Durability of the Bond between CFRP and Concrete Exposed to Thermal Cycles
Abstract
:1. Introduction
2. Experimental
2.1. Raw Materials
2.2. Single-Lap Shear Test
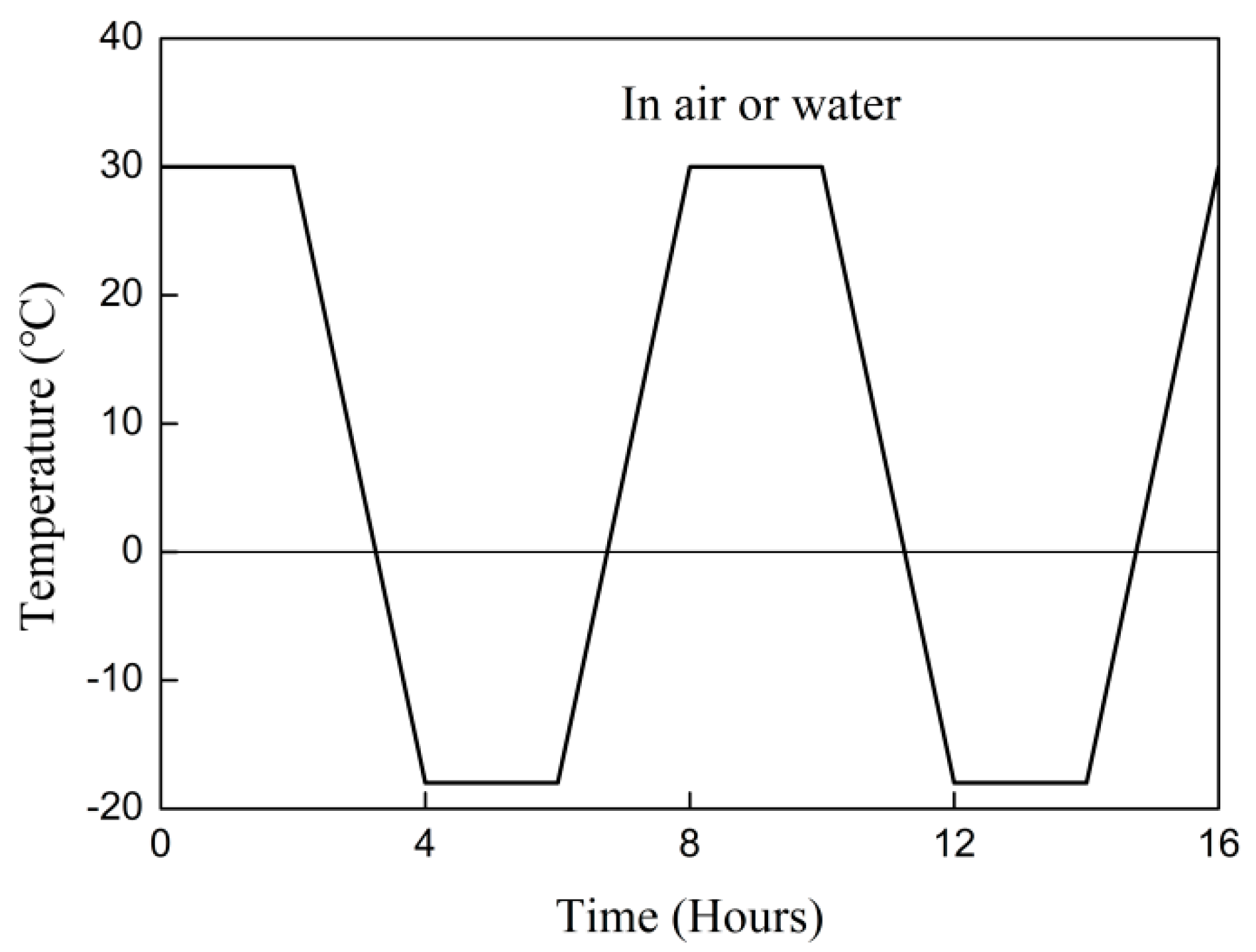
2.3. Exposure Conditions
3. Results and Discussion
3.1. Durability of Constituent Materials
3.2. Test Phenomena and Failure Modes
