Enhancing Interfacial Bonding and Tensile Strength in CNT-Cu Composites by a Synergetic Method of Spraying Pyrolysis and Flake Powder Metallurgy
Abstract
1. Introduction
2. Experiment
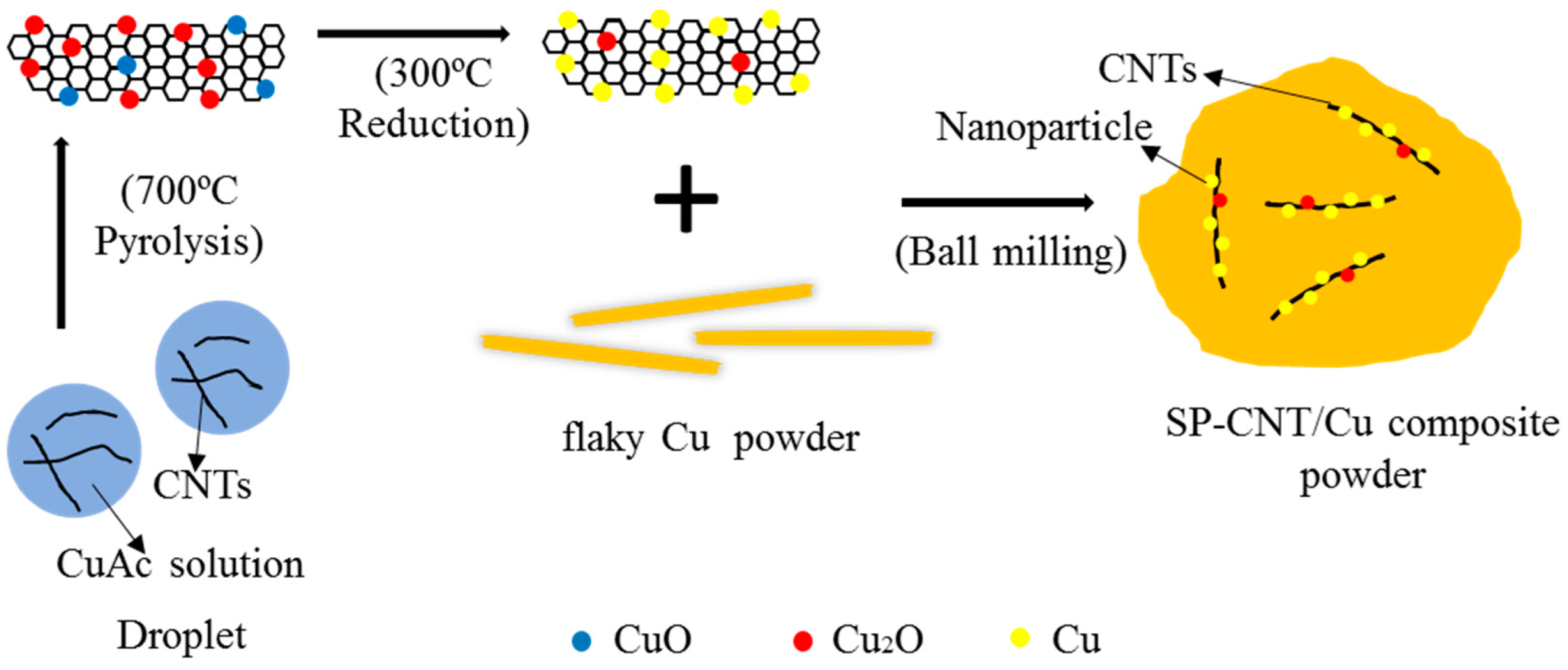
2.1. Preparation of CNT-Cu Oxide Composite Powder by Spraying Pyrolysis (SP)
2.2. Preparation of CNT-Cu Composites
2.3. Characterization Tests
3. Results and Discussion
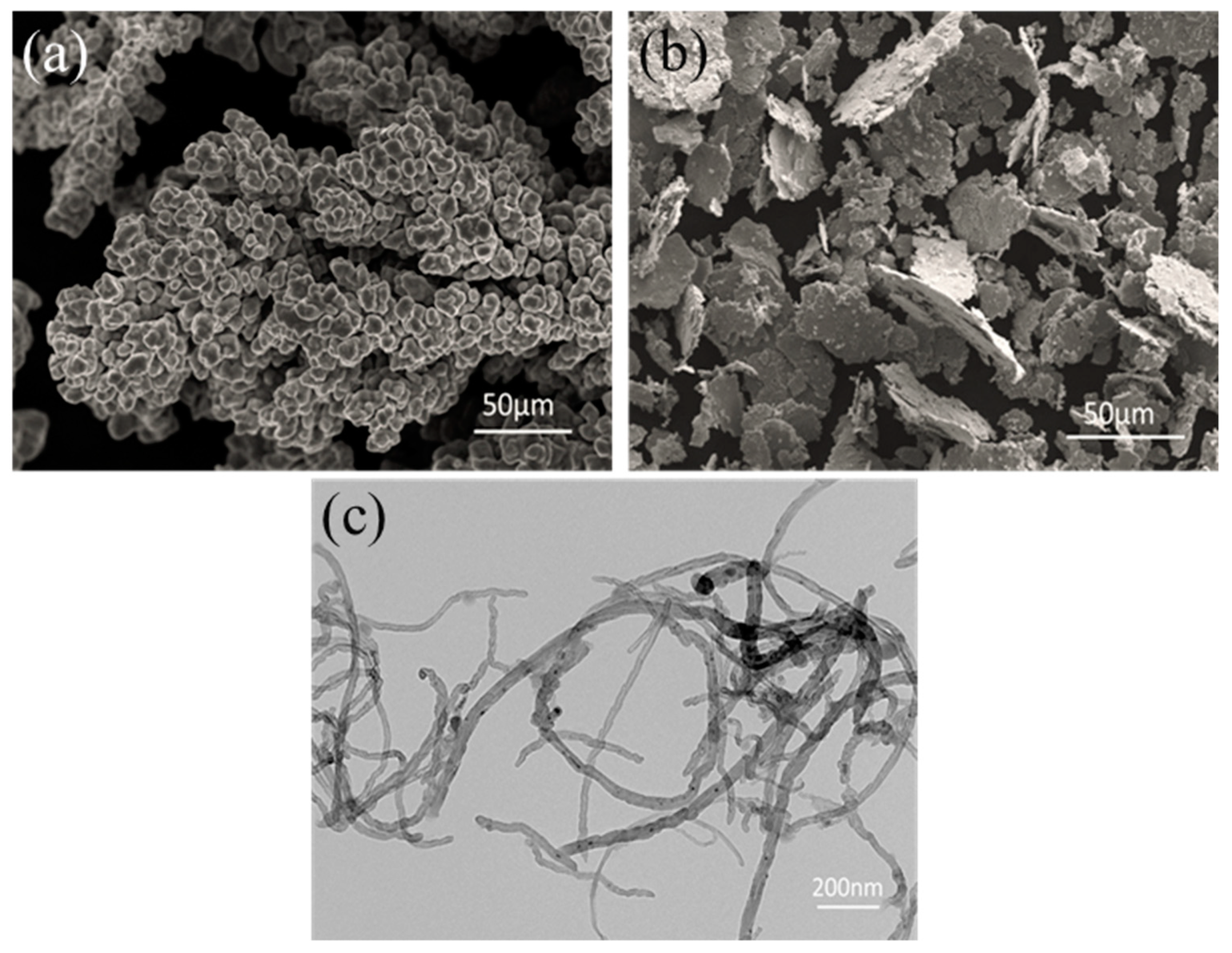
3.1. Starting Materials
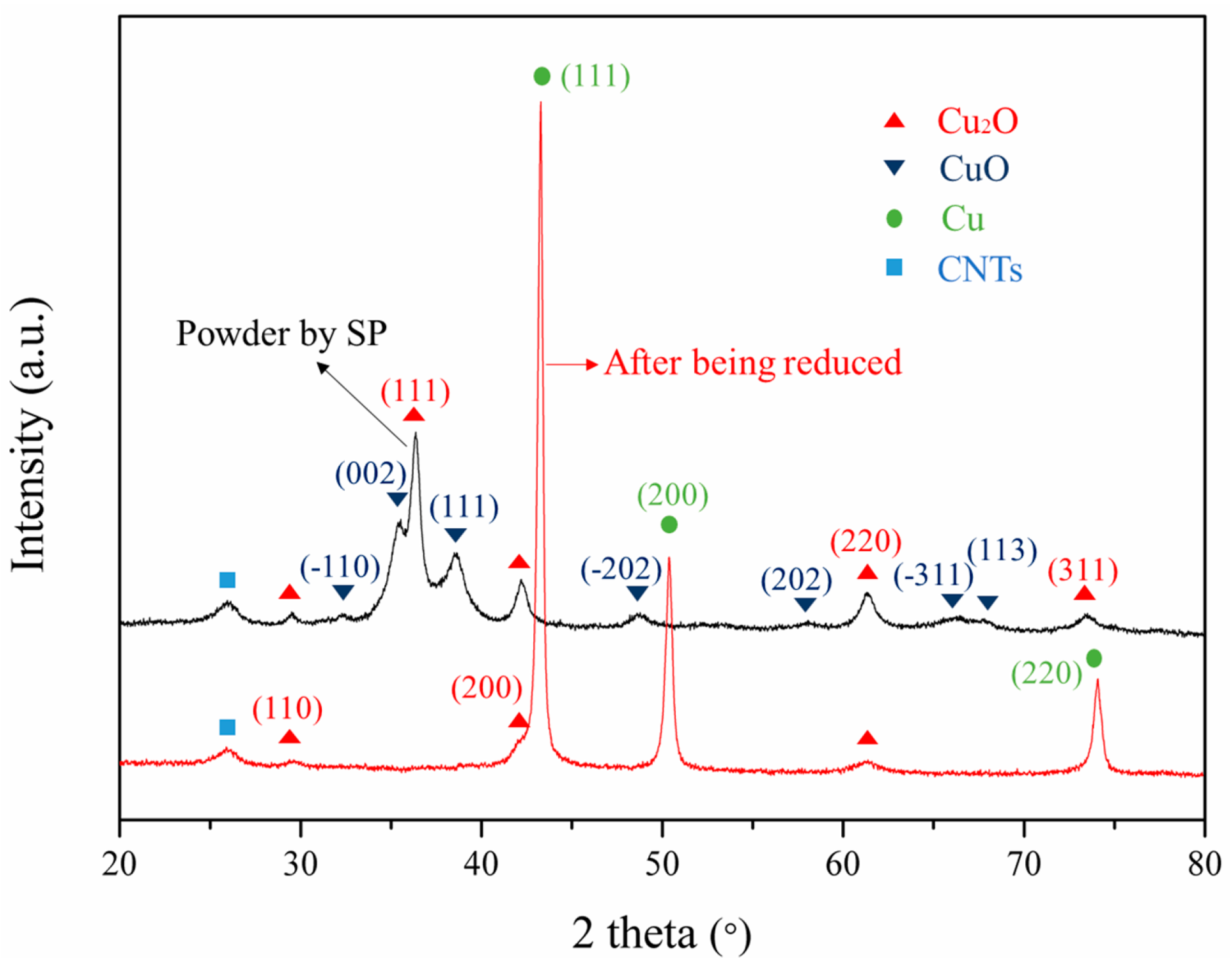
3.2. Composite Powders
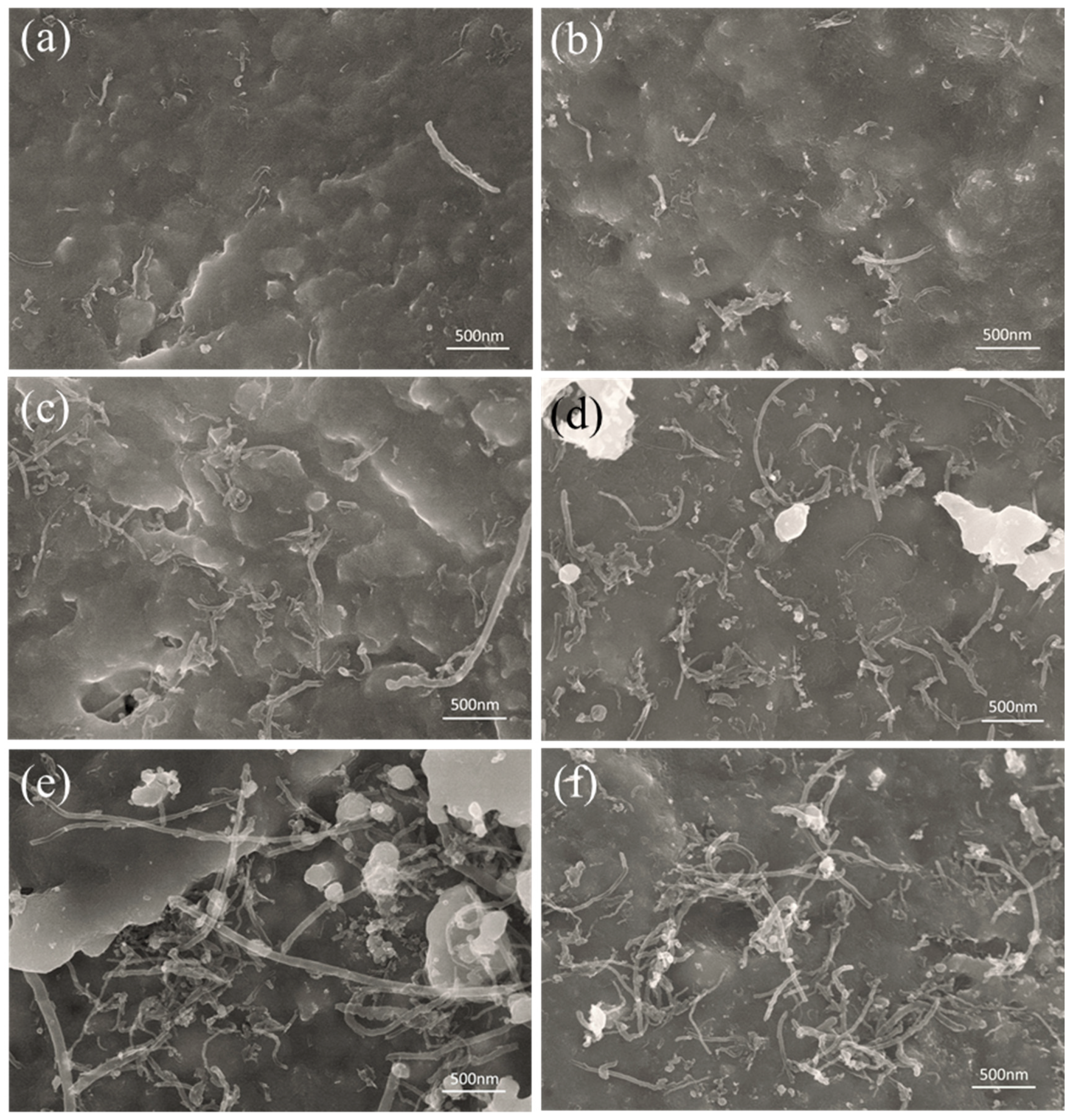
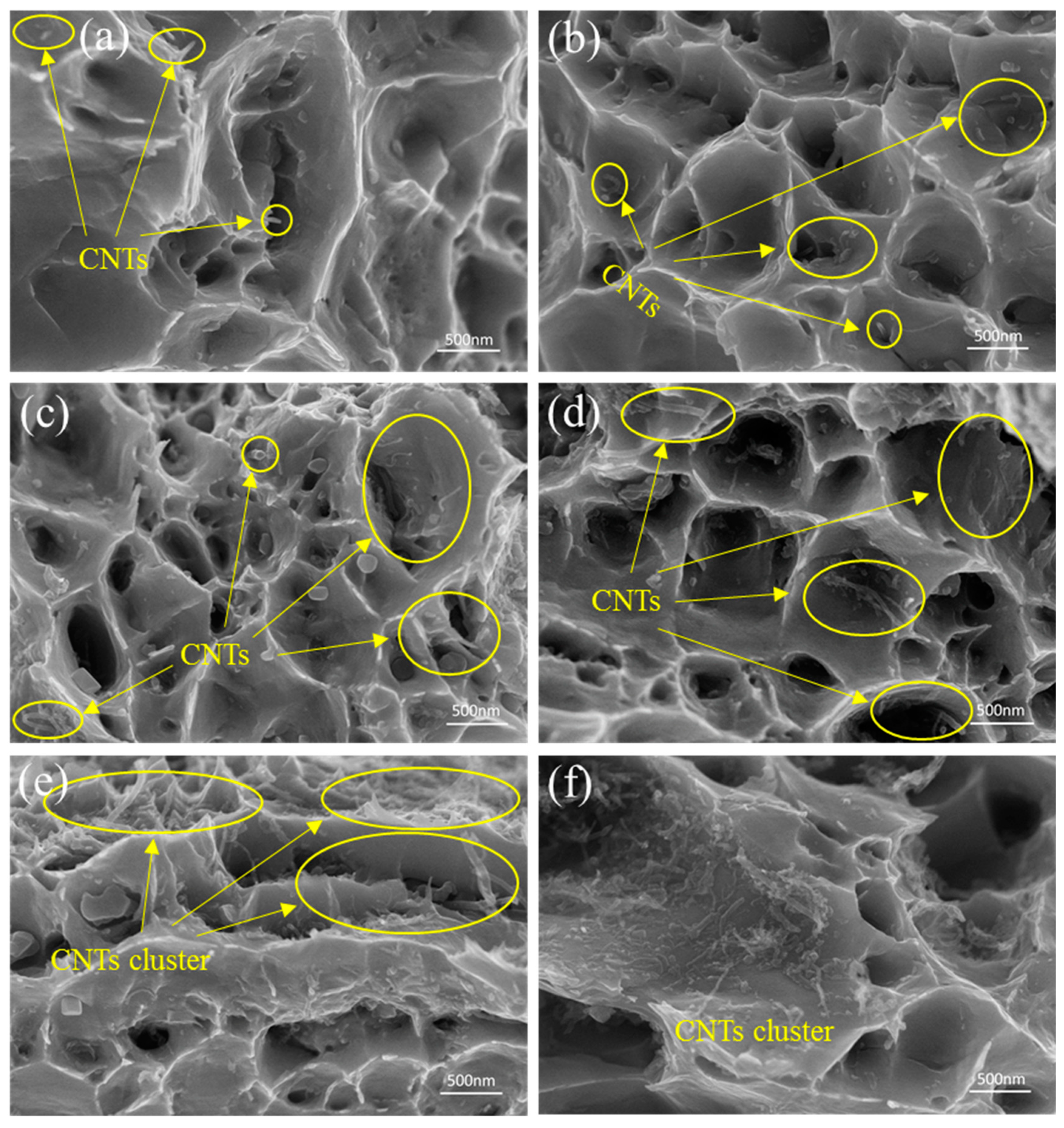
3.3. Tensile Property and Fracture Morphology
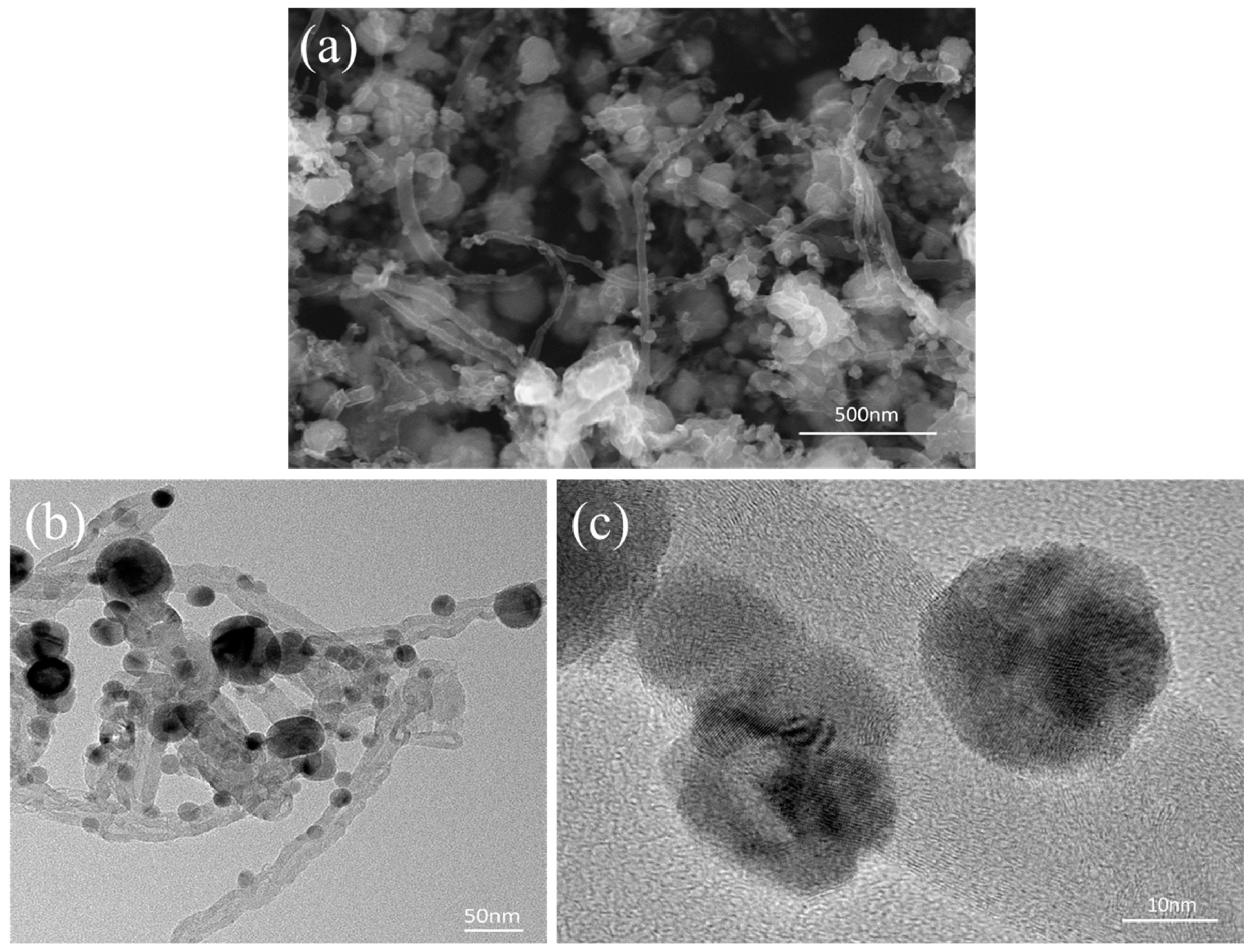
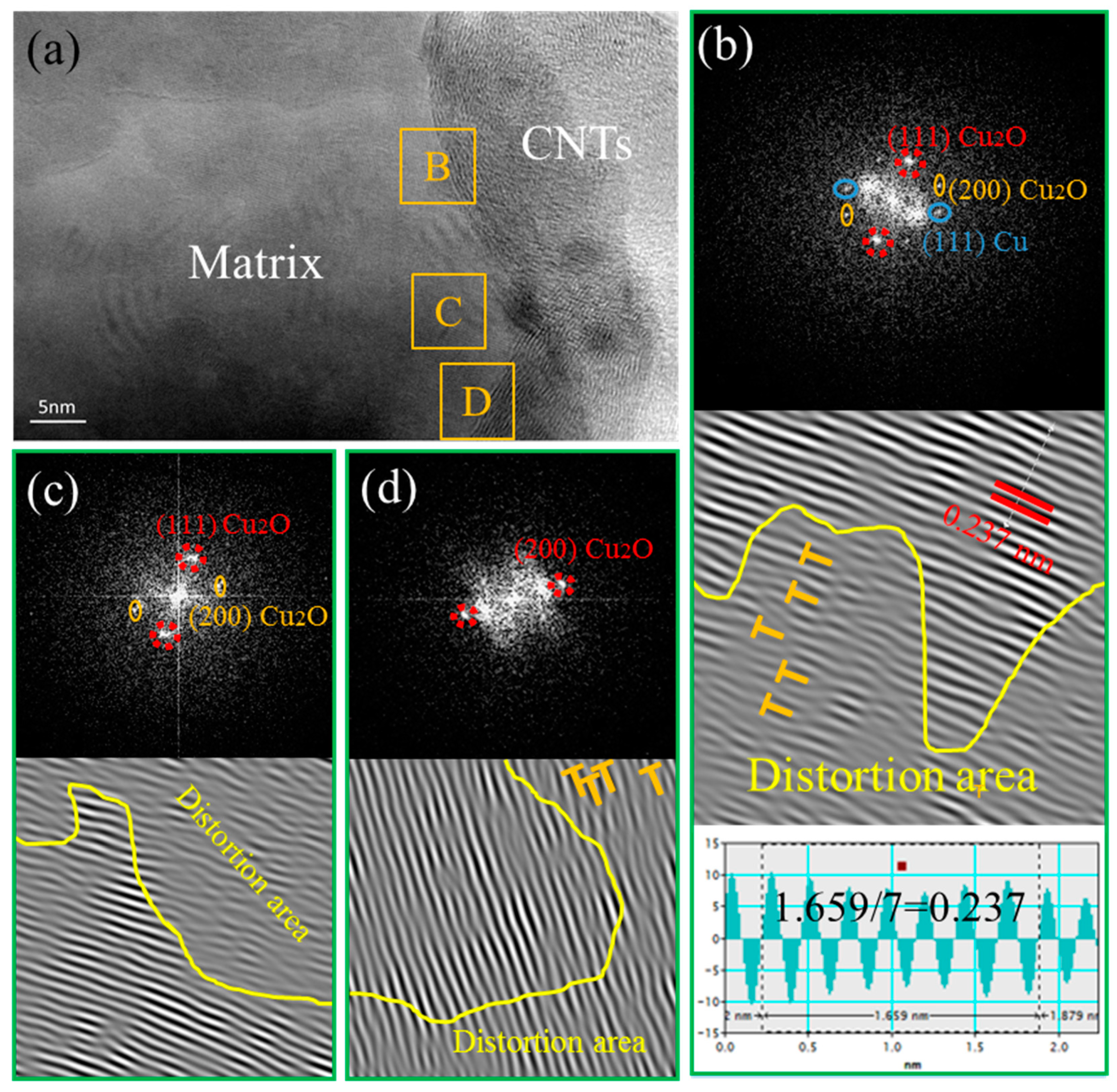
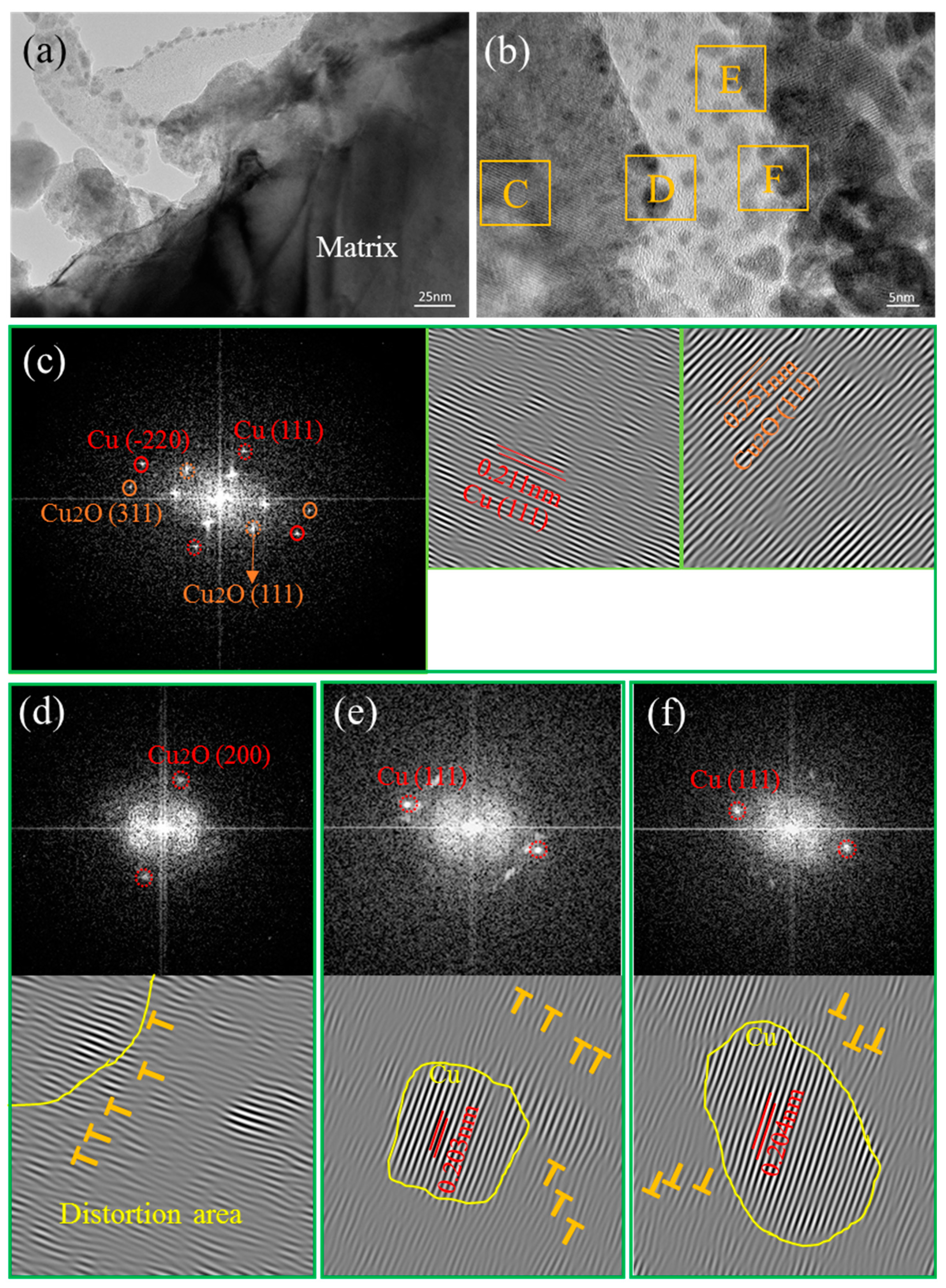
3.4. Interface Characteristics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bor, A.; Ichinkhorloo, B.; Uyanga, B.; Lee, J.; Choi, H. Cu/CNT nanocomposite fabrication with different raw material properties using a planetary ball milling process. Powder Technol. 2018, 323, 563–573. [Google Scholar] [CrossRef]
- Jo, H.R.; Kim, J.T.; Hong, S.H.; Kim, Y.S.; Park, H.J.; Park, W.J.; Park, J.M.; Kim, K.B. Effect of silicon on microstructure and mechanical properties of Cu-Fe alloys. J. Alloys Compd. 2017, 707, 184–188. