Improvement of ZrC/Zr Coating on the Interface Combination and Physical Properties of Diamond-Copper Composites Fabricated by Spark Plasma Sintering
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Preparation of Composite
2.2. Property Testing
3. Results and Discussion
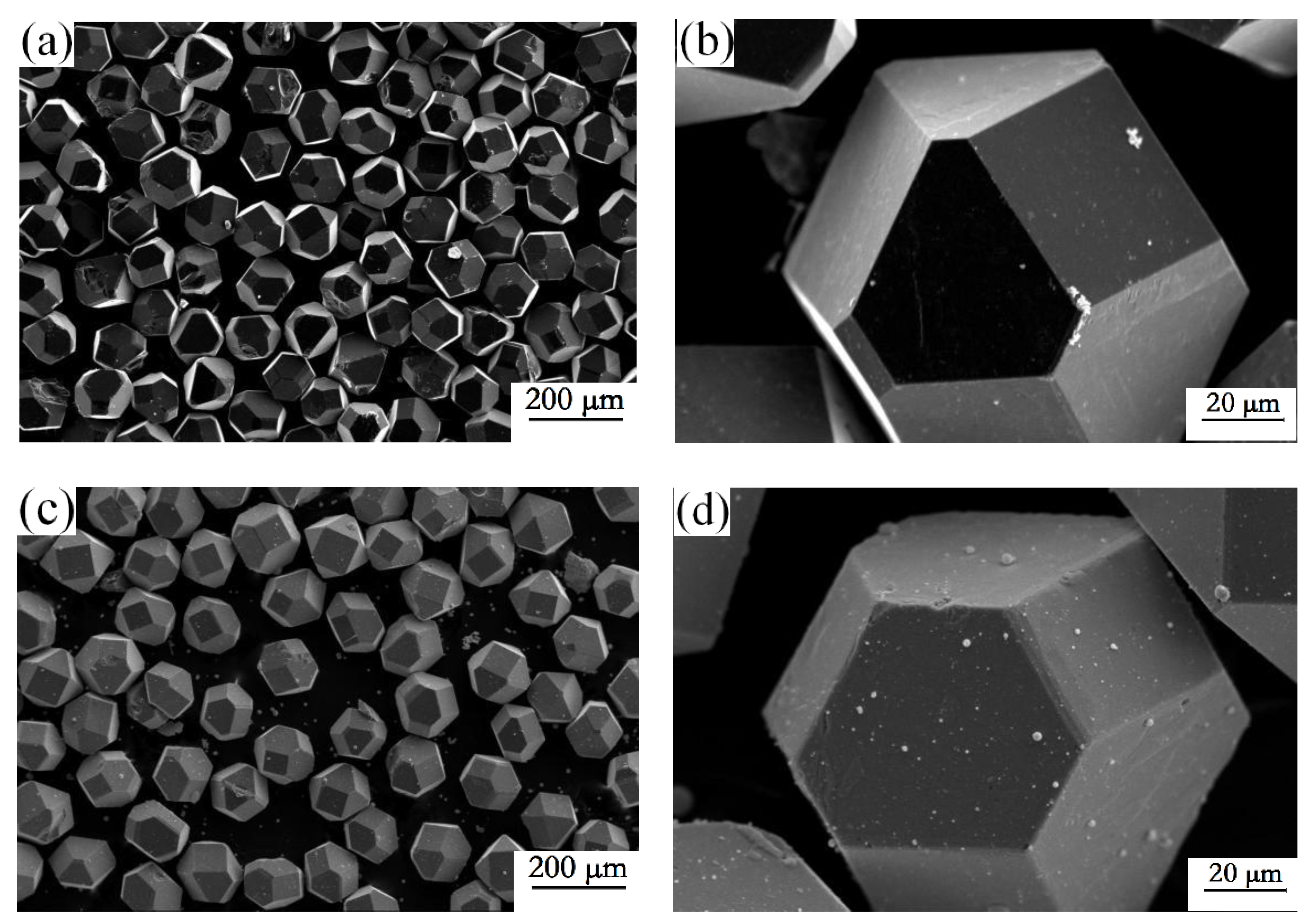
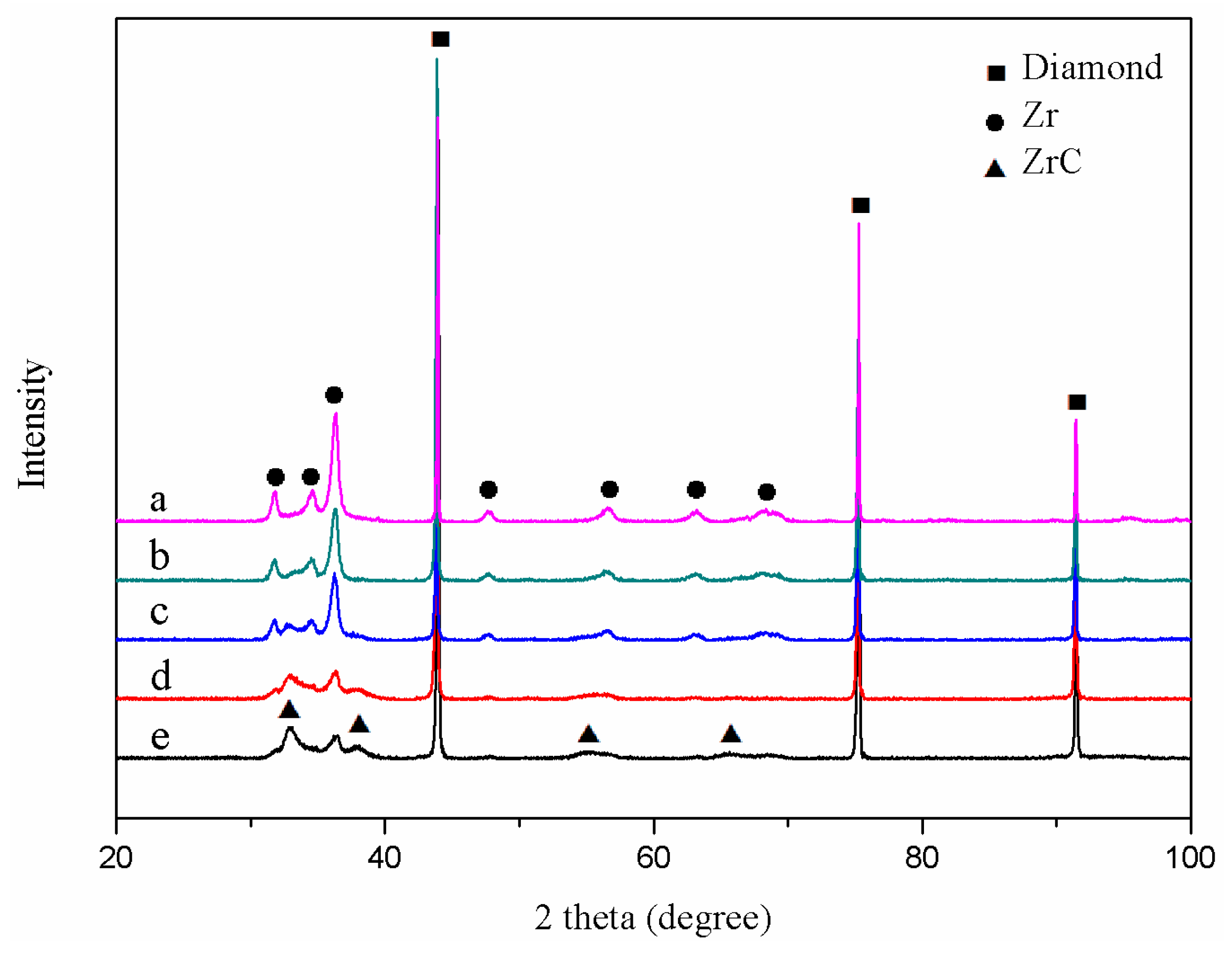
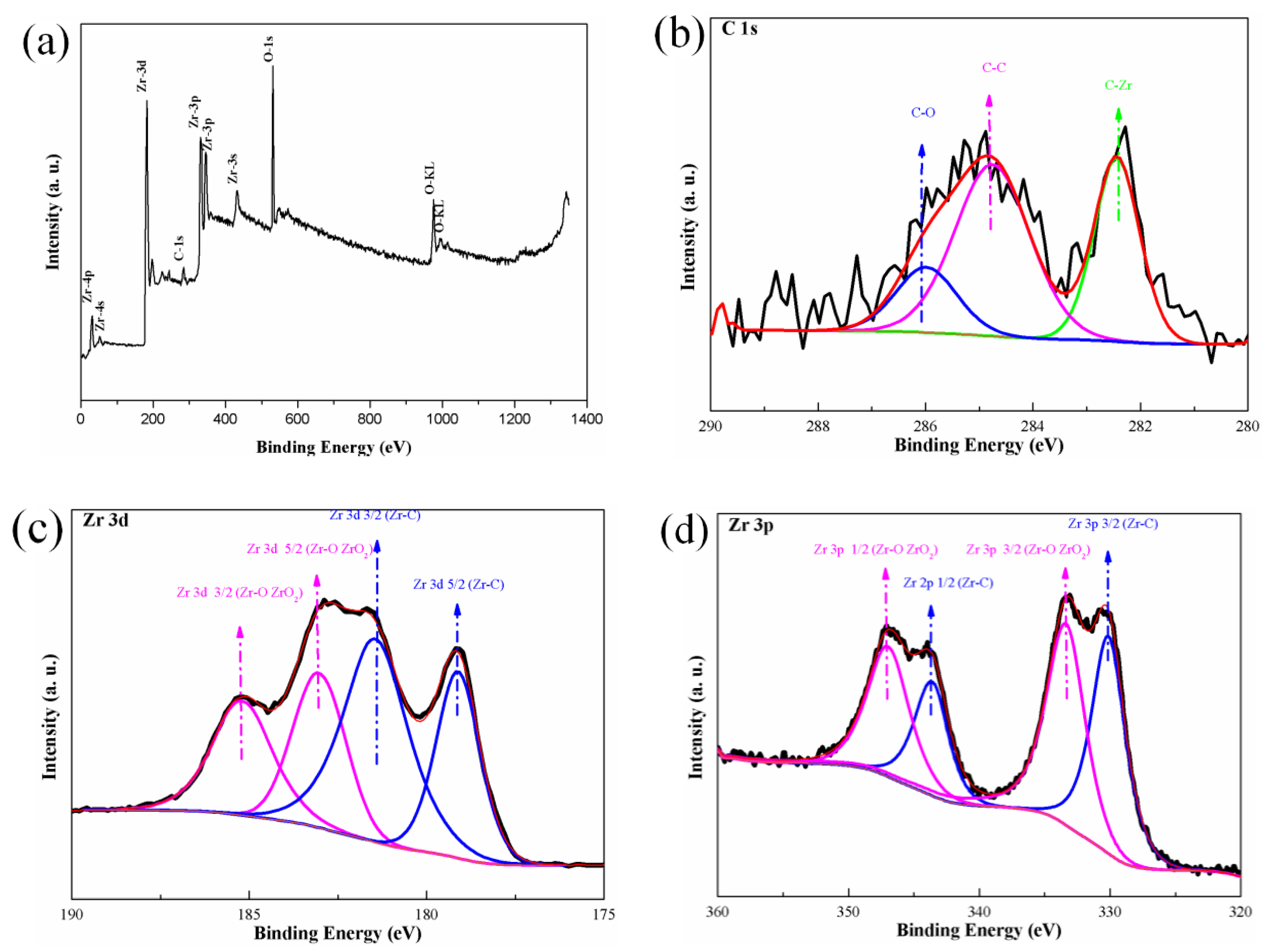
3.1. Characteristics of the Zirconium Carbide Coating
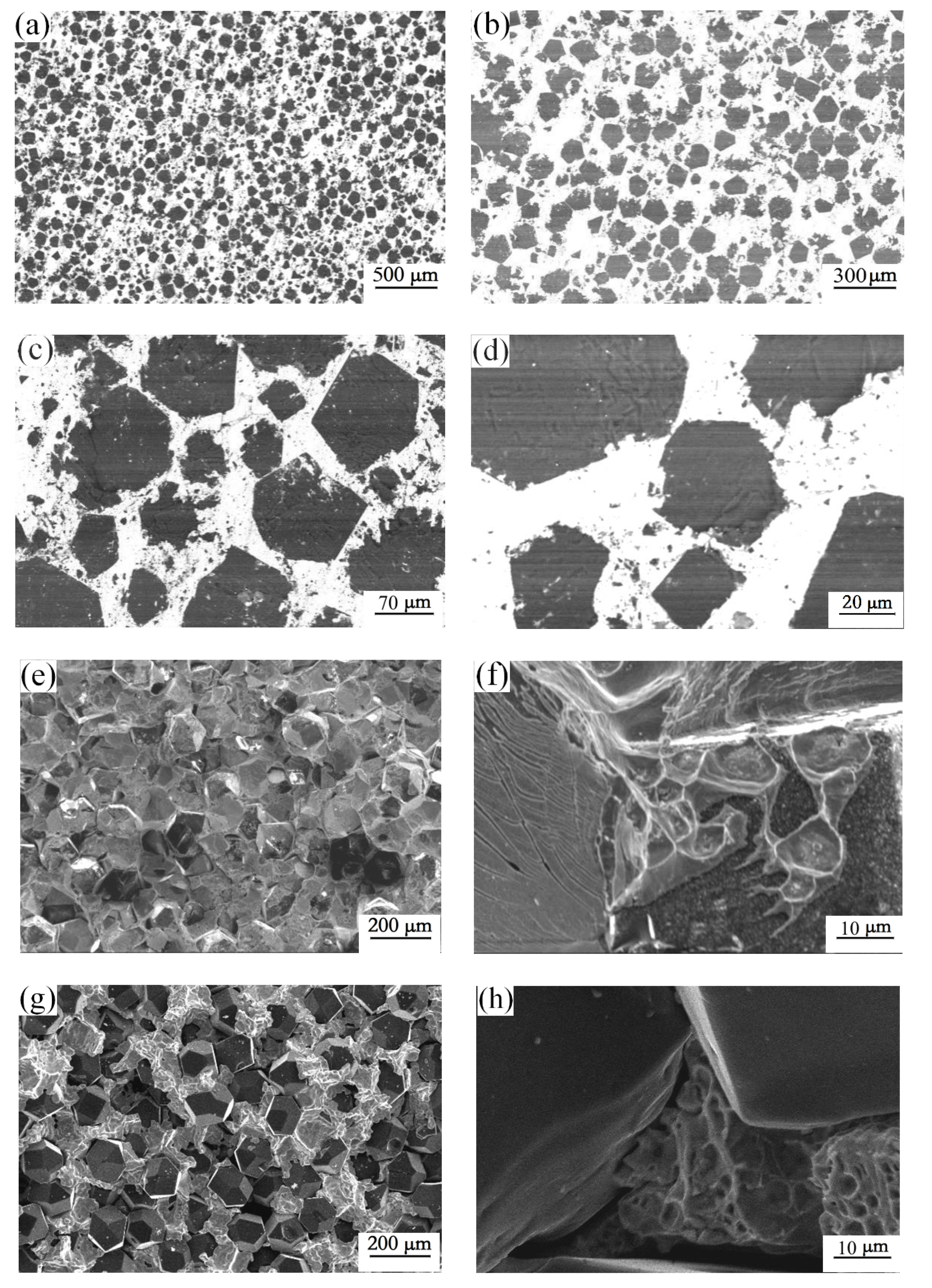
3.2. Microstructure of the Diamond-Copper Composites
3.3. Thermal Conductivity of Diamond-Copper Composites
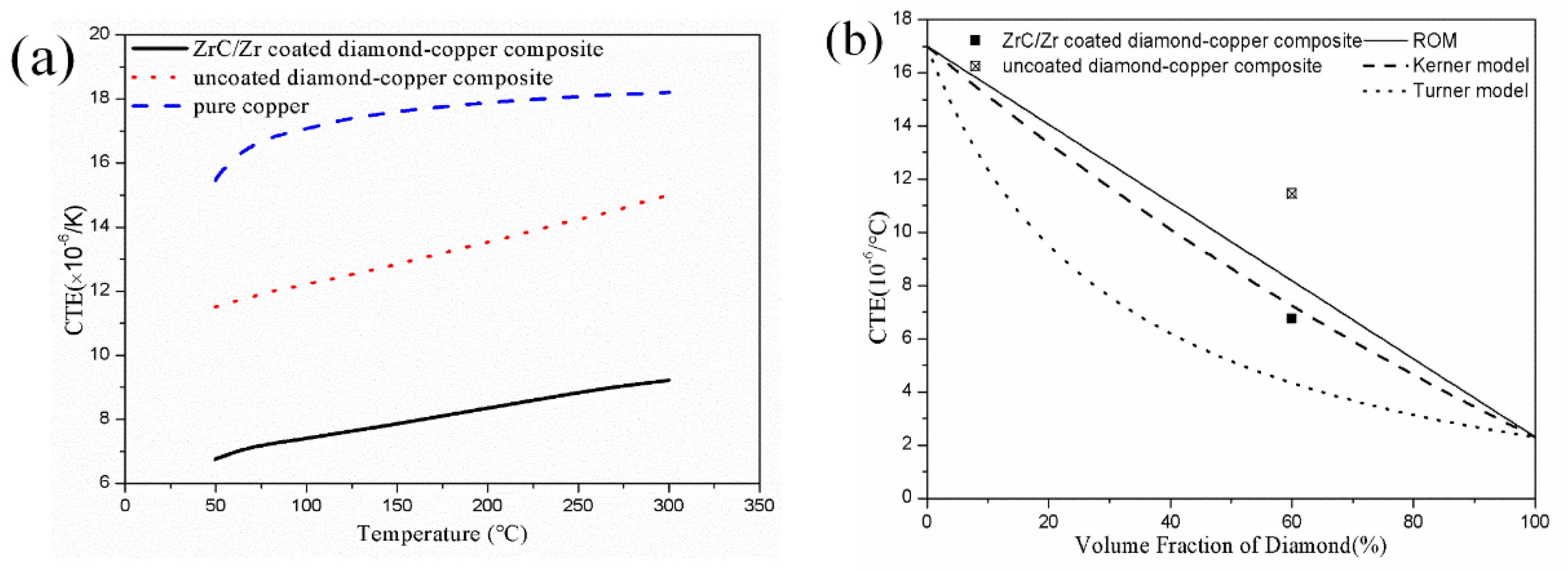
3.4. Coefficient of Thermal Expansion of the Diamond-Copper Composites
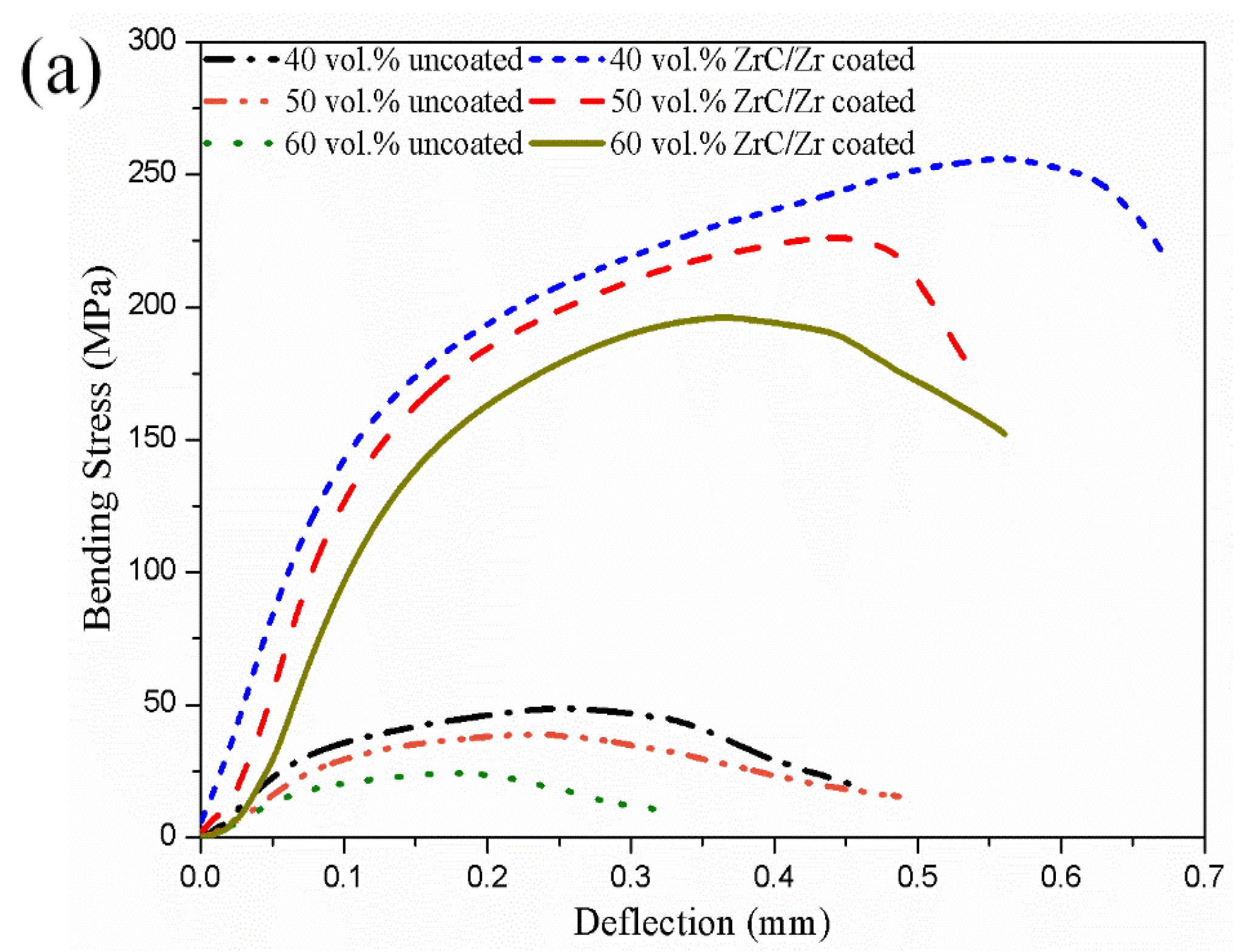
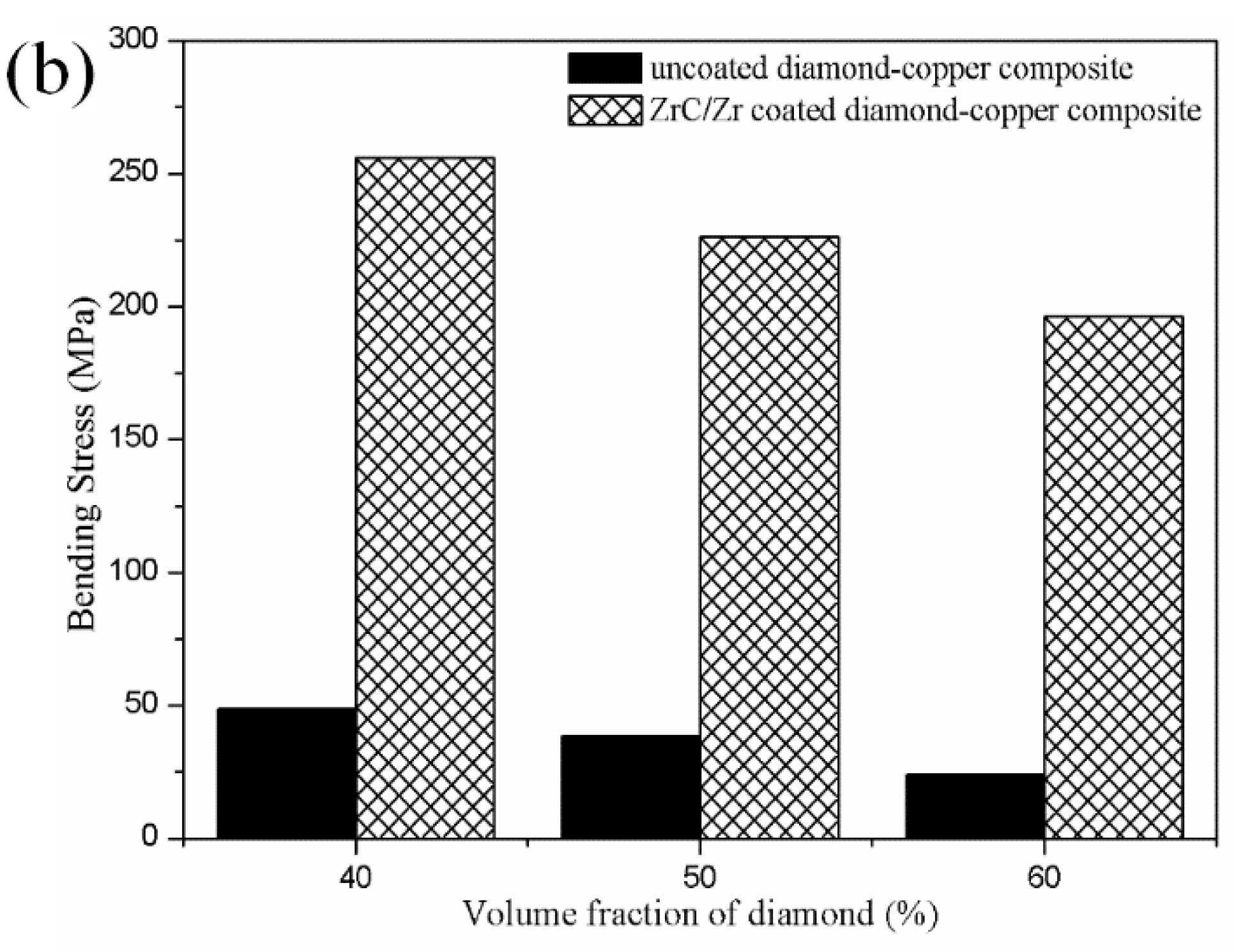
3.5. Mechanical Characterizations of the Diamond-Copper Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Niu, Y.R.; Lu, D.; Huang, L.P.; Zhao, J.; Zheng, X.B.; Chen, G. Comparison of W-Cu composite coatings fabricated by atmospheric and vacuum plasma spray processes. Vacuum 2015, 117, 98–103. [Google Scholar] [CrossRef]
