Influence of the Surface Roughness of PEEK GRF30 and Ti6Al4V SLM on the Viability of Primary Human Osteoblasts Determined by the MTT Test
Abstract
1. Introduction
2. Materials and Methods
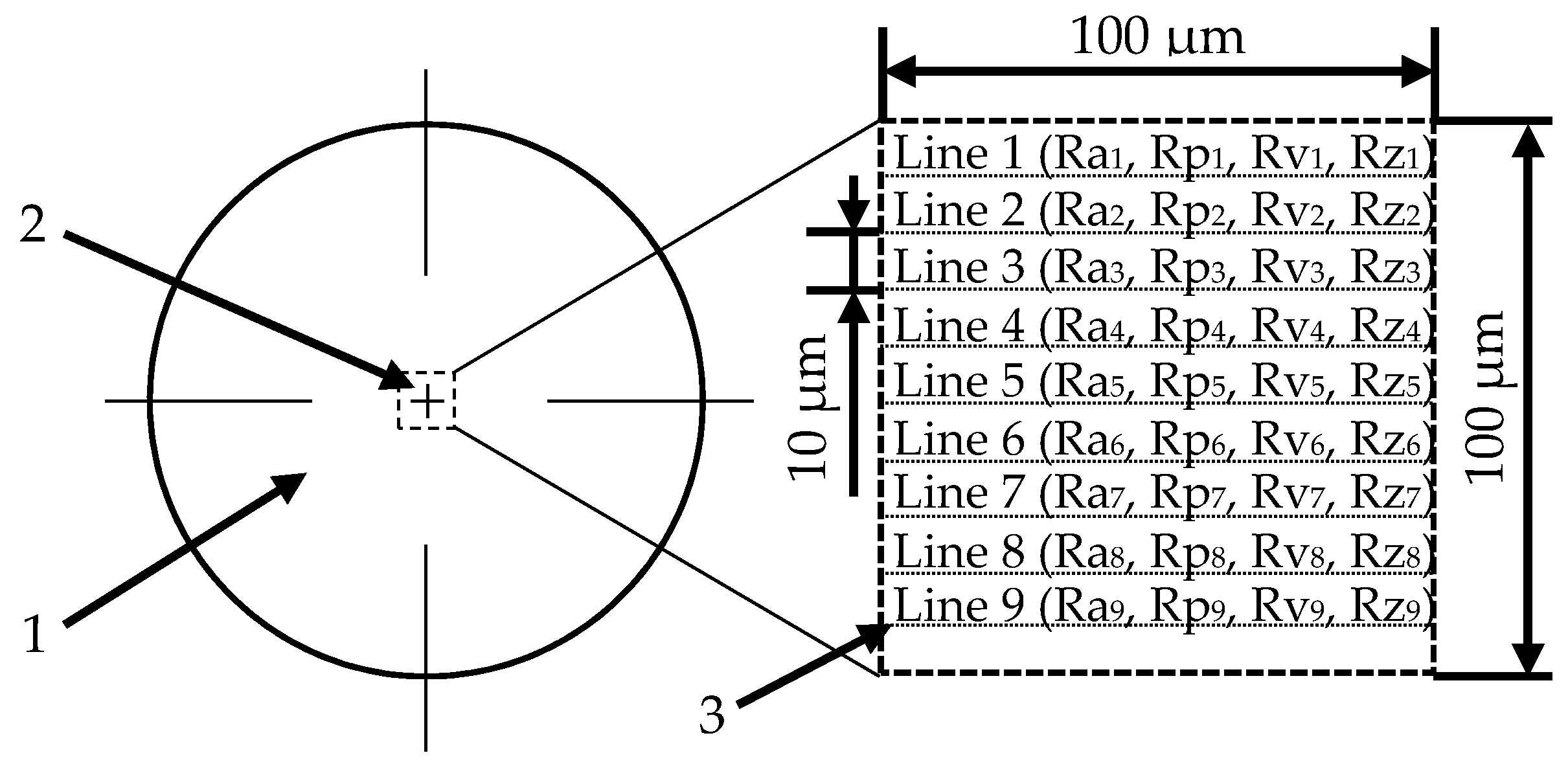
2.1. Preparation of Samples and Modifications of Their Surface
2.2. Evaluation of Primary Human Osteoblasts with the Use of MTT Test
3. Results and Discussion
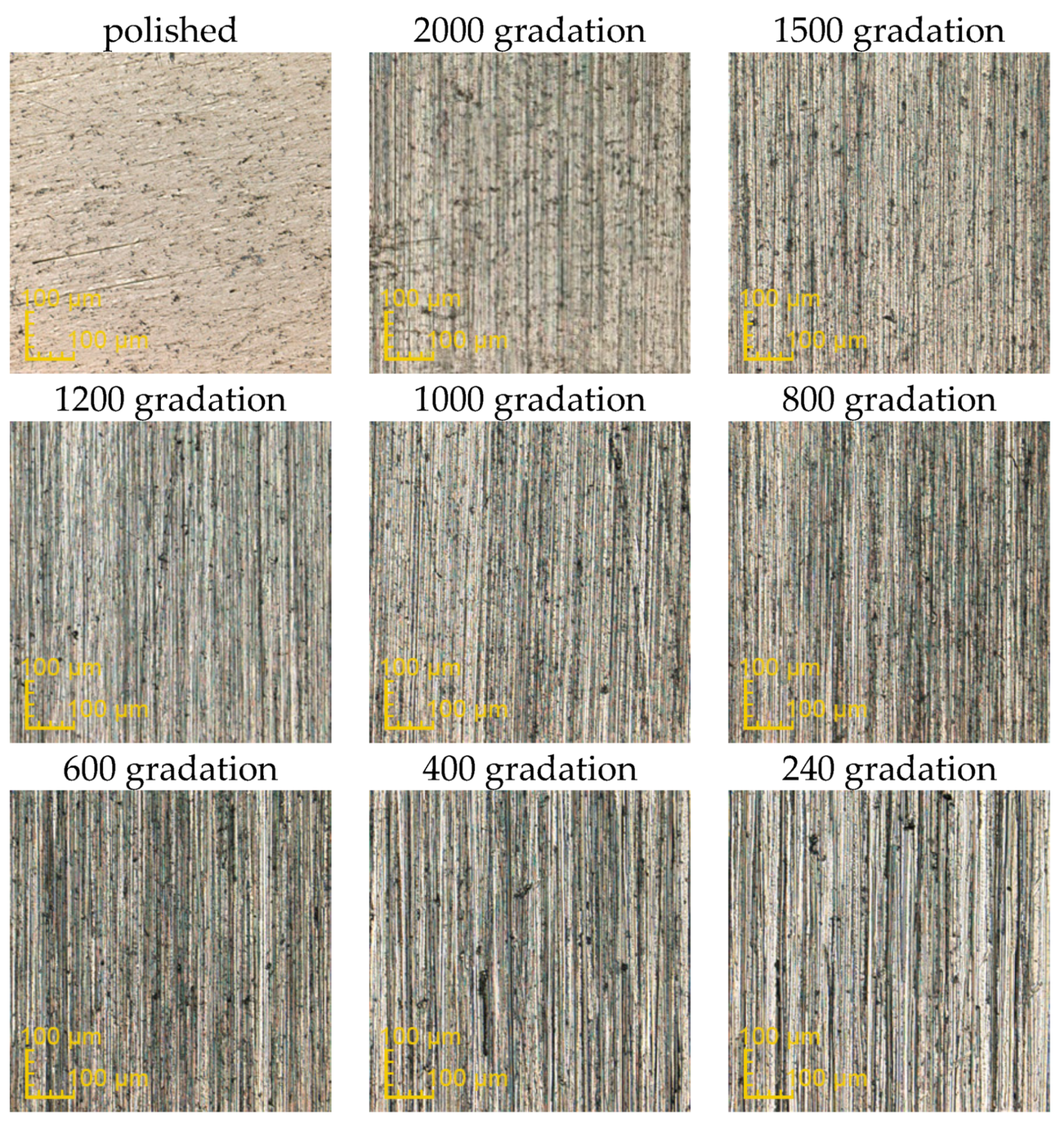
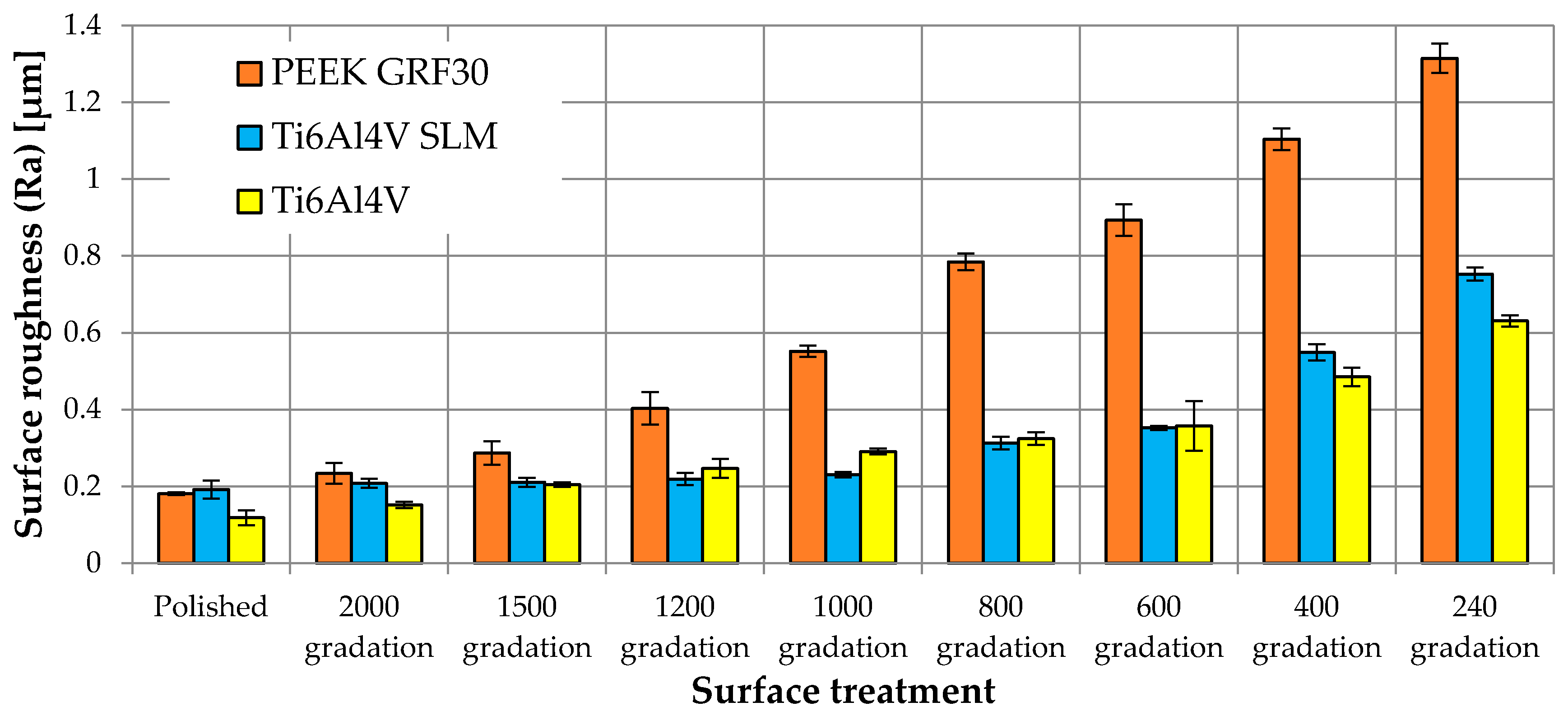
3.1. The Influence of Mechanical Treatment on Surface Roughness
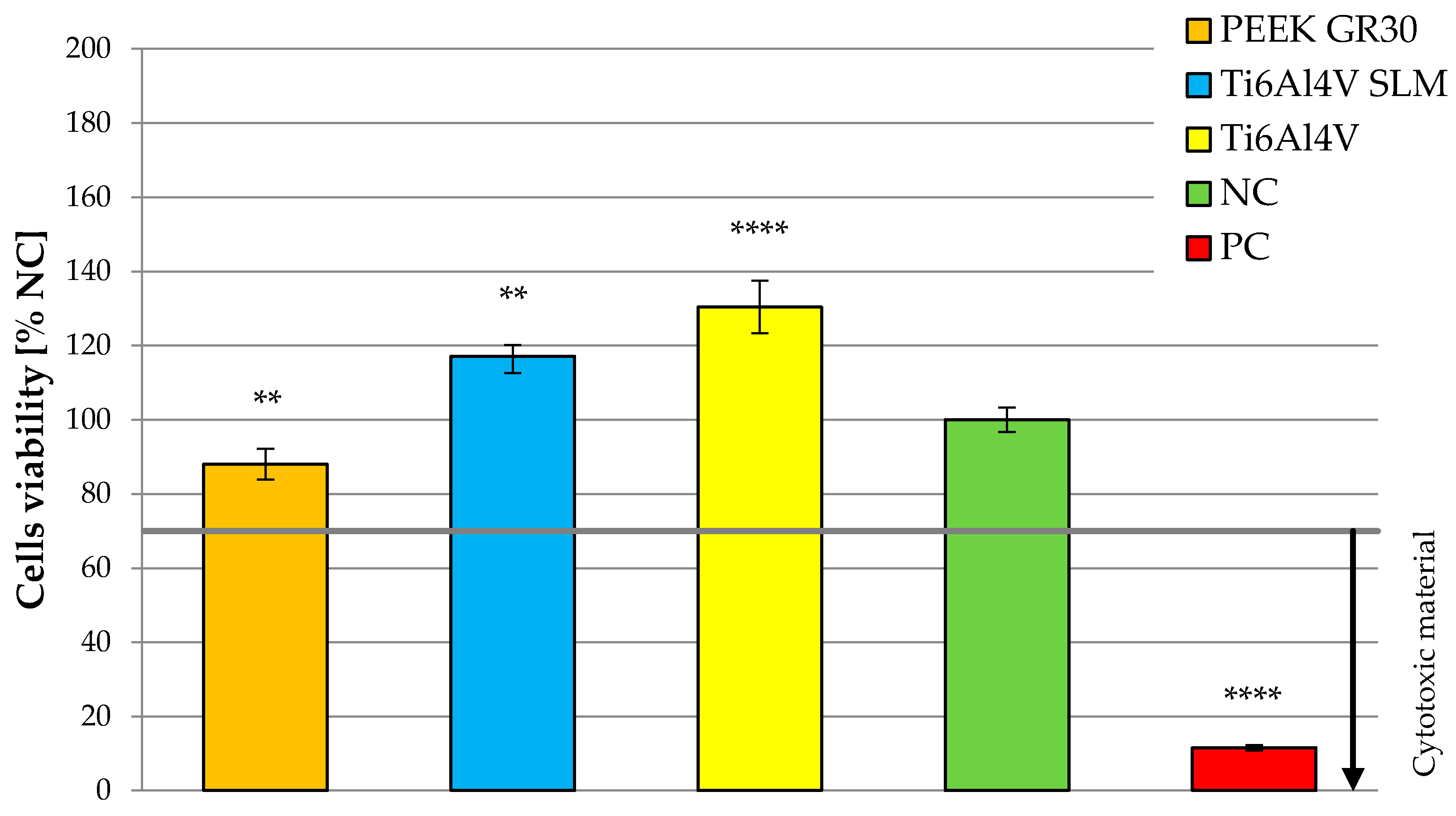
3.2. Determination of Osteoblasts Viability Using MTT Test
3.3. Future Work
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chandar, S.; Kotian, R.; Madhyastha, P.; Kabekkodu, S.P.; Rao, P. In vitro evaluation of cytotoxicity and corrosion behavior of commercially pure titanium and Ti-6Al-4V alloy for dental implants. J. Indian Prosthodont. Soc. 2017, 17, 35–40. [Google Scholar] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Berridge, M.V.; Tan, A.S. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, G.; Danti, S.; D’Alessandro, D.; Moscato, S.; Menciassi, A. Assessing cytotoxicity of boron nitride nanotubes: Interference with the MTT assay. Biochem. Biophys. Res. Commun. 2010, 394, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Bopp, S.K.; Lettieri, T. Comparison of four different colorimetric and fluorometric cytotoxicity assays in a zebrafish liver cell line. BMC Pharm. 2008, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Zhao, C.; Jing, Z. An evaluation of the colorimetric assays based on enzymatic reactions used in the measurement of human natural cytotoxicity. J. Immunol. Methods 2001, 251, 11–19. [Google Scholar] [CrossRef]
