Fabrication of Porous Materials by Spark Plasma Sintering: A Review
Abstract
1. Introduction
2. Fabrication of Porous Materials by Partial Densification and Sintering of Porous and Hollow Particles/Spheres
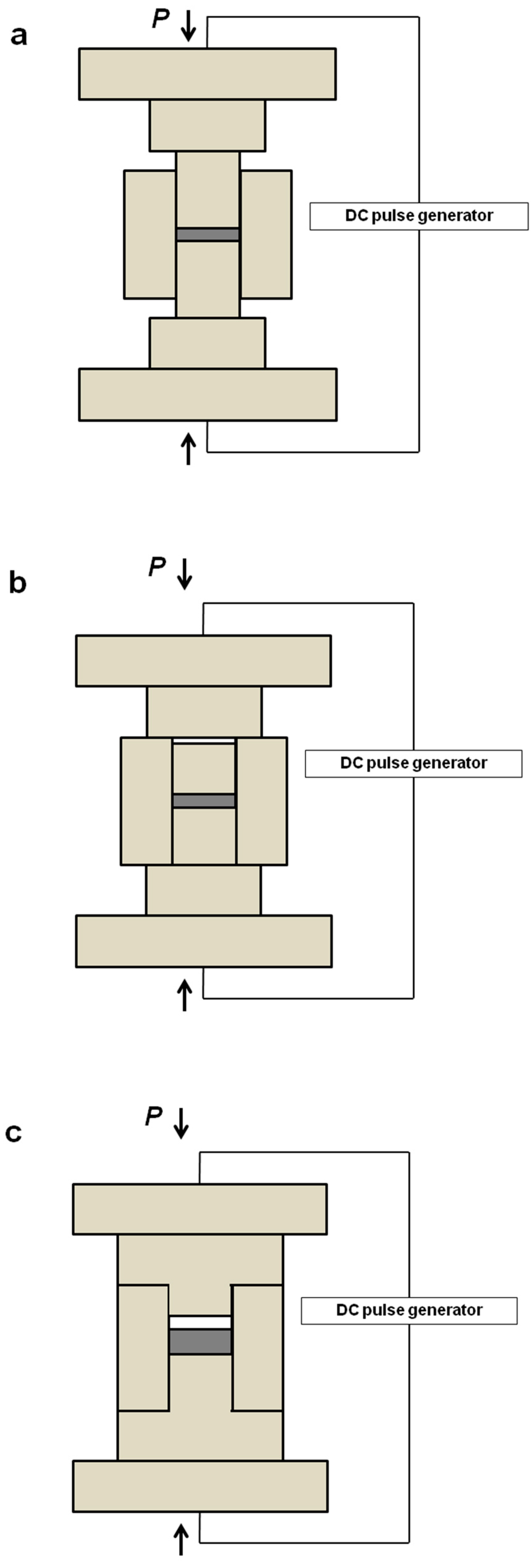
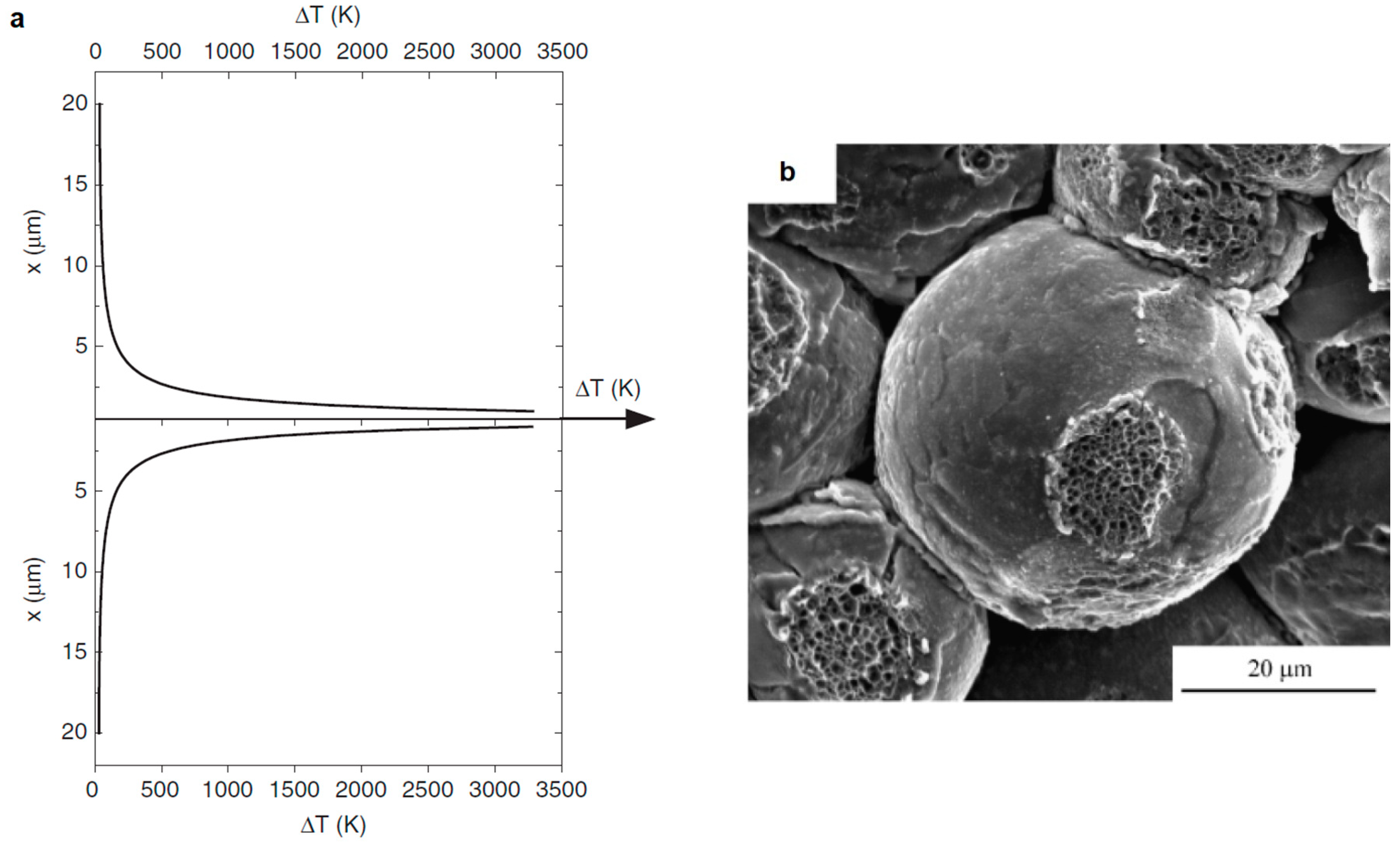
2.1. Development of Inter-Particle Contacts During SPS
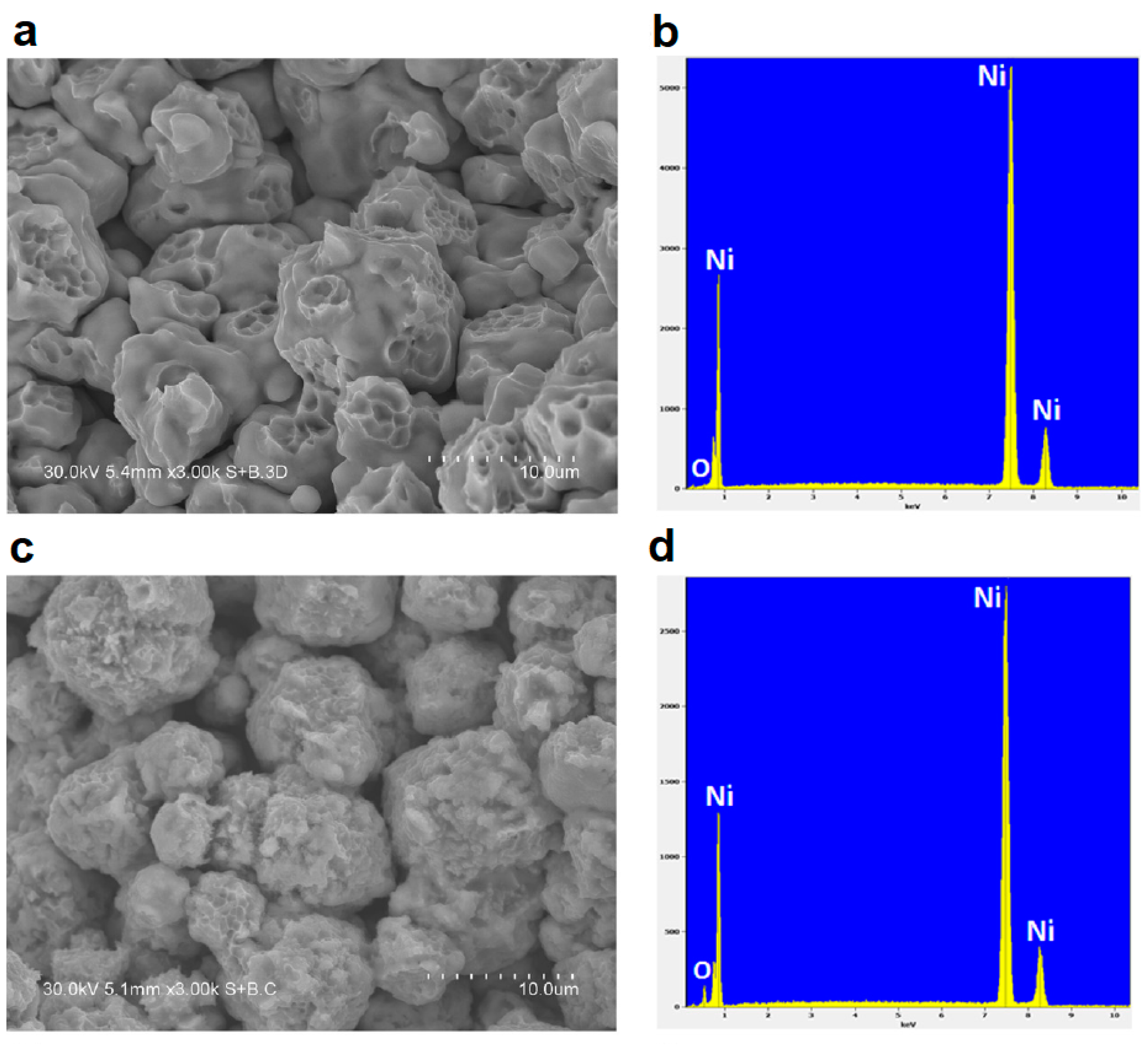
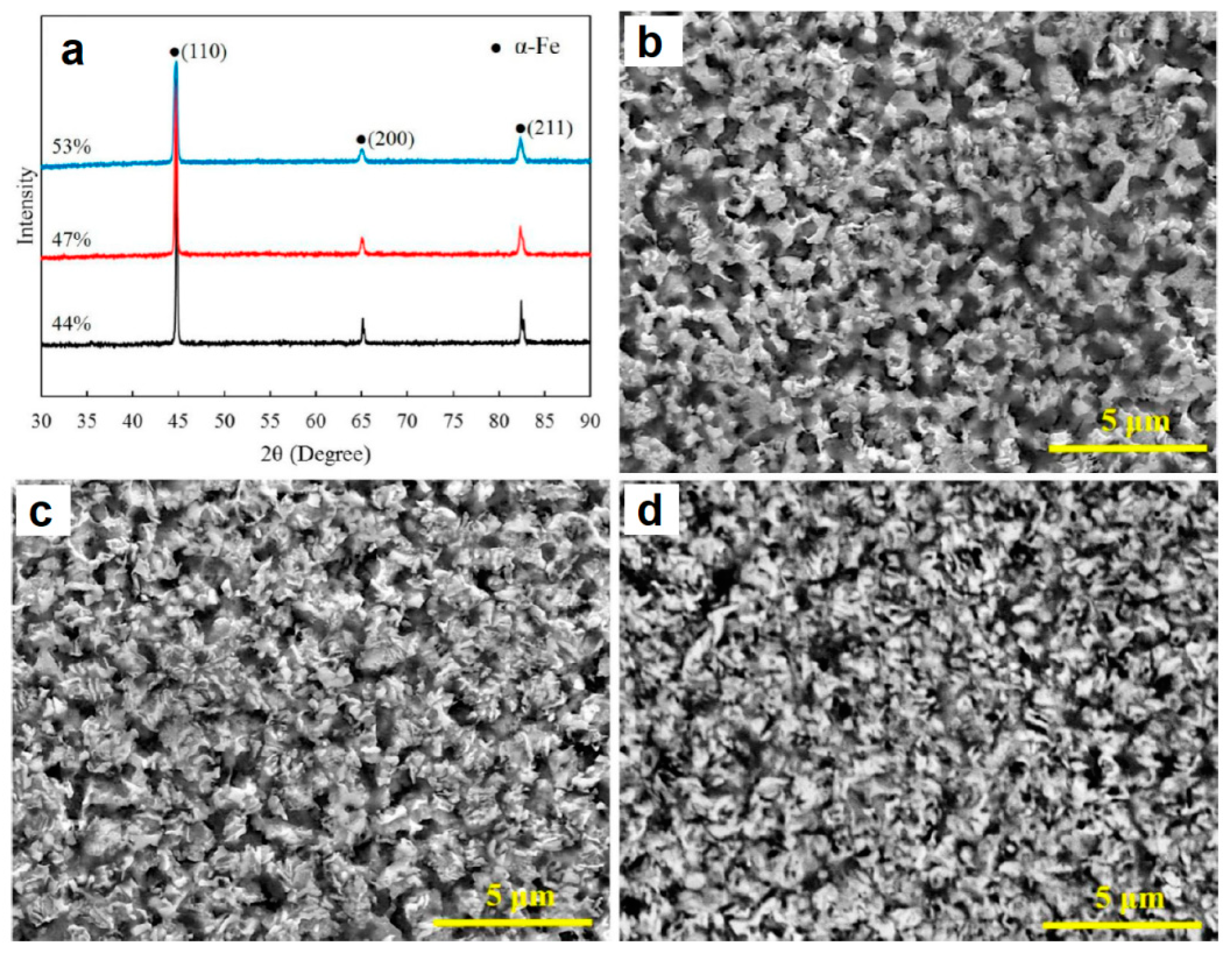
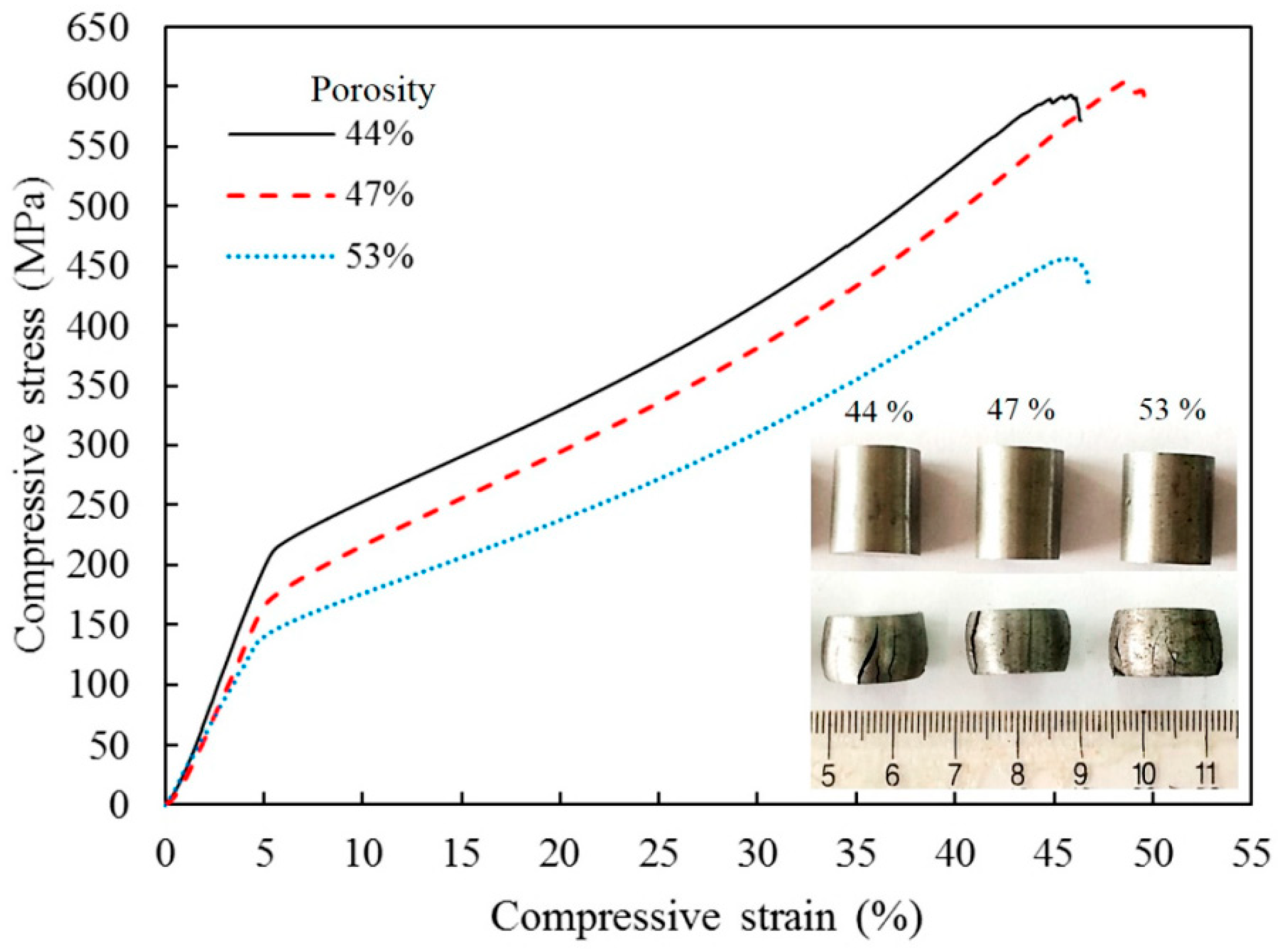
2.2. SPS of Porous Particles
2.3. SPS of Core–Shell Particles
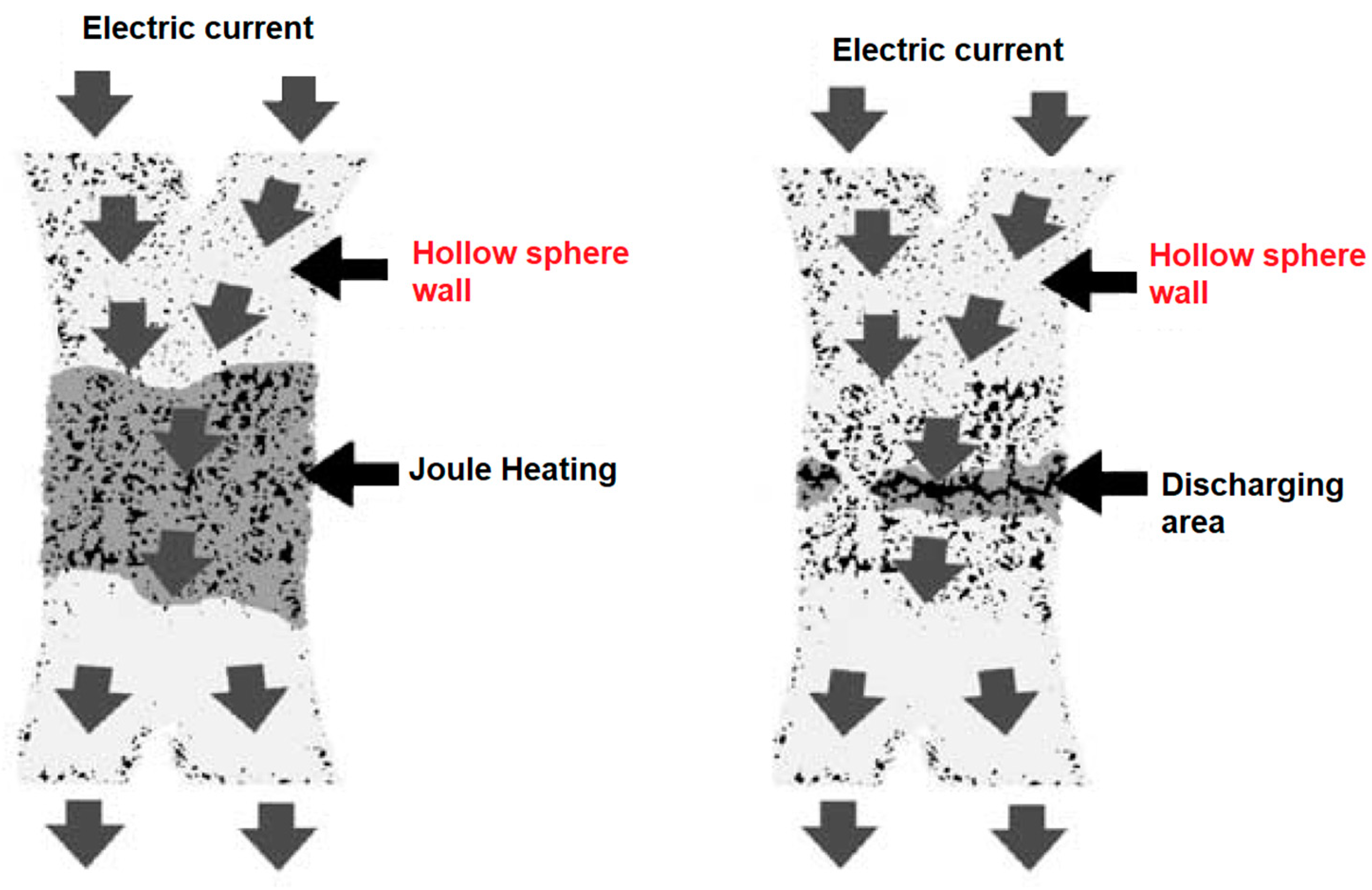
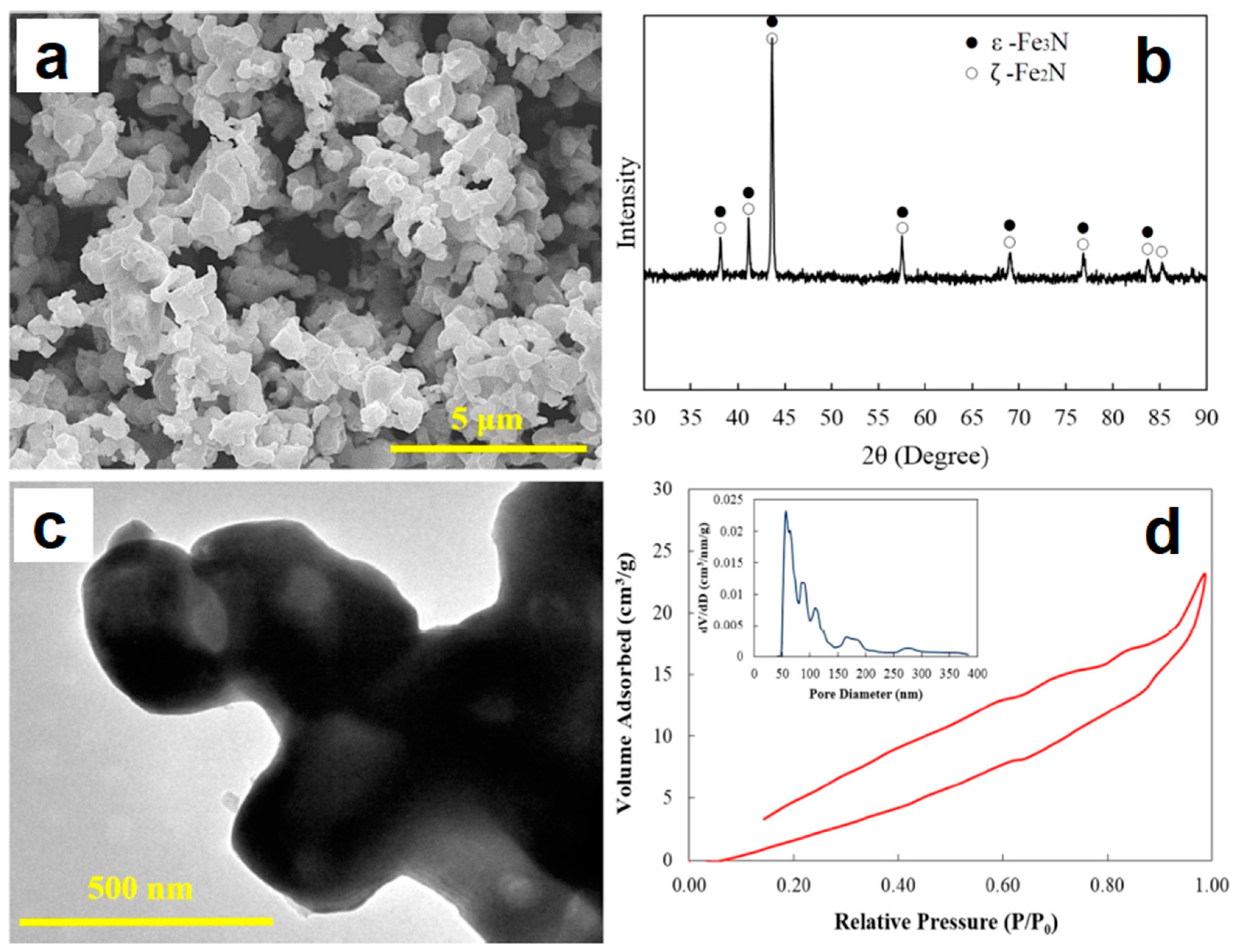
2.4. SPS of Hollow Particles and Spheres
2.5. Pressureless SPS of Pre-Compacted Porous Compacts
2.6. Reactive Sintering
2.7. Low-Temperature SPS
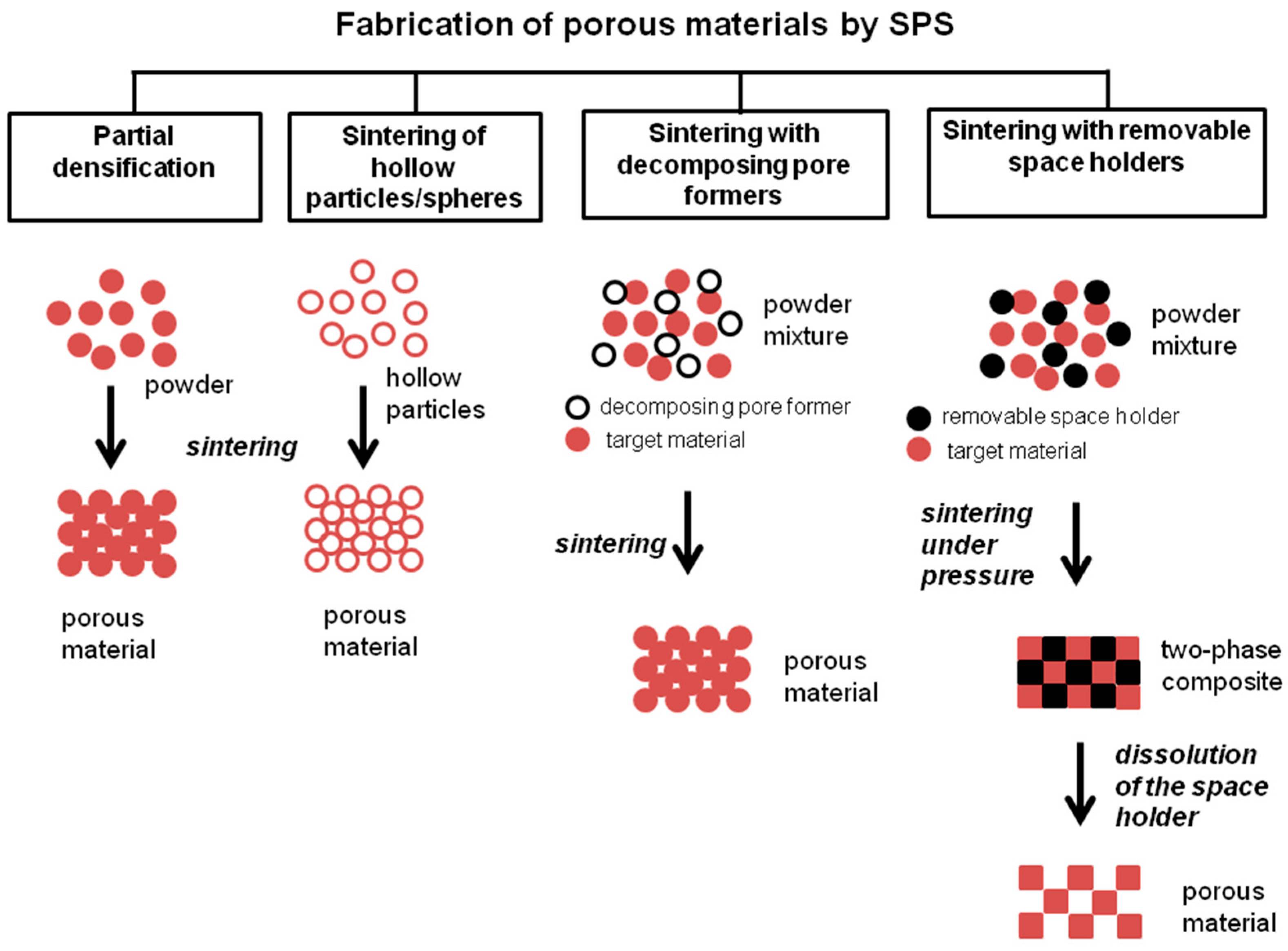
3. Fabrication of Porous Materials by SPS Using Space Holders Removed after Sintering or Decomposing During Sintering
3.1. Sintering with Space Holders Removable after Sintering
3.2. Removal of the Sacrificial Phase: Effect on the Target Phase
3.3. Interaction between the Space Holder and the Target Material
4. Challenges Associated with Processing of Porous Materials by SPS
5. Potential Applications of Porous Materials Obtained by SPS
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Banhart, J. Manufacture, characterisation and application of cellular metals and metal foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Oh, I.H.; Nomura, N.; Masahashi, N.; Hanada, S. Mechanical properties of porous titanium compacts prepared by powder sintering. Scr. Mater. 2003, 49, 1197–1202. [Google Scholar] [CrossRef]
- Kennedy, A. Porous metals and metal foams made from powders. In Powder Metallurgy; Kondoh, K., Ed.; IntechOpen: London, UK, 2012; pp. 31–46. [Google Scholar]
- Inagaki, M.; Qiu, J.; Guo, Q. Carbon foam: Preparation and application. Carbon 2015, 87, 128–152. [Google Scholar] [CrossRef]
- Hammel, E.C.; Ighodaro, O.L.-R.; Okoli, O.I. Processing and properties of advanced porous ceramics: An application based review. Ceram. Int. 2014, 40, 15351–15370. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Yavari, S.A.; Wauthle, R.; Pouran, B.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Additively manufactured open-cell porous biomaterials made from six different space-filling unit cells: The mechanical and morphological properties. Materials 2015, 8, 1871–1896. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.E.; Mabuchi, M.; Yamada, Y.; Shimojima, K.; Chino, Y.; Hosokawa, H.; Asahina, T. Processing of fine-grained aluminum foam by spark plasma sintering. J. Mater. Sci. Lett. 2003, 22, 1407–1409. [Google Scholar] [CrossRef]
- Belyavin, K.E.; Min’ko, D.V.; Kuznechik, O.O. Modeling of the process of the electric-discharge sintering of metal powder. J. Eng. Phys. Thermophys. 2004, 77, 628–637. [Google Scholar] [CrossRef]
- Minko, D.; Belyavin, K. A porous materials production with an electric discharge sintering. Int. J. Refract. Met. Hard Mater. 2016, 59, 67–77. [Google Scholar]
