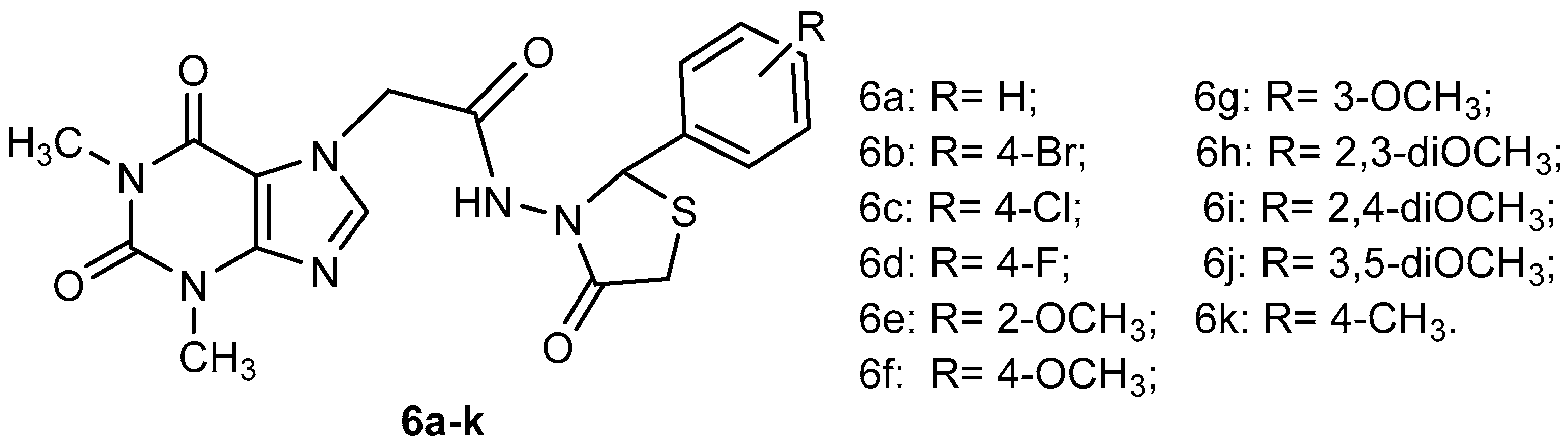
Formulation and Characterization of New Polymeric Systems Based on Chitosan and Xanthine Derivatives with Thiazolidin-4-One Scaffold
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Development of New Polymeric Systems Based on Chitosan
2.2.1. Preparation of Chitosan Microparticles
2.2.2. Preparation of Chitosan Microparticles Loaded with Xanthine Derivatives with Thiazolidine-4-One Scaffold (CS-XTDs)
2.3. Characterization of Chitosan Microparticles Loaded with Xanthine Derivatives with Thiazolidine-4-one Scaffold (CS-XTDs)
2.3.1. Particle Size and Morphology
2.3.2. Swelling Degree (SD)
2.3.3. Drug Loading and Entrapment Efficiency
2.3.4. In Vitro Release
2.3.5. Fourier Transform Infrared (FTIR) Spectroscopy
2.4. Biological Evaluation
2.4.1. Acute Toxicity Assay
2.4.2. Antibacterial/Antifungal Tests
The Agar Disc Diffusion Method.
The Broth Micro-Dilution Method.
3. Results and Discussions
3.1. New Polymeric Systems Based on Chitosan
3.1.1. Particle Size and Morphology
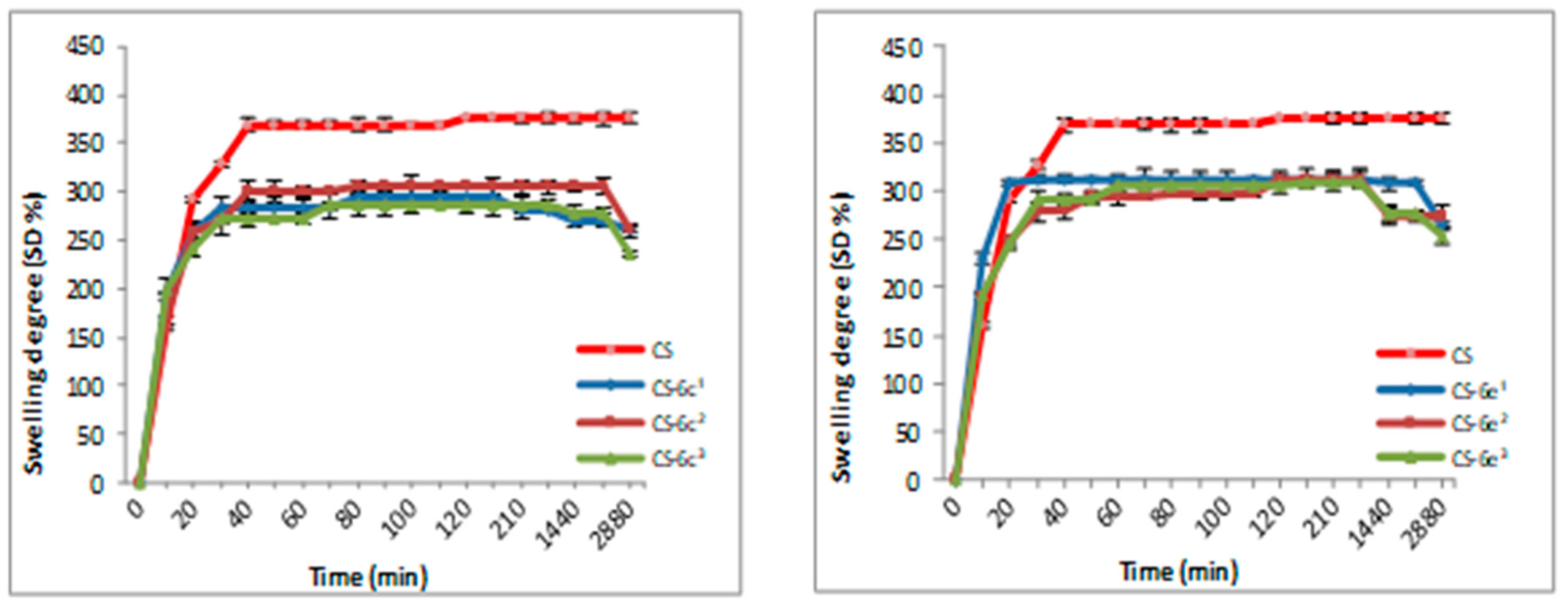
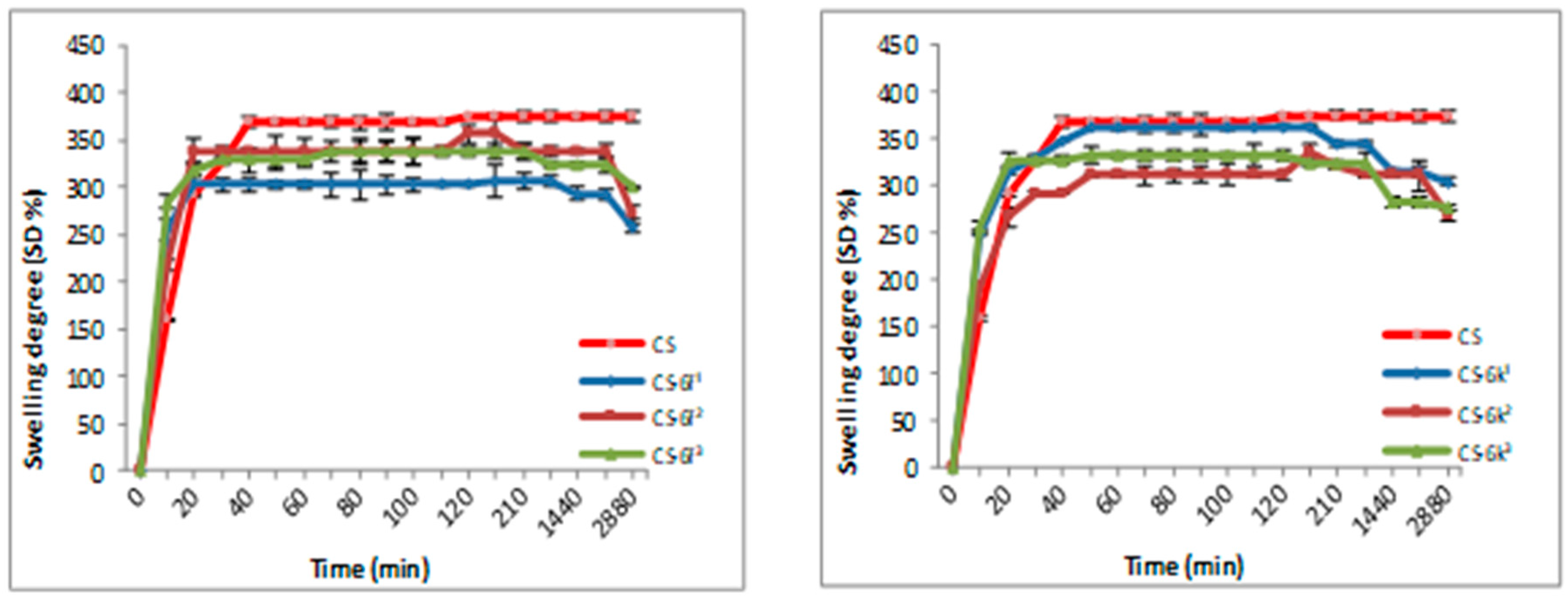
3.1.2. Swelling Degree (SD)
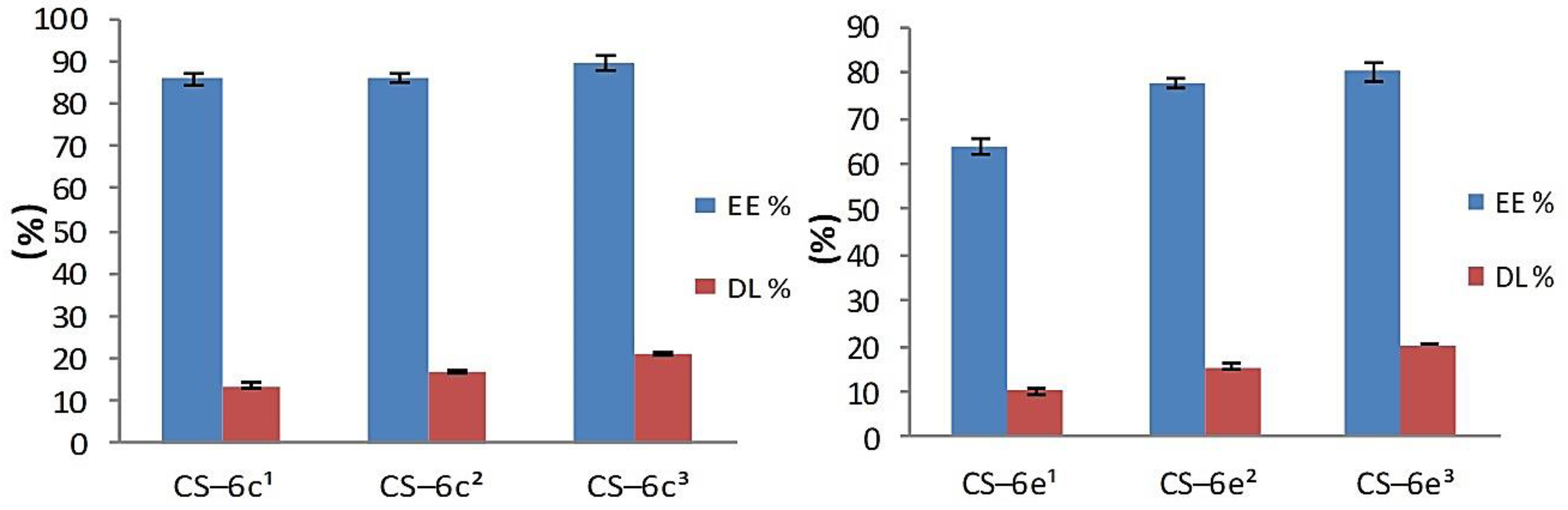
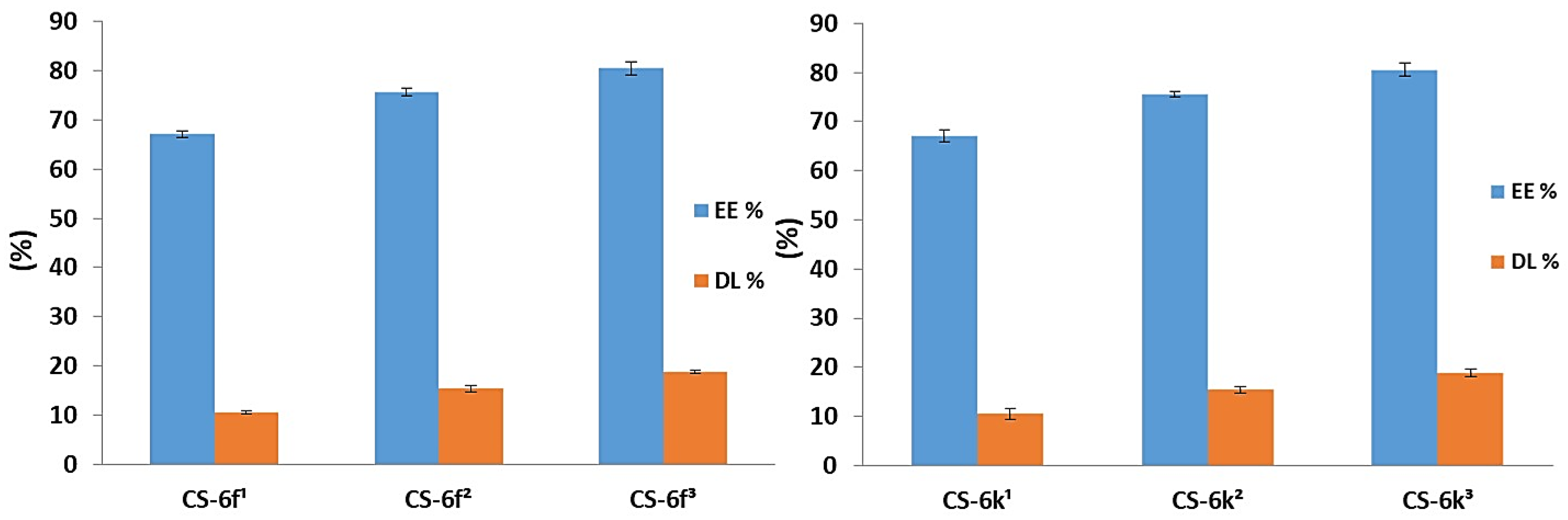
3.1.3. Drug Loading and Entrapment Efficiency
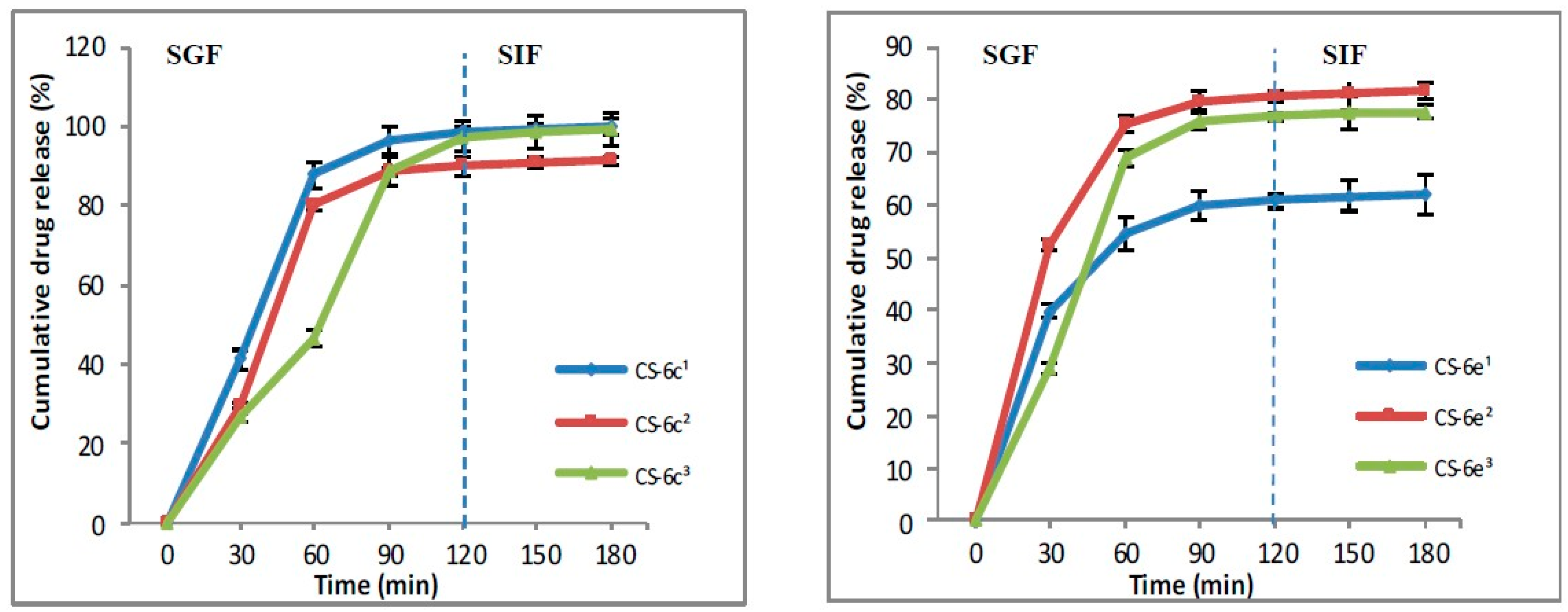
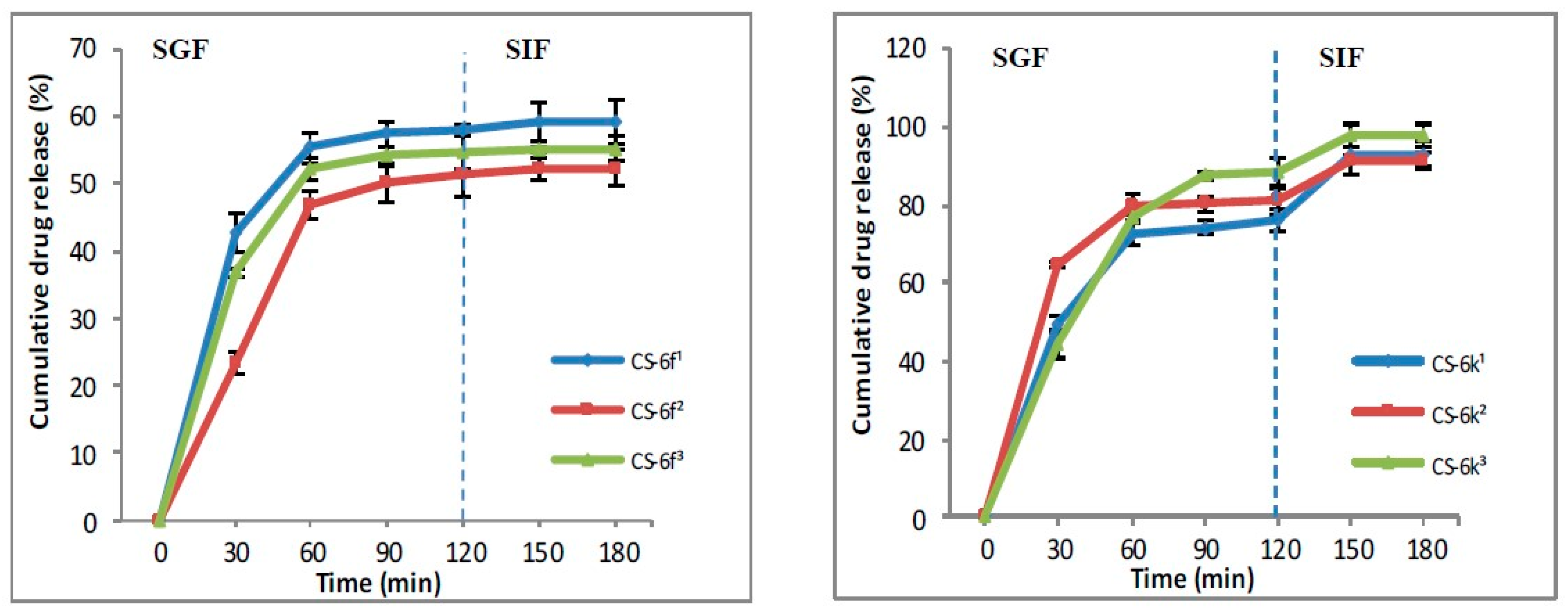
3.1.4. In Vitro Drug Release
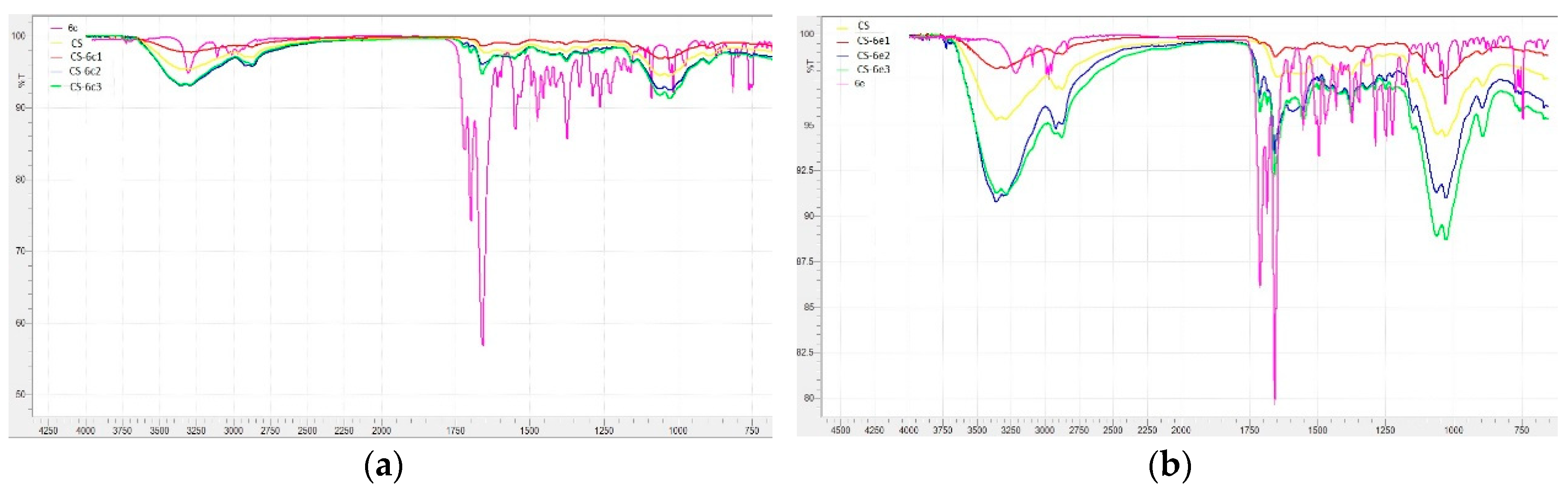
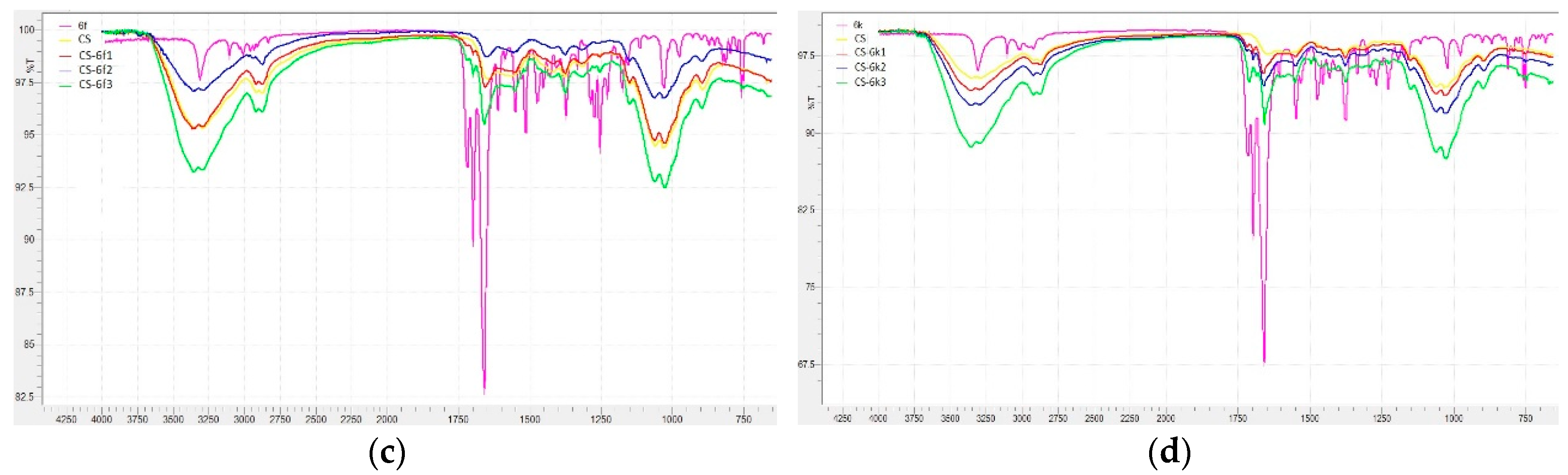
3.1.5. FTIR Analysis
3.2. Biological Evaluation
3.2.1. Toxicity Degree
3.2.2. Antibacterial/Antifungal Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) |
| bw | Body weight |
| CFU | Colony Forming Unit |
| CS | Chitosan |
| CS-XTDs | Chitosan microparticles loaded with xanthine derivatives with thiazolidine-4-one scaffold |
| DL | Drug loading |
| DMSO | Dimethyl sulfoxide |
| DPPH | 2,2-diphenyl-1-picryl-hydrazyl-hydrate |
| DR | Drug release |
| EE | Entrapment efficiency |
| FT-IR | Fourier transform infrared |
| LD50 | Lethal dose 50 |
| MBC | Minimum bactericidal concentration |
| MFC | Minimum fungicidal concentration |
| MIC | Minimum inhibitory concentration |
| SD | Swelling degree |
| SEM | Scanning electron microscopy |
| SGF | Simulated gastric fluid |
| SIF | Simulated intestinal fluid |
| TPP | Pentasodium tripolyphosphate |
| XTDs | Xanthine derivatives with thiazolidine-4-one scaffold |
References
- Martinez-Lopez, S.; Sarria, B.; Gomez-Juaristi, M.; Goya, L.; Mateos, R.; Bravo-Clemente, L. Theobromine, caffeine, and theophylline metabolites in human plasma and urine after consumption of soluble cocoa products with different methylxanthine contents. Food Res. Int. 2014, 63, 446–455. [Google Scholar] [CrossRef]