- Liu, Q.; Li, W.; Cao, L.; Wang, J.; Qu, Y.; Wang, X.; Qiu, R.; Di, X.; Wang, Z.; Liang, B. Response of MG63 Osteoblast Cells to Surface Modification of Ti-6Al-4V Implant. Alloy by Laser Interference Lithography. J. Bionic Eng. 2017, 14, 448–458. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Brown, S.E.; Wang, Z.; Krebsbach, P.H. Phenotypic characterization, osteoblastic differentiation, and bone regeneration capacity of human embryonic stem cell-derived mesenchymal stem cells. Stem Cells Dev. 2009, 18, 955–968. [Google Scholar] [CrossRef]
- Muszyński, S.; Tomaszewska, E.; Dobrowolski, P.; Kwiecień, M.; Wiącek, D.; Świetlicka, I.; Skibińska, M.; Szymańska-Chargot, M.; Orzeł, J.; Świetlicki, M.; et al. Analysis of bone osteometry, mineralization, mechanical and histomorphometrical properties of tibiotarsus in broiler chickens demonstrates a influence of dietary chickpea seeds (Cicer arietinum L.) inclusion as a primary protein source. PLoS ONE 2018, 13, e0208921. [Google Scholar]
- Rodan, S.B.; Imai, Y.; Thiede, M.A.; Wesolowski, G.; Thompson, D.; Bar-Shavit, Z.; Shull, S.; Mann, K.; Rodan, G.A. Characterization of a human osteosarcoma cell line (Saos-2) with osteoblastic properties. Cancer Res. 1987, 47, 4961–4966. [Google Scholar] [PubMed]
