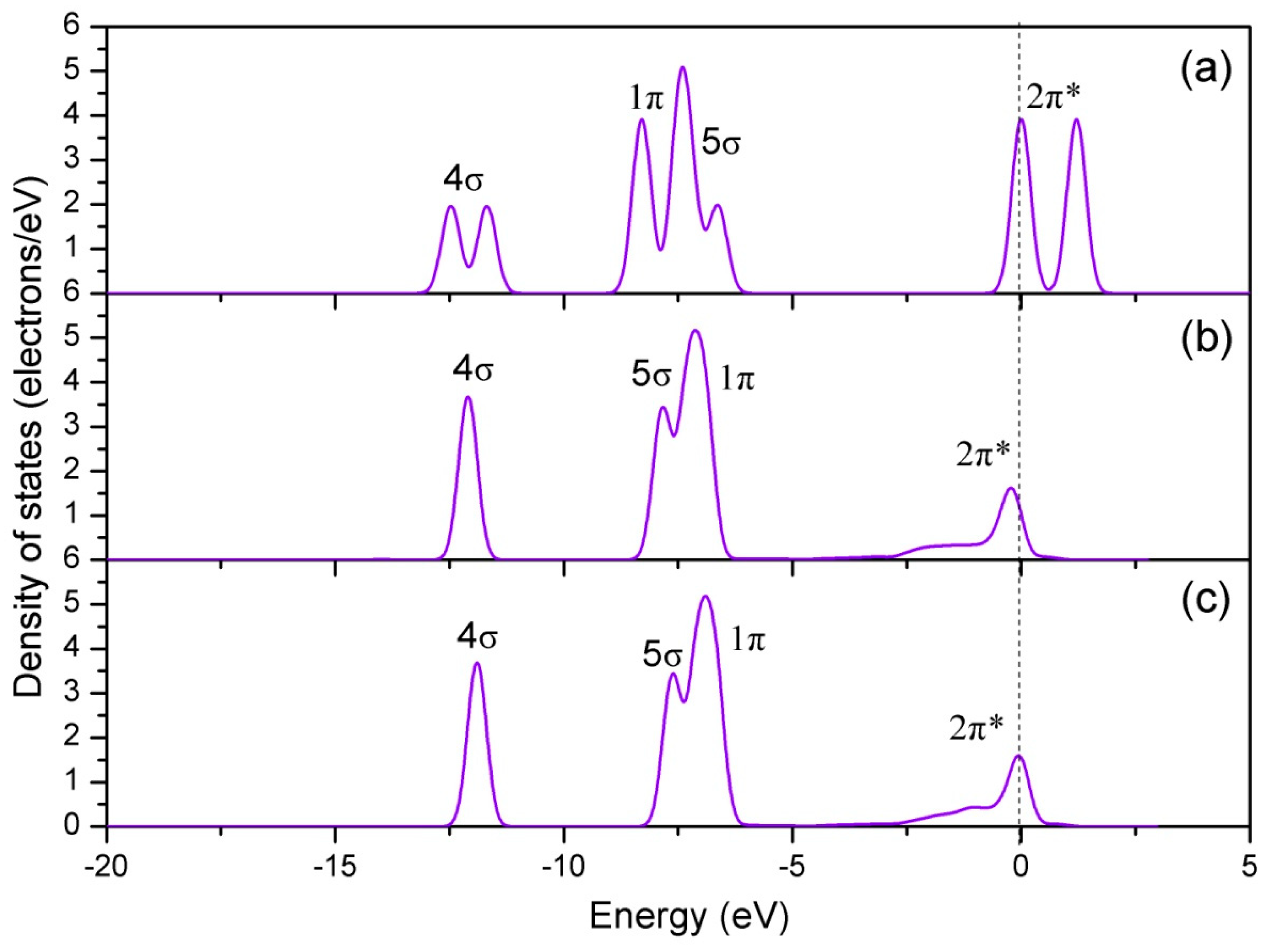
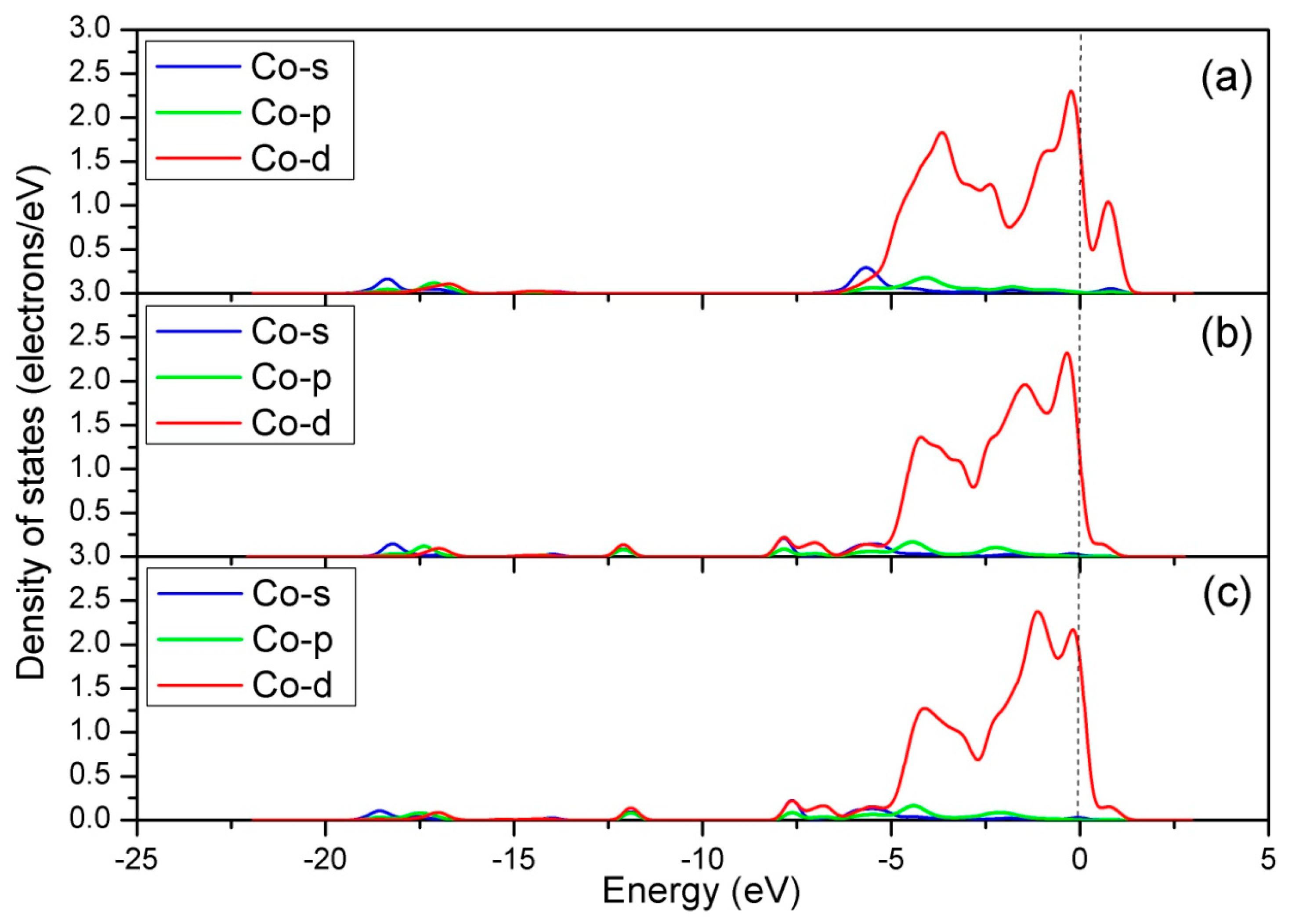
3.1.1. Calculation for the Perfect LaO-Terminated Surface
3.1.2. Calculation for the Perfect CoO2-Terminated Surface
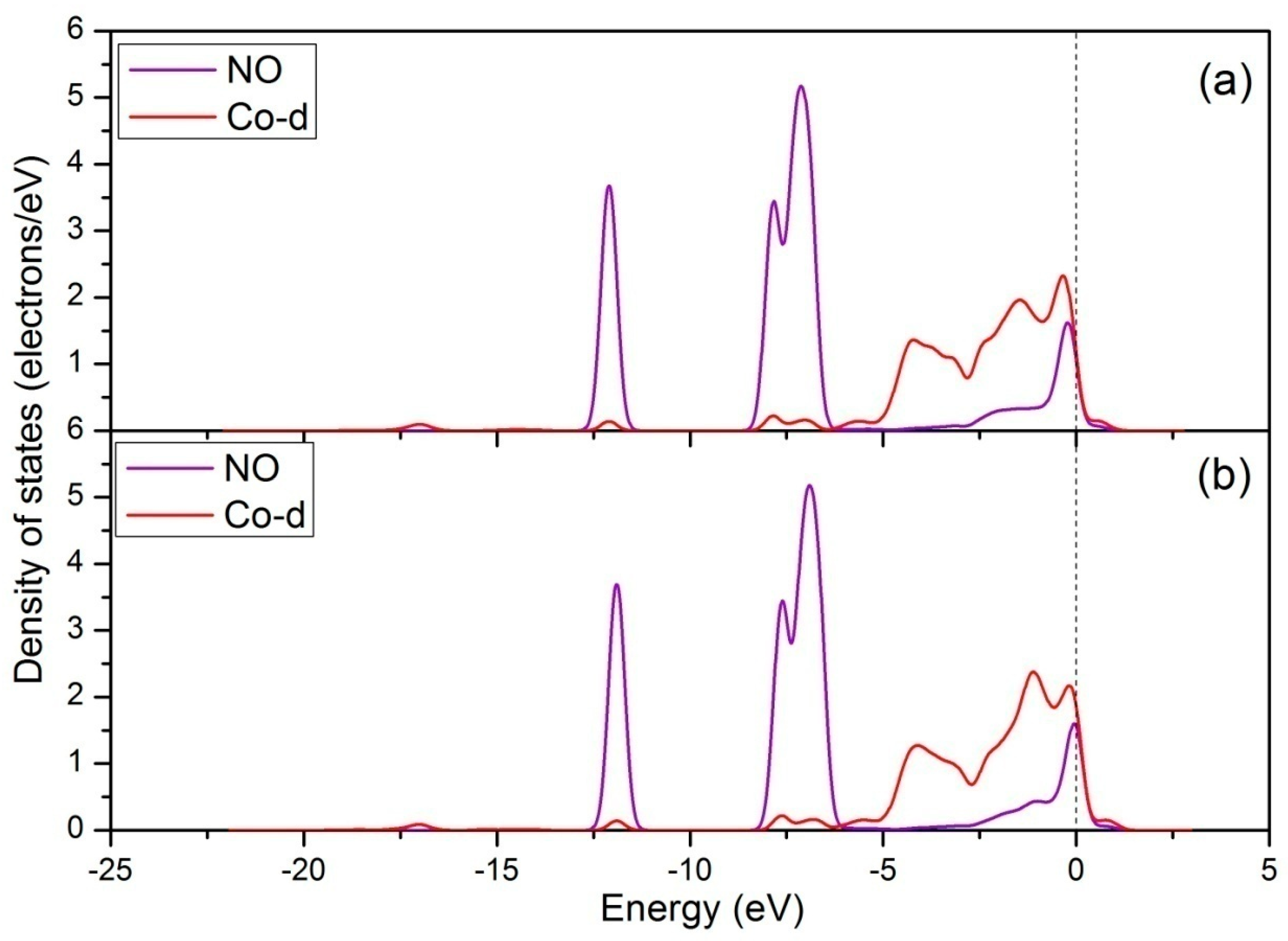
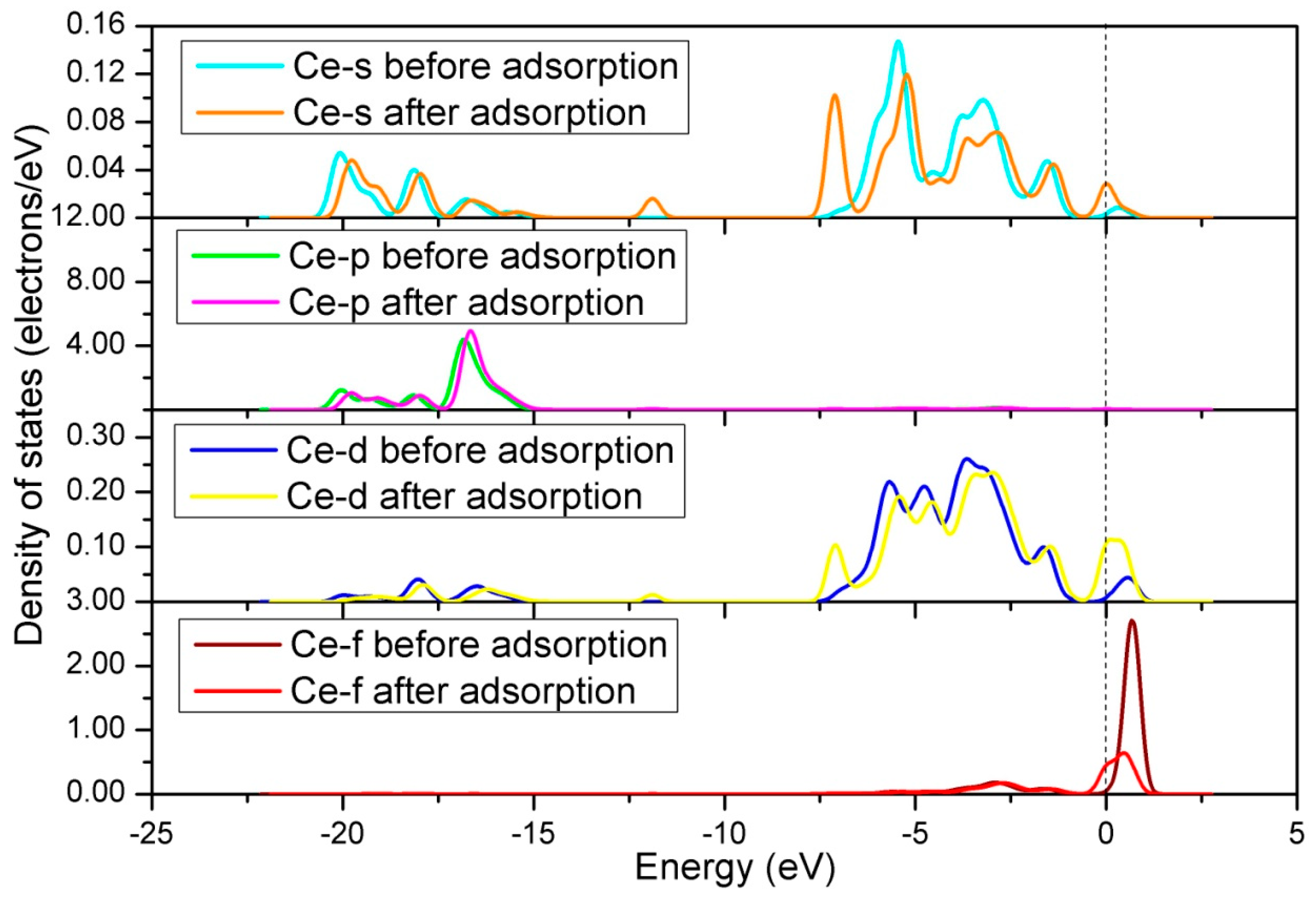
3.2. NO Adsorption on the (011) Surface of Ce-Doped LaCoO3
3.2.1. Calculation for the Ce-doped LaO-Terminated Surface
3.2.2. Calculation for the Ce-doped CoO2-Terminated Surface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Iwamoto, M.; Hamada, H. Removal of nitrogen monoxide from exhaust gases through novel catalytic processes. Catal. Today. 1991, 10, 57–71. [Google Scholar] [CrossRef]
- Ohtsuka, H. The selective catalytic reduction of nitrogen oxides by methane on noble metal-loaded sulfated zirconia. Appl. Catal. B Environ. 2001, 33, 325–333. [Google Scholar] [CrossRef]
- Takahashi, N.; Shinjoh, H.; Iijima, T.; Suzuki, T.; Yamazaki, K.; Yokota, K.; Suzuki, H.; Miyoshi, N.; Matsumoto, S.; Tanizawa, T.; et al. The new concept 3-way catalyst for automotive lean-burn engine: NOx storage and reduction catalyst. Catal. Today. 1996, 27, 63–69. [Google Scholar] [CrossRef]
- Chen, J.; Shen, M.; Wang, X.; Qi, G.; Wang, J.; Li, W. The influence of nonstoichiometry on LaMnO3 perovskite for catalytic NO oxidation. Appl. Catal. B-Environ. 2013, 134, 251–257. [Google Scholar] [CrossRef]
- Chen, J.; Shen, M.; Wang, X.; Wang, J.; Su, Y.; Zhao, Z. Catalytic performance of NO oxidation over LaMeO3 (Me = Mn, Fe, Co) perovskite prepared by the sol-gel method. Catal. Commun. 2013, 37, 105–108. [Google Scholar]