- Yurlova, M.S.; Grigoryev, E.G.; Olevsky, E.A.; Demenyuk, V.D. Electric pulse consolidation of tantalum anodes for electrolytic capacitors. Inorg. Mater. Appl. Res. 2015, 6, 267–274. [Google Scholar] [CrossRef]
- Kim, Y.H.; Cho, Y.J.; Lee, C.M.; Kim, S.J.; Lee, N.S.; Kim, K.B.; Jeon, E.C.; Sok, J.H.; Park, J.S.; Kwon, H.; et al. Self–assembled microporous Ti–6Al–4V implant compacts induced by electro–discharge sintering. Scr. Mater. 2007, 56, 449–451. [Google Scholar] [CrossRef]
- Lee, W.H.; Hyun, C.Y. Surface characteristics of self-assembled microporous Ti-6Al-4V compacts fabricated by electro-discharge-sintering in air. Appl. Surf. Sci. 2007, 253, 4649–4651. [Google Scholar] [CrossRef]
- Cho, J.Y.; Shin, J.S.; Jo, Y.J.; Lee, J.K.; Lee, M.H.; Lee, H.S.; Lee, W.H.; Kim, K.B. Formation of porous metallic glass compacts by electro-discharge sintering. J. Alloys Compd. 2011, 509, S184–S187. [Google Scholar] [CrossRef]
- Mironov, V.; Tatarinov, A.; Lapkovsky, V. Consolidation of metallic hollow spheres by electric sintering. IOP Conf. Ser. Mater. Sci. Eng. 2017, 218, 012009. [Google Scholar] [CrossRef]
- Olevsky, E.A.; Dudina, D.V. Microwave Sintering. In Field-Assisted Sintering: Science and Applications; Springer International Publishing: Berlin, Germany, 2018; pp. 237–274. ISBN 978-3-319-76031-5. [Google Scholar]
- Tokita, M. Trends in advanced SPS spark plasma sintering systems and technology. J. Soc. Powder Technol. Jpn. 1993, 30, 790–804. [Google Scholar] [CrossRef]
- Munir, Z.A.; Quach, D.; Ohyanagi, M. Electric Current Activation of Sintering: A review of the pulsed electric current sintering process. J. Am. Ceram. Soc. 2011, 94, 1–19. [Google Scholar] [CrossRef]
- Tokita, M. Spark plasma sintering (SPS) method, systems and applications. In Handbook of Advanced Ceramics: Materials, Applications, Processing and Properties, 2nd ed.; Somiya, S., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 1149–1178. [Google Scholar]
- Dudina, D.V.; Mukherjee, A.K. Reactive Spark Plasma Sintering: Successes and challenges of nanomaterial synthesis. J. Nanomater. 2013, 5. [Google Scholar] [CrossRef]
- Dudina, D.V.; Anisimov, A.G.; Mali, V.I.; Bulina, N.V.; Bokhonov, B.B. Smaller crystallites in sintered materials? A discussion of the possible mechanisms of crystallite size refinement during pulsed electric current-assisted sintering. Mater. Lett. 2015, 144, 168–172. [Google Scholar] [CrossRef]
- Bokhonov, B.B.; Dudina, D.V. Preparation of porous materials by Spark Plasma Sintering: Peculiarities of alloy formation during consolidation of Fe@Pt core–shell and hollow Pt(Fe) particles. J. Alloys Compd. 2017, 707, 233–237. [Google Scholar] [CrossRef]
- Trusov, G.V.; Tarasov, A.B.; Moskovskikh, D.O.; Rogachev, A.S.; Mukasyan, A.S. High porous cellular materials by spray solution combustion synthesis and spark plasma sintering. J. Alloys Compd. 2019, 779, 557–565. [Google Scholar] [CrossRef]
- Giuntini, D.; Wei, X.; Maximenko, A.L.; Wei, L.; Ilyina, A.M.; Olevsky, E.A. Initial stage of Free Pressureless Spark-Plasma Sintering of vanadium carbide: Determination of surface diffusion parameters. Int. J. Refract. Met. Hard Mater. 2013, 41, 501–506. [Google Scholar] [CrossRef]