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, J.; Jin, C.; Liu, Y. Anti-friction properties of Cu10Sn-based composite containing nanometer diamond particles. Wear 2000, 242, 202–206. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; He, Q. Influence of Cu on microstructure and wear resistance of TiC/TiB/TiN reinforced composite coating fabricated by laser cladding. Mater. Chem. Phys. 2012, 133, 741–745. [Google Scholar] [CrossRef]
- Peng, B.; Locascio, M.; Zapol, P.; Li, S.; Mielke, S.L.; Schatz, G.C.; Espinosa, H.D. Measurements of near-ultimate strength for multiwalled carbon nanotubes and irradiation-induced crosslinking improvement. Nat. Nanotechnol. 2008, 3, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.N. Carbon nanotubes: Properties and application. Mater. Sci. Eng. R 2004, 43, 61–102. [Google Scholar] [CrossRef]
- De Volder, M.F.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Aroca, A.S.; Pividal, J.F.R.; Gamez, M.L. Eehamcement of water diffusion and compression performance of crosslinked alginate films with a minuscule amount of graphene oxide. Sci. Rep. 2017, 7, 1–8. [Google Scholar]
- Gamez, M.L.; Aroca, A.S. Low-cast advance hydrogels of calcium alginate/carbon nanofibers with enhanced water diffusion and compression properties. Polymers 2018, 10, 405. [Google Scholar] [CrossRef]