- Weber, L.; Sinicco, G.; Molina, J.M. Influence of processing route on electrical and thermal conductivity of Al/SiC composites with bimodal particle distribution. J. Mater. Sci. 2010, 45, 2203–2209. [Google Scholar] [CrossRef]
- Celebi Efe, G.; Altinsoy, I.; Yener, T.; Ipek, M.; Zeytin, S.; Bindal, C. Characterization of cemented Cu matrix composites reinforced with SiC. Vacuum 2010, 85, 643–647. [Google Scholar]
- Zweben, C. Thermal materials solve power electronics challenges. Power Electron. Technol. 2006, 32, 40–47. [Google Scholar]
- Cho, J.; Goodson, K.E. Thermal transport: Cool electronics. Nat. Mater. 2015, 14, 136–137. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Morigami, H. Thermal properties of diamond/copper composite material. Microelectron. Reliab. 2004, 44, 303–308. [Google Scholar] [CrossRef]
- Lu, C.Y.; Tian, Y.; Shen, Y.F.; Feng, X.M.; Jiang, J. Thermal shock resistance and thermal conductivity of diamond-Cu composite coatings on Cu substrate via mechanical milling method. Surf. Coat. Technol. 2018, 352, 529–540. [Google Scholar] [CrossRef]
- Raza, K.; Khalid, F.A. Optimization of sintering parameters for diamond-copper composites in conventional sintering and their thermal conductivity. J. Alloys Compd. 2014, 615, 111–118. [Google Scholar] [CrossRef]