- Yamanoglu, R.; Gulsoy, N.; Olevsky, E.A.; Gulsoy, H.O. Production of porous Ti5Al2.5Fe alloy via pressureless spark plasma sintering. J. Alloys Compd. 2016, 680, 654–658. [Google Scholar] [CrossRef]
- Dudina, D.V.; Bokhonov, B.B. Elimination of oxide films during Spark Plasma Sintering of metallic powders: A case study using partially oxidized nickel. Adv. Powder Technol. 2017, 28, 641–647. [Google Scholar] [CrossRef]
- Aman, Y.; Garnier, V.; Djurado, E. Pressure-less spark plasma sintering effect on non-conventional necking process during the initial stage of sintering of copper and alumina. J. Mater. Sci. 2012, 47, 5766–5773. [Google Scholar] [CrossRef]
- Gelbstein, Y.; Haim, Y.; Kalabukhov, S.; Kasiyan, V.; Hartmann, S.; Rothe, S.; Frage, N. Correlation between thermal and electrical properties of Spark Plasma Sintered (SPS) porous copper. In Sintering Techniques of Materials; Lakshmanan, A., Ed.; IntechOpen: London, UK, 2015; pp. 155–166. ISBN 978-953-51-2033-9. [Google Scholar]
- Kim, S.-K.; Kim, H.-T.; Kim, J.-S.; Kwon, Y.-S. Production of metal powder compact with controlled porosity by spark plasma sintering process. In Proceedings of the KORUS-2000—The 4th Korea-Russia International Symposium on Science and Technology, Ulsan, Korea, 27 June–1 July 2000; pp. 286–290. [Google Scholar]
- Song, X.; Liu, X.; Zhang, J. Neck formation and self-adjusting mechanism of neck growth of conducting powders in Spark Plasma Sintering. J. Am. Ceram. Soc. 2006, 89, 494–500. [Google Scholar] [CrossRef]
- Shi, M.; Liu, S.; Wang, Q.; Yang, X.; Zhang, G. Preparation and properties of titanium obtained by Spark Plasma Sintering of a Ti powder–fiber mixture. Materials 2018, 11, 2510. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.Q.; Jiang, Y.H.; Zhou, R. Mechanical behaviors of porous Ti with high porosity and large pore size prepared by one-step spark plasma sintering technique. Vacuum 2015, 122, 187–194. [Google Scholar] [CrossRef]
- Ibrahim, A.; Zhang, F.; Otterstein, E.; Burkel, E. Processing of porous Ti and Ti5Mn foams by spark plasma sintering. Mater. Des. 2011, 32, 146–153. [Google Scholar] [CrossRef]
- Zhang, F.; Otterstein, E.; Burkel, E. Spark Plasma Sintering, microstructure, and mechanical properties of macroporous titanium foams. Adv. Eng. Mater. 2010, 12, 863–872. [Google Scholar] [CrossRef]
- Hakamada, M.; Yamada, Y.; Nomura, T.; Chen, Y.; Kusuda, H.; Mabuchi, M. Fabrication of porous aluminum by spacer method consisting of Spark Plasma Sintering and sodium chloride dissolution. Mater. Trans. 2005, 46, 2624–2628. [Google Scholar] [CrossRef]
- Hakamada, M.; Yamada, Y.; Nomura, T.; Kusuda, H.; Chen, Y.; Mabuchi, M. Effect of sintering temperature on compressive properties of porous aluminum produced by Spark Plasma Sintering. Mater. Trans. 2005, 46, 186–188. [Google Scholar] [CrossRef]
- Bokhonov, B.B.; Dudina, D.V. Recrystallisation-accompanied phase separation in Ag–Fe and Ag–Ni nanocomposites: A route to structure tailoring of nanoporous silver. RSC Adv. 2013, 3, 12655–12661. [Google Scholar] [CrossRef]