- Li, Z.; Meng, M.; Li, Q.; Xie, Y.; Hu, T.; Zhang, J. Fe-substituted nanometric La0.9K0.1Co1−xFexO3-δ perovskite catalysts used for soot combustion, NOx storage and simultaneous catalytic removal of soot and NOx. Chem. Eng. J. 2010, 164, 98–105. [Google Scholar] [CrossRef]
- Guo, X.; Meng, M.; Dai, F.; Li, Q.; Zhang, Z.; Jiang, Z.; Zhang, S.; Huang, Y. NOx-assisted soot combustion over dually substituted perovskite catalysts La1−xKxCo1−yPdyO3-δ. Appl. Catal. B-Environ. 2013, 142, 278–289. [Google Scholar] [CrossRef]
- Li, X.; Gao, H. Role of ceria in the improvement of NO removal of lanthanum-based perovskite-type catalysts. Rsc Adv. 2018, 8, 11778–11784. [Google Scholar] [CrossRef]
- Cohen, R.E.; Lin, Y. Prediction of a potential high-pressure structure of FeSiO3. Phys. Rev. B. 2014, 90, 140102. [Google Scholar] [CrossRef]
- You, R.; Zhang, Y.; Liu, D.; Meng, M.; Zheng, L.; Zhang, J.; Hu, T. YCeZrO Ternary Oxide Solid Solution Supported Nonplatinic Lean-Burn NOx Trap Catalysts Using LaCoO3 Perovskite as Active Phase. J. Phys. Chem. C 2014, 118, 25403–25420. [Google Scholar] [CrossRef]
- Li, X.-G.; Dong, Y.-H.; Xian, H.; Hernandez, W.Y.; Meng, M.; Zou, H.-H.; Ma, A.-J.; Zhang, T.-Y.; Jiang, Z.; Tsubaki, N.; Vernoux, P. De-NOx in alternative lean/rich atmospheres on La1−xSrxCoO3 perovskites. Energy Environ. Sci. 2011, 4, 3351–3354. [Google Scholar]
- Kim, C.H.; Qi, G.; Dahlberg, K.; Li, W. Strontium-Doped Perovskites Rival Platinum Catalysts for Treating NOx in Simulated Diesel Exhaust. Science 2010, 327, 1624–1627. [Google Scholar] [CrossRef] [PubMed]