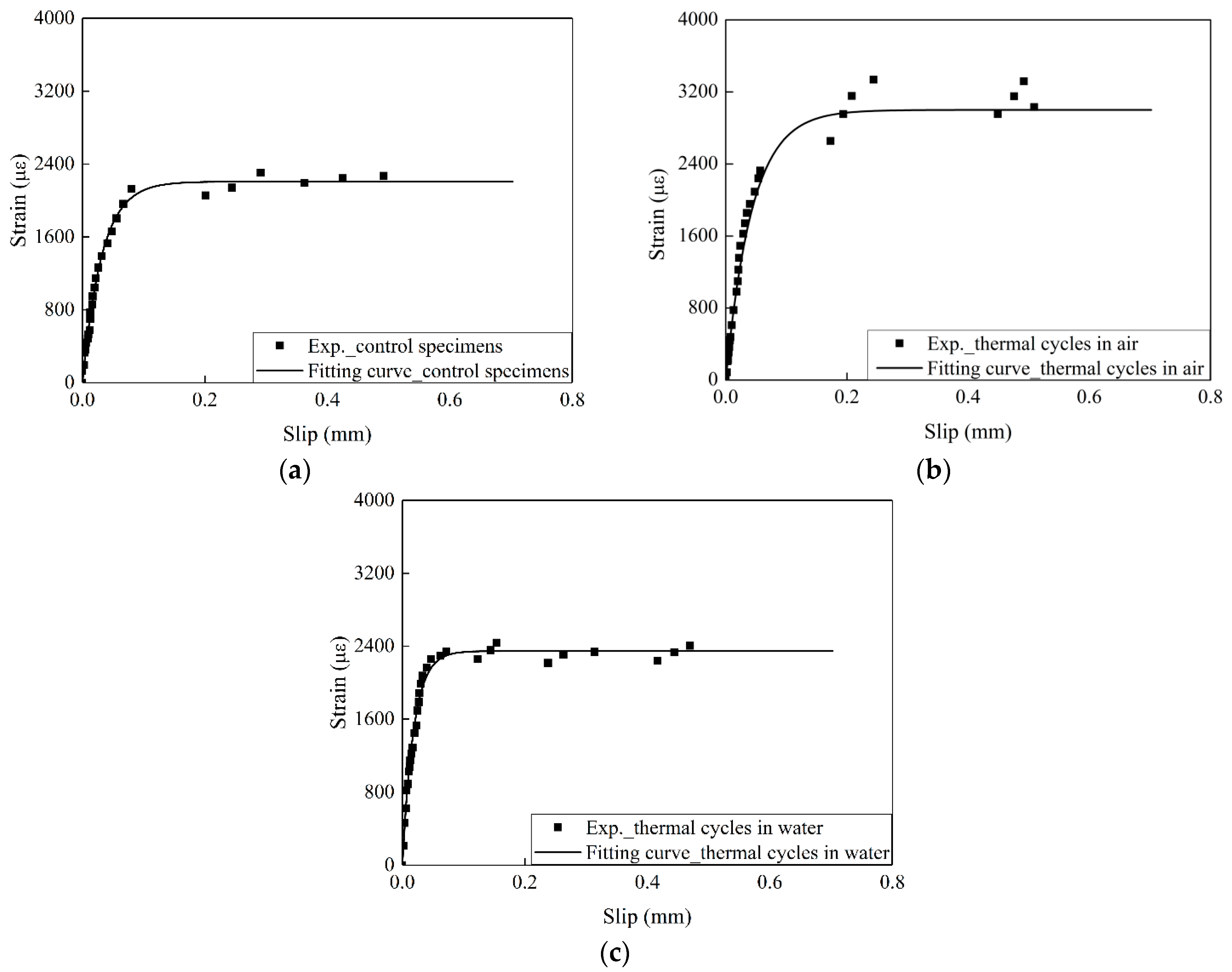
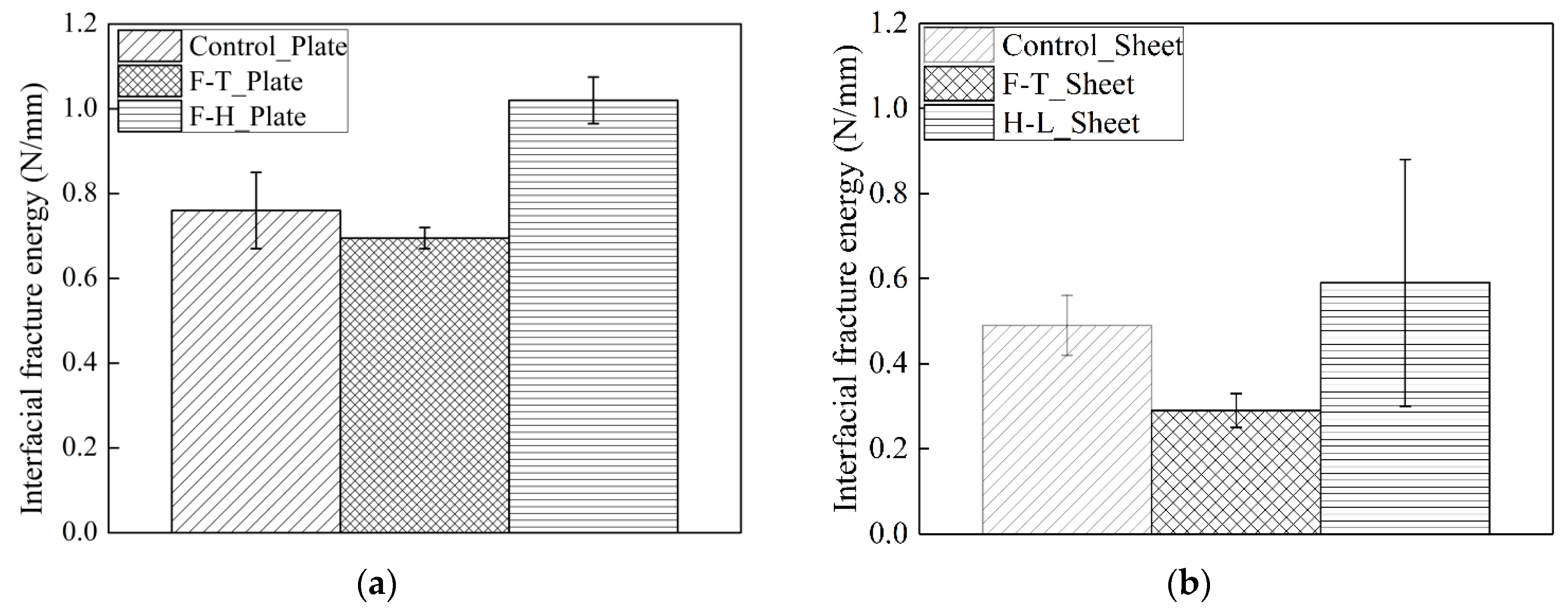
3.3. The Influence of the Exposure Conditions on the Interfacial Fracture Energy and Bond Stress–Slip Curve
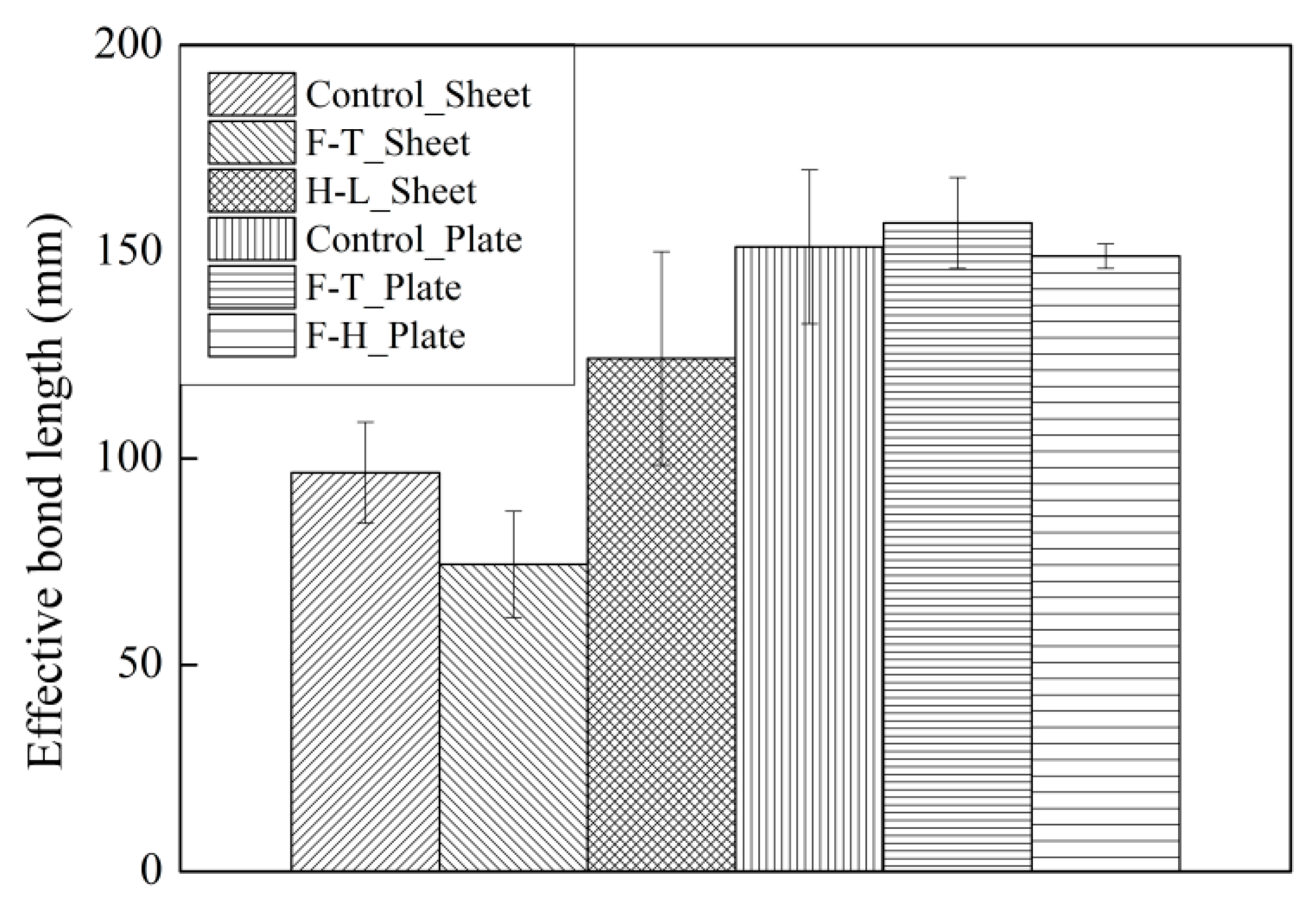
3.4. The Influence of Exposure Conditions on the Interfacial Effective Bond Length