- Chen, W.X.; Tu, J.P.; Wang, L.Y.; Gan, H.Y.; Xu, Z.D.; Zhang, X.B. Tribological application of carbon nanotubes in a metal-based composite coating and composites. Carbon 2003, 41, 215–222. [Google Scholar] [CrossRef]
- Song, J.L.; Chen, W.G.; Dong, L.L.; Wang, J.J.; Deng, N. An electroless plating and planetary ball milling process for mechanical properties enhancement of bulk CNTs/Cu composites. J. Alloys Compd. 2017, 720, 54–62. [Google Scholar] [CrossRef]
- Fan, G.; Jiang, Y.; Tan, Z.; Guo, Q.; Xiong, D.-B.; Su, Y.; Lin, R.; Hu, L.; Li, Z.; Zhang, D. Enhanced interfacial bonding and mechanical properties in CNT/Al composites fabricated by flake powder metallurgy. Carbon 2018, 130, 333–339. [Google Scholar] [CrossRef]
- Chu, K.; Jia, C.C.; Li, W.S. Mechanical and electrical properties of carbon-nanotube-reinforced Cu-Ti alloy matrix composites. Phys. Status Solidi (a) 2013, 210, 594–599. [Google Scholar] [CrossRef]
- Daoush, W.M.; Lim, B.K.; Mo, C.B.; Nam, D.H.; Hong, S.H. Electrical and mechanical properties of carbon nanotube reinforced copper nanocomposites fabricated by electroless deposition process. Mater. Sci. Eng. A 2009, 513, 247–253. [Google Scholar] [CrossRef]
- Sahraei, A.A.; Fathi, A.; Givi, M.K.B.; Pashaei, M.H. Fabricating and improving properties of copper matrix nanocomposites by electroless copper-coated MWCNTs. Appl. Phys. A 2014, 116, 1677–1686. [Google Scholar] [CrossRef]