- Onrubia, J.A.; Pereda-Ayo, B.; De-La-Torre, U.; Gonzalez-Velasco, J.R. Key factors in Sr-doped LaBO3 (B = Co or Mn) perovskites for NO oxidation in efficient diesel exhaust purification. Appl. Catal. B-Environ. 2017, 213, 198–210. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, C.; He, H.; Yu, Y.; Teraoka, Y. Catalytic oxidation of nitrogen monoxide over La1−xCexCoO3 perovskites. Catal. Today. 2007, 126, 400–405. [Google Scholar] [CrossRef]
- Forni, L.; Oliva, C.; Vatti, F.P.; Kandala, M.A.; Ezerets, A.M.; Vishniakov, A.V. LaCeCo perovskites as catalysts for exhaust gas depollution. Appl. Catal. B Environ. 1996, 7, 269–284. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, S.; Yang, Y.; Zheng, C.; Zhou, J.; Gao, X.; Tu, X. Enhanced performance for plasma-catalytic oxidation of ethyl acetate over La1−xCexCoO3+δ catalysts. Appl. Catal. B-Environ. 2017, 213, 97–105. [Google Scholar] [CrossRef]
- Zhao, B.; Ran, R.; Sun, L.; Yang, Z.; Wu, X.; Weng, D. A high-surface-area La-Ce-Mn mixed oxide with enhanced activity for CO and C3H8 oxidation. Catal. Commun. 2018, 105, 26–30. [Google Scholar] [CrossRef]
- Guguchia, Z.; Adachi, T.; Shermadini, Z.; Ohgi, T.; Chang, J.; Bozin, E.S.; Von Rohr, F.; Dos Santos, A.M.; Molaison, J.J.; Boehler, R.; et al. Pressure tuning of structure, superconductivity, and novel magnetic order in the Ce-underdoped electron-doped cuprate T′−Pr1.3−xLa0.7CexCuO4 (x = 0.1). Phys. Rev. B 2017, 96, 094515. [Google Scholar] [CrossRef]