- Singh, N.; Shreshtha, A.K.; Thakur, M.S.; Patra, S. Xanthine scaffold: Scope and potential in drug development. Heliyon 2018, 4, 00829. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.P.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. Structure-Bioactivity Relationships of Methylxanthines: Trying to Make Sense of All the Promises and the Drawbacks. Molecules 2016, 21, 974. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Calzetta, L.; Barnes, P.J.; Criner, G.J.; Martinez, F.J.; Papi, A.; Matera, M.G. Efficacy and safety profile of xanthines in COPD: A network meta-analysis. Eur. Respir. Rev. 2018, 27, 180010. [Google Scholar] [CrossRef] [PubMed]
- Lupascu, F.G.; Dash, M.; Samal, S.K.; Dubruel, P.; Lupusoru, C.E.; Lupusoru, R.V.; Dragostin, O.; Profire, L. Development, optimization and biological evaluation of chitosan scaffold formulations of new xanthine derivatives for treatment of type-2 diabetes mellitus. Eur. J. Pharm. Sci. 2015, 77, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.S.; Yao, Y.; Zheng, Y.C.; Feng, S.; Chang, J.; Yu, B.; Liu, H.M. Ligand-based design, synthesis and biological evaluation of xanthine derivatives as LSD1/KDM1A inhibitors. Eur. J. Med. Chem. 2019, 162, 555–567. [Google Scholar] [CrossRef]
- Ruddarraju, R.R.; Murugulla, A.C.; Kotla, R.; Chandra Babu Tirumalasetty, M.; Wudayagiri, R.; Donthabakthuni, S.; Maroju, R.; Baburao, K.; Parasa, L.S. Design, synthesis, anticancer, antimicrobial activities and molecular docking studies of theophylline containing acetylenes and theophylline containing 1,2,3-triazoles with variant nucleoside derivatives. Eur. J. Med. Chem. 2016, 123, 379–396. [Google Scholar] [CrossRef]
- Wang, X.; Han, C.; Xu, Y.; Wu, K.; Chen, S.; Hu, M.; Wang, L.; Ye, Y.; Ye, F. Synthesis and Evaluation of Phenylxanthine Derivatives as Potential Dual A2AR Antagonists/MAO-B Inhibitors for Parkinson’s Disease. Molecules 2017, 22, 1010. [Google Scholar] [CrossRef]
- Borde, R.M.; Gaikwad, M.A.; Waghmare, R.A.; Munde, A.S. Design, Synthesis and In-Vitro Anti-inflammatory, Antimicrobial Activities of Some Novel 2,3-Disubstituted-1,3-Thiazolidin-4-One Derivatives Containing Thiazole Moiety. J. Ultra Chem. 2018, 14, 104–114. [Google Scholar] [CrossRef]
- Da Silva, I.M.; da Silva Filho, J.; Santiago, P.B.; do Egito, M.S.; de Souza, C.A.; Gouveia, F.L.; Ximenes, R.M.; de Sena, K.X.; de Faria, A.R.; Brondani, D.J.; et al. Synthesis and antimicrobial activities of 5-Arylidene-thiazolidine-2,4-dione derivatives. Biomed. Res. Int. 2014, 2014, 316082. [Google Scholar] [CrossRef]