- Liu, L.; Bao, R.; Yi, J.H.; Fang, D. Fabrication of CNT/Cu composites with enhanced strength and ductility by SP combined with optimized SPS method. J. Alloys Compd. 2018, 47, 91–99. [Google Scholar] [CrossRef]
- Meng, L.; Wang, X.; Ning, J.; Hu, X.; Fan, G.; Wu, K. Beyond the dimensional limitation in bio-inspired composite: Insertion of carbon nanotubes induced laminated Cu composite and the simultaneously enhanced strength and toughness. Carbon 2018, 130, 222–232. [Google Scholar] [CrossRef]
- Jiang, L.; Fan, G.; Li, Z.; Kai, X.; Zhang, D.; Chen, Z.; Humphries, S.; Heness, G.; Yeung, W.Y. An approach to the uniform dispersion of a high volume fraction of carbon nanotubes in aluminum powder. Carbon 2011, 49, 1965–1971. [Google Scholar] [CrossRef]
- Li, Z.; Fan, G.; Tan, Z.; Li, Z.; Guo, Q.; Xiong, D.; Zhang, D. A versatial method for uniform dispersion of nanocarbons in metal matrix based on electrostatic interactions. Nano-Micro Lett. 2015, 8, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S. Thermoanalytical investigations of the decomposition course of copper oxysalts. J. Ther. Anal. 1995, 45, 1381–1392. [Google Scholar] [CrossRef]
- Cha, S.I.; Kim, K.T.; Arshad, S.N. Extraordinary strengthening effect of carbon nanotubes in metal-matrix nanocomposites processes by molecular-level mixing. Adv. Mater. 2005, 17, 1377–1381. [Google Scholar] [CrossRef]