- Zhang-Steenwinkel, Y.; Beckers, J.; Bliek, A. Surface properties and catalytic performance in CO oxidation of cerium substituted lanthanum–manganese oxides, Appl. Catal. Gen. 2002, 235, 79–92. [Google Scholar] [CrossRef]
- Białobok, B.; Trawczyński, J.; Miśta, W.; Zawadzki, M. Ethanol combustion over strontium- and cerium-doped LaCoO3 catalysts. Appl. Catal. B Environ. 2007, 72, 395–403. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Gao, H. Adsorption properties of NO molecules on the hexagonal LaCoO3 (001) surface: A density functional theory study. RSC Adv. 2017, 7, 34714–34721. [Google Scholar] [CrossRef]
- Penninger, M.W.; Kim, C.H.; Thompson, L.T.; Schneider, W.F. DFT Analysis of NO Oxidation Intermediates on Undoped and Doped LaCoO 3 Perovskite. J. Phys. Chem. C 2015, 119, 20488–20494. [Google Scholar] [CrossRef]
- Chen, Z.; Kim, C.H.; Thompson, L.T.; Schneider, W.F. LDA+U evaluation of the stability of low-index facets of LaCoO3 perovskite. Surf. Sci. 2014, 619, 71–76. [Google Scholar]
- Sun, L.; Hu, J.; Qin, H.; Zhao, M.; Fan, K. Influences of Ca Doping and Oxygen Vacancy upon Adsorption of CO on the LaFeO3 (010) Surface: A First-Principles Study. J. Phys. Chem. C 2011, 115, 5593–5598. [Google Scholar] [CrossRef]
- Sun, L.; Li, G.; Chen, W.; Hu, J. Adsorption of CO on the oxygen defective LaCoO3 (001) surface: A first-principles study. Comput. Mater. Sci. 2016, 115, 154–157. [Google Scholar]