- Rogachev, A.S.; Kolobov, Y.R.; Vadchenko, S.G.; Golosova, O.A.; Bozhko, S.A.; Moskovskikh, D.O. Preparation of titanium materials with gradient porosity by spark plasma sintering. Fund. Res. 2014, 12, 947–951. (In Russian) [Google Scholar]
- Hasebe, T.; Kobayashi, E.; Tezuka, H.; Sato, T. Effects of sintering conditions on mechanical properties of biomedical porous Ti produced by Spark Plasma Sintering. Jpn. J. Appl. Phys. 2013, 52, Number-1S. [Google Scholar] [CrossRef]
- Mandal, M.; Singh, D.; Gouthama; Murty, B.S.; Sangal, S.; Mondal, K. Porous copper template from partially spark plasma-sintered Cu–Zn aggregate via dezincification. Bull. Mater. Sci. 2014, 37, 743–752. [Google Scholar] [CrossRef]
- Cui, G.; Wei, X.; Olevsky, E.A.; German, R.M.; Chen, J. The manufacturing of high porosity iron with an ultra-fine microstructure via free pressureless Spark Plasma Sintering. Materials 2016, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Rechtin, J.; Torresani, E.; Ivanov, E.; Olevsky, E. Fabrication of titanium-niobium-zirconium-tantalum Alloy (TNZT) bioimplant components with controllable porosity by Spark Plasma Sintering. Materials 2018, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Fukuhara, M.; Louzguine-Luzgin, D.V.; Inoue, A. Ultrasonic characteristics of porous Zr55Cu30Al10Ni5 bulk metallic glass fabricated by spark plasma sintering. Intermetallics 2010, 18, 2014–2018. [Google Scholar] [CrossRef]
- Xie, G.; Zhang, W.; Louzguine-Luzgin, D.V.; Kimura, H.; Inoue, A. Microstructure and mechanical properties of porous Zr55Cu30Al10Ni5 bulk metallic glass fabricated by Spark Plasma Sintering process. Mater. Trans. 2007, 48, 1589–1594. [Google Scholar] [CrossRef]
- Nicula, R.; Lüthen, F.; Stir, M.; Nebe, B.; Burkel, E. Spark plasma sintering synthesis of porous nanocrystalline titanium alloys for biomedical applications. Biomol. Eng. 2007, 24, 564–567. [Google Scholar] [CrossRef]
- Fabrègue, D.; Mouawad, B.; Buttay, C.; Soueidan, M.; Lamontagne, A.; Forte, R.; Perez, M.; Courtois, L.; Landron, C.; Maire, É.; et al. Elaboration of architectured materials by Spark Plasma Sintering. Mater. Sci. Forum 2012, 706–709, 1885–1892. [Google Scholar]
- Zhang, L.; Zhang, Y.Q.; Jiang, Y.H.; Zhou, R. Superelastic behaviors of biomedical porous NiTi alloy with high porosity and large pore size prepared by spark plasma sintering. J. Alloys Compd. 2015, 644, 513–522. [Google Scholar] [CrossRef]
- Dudina, D.V.; Bokhonov, B.B.; Mukherjee, A.K. Formation of aluminum particles with shell morphology during pressureless Spark Plasma Sintering of Fe-Al mixtures: Current-related or Kirkendall effect? Materials 2016, 9, 375. [Google Scholar] [CrossRef] [PubMed]
- Dudina, D.V. Application of a spark plasma sintering facility for the heat treatment of compact and powder materials. Inorg. Mater. 2017, 53, 658–663. [Google Scholar] [CrossRef]
- Dudina, D.V.; Brester, A.E.; Anisimov, A.G.; Bokhonov, B.B.; Legan, M.A.; Novoselov, A.N.; Skovorodin, I.N.; Uvarov, N.F. Fast synthesis and consolidation of porous FeAl by pressureless Spark Plasma Sintering. IOP Conf. Ser. Mater. Sci. Eng. 2017, 218, 012003. [Google Scholar] [CrossRef]