- Samadhiya, P.; Sharma, R.; Srivastava, S.K.; Srivastava, S.D. Synthesis and biological evaluation of 4-thiazolidinone derivatives as antitubercular and antimicrobial agents. Arab. J. Chem. 2014, 7, 657–665. [Google Scholar] [CrossRef]
- Ramachandran, S.; Bharathi, B.; Lavanya, R.; Nandhini, R.; Sivaranjani, R.; Sundhararajan, R. Synthesis, characterisation, antimicrobial evaluation of 2-hydroxy phenyl thiazolidine-4-one derivative. Int. J. Pharm. Pharm. Sci. 2017, 6, 278–283. [Google Scholar] [CrossRef]
- Solankee, A. Synthesis, Characterization and in vitro antimicrobial activity of some new 2,3-disubstituted-4-thiazolidinone and 2,3-disubstituted-5-methyl-4-thiazolidinone derivatives as a biologically active scaffold. Int. J. Pharm. Pharm. Sci. 2016, 6, 386–399. [Google Scholar]
- Ray, M.; Pal, K.; Anis, A.; Banthia, A.K. Development and Characterization of Chitosan-Based Polymeric Hydrogel Membranes. Des. Monomers Polym. 2010, 13, 193–206. [Google Scholar] [CrossRef]
- Ferreira Tomaz, A.; Sobral de Carvalho, S.M.; Cardoso Barbosa, R.; L. Silva, S.M.; Sabino Gutierrez, M.A.; B. de Lima, A.G.; L. Fook, M.V. Ionically Crosslinked Chitosan Membranes Used as Drug Carriers for Cancer Therapy Application. Materials 2018, 11, 2051. [Google Scholar] [CrossRef] [PubMed]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Xie, J.; Li, A.; Li, J. Advances in pH-Sensitive Polymers for Smart Insulin Delivery. Macromol. Rapid. Commun. 2017, 38, 1700413. [Google Scholar] [CrossRef]
- Lizardi-Mendoza, J.; Argüelles-Monal, W.M.; Goycoolea, F.M. Chemical Characteristics and Functional Properties of Chitosan. In Chitosan in the Preservation of Agricultural Commodities; Bautista-Baños, S., Romanazzi, G., Jiménez-Aparicio, A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 3–31. [Google Scholar]
- O’Callaghan, K.A.M.; Kerry, J.P. Preparation of low- and medium-molecular weight chitosan nanoparticles and their antimicrobial evaluation against a panel of microorganisms, including cheese-derived cultures. Food Control 2016, 69, 256–261. [Google Scholar] [CrossRef]
- Xing, R.; He, X.; Liu, S.; Yu, H.; Qin, Y.; Chen, X.; Li, K.; Li, R.; Li, P. Antidiabetic Activity of Differently Regioselective Chitosan Sulfates in Alloxan-Induced Diabetic Rats. Mar. Drugs 2015, 13, 3072–3090. [Google Scholar] [CrossRef]