- Jagannatham, S.; Sankaran, S.; Haridoss, P. Electroless nickel plating of arc discharge synthesized carbon nanotubes for metal matrix composites. Appl. Surf. Sci. 2015, 324, 475–485. [Google Scholar]
- Lim, B.; Kim, C.; Kim, B. The effrcts of interfacial bonding on mechanical properties of single-walled carbon nanotube reinforced copper matrix nanocomposites. Nanotechnology 2006, 17, 5759–5764. [Google Scholar] [CrossRef]
- Bakshi, S.R.; Agarwal, A. An analysis of the factors affecting strengthening in carbon nanotube reinforced aluminum composites. Carbon 2011, 49, 533–544. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Zhao, K.; Xiao, B.L.; Wang, W.G.; Ma, Z.Y. Fabrication of CNT/Al composites with low damage to CNTs by a novel solution-assisted wet mixing combined with powder metallurgy processing. Mater. Des. 2016, 97, 424–430. [Google Scholar] [CrossRef]
- Moghadam, A.D.; Omrani, E.; Menezes, P.L.; Rohatgi, P.K. Mechanical and tribological properties of self-lubricating metal matrix nanocomposites reinforced by carbon nanotubes (CNTs) and graphene—A review. Compos. Part B: Eng. 2015, 77, 402–420. [Google Scholar]
- Goh, C.S.; Wei, J.; Lee, L.C. Development of novel carbon nanotube reinforced magnesium nanocomposites using the powder metallurgy technique. Nanotechnology 2005, 17, 7–12. [Google Scholar] [CrossRef]