- Fogh, J.; Fogh, J.M.; Orfeo, T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J. Natl. Cancer Inst. 1977, 59, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Postiglione, L.; Di Domenico, G.; Ramaglia, L.; Montagnani, S.; Salzano, S.; Di Meglio, F.; Sbordone, L.; Vitale, M.; Rossi, G. Behavior of SaOS-2 cells cultured on different titanium surfaces. J. Dent. Res. 2003, 82, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Macnair, R.; Rodgers, E.H.; Macdonald, C.; Wykman, A.; Goldie, I.; Grant, M.H. The response of primary rat and human osteoblasts and an immortalized rat osteoblast cell line to orthopaedic materials: Comparative sensitivity of several toxicity indices. J. Mater. Sci. Mater. Med. 1997, 8, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Pauksch, L.; Hartmann, S.; Rohnke, M.; Szalay, G.; Alt, V.; Schnettler, R.; Lips, K.S. Biocompatibility of silver nanoparticles and silver ions in primary human mesenchymal stem cells and osteoblasts. Acta Biomater. 2014, 10, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Sagomonyants, K.B.; Jarman-Smith, M.L.; Devine, J.N.; Aronow, M.S.; Gronowicz, G.A. The in vitro response of human osteoblasts to polyetheretherketone (PEEK) substrates compared to commercially pure titanium. Biomaterials 2008, 29, 1563–1572. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In search of an osteoblast cell model for in vitro research. Eur. Cells Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef]