- Dudina, D.V.; Legan, M.A.; Fedorova, N.V.; Novoselov, A.N.; Anisimov, A.G.; Esikov, M.A. Structural and mechanical characterization of porous iron aluminide FeAl obtained by pressureless Spark Plasma Sintering. Mater. Sci. Eng. A 2017, 695, 309–314. [Google Scholar] [CrossRef]
- Dudina, D.V.; Bokhonov, B.B.; Legan, M.A.; Novoselov, A.N.; Skovorodin, I.N.; Bulina, N.V.; Esikov, M.A.; Mali, V.I. Analysis of the formation of FeAl with a high open porosity during electric current-assisted sintering of loosely packed Fe-Al powder mixtures. Vacuum 2017, 146, 74–78. [Google Scholar] [CrossRef]
- Quan, Y.; Zhang, F.; Rebl, H.; Nebe, B.; Keßler, O.; Burkel, E. Ti6Al4V foams fabricated by spark plasma sintering with post-heat treatment. Mater. Sci. Eng. A 2013, 565, 118–125. [Google Scholar] [CrossRef]
- Nilsén, F.; Lehtonen, J.; Ge, Y.; Aaltio, I.; Hannula, S.-P. Highly porous spark plasma sintered Ni-Mn-Ga structures. Scr. Mater. 2017, 139, 148–151. [Google Scholar]
- Du, H.; Liu, X.W.; Li, J.; Tao, P.; Jiang, J.; Sun, R.; Fan, Z.T. Use of Spark Plasma Sintering for fabrication of porous titanium aluminide alloys from elemental powders. Mater. Manuf. Proc. 2016, 31, 725–732. [Google Scholar] [CrossRef]
- Khor, K.A.; Yu, L.G.; Andersen, O.; Stephani, G. Effect of spark plasma sintering (SPS) on the microstructure and mechanical properties of randomly packed hollow sphere (RHS) cell wall. Mater. Sci. Eng. A 2003, 356, 130–135. [Google Scholar] [CrossRef]
- Stingaciu, M.; Zhu, B.; Singh, M.; Johnsson, M. Single-component fuel cells fabricated by spark plasma sintering. RSC Adv. 2012, 2, 12140–12143. [Google Scholar] [CrossRef]
- Ning, H.; Mastrorillo, G.D.; Grasso, S.; Du, B.; Mori, T.; Hu, C.; Xu, Y.; Simpson, K.; Maizza, G.; Reece, M.J. Enhanced thermoelectric performance of porous magnesium tin silicide prepared using pressure-less spark plasma sintering. J. Mater. Chem. A 2015, 3, 17426–17432. [Google Scholar] [CrossRef]
- Ortali, C.; Julien, I.; Vandenhende, M.; Drouet, C.; Champion, E. Consolidation of bone-like apatite bioceramics by spark plasma sintering of amorphous carbonated calcium phosphate at very low temperature. J. Eur. Ceram. Soc. 2018, 38, 2098–2109. [Google Scholar] [CrossRef]
- Sairam, K.; Sonber, J.K.; Murthy, T.S.R.C.; Sahu, A.K.; Bedse, R.D.; Chakravartty, J.K. Pressureless sintering of chromium diboride using spark plasma sintering facility. Int. J. Refract. Met. Hard Mater. 2016, 58, 165–171. [Google Scholar] [CrossRef]
- Vasiliev, P.O.; Shen, Z.J.; Hodgkins, R.P.; Bergstrom, L. Meso/macroporous, mechanically stable silica monoliths of complex shape by controlled fusion of mesoporous spherical particles. Chem. Mater. 2006, 18, 4933–4938. [Google Scholar] [CrossRef]
- Dibandjo, P.; Bois, L.; Estournes, C.; Durand, B.; Miele, P. Silica, carbon and boron nitride monoliths with hierarchical porosity prepared by Spark Plasma Sintering process. Microporous Mesoporous Mater. 2008, 111, 643–648. [Google Scholar] [CrossRef]