- Nam, D.H.; Cha, S.I.; Lim, B.K.; Park, H.M.; Han, D.S.; Hong, S.H. Synergistic strengthening by load transfer mechanism and grain refinement of CNT/Al–Cu composites. Carbon 2012, 50, 2417–2423. [Google Scholar] [CrossRef]
- George, R.; Kashyap, K.T.; Rahul, R.; Yamdagni, S. Strengthening in carbon nanotube/aluminium (CNT/Al) composites. Scr. Mater. 2005, 53, 1159–1163. [Google Scholar] [CrossRef]
- Chen, B.; Li, S.; Imai, H.; Jia, L.; Umeda, J.; Takahashi, M.; Kondoh, K. Load transfer strengthening in carbon nanotubes reinforced metal matrix composites via in-situ tensile tests. Compos. Sci. Technol. 2015, 113, 1–8. [Google Scholar] [CrossRef]
- File, P.D. JCPDS International, Centre for Diffraxtion Data; PCDFWIN V 23; ICDD: Newtown Square, PA, USA, 2002. [Google Scholar]
- Hull, D.; Bacon, D.J. Introduction to Dislocations, 5th ed.; Butterworth-Heinemann: Oxford, UK, 2002. [Google Scholar]
- Kim, K.T.; Cha, S.I.; Gemming, T. The role of interfacial oxygen atoms in the enhanced mechanical properties of carbon-nanotube-reinforced metal matrix nanocomposites. Small 2008, 11, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.H.; Zhou, G.H.; Liao, L.; Liu, Y.; Luo, L. Interfacial structure in AZ91 alloy composites reinforced by grapherne nanosheets. Carbon 2018, 127, 177–186. [Google Scholar] [CrossRef]