- Carlotto, S.; Natile, M.M.; Glisenti, A.; Paul, J.-F.; Blanck, D.; Vittadini, A. Energetics of CO oxidation on lanthanide-free perovskite systems: the case of Co-doped SrTiO3. Phys. Chem. Chem. Phys. 2016, 18, 33282–33286. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Z.; Wen, Y.; Chen, R.; Shan, B. Surface stabilities and NO oxidation kinetics on hexagonal-phase LaCoO3 facets: a first-principles study. Catal. Sci. Technol. 2014, 4, 3687–3696. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, X.; Wu, C.; Wen, Y.; Xue, Y.; Chen, R.; Zhang, Z.; Shan, B.; Yin, H.; Wang, W.G. NO oxidation catalysis on copper doped hexagonal phase LaCoO3: a combined experimental and theoretical study. Phys. Chem. Chem. Phys. 2014, 16, 5106–5112. [Google Scholar] [CrossRef]
- Wang, L.; Chen, W. Density functional theory for adsorption of HCHO on the FeO(100) surface. J. Nat. Gas Chem. 2010, 19, 21–24. [Google Scholar] [CrossRef]
- Giordano, L.; Del Vitto, A.; Pacchioni, G.; Maria Ferrari, A. CO adsorption on Rh, Pd and Ag atoms deposited on the MgO surface: a comparative ab initio study. Surf. Sci. 2003, 540, 63–75. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, Y.-P.; Li, L.; Wang, T. Ab Initio Study of ZnO-Based Gas-Sensing Mechanisms: Surface Reconstruction and Charge Transfer. J. Phys. Chem. C 2009, 113, 6107–6113. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol 3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, E.; Sun, L.; Hu, J. Adsorption of NO on the SrFeO3 (001) surface: A DFT study, Comput. Mater. Sci. 2015, 102, 135–139. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Li, Z. First principle study of SrTiO3 (001) surface and adsorption of NO on SrTiO3 (001). Appl. Surf. Sci. 2007, 253, 8345–8351. [Google Scholar] [CrossRef]