- He, J.; Wang, X.; Zhang, Y.; Zhao, Y.; Zhang, H. Thermal conductivity of Cu-Zr/diamond composites produced by high temperature-high pressure method. Compos. Part B Eng. 2015, 68, 22–26. [Google Scholar] [CrossRef]
- Li, J.W.; Wang, X.T.; Qiao, Y.; Zhang, Y.; He, Z.B.; Zhang, H.L. High thermal conductivity through interfacial layer optimization in diamond particles dispersed Zr-alloyed Cu matrix composites. Scr. Mater. 2015, 109, 72–75. [Google Scholar] [CrossRef]
- He, J.; Zhang, H.; Zhang, Y.; Zhao, Y.; Wang, X. Effect of boron addition on interface microstructure and thermal conductivity of Cu/diamond composites produced by high temperature-high pressure method. Phys. Status Solidi A 2014, 211, 587–594. [Google Scholar] [CrossRef]
- Li, J.W.; Zhang, H.L.; Wang, L.H.; Che, Z.F.; Zhang, Y.; Wang, J.G.; Kim, M.J.; Wang, X.T. Optimized thermal properties in diamond particles reinforced copper-titanium matrix composites produced by gas pressure infiltration. Composites Part A 2016, 91, 189–194. [Google Scholar] [CrossRef]
- Chung, C.Y.; Lee, M.T.; Tsai, M.Y.; Chu, C.H.; Lin, S.J. High thermal conductive diamond/Cu-Ti composites fabricated by pressureless sintering technique. Appl. Therm. Eng. 2014, 69, 208–213. [Google Scholar] [CrossRef]