- Barakat, N.S.; Almurshedi, A.S. Design and development of gliclazide-loaded chitosan microparticles for oral sustained drug delivery: in-vitro/in-vivo evaluation. J. Pharm. Pharmacol. 2011, 63, 169–178. [Google Scholar] [CrossRef]
- Perumal, V.; Manickam, T.; Bang, K.S.; Velmurugan, P.; Oh, B.T. Antidiabetic potential of bioactive molecules coated chitosan nanoparticles in experimental rats. Int. J. Biol. Macromol. 2016, 92, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, S.; An, F.; Ma, S.; Cheng, Y.; Liu, W.; Xu, F.; Li, M. Water soluble chitosans shows anti-cancer effect in mouse H22 liver cancer by enhancing the immune response. Int. J. Clin. Exp. Med. 2016, 9, 164–171. [Google Scholar]
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018, 2018, 1708172. [Google Scholar] [CrossRef]
- Ghosh, S.; Bhattacharyya, S.; Rashid, K.; Sil, P.C. Curcumin protects rat liver from streptozotocin-induced diabetic pathophysiology by counteracting reactive oxygen species and inhibiting the activation of p53 and MAPKs mediated stress response pathways. Toxicol. Rep. 2015, 2, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Kalam, M.A. Development of chitosan nanoparticles coated with hyaluronic acid for topical ocular delivery of dexamethasone. Int. J. Biol. Macromol. 2016, 89, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Panith, N.; Wichaphon, J.; Lertsiri, S.; Niamsiri, N. Effect of physical and physicochemical characteristics of chitosan on fat-binding capacities under in vitro gastrointestinal conditions. LWT Food Sci. Technol. 2016, 71, 25–32. [Google Scholar] [CrossRef]
- Li, J.; Xie, B.; Xia, K.; Li, Y.; Han, J.; Zhao, C. Enhanced Antibacterial Activity of Silver Doped Titanium Dioxide-Chitosan Composites under Visible Light. Materials 2018, 11, 1403. [Google Scholar] [CrossRef]
- Benito-Pena, E.; González-Vallejo, V.; Rico-Yuste, A.; Barbosa-Pereira, L.; Cruz, J.M.; Bilbao, A.; Alvarez-Lorenzo, C.; Moreno-Bondi, M.C. Molecularly imprinted hydrogels as functional active packaging materials. Food Chem. 2016, 190, 487–494. [Google Scholar] [CrossRef]
- Sung, S.Y.; Sin, L.T.; Tee, T.T.; Bee, S.T.; Rahmat, A.R.; Rahman, W.A.W.A.; Tan, A.C.; Vikhraman, M. Antimicrobial agents for food packaging applications. Trends Food Sci. Technol. 2013, 33, 110–123. [Google Scholar] [CrossRef]
- Limbo, S.; Mousavi Khaneghah, A. Active packaging of foods and its combination with electron beam processing. In Electron Beam Pasteurization and Complementary Food Processing Technologies; Pillai, S., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 195–217. [Google Scholar]
