Structural Analysis of Calcium Phosphate-Based Submicrospheres with Internally-Crystallized Iron Oxide Nanoparticles Fabricated by a Laser-Assisted Precipitation Process
Abstract
:1. Introduction
2. Materials and Methods
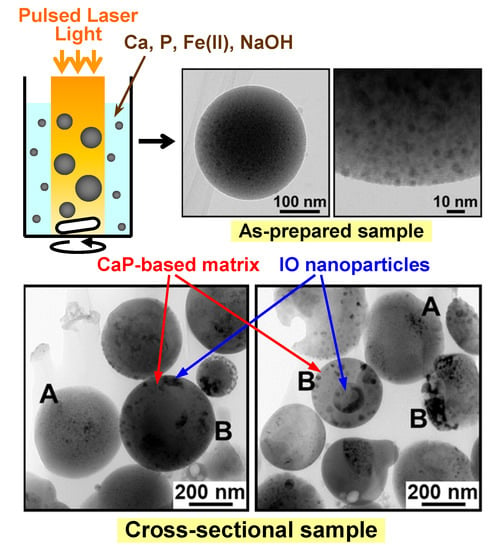
2.1. Preparation of the IO–CaP Submicrospheres
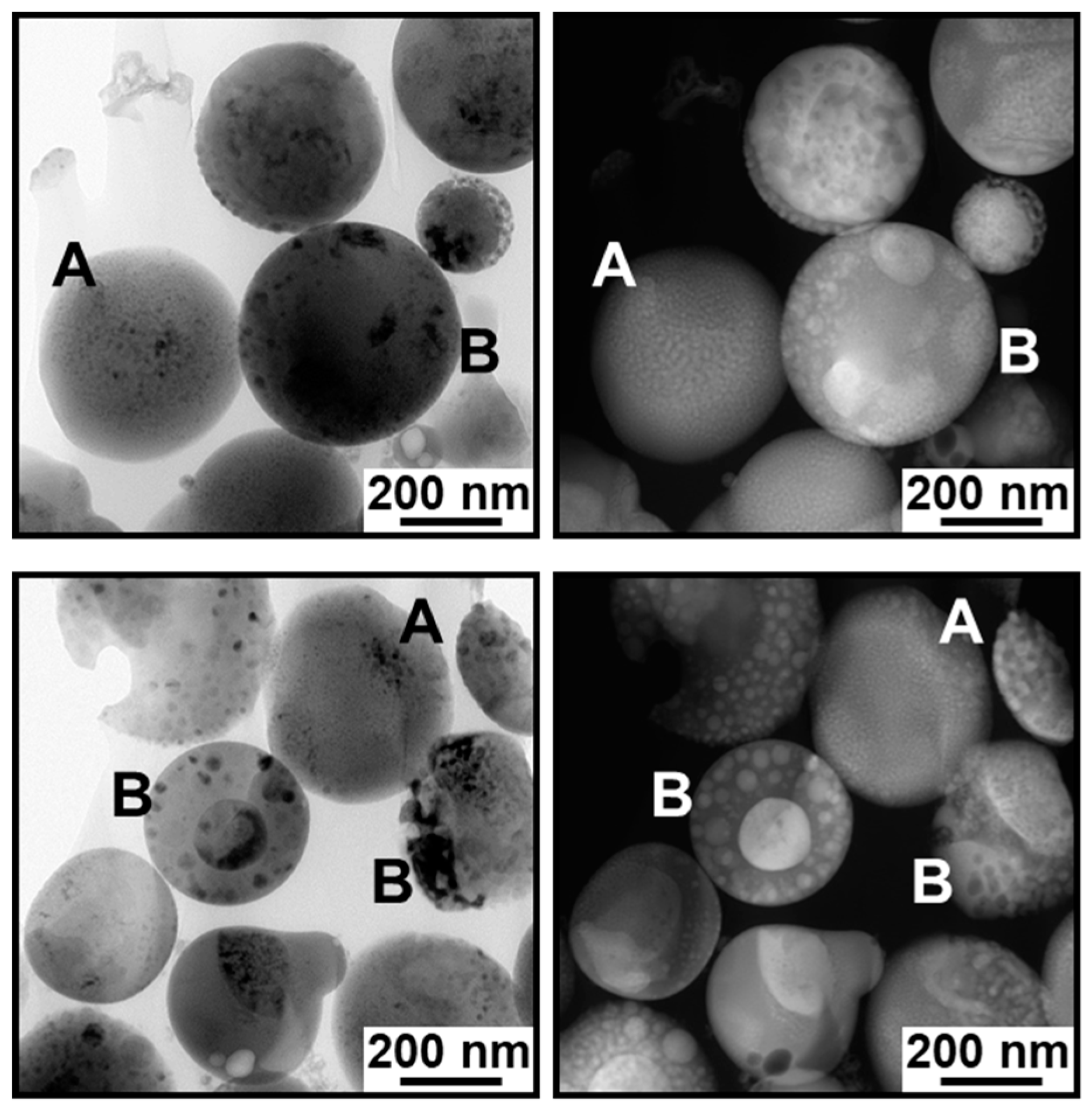
2.2. Cross-Sectional Analysis of the IO–CaP Submicrospheres
3. Results
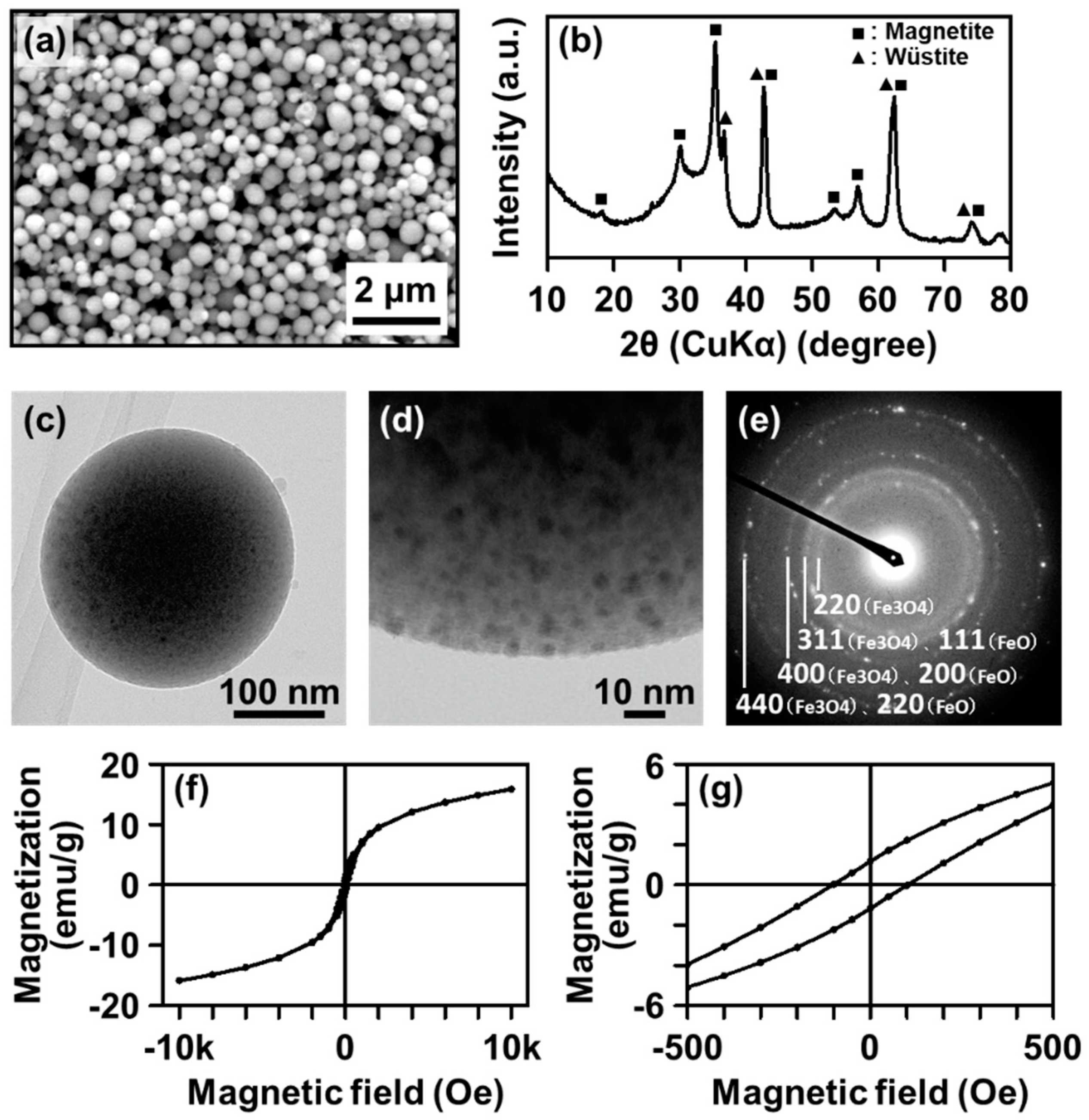
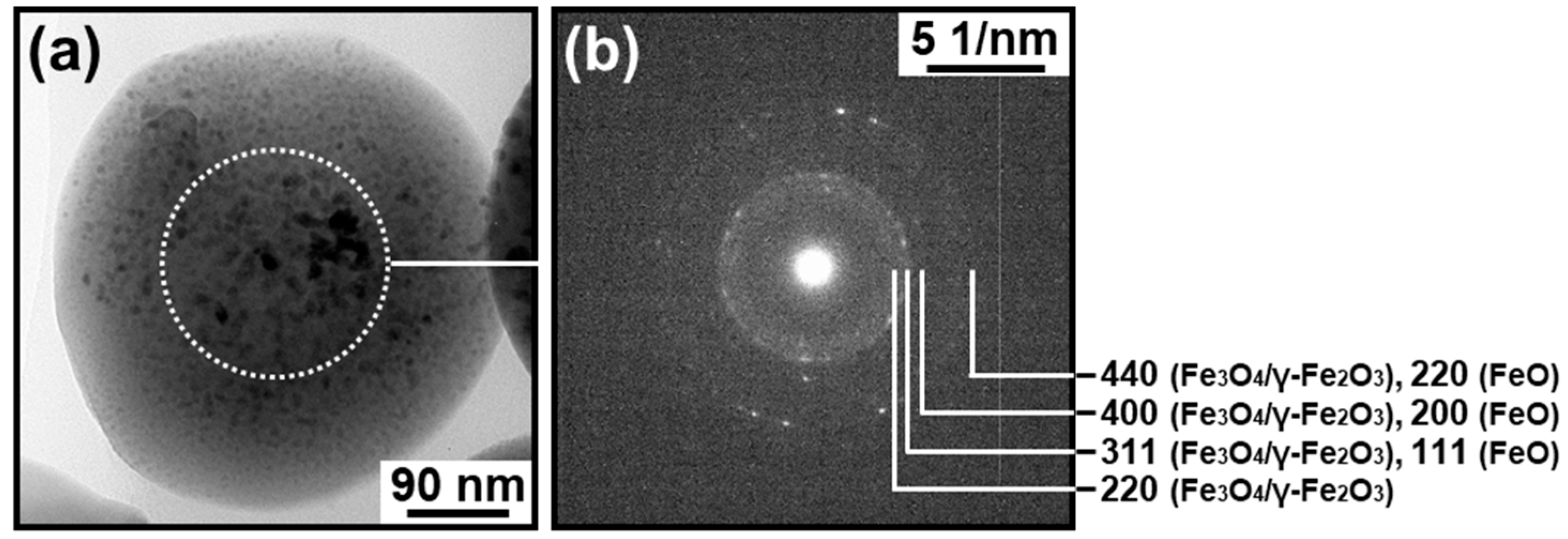
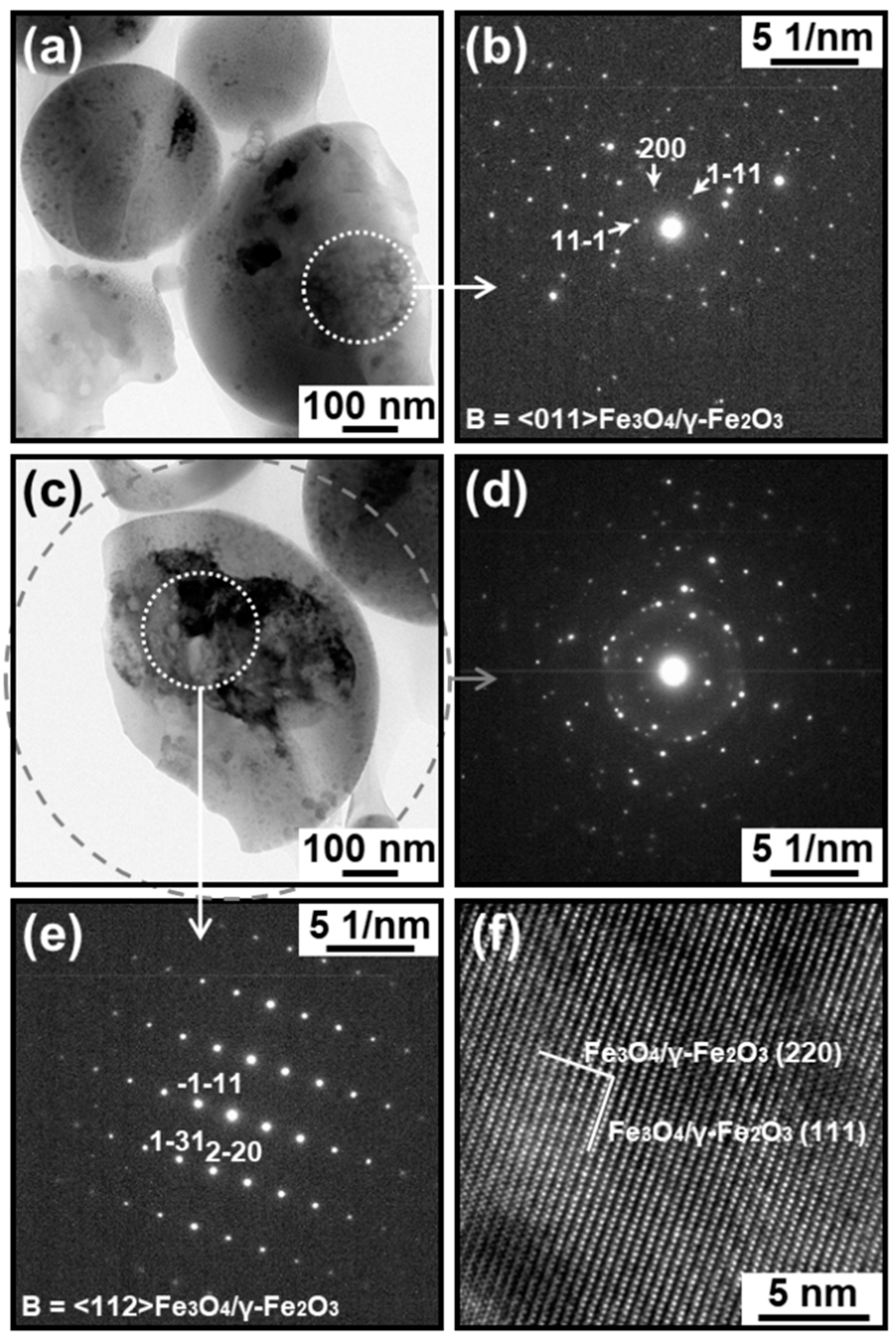
3.1. TEM and HAADF-STEM Observation
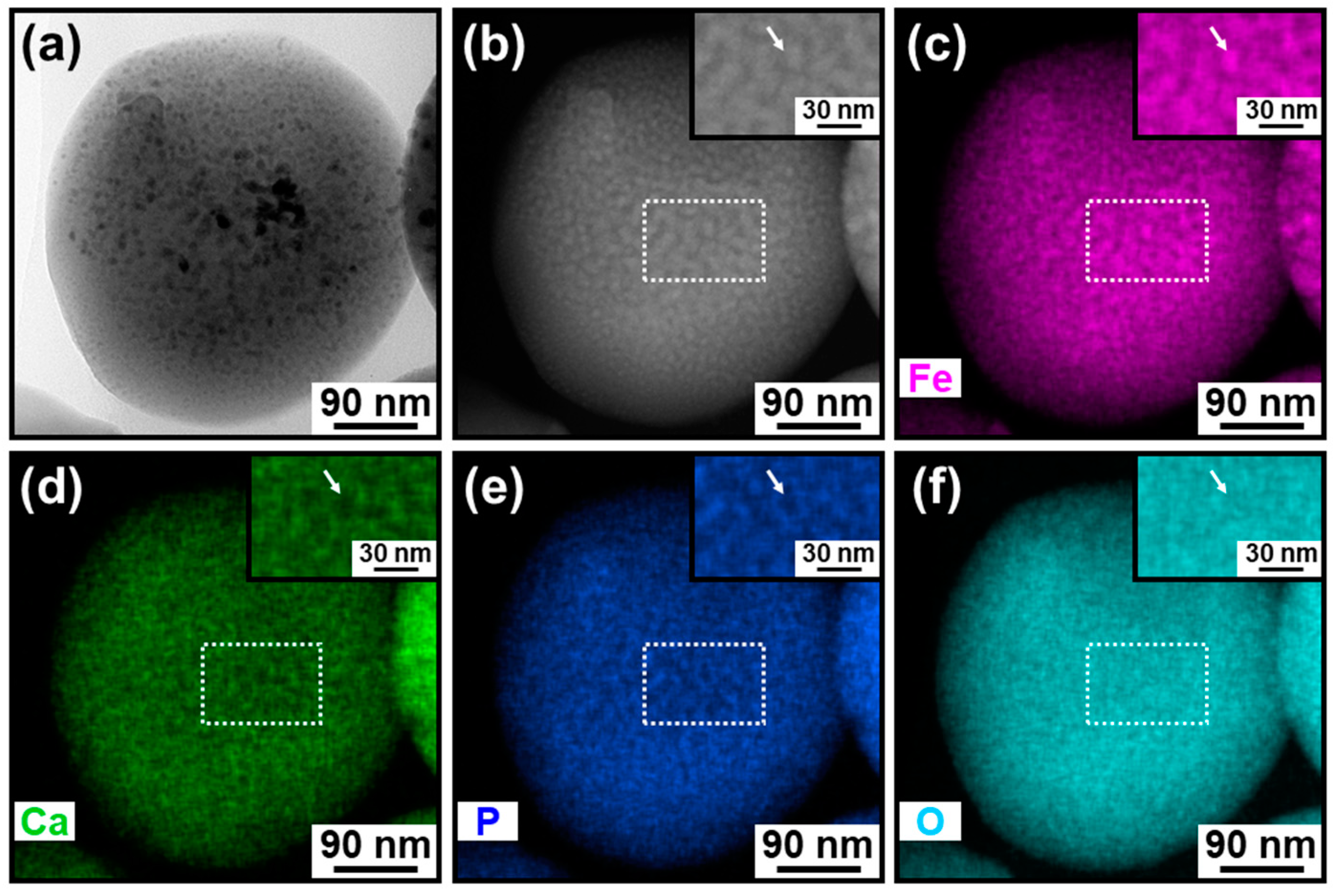
3.2. Elemental Analysis
3.3. Electron Diffraction Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef] [PubMed]
- Dulinska-Litewka, J.; Lazarczyk, A.; Halubiec, P.; Szafranski, O.; Karnas, K.; Karewicz, A. Superparamagnetic iron oxide nanoparticles—Current and prospective medical applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palanisamy, S.; Wang, Y.M. Superparamagnetic iron oxide nanoparticulate system: Synthesis, targeting, drug delivery and therapy in cancer. Dalton Trans. 