- Zhang, X.M.; Guo, H.; Yin, F.Z.; Fan, Y.M.; Zhang, Y.Z. Interfacial microstructure and properties of diamond/Cu-xCr composites for electronic packaging applications. Rare Met. 2011, 30, 94–98. [Google Scholar] [CrossRef]
- Weber, L.; Tavangar, R. On the influence of active element content on the thermal conductivity and thermal expansion of Cu-X (X = Cr, B) diamond composites. Scr. Mater. 2007, 57, 988–991. [Google Scholar] [CrossRef]
- Schubert, T.; Ciupiński, Ł.; Zieliński, W.; Michalski, A.; Weiβgärber, T.; Kieback, B. Interfacial characterization of Cu/diamond composites prepared by powder metallurgy for heat sink applications. Scr. Mater. 2008, 58, 263–266. [Google Scholar] [CrossRef]
- Hu, H.B.; Kong, J. Improved Thermal Performance of Diamond-Copper Composites with Boron Carbide Coating. J. Mater. Eng. Perform. 2014, 23, 651–657. [Google Scholar] [CrossRef]
- Hell, J.; Chirtoc, M.; Neubauer, E.; Zellhofer, K. Characterisation of sputter deposited niobium and boron interlayer in the copper-diamond system. Surf. Coat. Technol. 2012, 208, 24–31. [Google Scholar] [CrossRef]