- Mousavi Khaneghah, A.; Hashemi, S.M.B.; Limbo, S. Antimicrobial agents and packaging systems in antimicrobial active food packaging: An overview of approaches and interactions. Food Bioprod. Process. 2018, 111, 1–19. [Google Scholar] [CrossRef]
- Diblan, S.; Kaya, S. Antimicrobials used in active packaging films. Food Health 2018, 4, 63–79. [Google Scholar] [CrossRef]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial food packaging: Potential and pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan based antimicrobial films for food packaging applications. E-Polymers 2008, 8, 093. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Hashemi, S.M.B.; Eş, I.; Fracassetti, D.; Limbo, S. Efficacy of Antimicrobial Agents for Food Contact Applications: Biological Activity, Incorporation into Packaging, and Assessment Methods: A Review. J. Food Prot. 2018, 81, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.D.; Niu, Y.S.; Yue, P.P.; Hu, Y.; Bian, J.; Li, M.F.; Peng, F.; Sun, R.C. Composite Film Based on Pulping Industry Waste and Chitosan for Food Packaging. Materials 2018, 11, 2264. [Google Scholar] [CrossRef] [PubMed]
- Constantin, S.; Lupascu, F.G.; Apotrosoaei, M.; Vasincu, I.M.; Lupascu, D.; Buron, F.; Routier, S.; Profire, L. Synthesis and biological evaluation of the new 1,3-dimethylxanthine derivatives with thiazolidine-4-one scaffold. Chem. Cent. J. 2017, 11, 12–25. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Si, S.; Wang, L.; Shi, C.; Sun, Y.; Liang, Z.; Mao, S. Effect of formulation variables on in vitro release of a water-soluble drug from chitosan-sodium alginate matrix tablets. Asian J. Pharm. Sci. 2014, 10, 314–321. [Google Scholar] [CrossRef]
- Venkatesan, B.; Tumala, A.; Subramanian, V.; Vellaichamy, E. Data on synthesis and characterization of chitosan nanoparticles for in vivo delivery of siRNA-Npr3: Targeting NPR-C expression in the heart. Data Brief 2016, 8, 441–447. [Google Scholar] [CrossRef]
- Papadimitriou, S.; Bikiaris, D. Novel self-assembled core-shell nanoparticles based on crystalline amorphous moieties of aliphatic copolyesters for efficient controlled drug release. J. Control Release 2009, 138, 177–184. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Divya, P.L.; Nima, J. Synthesis and characterization of novel drug delivery system using modified chitosan based hydrogel grafted with cyclodextrin. Chem. Eng. J. 2016, 284, 1259–1269. [Google Scholar] [CrossRef]