- Thornton, G.; Tofield, B.; Hewat, A. A neutron diffraction study of LaCoO3 in the temperature range 4.2 < T < 1248 K. J. Solid State Chem. 1986, 61, 301–307. [Google Scholar]
- Boateng, I.W.; Tia, R.; Adei, E.; Dzade, N.Y.; Catlow, C.R.A.; De Leeuw, N.H. A DFT plus U investigation of hydrogen adsorption on the LaFeO3(010) surface. Phys. Chem. Chem. Phys. 2017, 19, 7399–7409. [Google Scholar] [CrossRef]
- Kizaki, H.; Kusakabe, K. DFT-GGA study of NO adsorption on the LaO (001) surface of LaFeO3. Surf. Sci. 2012, 606, 337–343. [Google Scholar]
- Zhou, Y.; Lu, Z.; Guo, P.; Tian, Y.; Huang, X.; Su, W. First-principles study on the catalytic role of Ag in the oxygen adsorption of LaMnO3 (0 0 1) surface. Appl. Surf. Sci. 2012, 258, 2602–2606. [Google Scholar] [CrossRef]
- Carlotto, S.; Natile, M.M.; Glisenti, A.; Vittadini, A. Adsorption of CO and formation of carbonates at steps of pure and Co-doped SrTiO 3 surfaces by DFT calculations. Appl. Surf. Sci. 2016, 364, 522–527. [Google Scholar] [CrossRef]
- Asthagiri, A.; Sholl, D. DFT study of Pt adsorption on low index SrTiO surfaces: SrTiO (100), SrTiO (111) and SrTiO (110). Surf. Sci. 2005, 581, 66–87. [Google Scholar] [CrossRef]
- Read, M.S.D.; Islam, M.S.; Watson, G.W.; King, F.; Hancock, F.E. Defect chemistry and surface properties of LaCoO3. J. Mater. Chem. 2000, 10, 2298–2305. [Google Scholar] [CrossRef]
- Johnson, R. Computational Chemistry Comparison and Benchmark Database, NIST Standard Reference Database 101. Available online: http://cccbdb.nist.gov/ (accessed on 19 April 2018).