- Li, J.W.; Zhang, H.L.; Zhang, Y.; Che, Z.F.; Wang, X.T. Microstructure and thermal conductivity of Cu/diamond composites with Ti-coated diamond particles produced by gas pressure infiltration. J. Alloys Compd. 2015, 647, 941–946. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.L.; Wu, J.H.; Wang, X.T. Enhanced thermal conductivity in copper matrix composites reinforced with titanium-coated diamond particles. Scr. Mater. 2011, 65, 1097–1100. [Google Scholar] [CrossRef]
- Chu, K.; Liu, Z.F.; Jia, C.C.; Chen, H.; Liang, X.B.; Gao, W.J.; Tian, W.H.; Guo, H. Thermal conductivity of SPS consolidated Cu/diamond composites with Cr-coated diamond particles. J. Alloys Compd. 2010, 490, 453–458. [Google Scholar] [CrossRef]
- Ren, S.B.; Shen, X.Y.; Guo, C.Y.; Liu, N.; Zang, J.B.; He, X.B.; Qu, X.H. Effect of coating on the microstructure and thermal conductivities of diamond-Cu composites prepared by powder metallurgy. Compos. Sci. Technol. 2011, 71, 1550–1555. [Google Scholar] [CrossRef]
- Ma, S.D.; Zhao, N.Q.; Shi, C.S.; Liu, E.Z.; He, C.N.; He, F.; Ma, L.Y. Mo2C coating on diamond: Different effects on thermal conductivity of diamond/Al and diamond/Cu composites. Appl. Surf. Sci. 2017, 402, 372–383. [Google Scholar] [CrossRef]