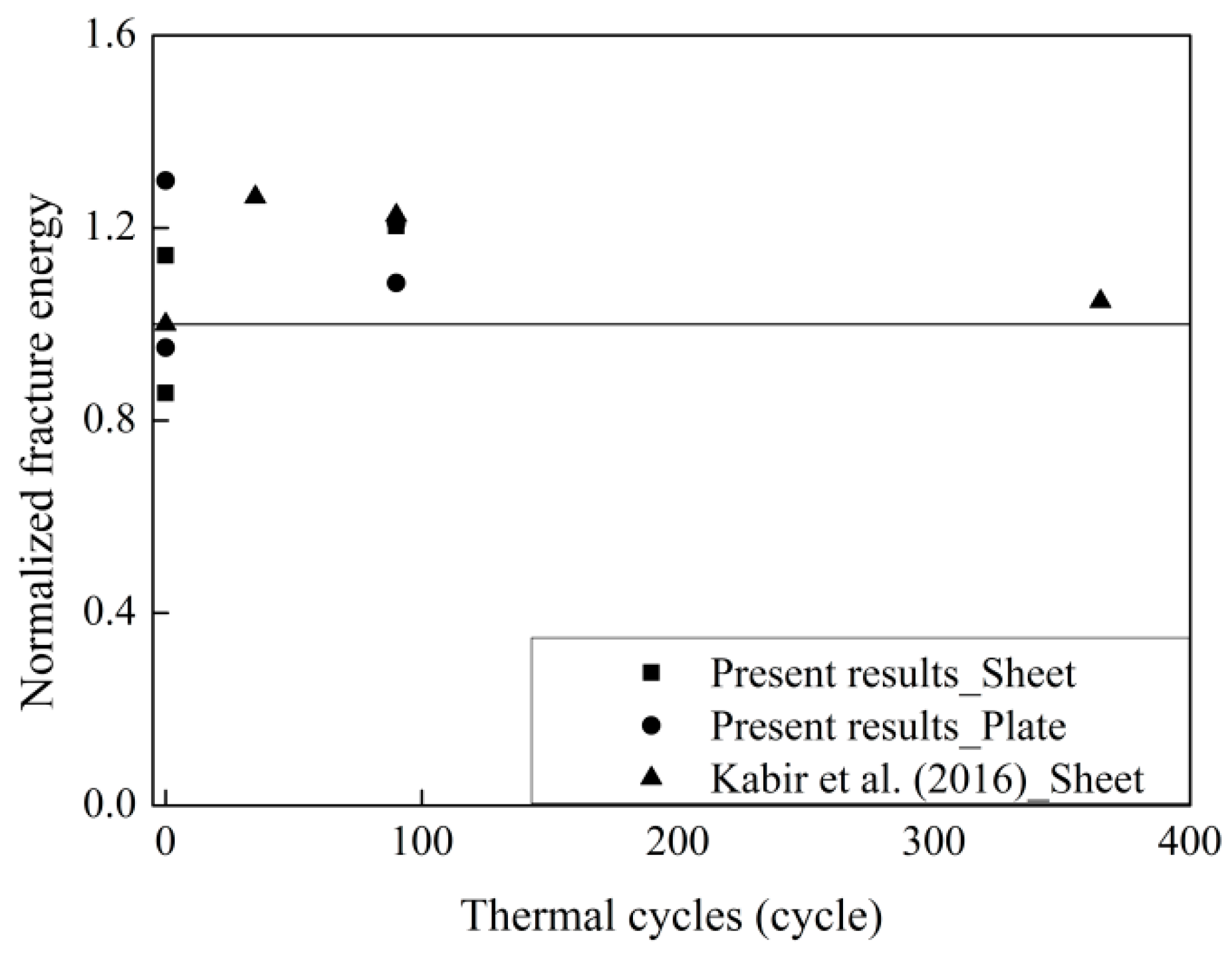
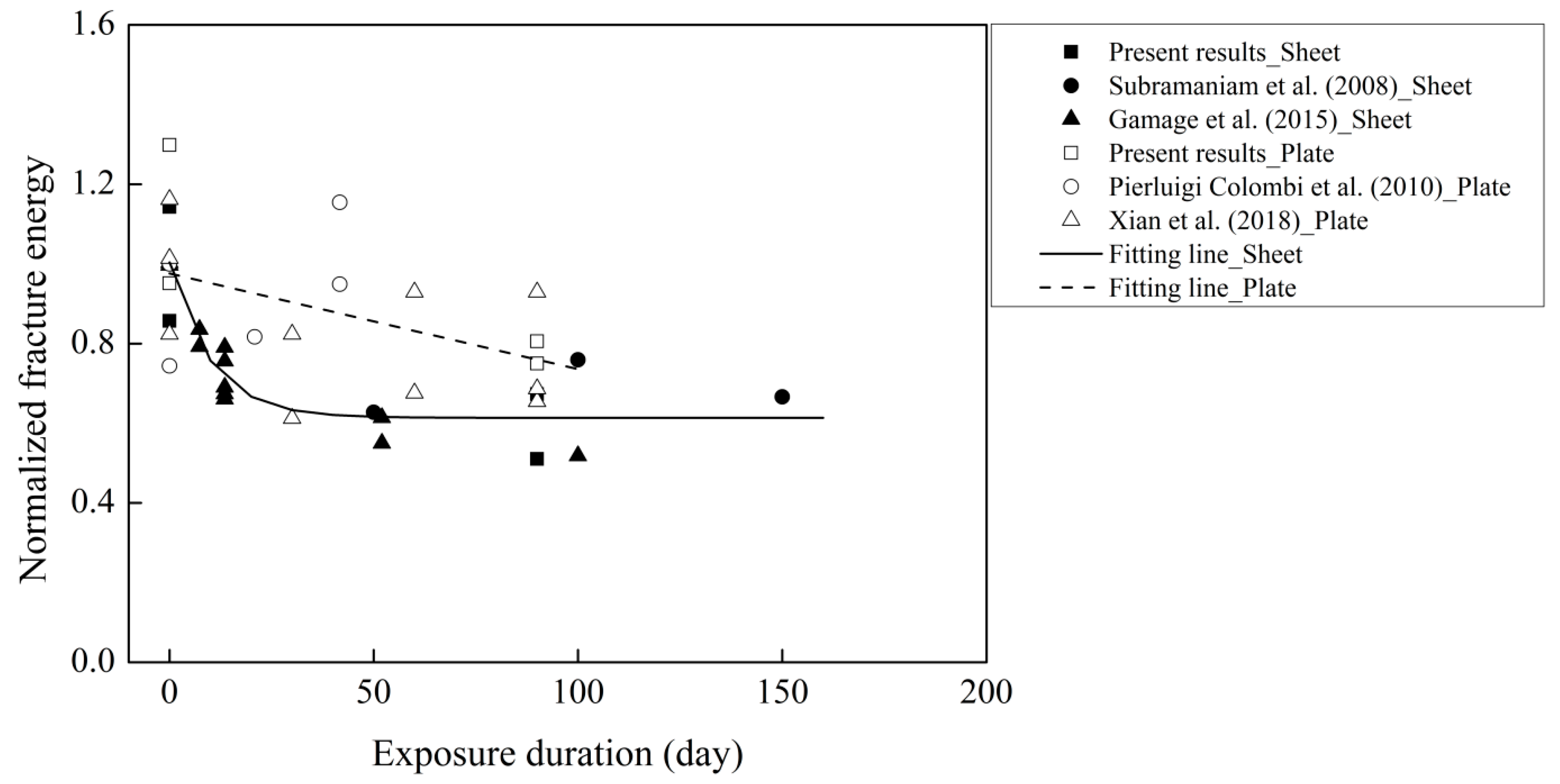
3.5. The Evolution of Fracture Energy Exposed to Thermal Cycles
4. Conclusions
- Thermal cycles increase the interfacial fracture energy. The increase in the fracture energy of CFRP sheet–concrete is smaller than that of CFRP plate–concrete.
- The failure modes of both CFRP sheet–concrete and CFRP plate–concrete are still concrete cohesive failure. The thermal cycles in water change the failure mode of both CFRP sheet–concrete and CFRP plate–concrete from concrete cohesive failure to interfacial debonding between the adhesive layer and concrete.
- The degradation of the interfacial bond mainly results from water moisture with 270 thermal cycles in water. The internal stress induced by thermal cycles in air has insignificant effects on the interfacial fracture energy.
- A reduction factor (0.7) for a CFRP–concrete structure for thermal cycles in water was proposed.