- Dash, M.; Chiellinia, F.; Ottenbriteb, R.M.; Chiellinia, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Oprea, A.M.; Nistor, M.T.; Popa, M.I.; Lupusoru, C.E.; Vasile, C. In vitro and in vivo theophylline release from cellulose/chondroitin sulfate hydrogels. Carbohydr. Polym. 2012, 90, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Deora, P.S.; Mishra, C.K.; Mavani, P.; Asha, R.; Shrivastava, B.; Rajesh, K.N. Effective alternative methods of LD50 help to save number of experimental animals. J. Chem. Pharm. Res. 2010, 2, 450–453. [Google Scholar]
- Yadav, R.; Bansal, R.; Rohilla, S.; Kachler, S.; Klotz, K.N. Synthesis and pharmacological characterization of novel xanthine carboxylatae amides as A2A adenosine receptor ligands exhibiting bronchospasmolytic activity. Bioorg. Chem. 2016, 65, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Koeth, L.M.; DiFranco-Fisher, J.M.; Scangarella-Oman, N.E.; Miller, L.A. Analysis of MIC and disk diffusion testing variables for gepotidacin and comparator agents against select bacterial pathogens. J. Clin. Microbiol. 2017, 55, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar] [PubMed]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure andinteractions in covalently and ionically crosslinked chitosan hydrogels forbiomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Grenha, A. Chitosan nanoparticles: A survey of preparation methods. J. Drug Target. 2012, 20, 291–300. [Google Scholar] [CrossRef]
- Onishi, H. Chitosan microparticles. J. Drug Deliv. Sci. Tech. 2010, 20, 15–22. [Google Scholar] [CrossRef]
- Ko, J.A.; Park, H.J.; Hwang, S.J.; Park, J.B.; Lee, J.S. Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int. J. Pharm. 2002, 249, 165–174. [Google Scholar] [CrossRef]
- Dinu-Pîrvu, C.; Simona Ivan, S. A Study ofthe Influence of Crosslinking Degree Onthe Physicochemical Properties of Gelatin Microparticles. Cellulose Chem. Technol. 2013, 47, 721–726. [Google Scholar]
- Heydari, M.; Moheb, A.; Ghiaci, M.; Masoomi, M. Effect of Cross-Linking Time on the Thermal and Mechanical Properties and PervaporationPerformance of Poly(vinyl alcohol) Membrane Cross-Linked with Fumaric Acid Used for Dehydration of Isopropanol. J. Appl. Polym. Sci. 2013, 128, 1640–1651. [Google Scholar]
- Priimagi, A.; Cavallo, G.; Metrangolo, P.; Resnati, G. The Halogen Bond in the Design of Functional Supramolecular Materials: Recent Advances. Acc. Chem. Res. 2013, 46, 2686–2695. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.S. Handbook of Food Toxicology; Marcel Dekker: New York, NY, USA, 2002; ISBN 0-8247-0760-5. [Google Scholar]
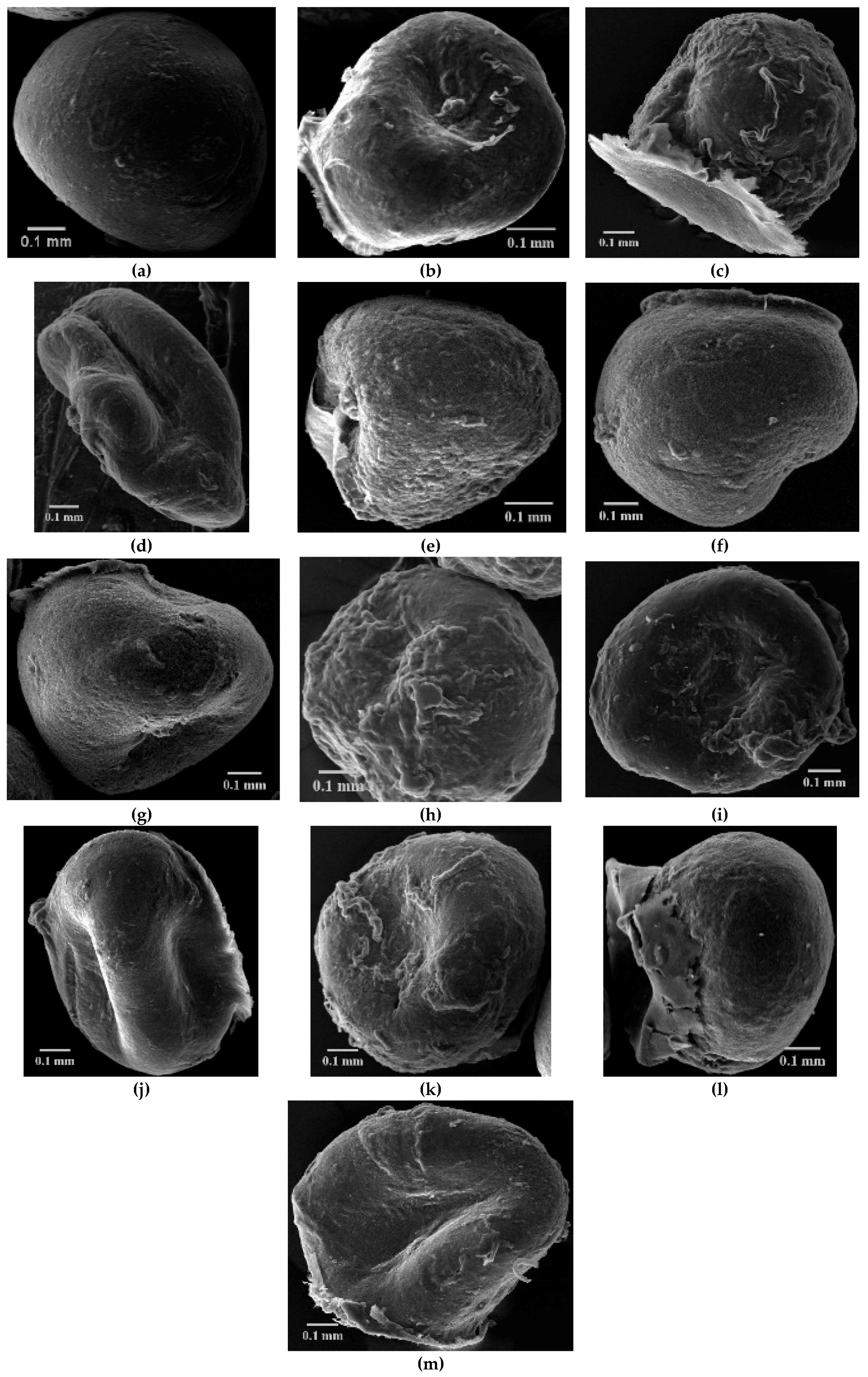
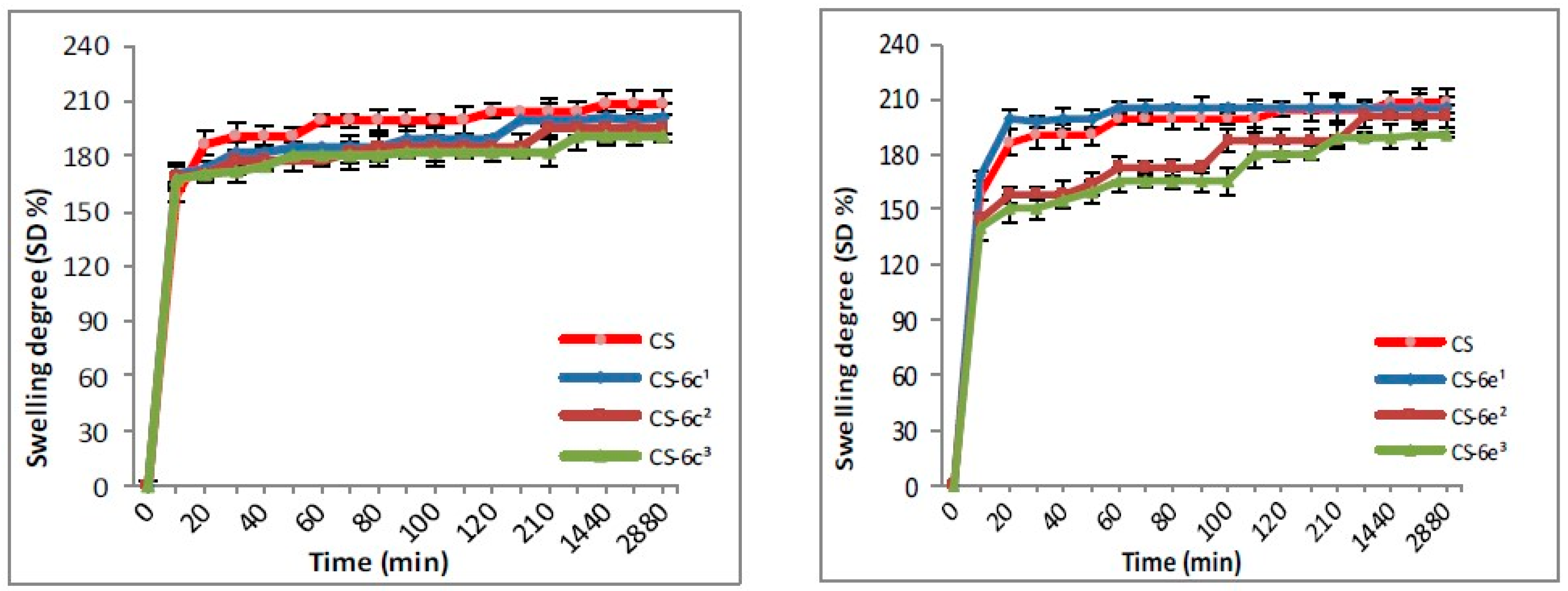