- Sun, L.; Hu, J.; Gao, F.; Zhang, Y.; Qin, H. First-principle study of NO adsorption on the LaFeO3 (010) surface. Phys. B-Condens. Matter. 2011, 406, 4105–4108. [Google Scholar] [CrossRef]
- Sorescu, D.C.; Rusu, C.N.; Yates, J.T. Adsorption of NO on the TiO2 (110) Surface: An Experimental and Theoretical Study. J. Phys. Chem. B. 2000, 104, 4408–4417. [Google Scholar] [CrossRef]
- Sun, L.; Li, G.; Chen, W.; Luo, F.; Hu, J.; Qin, H. Adsorption of CO on the LaCoO3 (001) surface by density functional theory calculation. Appl. Surf. Sci. 2014, 309, 128–132. [Google Scholar] [CrossRef]
- Liu, X.; Hu, J.; Cheng, B.; Qin, H.; Zhao, M.; Yang, C. First-principles study of O2 adsorption on the LaFeO3 (010) surface. Sens. Actuators B Chem. 2009, 139, 520–526. [Google Scholar] [CrossRef]
- Hoffmann, R. A chemical and theoretical way to look at bonding on surfaces. Rev. Mod. Phys. 1988, 60, 601–628. [Google Scholar] [CrossRef]
- Ding, K.; Li, J.; Zhang, Y. Cu and NO coadsorption on TiO2(110) surface: A density functional theory study. J. Mol. Struct. THEOCHEM 2005, 728, 123–127. [Google Scholar] [CrossRef]
- Fan, C.; Xiao, W.-D. Origin of site preference of CO and NO adsorption on Pd(111) at different coverages: A density functional theory study. Comput. Theor. Chem. 2013, 1004, 22–30. [Google Scholar] [CrossRef]
- Zeng, Z.-H.; Da Silva, J.L.F.; Li, W.-X. Theory of nitride oxide adsorption on transition metal (111) surfaces: A first-principles investigation. Phys. Chem. Chem. Phys. 2010, 12, 2459. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, F.; Tang, T.C.; He, J.P.; Wang, L. Theoretical analysis and comparation study of multiple-scattering cluster method: For CO/NiO(100) and NO/NiO(100) adsorption systems. Acta Phys. Sin. 2000, 49, 570–576. [Google Scholar]