- Akhtar, F.; Vasiliev, P.O.; Bergström, L. Hierarchically porous ceramics from diatomite powders by pulsed current processing. J. Am. Ceram. Soc. 2009, 92, 338–343. [Google Scholar] [CrossRef]
- Vasiliev, P.; Akhtar, F.; Grins, J.; Mouzon, J.; Andersson, C.; Hedlund, J.; Bergström, L. Strong hierarchically porous monoliths by pulsed current processing of zeolite powder assemblies. Appl. Mater. Interfaces 2010, 2, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Xiao, C.; Shen, Z. Nano pores evolution in hydroxyapatite microsphere during Spark Plasma Sintering. Sci. Sint. 2011, 43, 39–46. [Google Scholar] [CrossRef]
- Manukyan, K.V.; Yeghishyan, A.V.; Shuck, C.E.; Moskovskikh, D.O.; Rouvimov, S.; Wolf, E.E.; Mukasyan, A.S. Mesoporous metal-silica materials: Synthesis, catalytic and thermal properties. Microporous Mesoporous Mater. 2018, 257, 175–184. [Google Scholar] [CrossRef]
- Yuan, H.; Li, J.; Shen, Q.; Zhang, L. In situ synthesis and sintering of ZrB2 porous ceramics by the spark plasma sintering–reactive synthesis (SPS–RS) method. Int. J. Refract. Met. Hard Mater. 2012, 34, 3–7. [Google Scholar] [CrossRef]
- Bradbury, W.L.; Olevsky, E.A. Production of SiC–C composites by free-pressureless spark plasma sintering (FPSPS). Scr. Mater. 2010, 63, 77–80. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Papynov, E.K.; Shichalin, O.O.; Golub, A.V.; Mayorov, V.Y.; Avramenko, V.A.; Sevastyanov, V.G.; Kuznetsov, N.T. Preparation of porous SiC-ceramics by sol–gel and spark plasma sintering. J. Sol-Gel Sci. Technol. 2017, 82, 748–759. [Google Scholar] [CrossRef]
- Chakravarty, D.; Ramesh, H.; Rao, T.N. High strength porous alumina by spark plasma sintering. J. Eur. Ceram. Soc. 2009, 29, 1361–1369. [Google Scholar] [CrossRef]
- Shin, H.C.; Cho, W.-S.; Shin, S.-Y.; Kim, J.-G. Fabrication of porous alumina ceramics by Spark Plasma Sintering. J. Korean Ceram. Soc. 2002, 39, 1183–1189. [Google Scholar]
- Papynov, E.K.; Mayorov, V.Y.; Portnyagin, A.S.; Shichalin, O.O.; Kobylyakov, S.P.; Kaidalova, T.A.; Nepomnyashiy, A.V.; Sokol’nitskaya, T.A.; Zub, Y.L.; Avramenko, V.A. Application of carbonaceous template for porous structure control of ceramic composites based on synthetic wollastonite obtained via Spark Plasma Sintering. Ceram. Int. 2015, 41, 1171–1176. [Google Scholar] [CrossRef]
- Zhang, L.; He, Z.Y.; Zhang, Y.Q.; Jiang, Y.H.; Zhou, R. Rapidly sintering of interconnected porous Ti-HA biocomposite with high strength and enhanced bioactivity. Mater. Sci. Eng. C 2016, 67, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Ukhina, A.V.; Dudina, D.V.; Anisimov, A.G.; Mali, V.I.; Bulina, N.V.; Bataev, I.A.; Skovorodin, I.N.; Bokhonov, B.B. Porous electrically conductive materials produced by Spark Plasma Sintering and hot pressing of nanodiamonds. Ceram. Int. 2015, 41, 12459–12463. [Google Scholar] [CrossRef]
- Chakravarty, D.; Tiwary, C.S.; Woellner, C.F.; Radhakrishnan, S.; Vinod, S.; Ozden, S.; da Silva Autreto, P.A.; Bhowmick, S.; Asif, S.; Mani, S.A.; et al. 3D Porous graphene by low-temperature plasma welding for bone implants. Adv. Mater. 2016, 28, 8959–8967. [Google Scholar] [CrossRef]