Author Contributions
Funding
Conflicts of Interest
References
- Chen, G.; Teng, J.; Chen, J. Finite-element modeling of intermediate crack debonding in FRP-plated RC beams. J. Compos. Constr. 2010, 15, 339–353. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, J.-F. Concrete damage plasticity model for modeling FRP-to-concrete bond behavior. J. Compos. Constr. 2014, 19, 04014026. [Google Scholar] [CrossRef]
- Yao, J.; Teng, J.G.; Chen, J.F. Experimental study on FRP-to-concrete bonded joints. Compos. Part B 2005, 36, 99–113. [Google Scholar] [CrossRef]
- Shrestha, J.; Ueda, T.; Zhang, D.W. Durability of FRP concrete bonds and its constituent properties under the influence of moisture conditions. J. Mater. Civil. Eng. 2014, 27, A4014009. [Google Scholar] [CrossRef]
- Pan, Y.; Xian, G.; Li, H. Effects of freeze-thaw cycles on the behavior of the bond between CFRP plates and concrete substrates. J. Compos. Constr. 2018, 22, 04018011. [Google Scholar] [CrossRef]
- Jeong, Y.; Lopez, M.M.; Bakis, C.E. Effects of temperature and sustained loading on the mechanical response of CFRP bonded to concrete. Constr. Build. Mater. 2016, 124, 442–452. [Google Scholar] [CrossRef]
- Ferrier, E.; Rabinovitch, O.; Michel, L. Mechanical behavior of concrete–resin/adhesive–FRP structural assemblies under low and high temperatures. Constr. Build. Mater. 2016, 127, 1017–1028. [Google Scholar] [CrossRef]
- ACI Committee 440. Guide for the Design and Construction of Externally Bonded FRP Systems for Strengthening Concrete Structures; ACI Committee 440: Farmington Hills, MI, USA, 2008. [Google Scholar]
- Klamer, E.L.; Hordijk, D.A.; Janssen, H.J. The influence of temperature on the debonding of externally bonded CFRP. Spec. Issue ACI 2005, 230, 1551–1570. [Google Scholar]
- Kabir, M.I.; Samali, B.; Shrestha, R. Fracture properties of CFRP-concrete bond subjected to three environmental conditions. J. Compos. Constr. 2016, 20, 04016010. [Google Scholar] [CrossRef]
- Gamage, J.C.P.H.; Al-Mahaidi, R.; Wong, M.B. Integrity of CFRP-concrete bond subjected to long-term cyclic temperature and mechanical stress. Compos. Struct. 2016, 149, 423–433. [Google Scholar] [CrossRef]
- Yun, Y.; Wu, Y.-F. Durability of CFRP–concrete joints under freeze–thaw cycling. Cold Reg. Sci. Technol. 2011, 65, 401–412. [Google Scholar] [CrossRef]
- Ortega, J.M.; Sánchez, I.; Climent, M.A. Impedance spectroscopy study of the effect of environmental conditions in the microstructure development of OPC and slag cement mortars. Arch. Civ. Mech. Eng. 2015, 15, 569–583. [Google Scholar] [CrossRef]
- Joshaghani, A.; Balapour, M.; Ramezanianpour, A.A. Effect of controlled environmental conditions on mechanical, microstructural and durability properties of cement mortar. Constr. Build. Mater. 2018, 164, 134–149. [Google Scholar] [CrossRef]
- Ramezanianpour, A.A.; Malhotra, V.M. Effect of curing on the compressive strength, resistance to chloride-ion penetration and porosity of concretes incorporating slag, fly ash or silica fume. Cem. Concr. Compos. 1995, 17, 125–133. [Google Scholar] [CrossRef]
- Williams, M.; Ortega, J.M.; Sánchez, I.; Cabeza, M.; Climent, M.Á. Non-Destructive Study of the Microstructural Effects of Sodium and Magnesium Sulphate Attack on Mortars Containing Silica Fume Using Impedance Spectroscopy. Appl. Sci. 2017, 7, 648. [Google Scholar] [CrossRef]
- Zhou, C.; Ren, F.; Zeng, Q.; Xiao, L.; Wang, W. Pore-size resolved water vapor adsorption kinetics of white cement mortars as viewed from proton NMR relaxation. Cem. Concr. Res. 2018, 105, 31–43. [Google Scholar] [CrossRef]
- Colombi, P.; Fava, G.; Poggi, C. Bond strength of CFRP–concrete elements under freeze–thaw cycles. Compos. Struct. 2010, 92, 973–983. [Google Scholar] [CrossRef]
- Zhou, A.; Büyüköztürk, O.; Lau, D. Debonding of concrete-epoxy interface under the coupled effect of moisture and sustained load. Cem. Concr. Compos. 2017, 80, 287–297. [Google Scholar] [CrossRef]
- Pan, Y.F.; Xian, G.J.; Silva, M.A. Effects of water immersion on the bond behavior between CFRP plates and concrete substrate. Constr. Build. Mater. 2015, 101, 326–337. [Google Scholar] [CrossRef]