2019, 48, 9490–9515. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Andujar, C.; Walter, A.; Cotin, G.; Bordeianu, C.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Design of iron oxide-based nanoparticles for MRI and magnetic hyperthermia. Nanomedicine 2016, 11, 1889–1910. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yang, L.; Gao, J.; Chen, X. Structure-relaxivity relationships of magnetic nanoparticles for magnetic resonance imaging. Adv. Mater. 2019, 31, 1804567. [Google Scholar] [CrossRef]
- Li, Z.; Kawashita, M.; Araki, N.; Mitsumori, M.; Hiraoka, M.; Doi, M. Magnetite nanoparticles with high heating efficiencies for application in the hyperthermia of cancer. Mater. Sci. Eng. C 2010, 30, 990–996. [Google Scholar] [CrossRef]
- Hou, C.H.; Hou, S.M.; Hsueh, Y.S.; Lin, J.; Wu, H.C.; Lin, F.H. The in vivo performance of biomagnetic hydroxyapatite nanoparticles in cancer hyperthermia therapy. Biomaterials 2009, 30, 3956–3960. [Google Scholar] [CrossRef]
- Xu, Y.J.; Dong, L.; Lu, Y.; Zhang, L.C.; An, D.; Gao, H.L.; Yang, D.M.; Hu, W.; Sui, C.; Xu, W.P.; et al. Magnetic hydroxyapatite nanoworms for magnetic resonance diagnosis of acute hepatic injury. Nanoscale 2016, 8, 1684–1690. [Google Scholar] [CrossRef]
- Adamiano, A.; Iafisco, M.; Sandri, M.; Basini, M.; Arosio, P.; Canu, T.; Sitia, G.; Esposito, A.; Iannotti, V.; Ausanio, G.; et al. On the use of superparamagnetic hydroxyapatite nanoparticles as an agent for magnetic and nuclear in vivo imaging. Acta Biomater. 2018, 73, 458–469. [Google Scholar] [CrossRef] [Green Version]
- Uskokovic, V.; Uskokovic, D.P. Nanosized hydroxyapatite and other calcium phosphates: Chemistry of formation and application as drug and gene delivery agents. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 96, 152–191. [Google Scholar] [CrossRef] [Green Version]
- Syamchand, S.S.; Sony, G. Multifunctional hydroxyapatite nanoparticles for drug delivery and multimodal molecular imaging. Microchim. Acta 2015, 182, 1567–1589. [Google Scholar] [CrossRef]