- Bokhonov, B.B.; Dudina, D.V.; Ukhina, A.V.; Korchagin, M.A.; Bulina, N.V.; Mali, V.I.; Anisimov, A.G. Formation of self-supporting porous graphite structures by Spark Plasma Sintering of nickel-amorphous carbon mixtures. J. Phys. Chem. Solids 2015, 76, 192–202. [Google Scholar] [CrossRef]
- Dudina, D.V.; Ukhina, A.V.; Bokhonov, B.B.; Mali, V.I.; Anisimov, A.G.; Bulina, N.V.; Skovorodin, I.N. Nickel-graphite composites of variable architecture by graphitization-accompanied Spark Plasma Sintering and hot pressing and their response to phase separation. Sci. Sint. 2015, 47, 237–248. [Google Scholar] [CrossRef]
- Dudina, D.V.; Ukhina, A.V.; Bokhonov, B.B.; Korchagin, M.A.; Bulina, N.V.; Kato, H. The influence of the formation of Fe3C on graphitization in a carbon-rich iron-amorphous carbon mixture processed by Spark Plasma Sintering and annealing. Ceram. Intl. 2017, 43, 11902–11906. [Google Scholar] [CrossRef]
- Bokhonov, B.B.; Korchagin, M.A.; Ukhina, A.V.; Dudina, D.V. Structural and morphological transformations in cobalt-carbon mixtures during ball milling, annealing and Spark Plasma Sintering. Vacuum 2018, 157, 210–215. [Google Scholar] [CrossRef]
- Ye, Y.; Li, X.; Hu, K.; Lai, Y.; Li, Y. The influence of premolding load on the electrical behavior in the initial stage of electric current activated sintering of carbonyl iron powders. J. Appl. Phys. 2013, 113, 214902. [Google Scholar] [CrossRef]
- Collard, C.; Trzaska, Z.; Durand, L.; Chaix, J.M.; Monchoux, J.P. Theoretical and experimental investigations of local overheating at particle contacts in spark plasma sintering. Powder Technol. 2017, 321, 458–470. [Google Scholar] [CrossRef]
- Kuz’mov, A.V.; Olevskii, E.A.; Aleksandrova, E.V. Effect of micrononuniform heating of powder in field-assisted sintering on shrinkage kinetics. Powder Metall. Metal Ceram. 2013, 51, 657–665. [Google Scholar]
- Falcon, E.; Castaing, B. Electrical conductivity in granular media and Branly’s coherer: a simple experiment. Am. J. Phys. 2005, 73, 302–307. [Google Scholar] [CrossRef]
- Guyot, P.; Rat, V.; Coudert, J.F.; Maître, A.; Pradeilles, N. Does the Branly effect occur in spark plasma sintering. J. Phys. D Appl. Phys. 2012, 45, 092001. [Google Scholar] [CrossRef]
- Sanyal, U.; Davis, D.T.; Jagirdar, B.R. Bimetallic core–shell nanocomposites using weak reducing agent and their transformation to alloy nanostructures. Dalton Trans. 2013, 42, 7147–7157. [Google Scholar] [CrossRef]
- Liu, C.; Wu, X.; Klemmer, T.; Shukla, N.; Weller, D.; Roy, A.G.; Tanase, M.; Laughlin, D. Reduction of sintering during annealing of FePt Nanoparticles coated with iron oxide. Chem. Mater. 2005, 17, 620–625. [Google Scholar] [CrossRef]
- Handwerker, C.A.; Kim, S.J.; Stach, E.A. Shell activated sintering of core–shell particles. US Patent 20140370322, 2011. [Google Scholar]
- Dudina, D.V.; Ukhina, A.V.; Brester, A.E.; Mali, V.I.; Anisimov, A.G.; Bokhonov, B.B. Inter-particle interactions in partially densified compacts of electrically conductive materials during spark plasma sintering. In Proceedings of the 11 International Forum on Strategic Technology (IFOST 2016), Novosibirsk, Russia, 1–3 June 2016; pp. 139–143. [Google Scholar]