- Au, C.; Büyüköztürk, O. Peel and shear fracture characterization of debonding in FRP plated concrete affected by moisture. J. Compos. Constr. 2006, 10, 35–47. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Tensile Properties of Polymer Matrix Composite Materials; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- C.o. China. Standard for Test Method of Mechanical Properties on Ordinary Concrete; China Standard Publishing House: Beijing, China, 2002. [Google Scholar]
- ACI. Building Code Requirements for Structural Concrete and Commentary; American Concrete Institute: Farmington Hills, MI, USA, 2008. [Google Scholar]
- Chen, J.F.; Teng, J.G. Anchorage strength models for FRP and steel plates bonded to concrete. J. Struct. Eng. 2001, 127, 784–791. [Google Scholar] [CrossRef]
- Lu, Z.; Xian, G.; Li, H. Effects of exposure to elevated temperatures and subsequent immersion in water or alkaline solution on the mechanical properties of pultruded BFRP plates. Compos. Part B 2015, 77, 421–430. [Google Scholar] [CrossRef]
- Xian, G.J.; Karbhari, V.M. DMTA based investigation of hygrothermal ageing of an epoxy system used in rehabilitation. J. Appl. Polym. Sci. 2007, 104, 1084–1094. [Google Scholar] [CrossRef]
- Shen, C.-H.; Springer, G.S. Moisture absorption and desorption of composite materials. J. Compos. Mater. 1976, 10, 2–20. [Google Scholar] [CrossRef]
- Hong, B.; Xian, G. Ageing of a thermosetting polyurethane and its pultruded carbon fiber plates subjected to seawater immersion. Constr. Build. Mater. 2018, 165, 514–522. [Google Scholar] [CrossRef]
- Dai, J.G.; Ueda, T.; Sato, Y. Development of the nonlinear bond stress-slip model of fiber reinforced plastics sheet-concrete interfaces with a simple method. J. Compos. Constr. 2005, 9, 52–62. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, T.; Li, S.; Liu, Z. Bond behavior of wet-bonded carbon fiber-reinforced polymer-concrete interface subjected to moisture. Int. J. Polym. Sci. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Shrestha, J.; Zhang, D.W.; Ueda, T. Durability performances of carbon fiber–reinforced polymer and concrete-bonded systems under moisture conditions. J. Compos. Constr. 2016, 20, 04016023. [Google Scholar] [CrossRef]
- Lau, D. Adhesion between organic and inorganic materials at nanoscale under the effect of moisture. In Proceedings of the 2012 IEEE 6th International Conference on Nano/Molecular Medicine and Engineering (NANOMED), Bangkok, Thailand, 4–7 November 2012. [Google Scholar]
- Subramaniam, K.V.; Ali-Ahmad, M.; Ghosn, M. Freeze–thaw degradation of FRP–concrete interface: Impact on cohesive fracture response. Eng. Fract. Mech. 2008, 75, 3924–3940. [Google Scholar] [CrossRef]
- Liang, H.; Li, S.; Lu, Y.; Yang, T. Reliability analysis of bond behaviour of cfrp–concrete interface under wet–dry cycles. Materials 2018, 11, 741. [Google Scholar] [CrossRef] [PubMed]
- Büyüköztürk, O.; Buehler, M.J.; Lau, D.; Tuakta, C. Structural solution using molecular dynamics: Fundamentals and a case study of epoxy-silica interface. Int. J. Solids. Struct. 2011, 48, 2131–2140. [Google Scholar] [CrossRef]






| Materials | CFRP Plate–Concrete | CFRP Sheet–Concrete |
|---|---|---|
| Primer | Epoxy | Epoxy |
| Resin | - | Epoxy |
| Adhesive | Adhesive | - |
| Concrete | = 42.1 MPa | = 42.1 MPa |
| Parameter | Epoxy | Adhesive |
|---|---|---|
| Diffusivity coefficient (×10−8 mm2/s) | 6.0 | 7.0 |
| Equilibrium moisture (%) | 2.40 | 3.53 |
| Parameter | CFRP Sheet | CFRP Plate |
|---|---|---|
| Fiber volume fraction | 0.3 | 0.67 |
| Diffusivity coefficient (×10−8 mm2/s) | 2.3 | 0.5 |
| Equilibrium moisture (%) | 1.68 | 0.8 |
| Materials | Control | Cov. | In Air | Cov. | In Water | Cov. |
|---|---|---|---|---|---|---|
| Epoxy | 3.2 | 0.07 | 3.6 | 0.16 | 2.9 | 0.04 |
| Adhesive | 3.5 | 0.10 | 3.7 | 0.30 | 3.0 | 0.12 |
| CFRP sheet | 241.2 | 5.2 | 250.3 | 9.8 | 218.9 | 8.4 |
| CFRP plate | 176.5 | 3.5 | 178 | 8.3 | 173.0 | 5.1 |