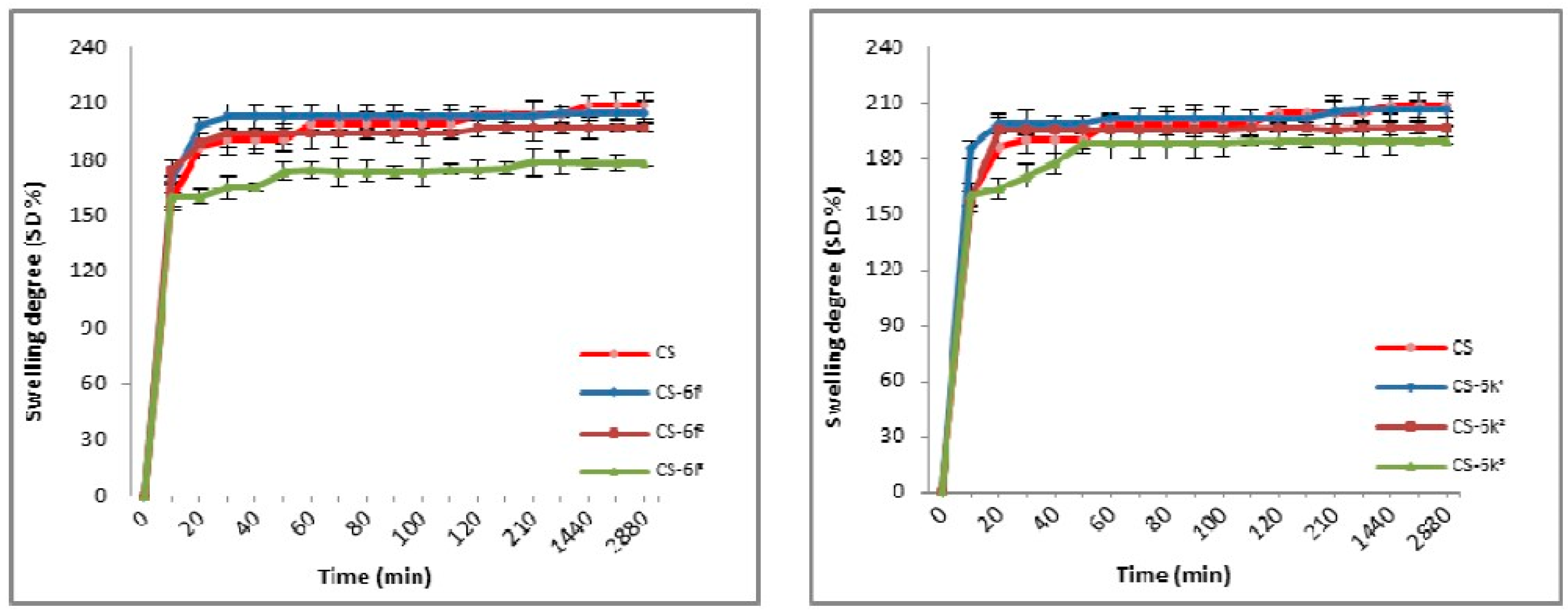
| CS/CS-XTDs | Size in Wet State (µm) | Size in Dry State (µm) | CS-XTDs | Size in Wet State (µm) | Size in Dry State (µm) |
|---|---|---|---|---|---|
| CS-6c1 | 904 ± 4.2 | 638 ± 3.1 | CS-6f1 | 887 ± 2.1 | 614 ± 3.1 |
| CS-6c2 | 897 ± 7.2 | 762 ± 2.5 | CS-6f2 | 921 ± 4.0 | 675 ± 5.0 |
| CS-6c3 | 951 ± 3.1 | 855 ± 2.1 | CS-6f3 | 947 ± 3.2 | 751 ± 6.4 |
| CS-6e1 | 858 ± 3.1 | 632 ± 3.5 | CS-6k1 | 929 ± 6.0 | 639 ± 7.0 |
| CS-6e2 | 923 ± 3.5 | 634 ± 4.7 | CS-6k2 | 912 ± 5.0 | 688 ± 4.0 |
| CS-6e3 | 953 ± 2.9 | 646 ± 4.0 | CS-6k3 | 918 ± 2.1 | 728 ± 4.7 |
| CS | 825 ± 3.5 | 611 ± 3.1 | - | - | - |
| CS-XTDs | SD (%) | CS-XTDs | SD (%) | CS-XTDs | SD (%) | CS-XTDs | SD (%) |
|---|---|---|---|---|---|---|---|
| CS-6c1 | 200 ± 2.7 * | CS-6e1 | 205 ± 3.6 | CS-6f1 | 205 ± 4.5 | CS-6k1 | 206 ± 2.9 |
| CS-6c2 | 195 ± 2.2 * | CS-6e2 | 201 ± 1.7 * | CS-6f2 | 197 ± 3.6 * | CS-6k2 | 196 ± 5.2 * |
| CS-6c3 | 190 ± 4.1 * | CS-6e3 | 190 ± 2.6 * | CS-6f3 | 178 ± 3.1 * | CS-6k3 | 189 ± 3.1 * |
| CS | 209 ± 3.5 | - | - | - | - | - | - |
| Sample | Diameter of Inhibition Area*(mm) | |||||
|---|---|---|---|---|---|---|
| Bacterial Strains | Yeasts Strains | |||||
| SA | SL | EC | CA | CG | CP | |
| 6a | 15.2 ± 0.23 | 20.1 ± 0.17 | 12.2 ± 0.35 | 11.1 ± 0.36 | 12.2 ± 0.22 | 12.2 ± 0.24 |
| 6b | 15.3 ± 0.40 | 19.1 ± 0.28 | 11.1 ± 0.23 | 10.1 ± 0.21 | 10.8 ± 0.37 | 10.3 ± 0.31 |
| 6c | 14.9 ± 0.21 | 19.0 ± 0.13 | 10.4 ± 0.15 | 15.2 ± 0.21 | 12.1 ± 0.42 | 11.2 ± 0.24 |
| 6d | 17.1 ± 0.24 | 18.1 ± 0.42 | 11.2 ± 0.47 | 10.0 ± 0.11 | 11.0 ± 0.46 | 11.3 ± 0.32 |
| 6e | 14.0 ± 0.38 | 18.8 ± 0.29 | 11.1 ± 0.14 | 10.1 ± 0.28 | 10.9 ± 0.26 | 14.2 ± 0.12 |
| 6f | 15.0 ± 0.50 | 20.1 ± 0.43 | 11.2 ± 0.35 | 12.1 ± 0.07 | 10.0 ± 0.06 | 15.2 ± 0.11 |
| 6g | 15.5 ± 0.27 | 20.0 ± 0.31 | 12.1 ± 0.26 | 11.3 ± 0.23 | 16.1 ± 0.28 | 15.4 ± 0.31 |
| 6h | 15.4 ± 0.34 | 19.2 ± 0.09 | 12.0 ± 0.06 | 15.2 ± 0.26 | 19.1 ± 0.17 | 12.0 ± 0.14 |
| 6i | 15.4 ± 0.25 | 19.1 ± 0.11 | 12.1 ± 0.04 | 12.5 ± 0.10 | 15.0 ± 0.24 | 12.1 ± 0.23 |
| 6j | 15.2 ± 0.14 | 19.1 ± 0.17 | 12.2± 0.26 | 12.2 ± 0.26 | 15.3 ± 0.09 | 12.1 ± 0.24 |
| 6k | 14.3 ± 0.19 | 17.8 ± 0.23 | 11.0 ± 0.31 | 10.3 ± 0.23 | 13.2 ± 0.11 | 11.0 ± 0.13 |
| CS-6c3 | 16.4 ± 0.41 | 21.0 ± 0.23 | 12.3 ± 0.21 | 17.4 ± 0.29 | 14.3 ± 0.38 | 13.2 ± 0.31 |
| CS-6e3 | 15.2 ± 0.23 | 20.4 ± 0.21 | 13.4 ± 0.25 | 12.5 ± 0.21 | 12.6 ± 0.21 | 15.4 ± 0.26 |
| CS-6f3 | 21.2 ± 0.43 | 25.1 ± 0.28 | 14.7 ± 0.38 | 16.7 ± 0.42 | 12.3 ± 0.51 | 18.4 ± 0.42 |
| CS-6k3 | 17.1 ± 0.32 | 22.4 ± 0.18 | 15.2 ± 0.18 | 14.6 ± 0.21 | 16.3 ± 0.38 | 17.2 ± 0.35 |