- Qi, C.; Lin, J.; Fu, L.H.; Huang, P. Calcium-based biomaterials for diagnosis, treatment, and theranostics. Chem. Soc. Rev. 2018, 47, 357–403. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Musetti, S.; Fu, L.H.; Zhu, Y.J.; Huang, L. Biomolecule-assisted green synthesis of nanostructured calcium phosphates and their biomedical applications. Chem. Soc. Rev. 2019, 48, 2698–2737. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Masse, S.; Zhang, H.; Helary, C.; Li, L.; Coradin, T. Surface reactivity of hydroxyapatite nanocoatings deposited on iron oxide magnetic spheres toward toxic metals. J. Colloid Interface. Sci. 2014, 417, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondal, S.; Manivasagan, P.; Bharathiraja, S.; Moorthy, M.S.; Nguyen, V.T.; Kim, H.H.; Nam, S.Y.; Lee, K.D.; Oh, J. Hydroxyapatite coated iron oxide nanoparticles: A promising nanomaterial for magnetic hyperthermia cancer treatment. Nanomaterials 2017, 7, 426. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.C.; Wang, T.W.; Sun, J.S.; Wang, W.H.; Lin, F.H. A novel biomagnetic nanoparticle based on hydroxyapatite. Nanotechnology 2007, 18, 165601. [Google Scholar] [CrossRef]
- Ansar, E.B.; Ajeesh, M.; Yokogawa, Y.; Wunderlich, W.; Varma, H. Synthesis and characterization of iron oxide embedded hydroxyapatite bioceramics. J. Am. Ceram. Soc. 2012, 95, 2695–2699. [Google Scholar] [CrossRef]
- Nakamura, M.; Oyane, A.; Sakamaki, I.; Ishikawa, Y.; Shimizu, Y.; Kawaguchi, K. Laser-assisted one-pot fabrication of calcium phosphate-based submicrospheres with internally crystallized magnetite nanoparticles through chemical precipitation. Phys. Chem. Chem. Phys. 2015, 17, 8836–8842. [Google Scholar] [CrossRef]
- Nakamura, M.; Oyane, A. Physicochemical fabrication of calcium phosphate-based thin layers and nanospheres using laser processing in solutions. J. Mater. Chem. B 2016, 4, 6289–6301. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.Y.; Yang, Z. Transmission electron microscopy (TEM). In Membrane Characterization, 1st ed.; Hilal, N., Ismail, A.F., Matsuura, T., Oatley-Radcliffe, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 8; pp. 145–159. [Google Scholar]