- Shen, X.Y.; He, X.B.; Ren, S.B.; Zhang, H.M.; Qu, X.H. Effect of molybdenum as interfacial element on the thermal conductivity of diamond/Cu composites. J. Alloys Compd. 2012, 529, 134–139. [Google Scholar] [CrossRef]
- Kang, Q.P.; He, X.B.; Ren, S.B.; Zhang, L.; Wu, M.; Liu, T.T.; Liu, Q.; Guo, C.Y.; Qu, X.H. Preparation of high thermal conductivity copper-diamond composites using molybdenum carbide-coated diamond particles. J. Mater. Sci. 2013, 48, 6133–6140. [Google Scholar] [CrossRef]
- Hell, J.; Horkel, M.; Neubauer, E.; Eisenmenger-Sittner, C. Construction and characterization of a sputter deposition system for coating granular materials. Vacuum 2010, 84, 453–457. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, R.C.; Cai, Z.Y.; Peng, C.Q.; Wang, N.G. Low-temperature densification of diamond/Cu composite prepared from dual-layer diamond particles. J. Mater. Sci. 2015, 26, 185–190. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, R.C.; Cai, Z.Y.; Peng, C.Q.; Feng, Y.; Zhang, L. Effects of dual-layer coatings on microstructure and thermal conductivity of diamond/Cu composites prepared by vaccum hot pressing. Surf. Coat. Technol. 2015, 277, 299–307. [Google Scholar] [CrossRef]