| CS | 12 ± 0.35 | 11 ± 0.26 | 9 ± 0.41 | - | - | - |
| Ca | 25.1 ± 0.08 | 25.0 ± 0.1 | 28.9 ± 0.18 | - | - | - |
| Ab | 27.1 ± 0.12 | 31.8 ± 0.15 | 21.0 ± 0.21 | - | - | - |
| Nc | - | - | - | 20.1 ± 0.11 | 21.0 ± 0.14 | 20.1 ± 0.09 |
| Sample | Staphylococcus Aureus ATCC 25923 | Escherichia Coli ATCC 25922 | Candida Albicans ATCC 90028 | |||
|---|---|---|---|---|---|---|
| MIC * | MBC * | MIC * | MBC * | MIC * | MBC * | |
| 6a | 0.625 | 10 | 1.25 | 1.25 | 1.25 | 1.25 |
| 6b | 2.5 | 10 | 2.5 | 2.5 | 1.25 | 1.25 |
| 6c | 2.5 | 10 | 1.25 | 1.25 | 1.25 | 2.5 |
| 6d | 0.3125 | 10 | 1.25 | 2.5 | 2.5 | 2.5 |
| 6e | 1.25 | 10 | 1.25 | 1.25 | 1.25 | 2.5 |
| 6f | 0.625 | 10 | 1.25 | 2.5 | 2.5 | 2.5 |
| 6g | 0.625 | 10 | 1.25 | 2.5 | 2.5 | 2.5 |
| 6h | 1.25 | 5 | 2.5 | 2.5 | 2.5 | 2.5 |
| 6i | 0.625 | 10 | 2.5 | 2.5 | 2.5 | 2.5 |
| 6j | 0.625 | 10 | 1.25 | 2.5 | 2.5 | 2.5 |
| 6k | 1.25 | 10 | 0.625 | 2.5 | 0.625 | 1.25 |
| A | 1 1 | 2 1 | 2 1 | 4 1 | - | - |
| N | - | - | - | - | 8 1 | 16 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantin, S.M.; Buron, F.; Routier, S.; Vasincu, I.M.; Apotrosoaei, M.; Lupașcu, F.; Confederat, L.; Tuchilus, C.; Constantin, M.T.; Sava, A.; et al. Formulation and Characterization of New Polymeric Systems Based on Chitosan and Xanthine Derivatives with Thiazolidin-4-One Scaffold. Materials 2019, 12, 558. https://doi.org/10.3390/ma12040558
Constantin SM, Buron F, Routier S, Vasincu IM, Apotrosoaei M, Lupașcu F, Confederat L, Tuchilus C, Constantin MT, Sava A, et al. Formulation and Characterization of New Polymeric Systems Based on Chitosan and Xanthine Derivatives with Thiazolidin-4-One Scaffold. Materials. 2019; 12(4):558. https://doi.org/10.3390/ma12040558
Chicago/Turabian StyleConstantin, Sandra Madalina, Frederic Buron, Sylvain Routier, Ioana Mirela Vasincu, Maria Apotrosoaei, Florentina Lupașcu, Luminița Confederat, Cristina Tuchilus, Marta Teodora Constantin, Alexandru Sava, and et al. 2019. "Formulation and Characterization of New Polymeric Systems Based on Chitosan and Xanthine Derivatives with Thiazolidin-4-One Scaffold" Materials 12, no. 4: 558. https://doi.org/10.3390/ma12040558
APA StyleConstantin, S. M., Buron, F., Routier, S., Vasincu, I. M., Apotrosoaei, M., Lupașcu, F., Confederat, L., Tuchilus, C., Constantin, M. T., Sava, A., & Profire, L. (2019). Formulation and Characterization of New Polymeric Systems Based on Chitosan and Xanthine Derivatives with Thiazolidin-4-One Scaffold. Materials, 12(4), 558. https://doi.org/10.3390/ma12040558