- El Hadad, A.A.; Peón, E.; García-Galván, F.R.; Barranco, V.; Parra, J.; Jiménez-Morales, A.; Galván, J.C. Biocompatibility and Corrosion Protection Behaviour of Hydroxyapatite Sol-Gel-Derived Coatings on Ti6Al4V Alloy. Materials 2017, 10, 94. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. BioMed. Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef]
- Fitzpatrick, N.; Smith, T.J.; Pendegrass, C.J.; Yeadon, R.; Ring, M.; Goodship, A.E.; Blunn, G.W. Intraosseous transcutaneous amputation prosthesis (ITAP) for limb salvage in 4 dogs. Vet. Surg. 2011, 40, 909–925. [Google Scholar] [CrossRef]
- Caouette, C.; Yahia, L.H.; Bureau, M.N. Reduced stress shielding with limited micromotions using a carbon fibre composite biomimetic hip stem: A finite element model. Proc. Inst. Mech. Eng. H 2011, 225, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Panayotov, I.V.; Orti, V.; Cuisinier, F.; Yachouh, J. Polyetheretherketone (PEEK) for medical applications. J. Mater. Sci. Mater. Med. 2016, 27, 118. [Google Scholar] [CrossRef] [PubMed]
- Ran, Q.; Yang, W.; Hu, Y.; Shen, X.; Yu, Y.; Xiang, Y.; Cai, K. Osteogenesis of 3D printed porous Ti6Al4V implants with different pore sizes. J. Mech. Behav. Biomed. Mater. 2018, 84, 1–11. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.A.; Ghazy, M.; Youssef, Y.M.; Essa, K. Optimization of SLM process parameters for Ti6Al4V medical implants. Rapid Prototyp. J. 2018, 25, 433–447. [Google Scholar] [CrossRef]
- Katzer, A.; Marquardt, H.; Westendorf, J.; Wening, J.; Von Foerster, G. Polyetheretherketone—cytotoxicity and mutagenicity in vitro. Biomaterials 2002, 23, 1749–1759. [Google Scholar] [CrossRef]