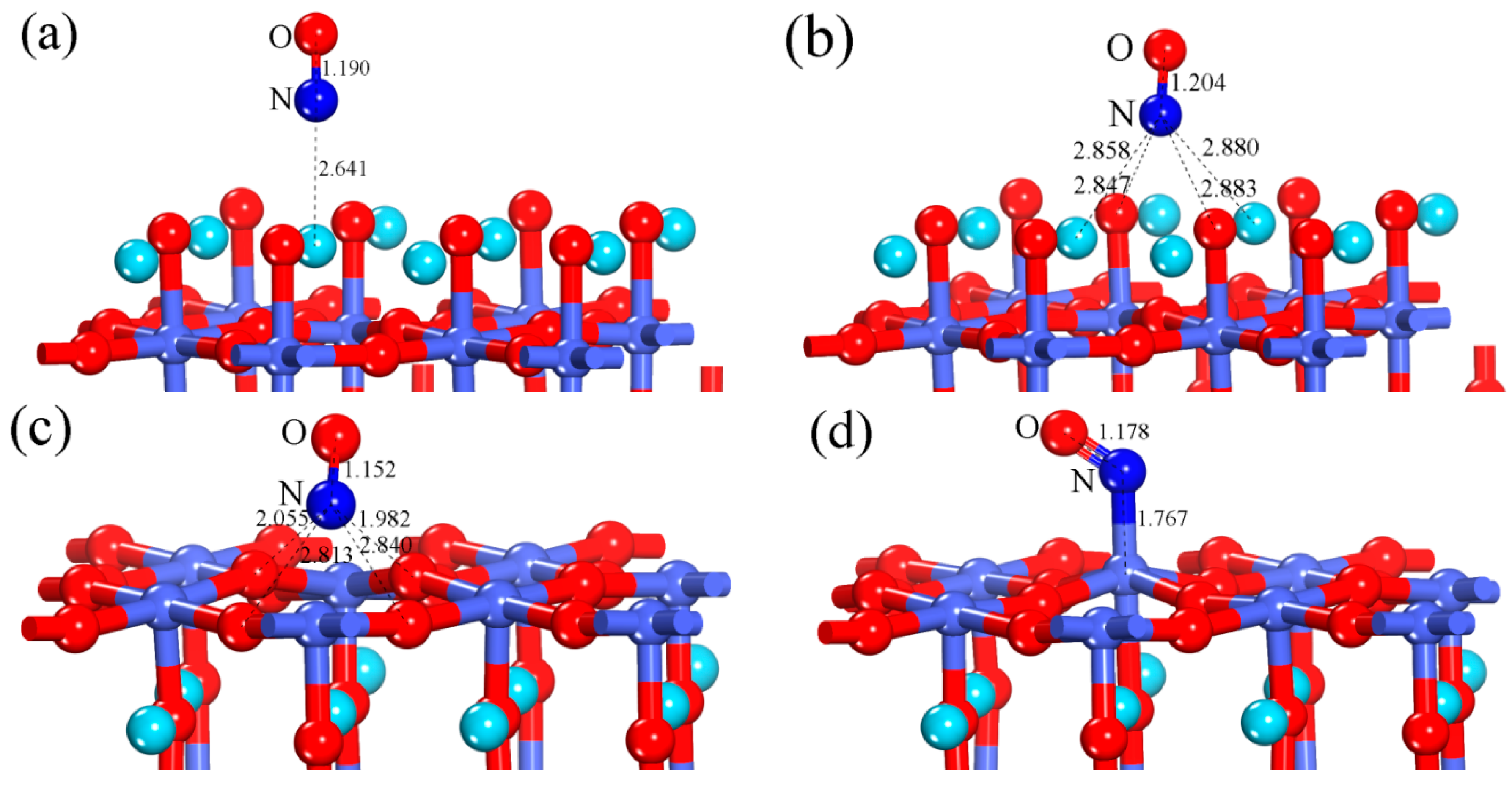
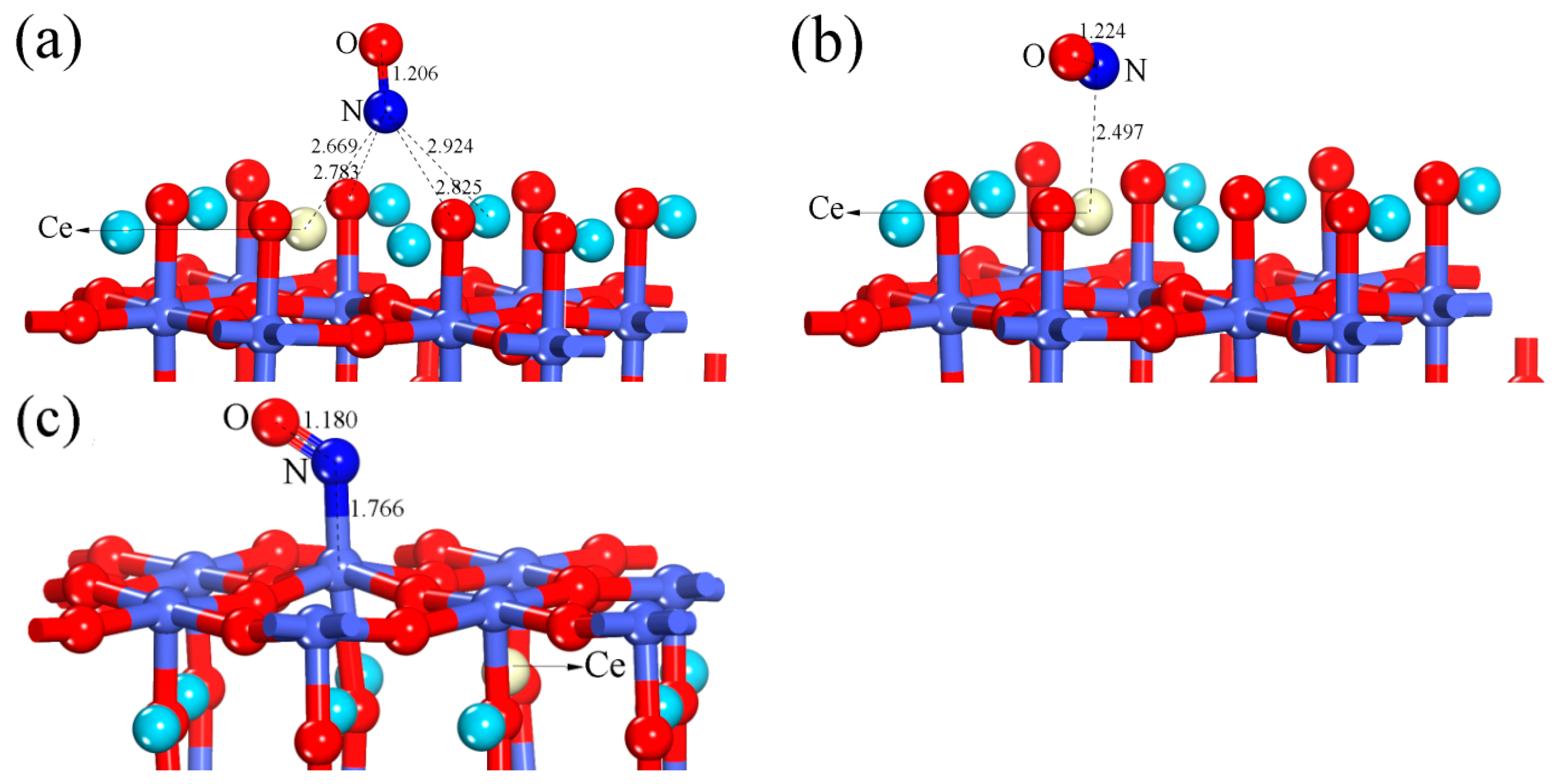

| Configuration | dN-O | ΔdN-O (Å) | d (Å) | qNO (e) | Eads (eV) |
|---|---|---|---|---|---|
| Perfect LaO-terminated surface (hollow-NO) | 1.204 | 0.041 | 2.847 | −0.295 | 0.593 |
| Perfect CoO2-terminated surface (Co-NO) | 1.178 | 0.015 | 1.767 | 0.126 | 1.302 |
| Ce-doped LaO-terminated surface (hollow-NO) | 1.206 | 0.043 | 2.669 | −0.317 | 0.849 |
| Ce-doped LaO-terminated surface (Ce-NO) | 1.224 | 0.061 | 2.497 | −0.371 | 0.797 |
| Ce-doped CoO2-terminated surface (Co-NO) | 1.18 | 0.017 | 1.766 | 0.104 | 1.380 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Gao, H. DFT Analysis of NO Adsorption on the Undoped and Ce-Doped LaCoO3 (011) Surface. Materials 2019, 12, 1379. https://doi.org/10.3390/ma12091379
Li X, Gao H. DFT Analysis of NO Adsorption on the Undoped and Ce-Doped LaCoO3 (011) Surface. Materials. 2019; 12(9):1379. https://doi.org/10.3390/ma12091379
Chicago/Turabian StyleLi, Xiaochen, and Hongwei Gao. 2019. "DFT Analysis of NO Adsorption on the Undoped and Ce-Doped LaCoO3 (011) Surface" Materials 12, no. 9: 1379. https://doi.org/10.3390/ma12091379
APA StyleLi, X., & Gao, H. (2019). DFT Analysis of NO Adsorption on the Undoped and Ce-Doped LaCoO3 (011) Surface. Materials, 12(9), 1379. https://doi.org/10.3390/ma12091379