- Li, L.B.; Bao, R.; Yi, J.H.; Liu, L.; Mao, S. Prepartion of CNTs/Cu nano composite powder with uniform dispersion and strong interface bonding by SP method. Powder Technol. 2018, 325, 107–112. [Google Scholar] [CrossRef]
- Chen, B.; Shen, J.; Ye, X.; Jia, L.; Li, S.; Umeda, J.; Takahashi, M.; Kondoh, K. Length effect of carbon nanotubes on the strengthening mechanisms in metal matrix composites. Acta Mater. 2017, 140, 317–325. [Google Scholar] [CrossRef]
- Munoz-morris, M.A.; Oca, C.G.; Morris, D.G. An analysis of strengthening mechanisms in a mechanically alloyed, oxide dispersion strengthened iron aluminide intermetallic. Acta Mater. 2002, 50, 2825–2836. [Google Scholar] [CrossRef]
- Park, J.G.; Keum, D.H.; Lee, Y.H. Strengthening mechanisms in carbon nanotube-reinforced aluminum composites. Carbon 2015, 95, 690–698. [Google Scholar] [CrossRef]
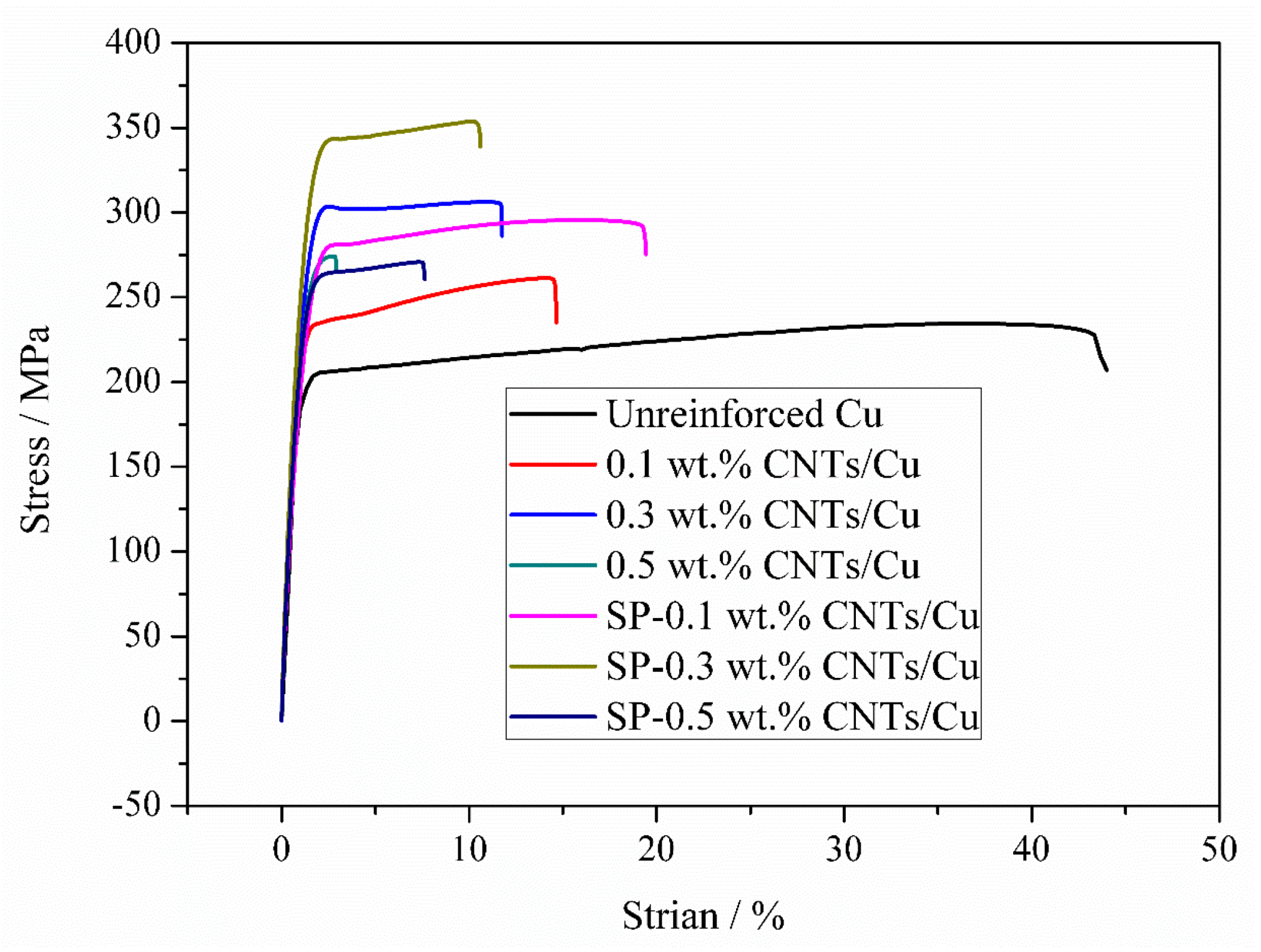

| Sample | Relative Density (%) | Hardness (HV) | UTS (MPa) | Elongation (%) | Enhancement (%) |
|---|---|---|---|---|---|
| Unreinforced Cu | 98.7 | 68.5 | 234.2 ± 6.0 | 43.3 ± 0.3 | -- |
| 0.1 wt % CNT-Cu | 98.6 | 87.1 | 261.2 ± 5.5 | 14.6 ± 0.3 | 11.5 |
| 0.3 wt % CNT-Cu | 97.7 | 95.3 | 306.2 ± 4.4 | 11.7 ± 0.5 | 30.7 |
| 0.5 wt % CNT-Cu | 96.1 | 98.8 | 274.1 ± 3.5 | 2.9 ± 1.0 | 17.1 |