| Specimens a | No. Cycles | Conditioning | Failure Mode | B (mm−1) | A (με) | Pu (kN) | Gf (N/mm) | Le (mm) |
|---|---|---|---|---|---|---|---|---|
| CSC-1 | 0 | - | Concrete | 27 | 3693 | 7.6 | 0.56 | 84 |
| CSC-2 | 0 | - | Concrete | 32 | 3222 | 6.6 | 0.42 | 109 |
| CSCA-1 | 270 | Air | Concrete | 40 | 3784 | 5.1 | 0.59 | 98 |
| CSCA-2 | 270 | Air | Concrete | 46 | 5336 | 5.8 | 1.17 | 150 |
| CSCW-1 | 270 | Water | Interface | 78 | 2476 | 7.7 | 0.25 | 87 |
| CSCW-2 | 270 | Water | Interface | 39 | 2849 | 10.9 | 0.33 | 62 |
| CPC-1 | 0 | - | Concrete | 22 | 2622 | 16.2 | 0.85 | - |
| CPC-2 | 0 | - | Concrete | 22 | 2339 | 14.4 | 0.67 | - |
| CPC-3 | 0 | - | Concrete | 15 | 3070 | 18.9 | 1.16 | 131 |
| CPC-4 | 0 | - | Concrete | 42 | 2462 | 15.2 | 0.75 | 176 |
| CPC-5 | 0 | - | Concrete | 33 | 2471 | 15.2 | 0.75 | 146 |
| CPCA-1 | 270 | Air | Concrete | 18 | 2954 | 18.2 | 1.08 | 149 |
| CPCA-2 | 270 | Air | Concrete | 26 | 2800 | 17.2 | 0.97 | 152 |
| CPCW-1 | 270 | Water | Interface | 34 | 2415 | 14.9 | 0.72 | 146 |
| CPCW-2 | 270 | Water | Interface | 36 | 2339 | 14.4 | 0.67 | 168 |
| Source of Data | Specimen | FRP Type | No. Cycles | Exposure | (MPa) | Eftf (GPa/mm) | Gf (N/mm) |
|---|---|---|---|---|---|---|---|
| Kabir et al. (2016) [10] | Control | Sheet | 0 | - | 36.6 | 52.9 | 1.57 |
| CT2 | Sheet | 35 | Air | 36.6 | 52.9 | 1.99 | |
| CT3 | Sheet | 90 | Air | 36.6 | 52.9 | 1.93 | |
| CT4 | Sheet | 335 | Air | 36.6 | 52.9 | 1.65 | |
| Pierluigi Colombi et al. (2010) [18] | P18A | Plate | 0 | - | 25.0 | 211.9 | 0.67 |
| P20A | Plate | 0 | - | 25.0 | 211.9 | 0.50 | |
| P2A | Plate | 100 | Water | 24.9 | 211.9 | 0.55 | |
| P4A | Plate | 200 | Water | 24.7 | 211.9 | 0.64 | |
| P6A | Plate | 200 | Water | 24.7 | 211.9 | 0.78 | |
| Subramaniam et al. (2008) [34] | 0 cycles | sheet | 0 | - | 38.0 | 39.1 | 1 |
| 100 cycles | sheet | 100 | Water | 38.0 | 41.8 | 0.92 | |
| 200 cycles | sheet | 200 | Water | 38.0 | 39.7 | 0.88 | |
| 300 cycles | sheet | 300 | Water | 38.0 | 41.5 | 0.83 | |
| Xian et al. (2018) [5] | F-T/W-0-1 | Plate | 0 | - | 44.1 | 246.4 | 1.1 |
| F-T/W-0-2 | Plate | 0 | - | 44.1 | 246.4 | 0.78 | |
| F-T/W-0-3 | Plate | 0 | - | 44.1 | 246.4 | 0.96 | |
| F-T-M-30-1 | Plate | 30 | 90% RH | 39.9 | 246.4 | 0.78 | |
| F-T-M-30-2 | Plate | 30 | 90% RH | 39.9 | 246.4 | 0.58 | |
| F-T-M-60-1 | Plate | 60 | 90% RH | 38.6 | 246.4 | 0.64 | |
| F-T-M-60-2 | Plate | 60 | 90% RH | 38.6 | 246.4 | 0.88 | |
| F-T-M-90-1 | Plate | 90 | 90% RH | 41.2 | 246.4 | 0.62 | |
| F-T-M-90-2 | Plate | 90 | 90% RH | 41.2 | 246.4 | 0.88 | |
| F-T-M-90-3 | Plate | 90 | 90% RH | 41.2 | 246.4 | 0.65 | |
| Gamage et al. (2015) [11] | A1-1 | sheet | 0 | 90% RH | 30 | 40.5 | 0.78 |
| A1-2 | sheet | 0 | 90% RH | 30 | 40.5 | 0.79 | |
| A2-1 | sheet | 44 | 90% RH | 30 | 40.5 | 0.62 | |
| A2-2 | sheet | 44 | 90% RH | 30 | 40.5 | 0.65 | |
| A4-4 | sheet | 81 | 90% RH | 30 | 40.5 | 0.59 | |
| A4-5 | sheet | 81 | 90% RH | 30 | 40.5 | 0.53 | |
| A4-6 | sheet | 81 | 90% RH | 30 | 40.5 | 0.62 | |
| A4-7 | sheet | 81 | 90% RH | 30 | 40.5 | 0.52 | |
| A4-8 | sheet | 81 | 90% RH | 30 | 40.5 | 0.54 | |
| A9-9 | sheet | 312 | 90% RH | 30 | 40.5 | 0.43 | |
| A9-10 | sheet | 312 | 90% RH | 30 | 40.5 | 0.48 | |
| A11 | sheet | 600 | 90% RH | 30 | 40.5 | 0.41 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Pan, Y.; Li, H.; Xian, G. Durability of the Bond between CFRP and Concrete Exposed to Thermal Cycles. Materials 2019, 12, 515. https://doi.org/10.3390/ma12030515
Liu S, Pan Y, Li H, Xian G. Durability of the Bond between CFRP and Concrete Exposed to Thermal Cycles. Materials. 2019; 12(3):515. https://doi.org/10.3390/ma12030515
Chicago/Turabian StyleLiu, Shuai, Yunfeng Pan, Hedong Li, and Guijun Xian. 2019. "Durability of the Bond between CFRP and Concrete Exposed to Thermal Cycles" Materials 12, no. 3: 515. https://doi.org/10.3390/ma12030515
APA StyleLiu, S., Pan, Y., Li, H., & Xian, G. (2019). Durability of the Bond between CFRP and Concrete Exposed to Thermal Cycles. Materials, 12(3), 515. https://doi.org/10.3390/ma12030515