- Fock, J.; Bogart, L.K.; González-Alonso, D.; Espeso, J.I.; Hansen, M.F.; Varón, M.; Frandsen, C.; Pankhurst, Q.A. On the ‘centre of gravity’ method for measuring the composition of magnetite/maghemite mixtures, or the stoichiometry of magnetite-maghemite solid solutions, via 57Fe Mössbauer spectroscopy. J. Phys. D Appl. Phys. 2017, 50, 265005. [Google Scholar] [CrossRef]
- Swiatkowska-Warkocka, Z.; Kawaguchi, K.; Wang, H.; Katou, Y.; Koshizaki, N. Controlling exchange bias in Fe3O4/FeO composite particles prepared by pulsed laser irradiation. Nanoscale Res. Lett. 2011, 6, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, Y.; Koshizaki, N.; Pyatenko, A. Submicrometer-sized spherical iron oxide particles fabricated by pulsed laser melting in liquid. IEEJ Trans. Electron. Inf. Syst. 2015, 135, 1066–1070. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, M.; Oyane, A. Structural Analysis of Calcium Phosphate-Based Submicrospheres with Internally-Crystallized Iron Oxide Nanoparticles Fabricated by a Laser-Assisted Precipitation Process. Materials 2019, 12, 4234. https://doi.org/10.3390/ma12244234
Nakamura M, Oyane A. Structural Analysis of Calcium Phosphate-Based Submicrospheres with Internally-Crystallized Iron Oxide Nanoparticles Fabricated by a Laser-Assisted Precipitation Process. Materials. 2019; 12(24):4234. https://doi.org/10.3390/ma12244234
Chicago/Turabian StyleNakamura, Maki, and Ayako Oyane. 2019. "Structural Analysis of Calcium Phosphate-Based Submicrospheres with Internally-Crystallized Iron Oxide Nanoparticles Fabricated by a Laser-Assisted Precipitation Process" Materials 12, no. 24: 4234. https://doi.org/10.3390/ma12244234
APA StyleNakamura, M., & Oyane, A. (2019). Structural Analysis of Calcium Phosphate-Based Submicrospheres with Internally-Crystallized Iron Oxide Nanoparticles Fabricated by a Laser-Assisted Precipitation Process. Materials, 12(24), 4234. https://doi.org/10.3390/ma12244234