- Barney, I.T.; Lennaerts, D.S.R.; Higgins, S.R.; Mukhopadhyay, S.M. Specific surface area of hierarchical graphitic substrates suitable for multi-functional applications. Mater. Lett. 2012, 88, 160–163. [Google Scholar] [CrossRef]

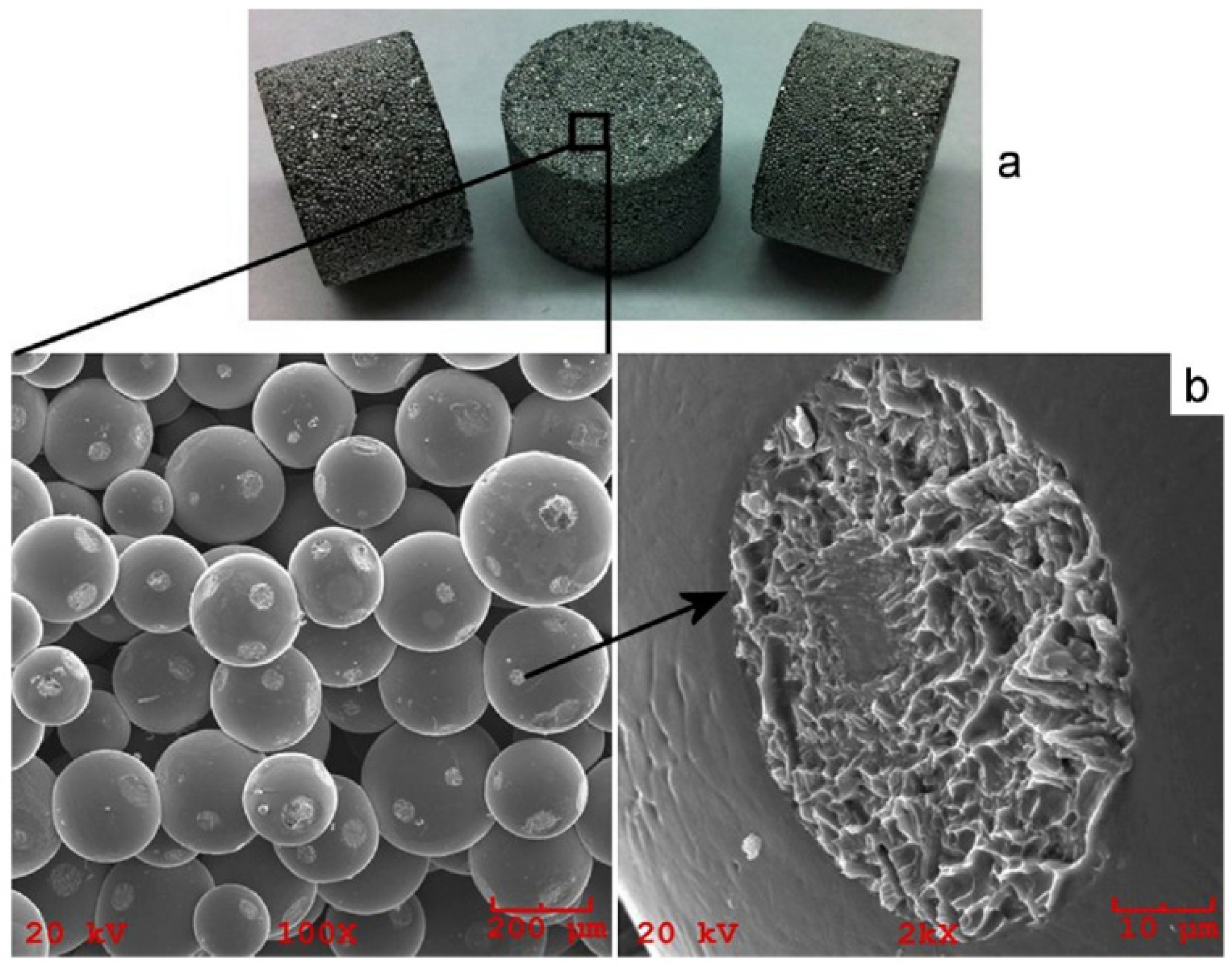
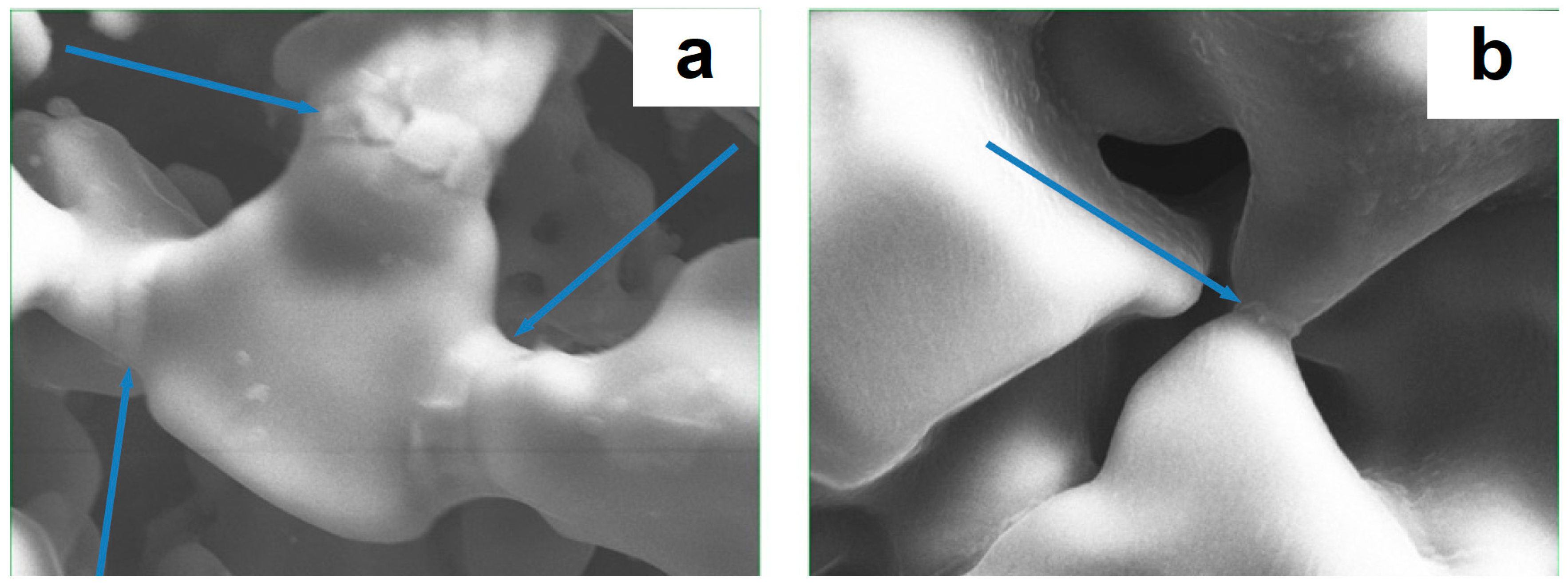
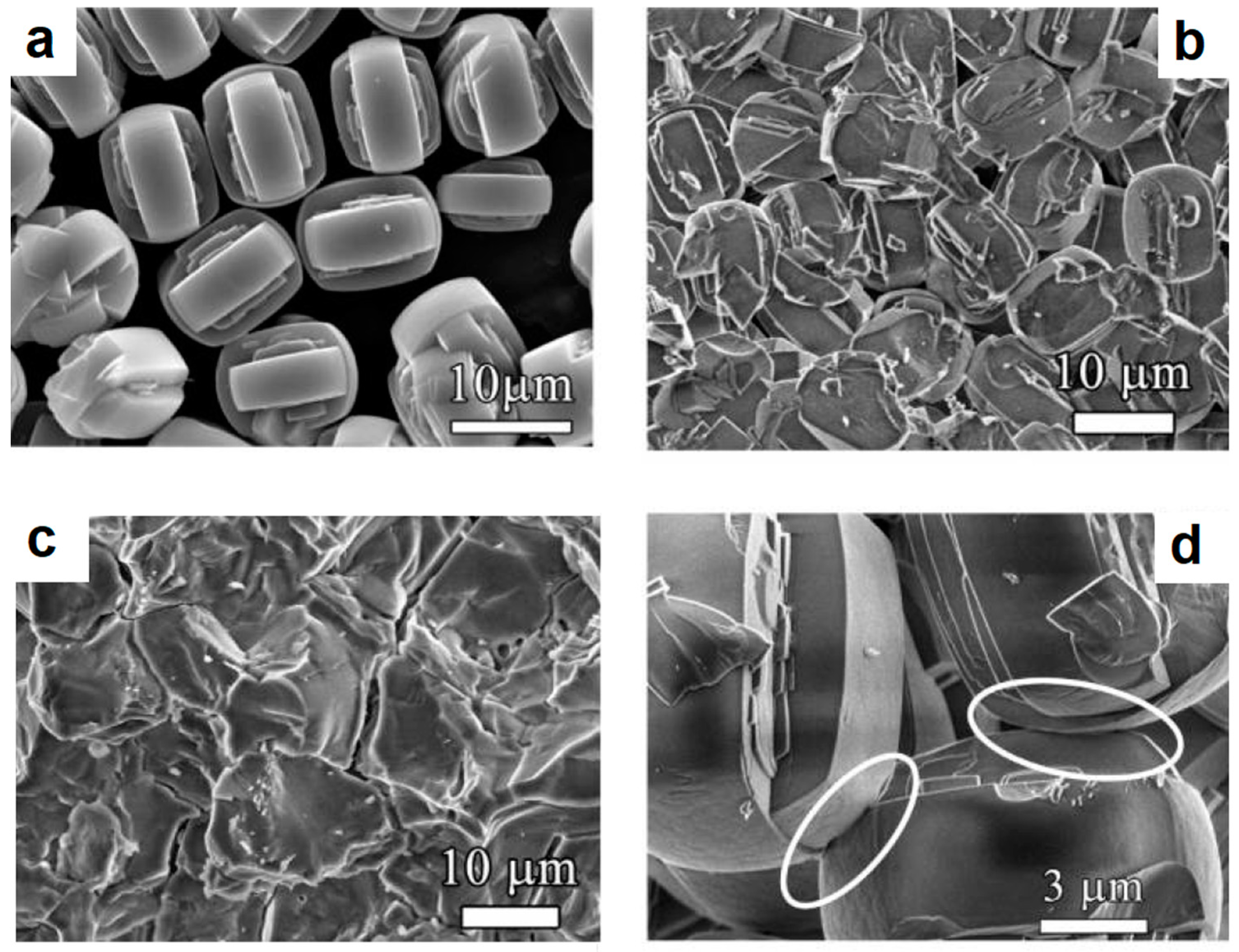
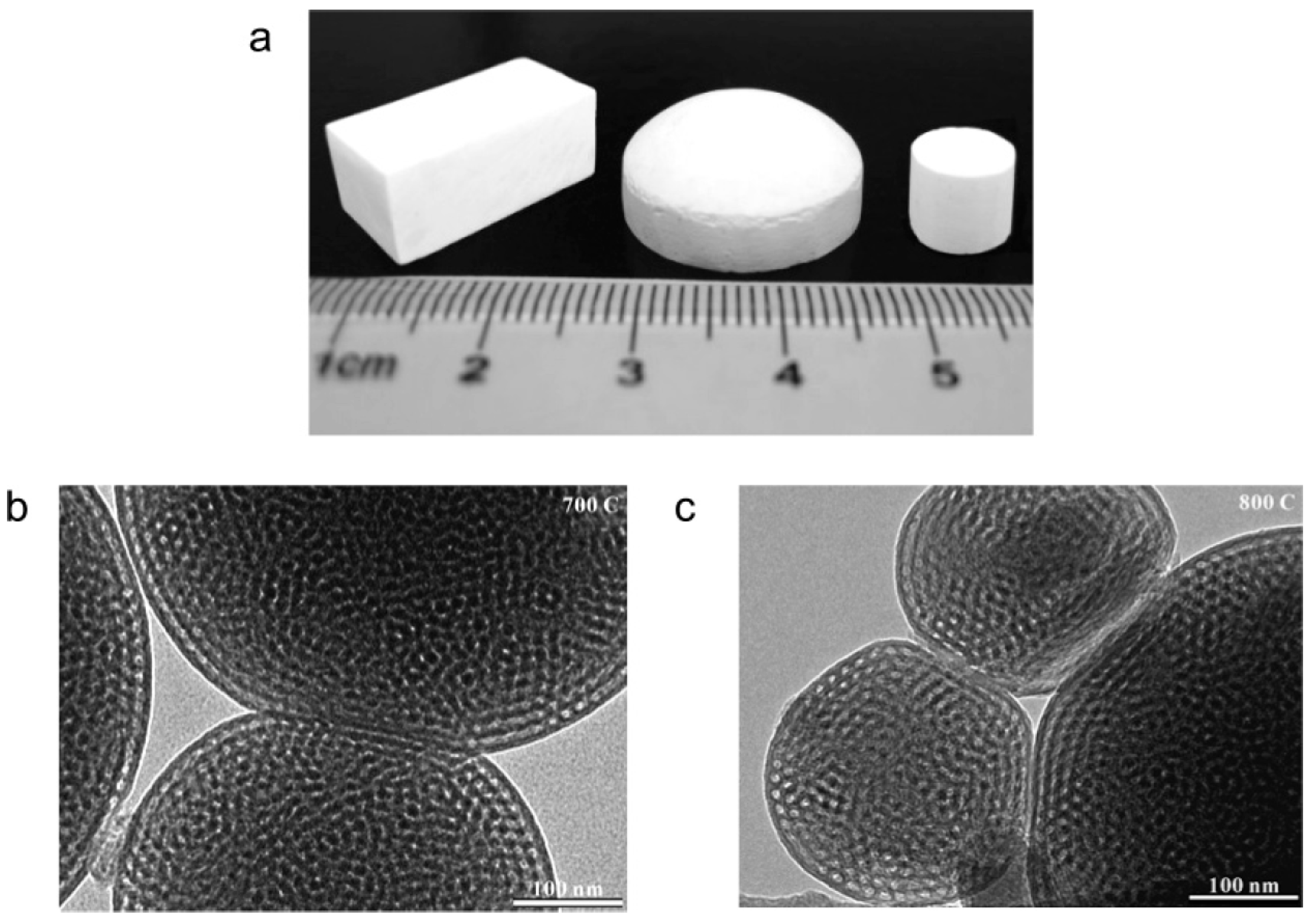
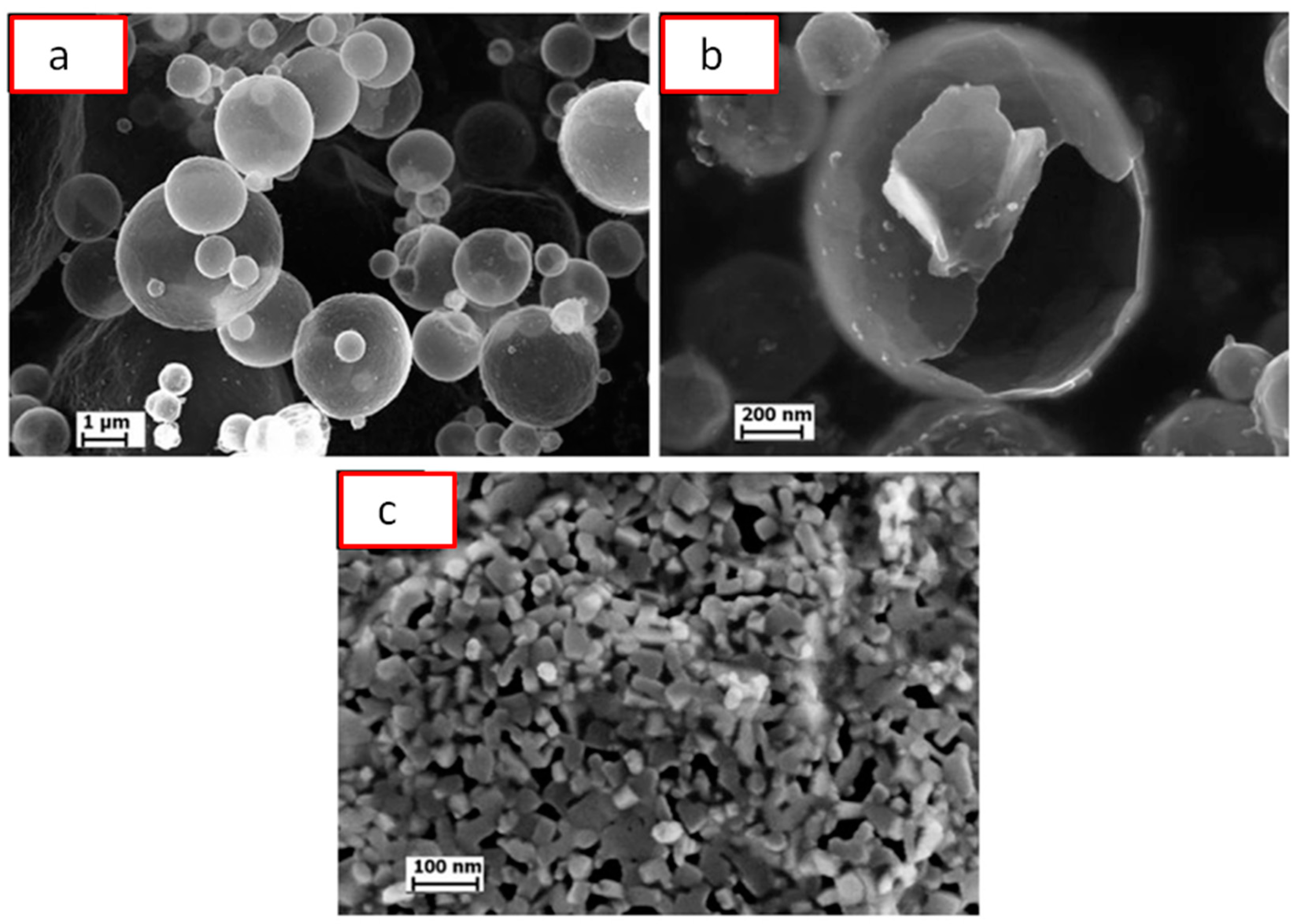
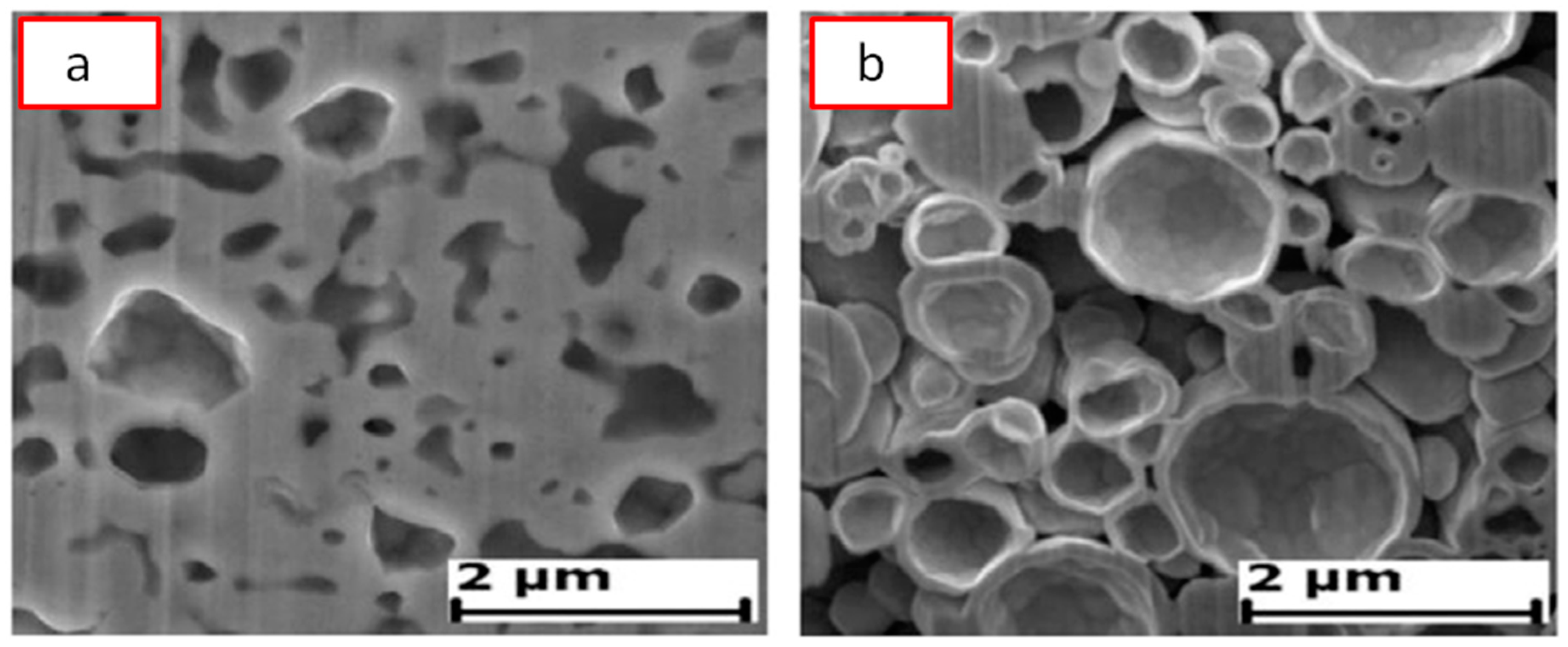
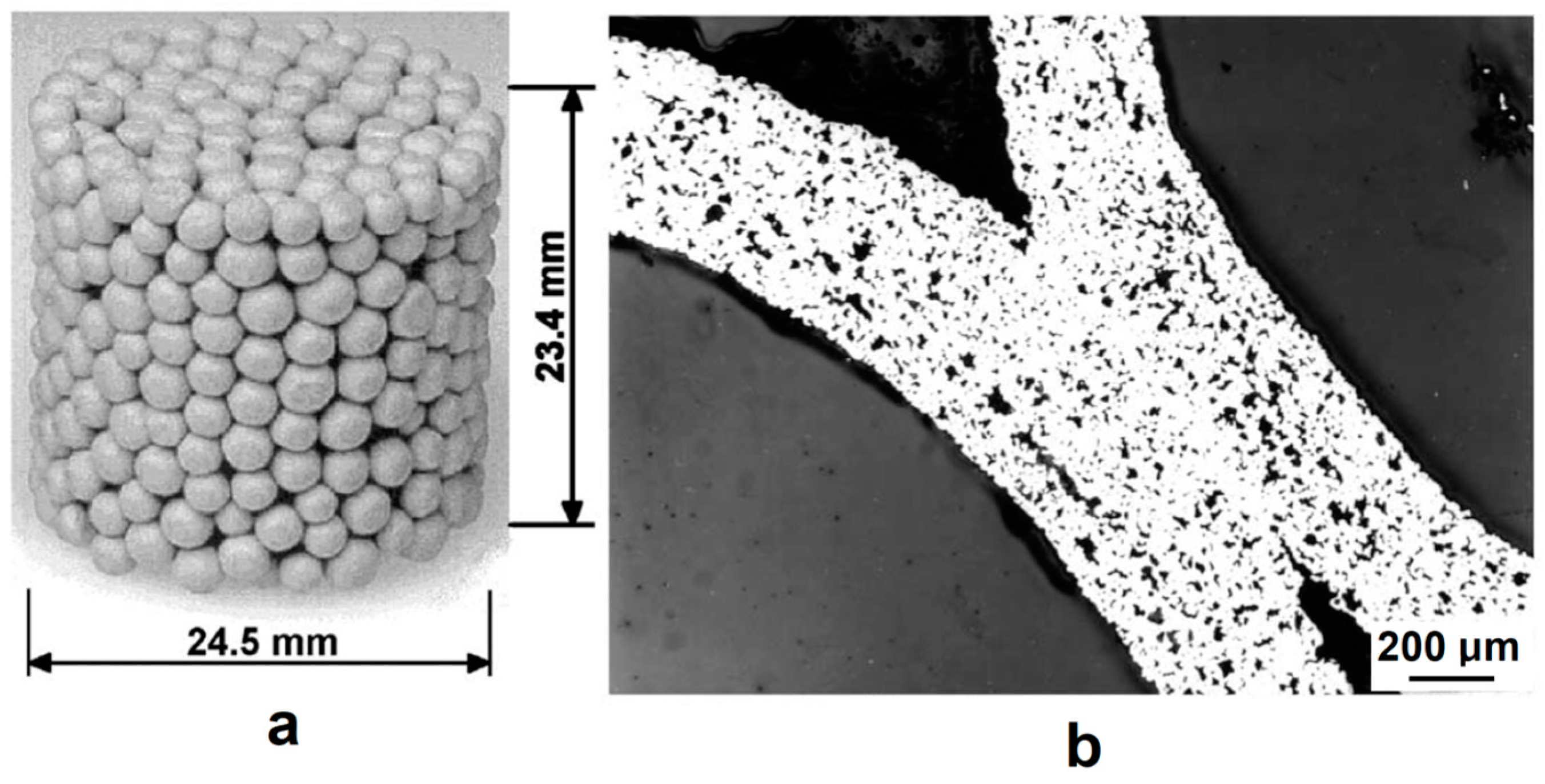
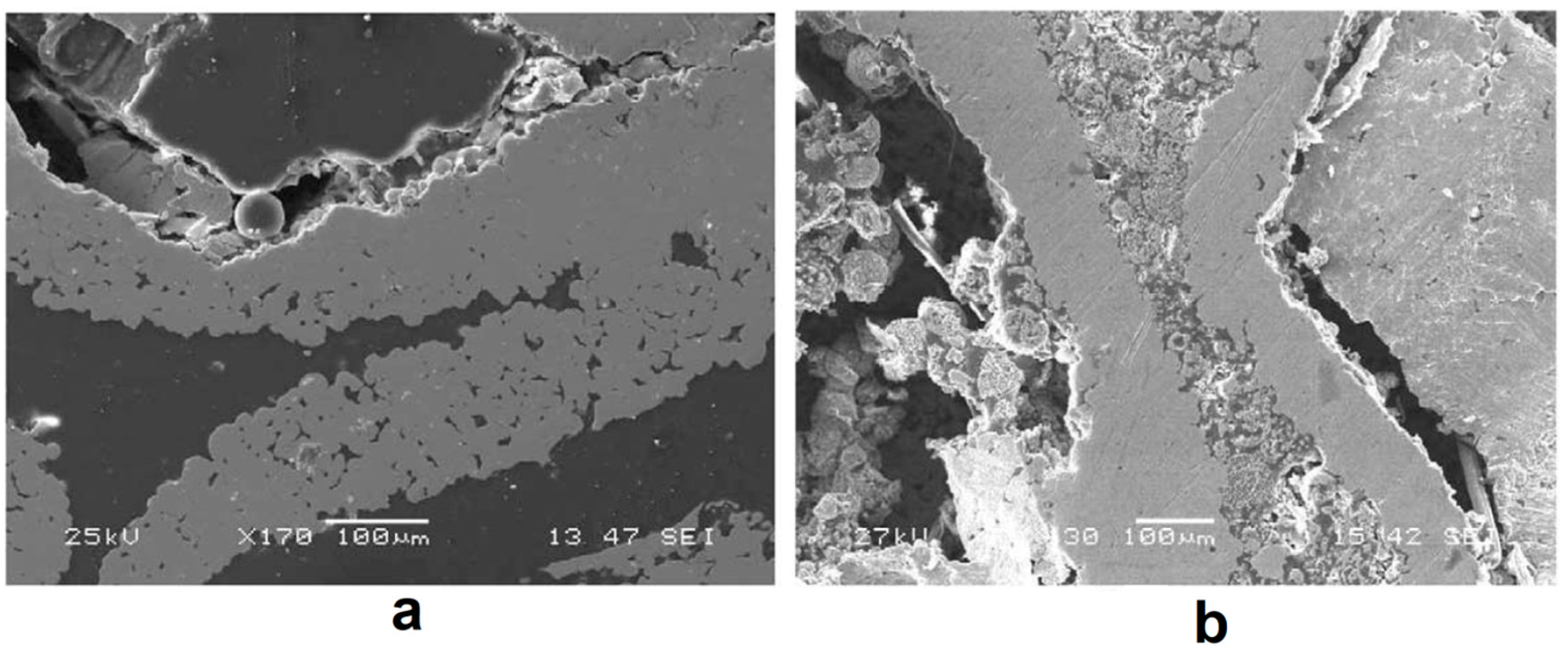
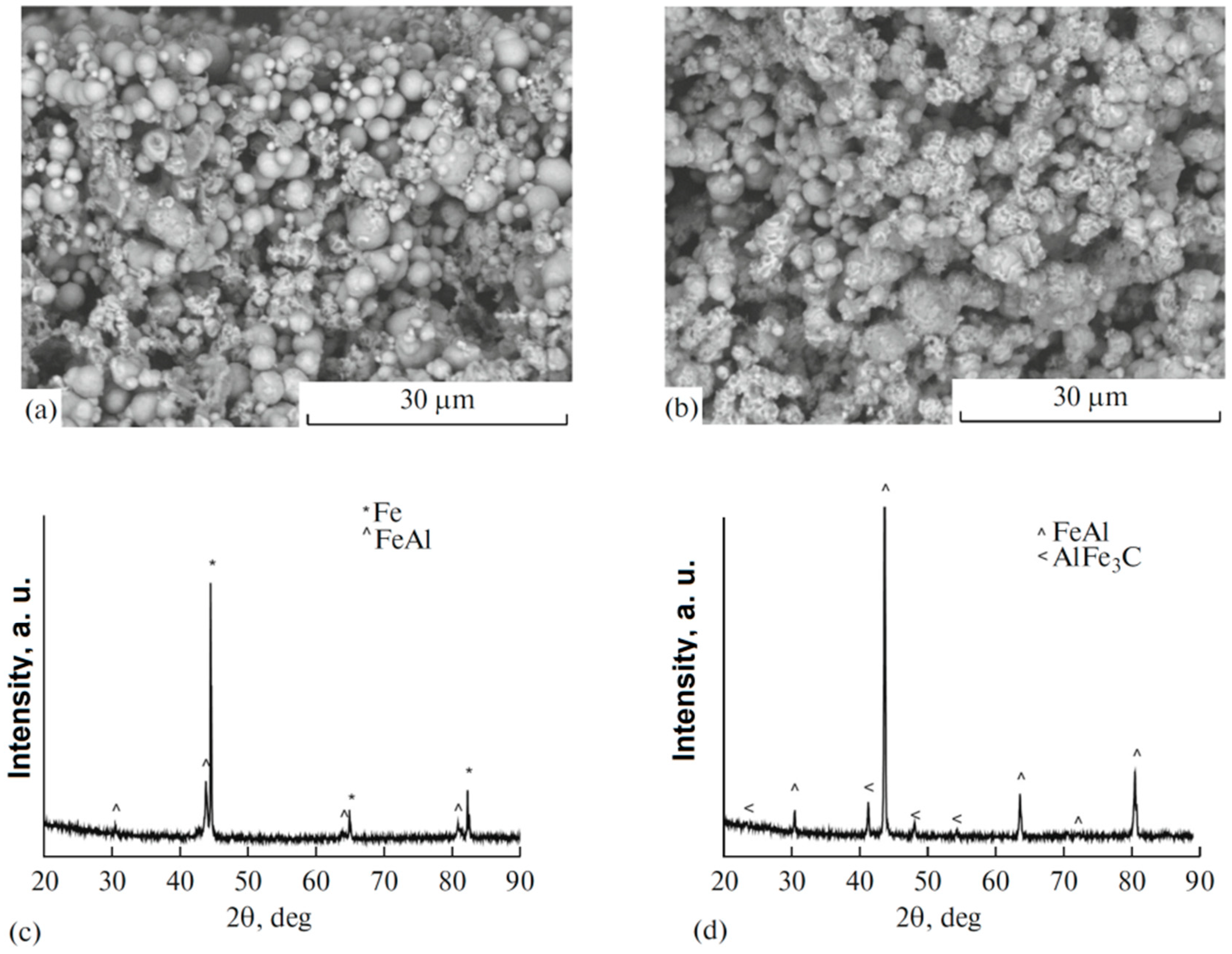
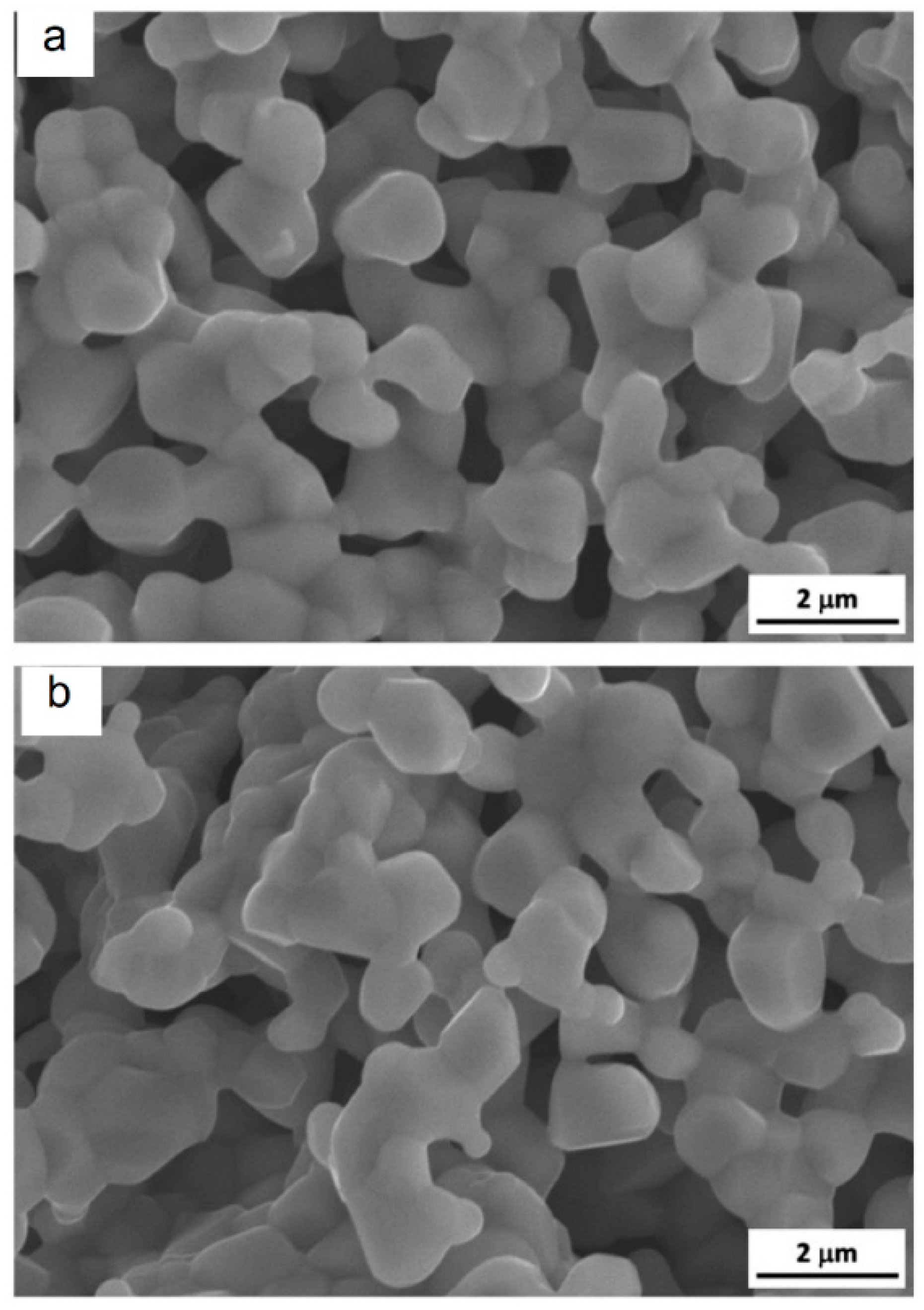
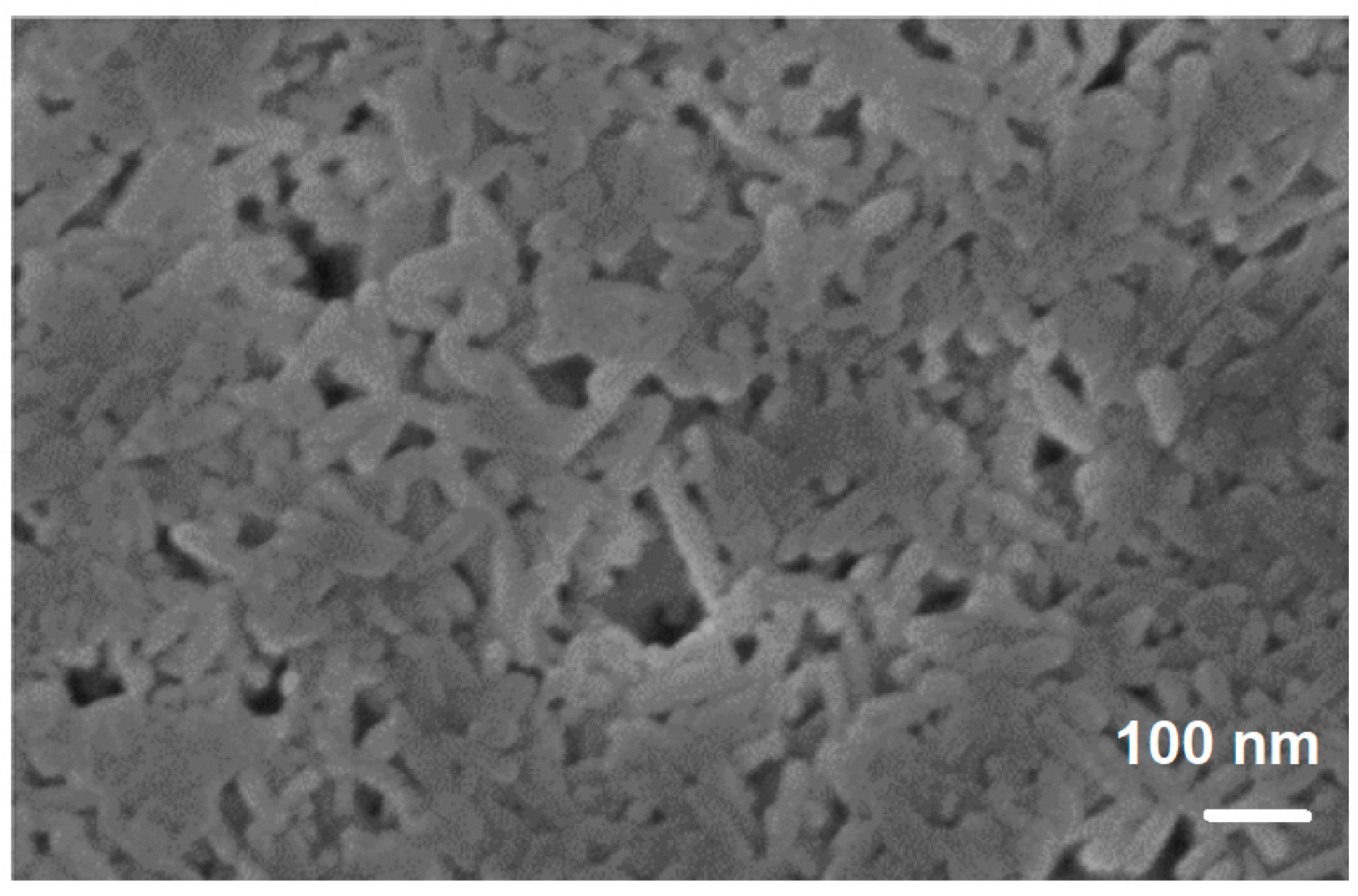

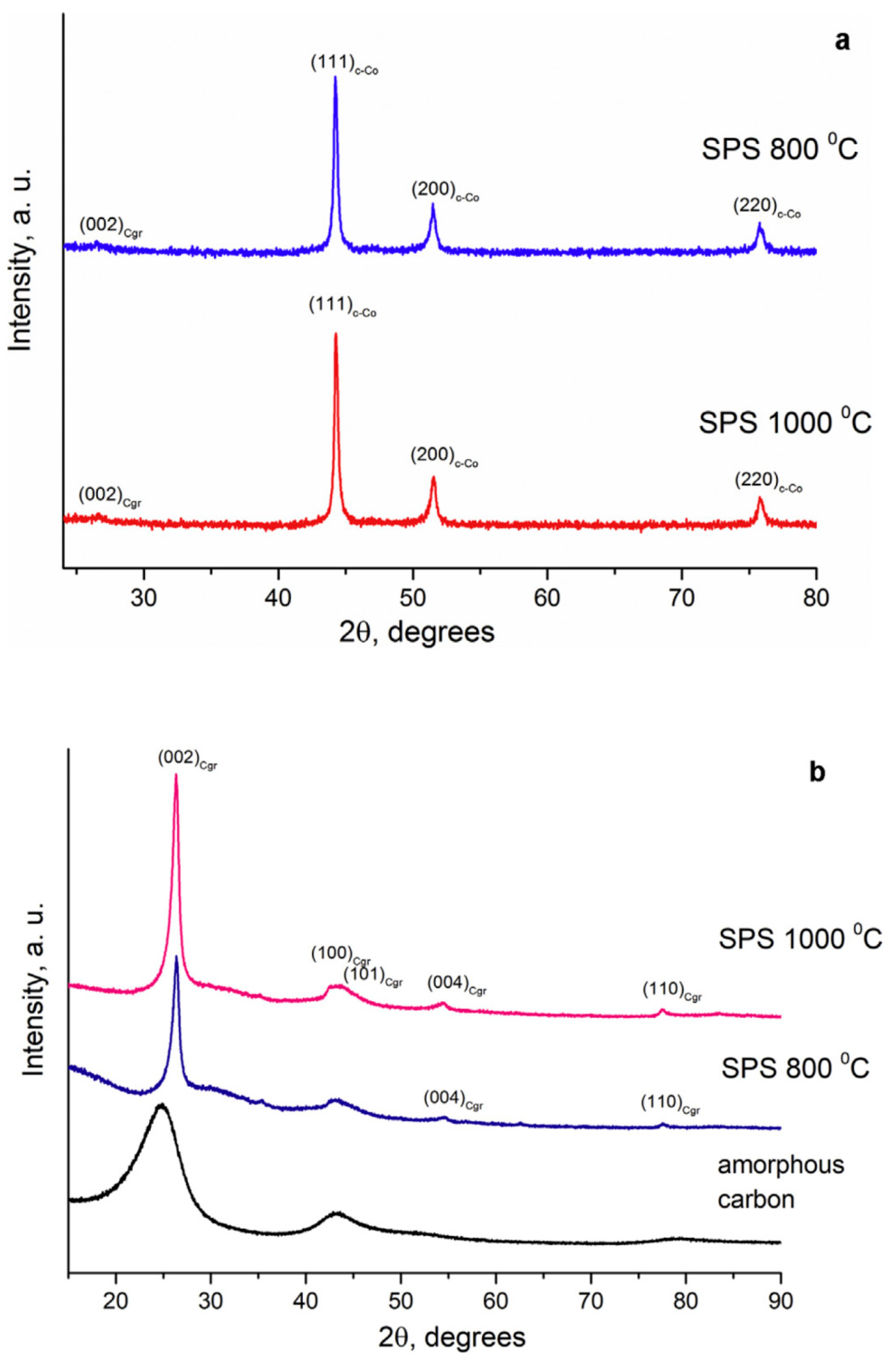

| Porous Material | Production Method | References |
|---|---|---|
| Metals | Partial densification during SPS (powders of conventional morphologies) | [20,25,26,27,28,29,30] |
| SPS with additives decomposing during sintering | [31,32] | |
| SPS under pressure with space holders removed after sintering | [33,34,35,36,37,38,39] | |
| SPS of hollow particles | [22] | |
| SPS of hollow particles of the material experiencing decomposition upon sintering | [40] | |
| Alloys and intermetallics | Partial densification during SPS (powders of conventional morphologies) | [24,41,42,43,44,45] |
| SPS with additives decomposing during sintering | [46] | |
| Chemical reaction and partial densification during SPS | [47,48,49,50,51] | |
| SPS under pressure with space holders removed after sintering | [52,53] | |
| SPS of reaction mixture under pressure with a space holder followed by post-treatment to conduct the synthesis | [54] | |
| SPS of hollow particles/spheres | [21,55] | |
| Ceramic materials and composites | Partial densification during SPS (powders of conventional morphologies) | [26,56,57,58,59] |
| SPS of porous particles | [60,61,62,63,64,65] | |
| Chemical reaction and partial densification during SPS | [66,67,68,69] | |
| SPS with a space holder | [70,71] | |
| SPS with additives decomposing during sintering | [72] | |
| Carbon materials | Partial densification during SPS | [73,74] |
| SPS under pressure with space holders removed after sintering | [75,76,77,78] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudina, D.V.; Bokhonov, B.B.; Olevsky, E.A. Fabrication of Porous Materials by Spark Plasma Sintering: A Review. Materials 2019, 12, 541. https://doi.org/10.3390/ma12030541
Dudina DV, Bokhonov BB, Olevsky EA. Fabrication of Porous Materials by Spark Plasma Sintering: A Review. Materials. 2019; 12(3):541. https://doi.org/10.3390/ma12030541
Chicago/Turabian StyleDudina, Dina V., Boris B. Bokhonov, and Eugene A. Olevsky. 2019. "Fabrication of Porous Materials by Spark Plasma Sintering: A Review" Materials 12, no. 3: 541. https://doi.org/10.3390/ma12030541
APA StyleDudina, D. V., Bokhonov, B. B., & Olevsky, E. A. (2019). Fabrication of Porous Materials by Spark Plasma Sintering: A Review. Materials, 12(3), 541. https://doi.org/10.3390/ma12030541