- Kang, Q.P.; He, X.B.; Ren, S.B.; Liu, T.T.; Liu, Q.; Wu, M.; Qu, X.H. Microstructure and thermal properties of copper-diamond composites with tungsten carbide coating on diamond particles. Mater. Charact. 2015, 105, 18–23. [Google Scholar] [CrossRef]
- Abyzov, A.M.; Kruszewski, M.J.; Ciupinski, L.; Mazukiewicz, M.; Michalski, A.; Kurzydlowski, K.J. Diamond-tungsten based coating-copper composites with high thermal conductivity produced by Pulse Plasma Sintering. Mater. Des. 2015, 76, 97–109. [Google Scholar] [CrossRef]
- Wang, H.Y.; Tian, J. Thermal conductivity enhancement in Cu/diamond composites with surface-roughened diamonds. Appl. Phys. A 2014, 116, 265–271. [Google Scholar] [CrossRef]
- Bai, H.; Ma, N.G.; Lang, J.; Zhu, C.X. Effect of a new pretreatment on the micro-structure and thermal conductivity of Cu/diamond composites. J. Alloys Compd. 2013, 580, 382–385. [Google Scholar] [CrossRef]
- Mizuuchi, K.; Inoue, K.; Agari, Y.; Yamada, S.; Sugioka, M.; Itami, M.; Kawahara, M.; Makino, Y. Consolidation and thermal conductivity of diamond particle dispersed copper matrix composites produced by spark plasma sintering (SPS). J. Jpn. Inst. Met. 2007, 71, 1066–1069. [Google Scholar] [CrossRef]