| SP-0.1 wt % CNT-Cu | 98.7 | 94.4 | 295.5 ± 4.8 | 19.4 ± 0.5 | 26.2 |
| SP-0.3 wt % CNT-Cu | 97.9 | 102.3 | 353.9 ± 4.8 | 10.6 ± 0.5 | 51.5 |
| SP-0.5 wt % CNT-Cu | 97.7 | 105.65 | 270.7 ± 5.0 | 7.6 ± 0.6 | 15.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Bao, R.; Yi, J.; Fang, D.; Tao, J.; Liu, Y. Enhancing Interfacial Bonding and Tensile Strength in CNT-Cu Composites by a Synergetic Method of Spraying Pyrolysis and Flake Powder Metallurgy. Materials 2019, 12, 670. https://doi.org/10.3390/ma12040670
Chen X, Bao R, Yi J, Fang D, Tao J, Liu Y. Enhancing Interfacial Bonding and Tensile Strength in CNT-Cu Composites by a Synergetic Method of Spraying Pyrolysis and Flake Powder Metallurgy. Materials. 2019; 12(4):670. https://doi.org/10.3390/ma12040670
Chicago/Turabian StyleChen, Xiangyang, Rui Bao, Jianhong Yi, Dong Fang, Jingmei Tao, and Yichun Liu. 2019. "Enhancing Interfacial Bonding and Tensile Strength in CNT-Cu Composites by a Synergetic Method of Spraying Pyrolysis and Flake Powder Metallurgy" Materials 12, no. 4: 670. https://doi.org/10.3390/ma12040670
APA StyleChen, X., Bao, R., Yi, J., Fang, D., Tao, J., & Liu, Y. (2019). Enhancing Interfacial Bonding and Tensile Strength in CNT-Cu Composites by a Synergetic Method of Spraying Pyrolysis and Flake Powder Metallurgy. Materials, 12(4), 670. https://doi.org/10.3390/ma12040670