- Bai, H.; Ma, N.G.; Lang, J.; Zhu, C.X.; Ma, Y. Thermal conductivity of Cu/diamond composites prepared by a new pretreatment of diamond powder. Composites Part B 2013, 52, 182–186. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; He, X.; Ren, S.; Wu, M.; Qu, X. Improvement of ZrC/Zr Coating on the Interface Combination and Physical Properties of Diamond-Copper Composites Fabricated by Spark Plasma Sintering. Materials 2019, 12, 475. https://doi.org/10.3390/ma12030475
Pan Y, He X, Ren S, Wu M, Qu X. Improvement of ZrC/Zr Coating on the Interface Combination and Physical Properties of Diamond-Copper Composites Fabricated by Spark Plasma Sintering. Materials. 2019; 12(3):475. https://doi.org/10.3390/ma12030475
Chicago/Turabian StylePan, Yanpeng, Xinbo He, Shubin Ren, Mao Wu, and Xuanhui Qu. 2019. "Improvement of ZrC/Zr Coating on the Interface Combination and Physical Properties of Diamond-Copper Composites Fabricated by Spark Plasma Sintering" Materials 12, no. 3: 475. https://doi.org/10.3390/ma12030475
APA StylePan, Y., He, X., Ren, S., Wu, M., & Qu, X. (2019). Improvement of ZrC/Zr Coating on the Interface Combination and Physical Properties of Diamond-Copper Composites Fabricated by Spark Plasma Sintering. Materials, 12(3), 475. https://doi.org/10.3390/ma12030475