- Lorber, V.; Paulus, A.C.; Buschmann, A.; Schmitt, B.; Grupp, T.M.; Jansson, V.; Utzschneider, S. Elevated cytokine expression of different PEEK wear particles compared to UHMWPE in vivo. J. Mater. Sci. Mater. Med. 2014, 25, 141–149. [Google Scholar] [CrossRef]
- Pace, N.; Marinelli, M.; Spurio, S. Technical and histologic analysis of a retrieved carbon fiber-reinforced poly-ether-ether-ketone composite alumina-bearing liner 28 months after implantation. J. Arthroplast. 2008, 23, 151–155. [Google Scholar] [CrossRef]
- Stratton-Powell, A.A.; Pasko, K.M.; Brockett, C.L.; Tipper, J.L. The Biologic Response to Polyetheretherketone (PEEK) Wear Particles in Total Joint Replacement: A Systematic Review. Clin. Orthop. Relat. Res. 2016, 474, 2394–2404. [Google Scholar] [CrossRef]
- Utzschneider, S.; Becker, F.; Grupp, T.M.; Sievers, B.; Paulus, A.; Gottschalk, O.; Jansson, V. Inflammatory response against different carbon fiber-reinforced PEEK wear particles compared with UHMWPE in vivo. Acta. Biomater. 2010, 6, 4296–4304. [Google Scholar] [CrossRef]
- Tomaszewski, P.K.; van Diest, M.; Bulstra, S.K.; Verdonschot, N.; Verkerke, G.J. Numerical analysis of an osseointegrated prosthesis fixation with reduced bone failure risk and periprosthetic bone loss. J. Biomech. 2012, 45, 1875–1880. [Google Scholar] [CrossRef]
- Mierzejewska, Ż.A. Effect of Laser Energy Density, Internal Porosity and Heat Treatment on Mechanical Behavior of Biomedical Ti6Al4V Alloy Obtained with DMLS Technology. Materials 2019, 12, 2331. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, B.; Maj, P.; Krawczyńska, A.; Różniatowski, K.; Zdunek, J.; Kurzydłowski, K.J.; Święszkowski, W. Microstructure and mechanical properties investigation of CP titanium processed by selective laser melting (SLM). J. Mater. 2017, 241, 13–23. [Google Scholar] [CrossRef]
- Calignano, F.; Galati, M.; Iuliano, L.; Minetola, P. Design of Additively Manufactured Structures for Biomedical Applications: A Review of the Additive Manufacturing Processes Applied to the Biomedical Sector. J. Healthc. Eng. 2019, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sui, Q.; Li, P.; Wang, K.; Yin, X.; Liu, L.; Zhang, Y.; Wang, L. Effect of Build Orientation on the Corrosion Behavior and Mechanical Properties of Selective Laser Melted Ti-6Al-4V. Metals 2019, 9, 976. [Google Scholar] [CrossRef]
- Salou, L.; Hoornaert, A.; Louarn, G.; Layrolle, P. Enhanced osseointegration of titanium implants with nanostructured surfaces: An experimental study in rabbits. Acta. Biomater. 2015, 11, 494–502. [Google Scholar] [CrossRef]
- Ciocca, L.; Fantini, M.; De Crescenzio, F.; Corinaldesi, G.; Scotti, R. Direct metal laser sintering (DMLS) of a customized titanium mesh for prosthetically guided bone regeneration of atrophic maxillary arches. Med. Biol. Eng. Comput. 2011, 49, 1347–1352. [Google Scholar] [CrossRef]
- Salmi, M.; Tuomi, J.; Paloheimo, K.S.; Björkstrand, R.; Paloheimo, M.; Salo, J.; Mäkitie, A.A. Patient-specific reconstruction with 3D modeling and DMLS additive manufacturing. Rapid Prototyp. J. 2012, 18, 209–214. [Google Scholar] [CrossRef]
- Sidambe, A. Biocompatibility of advanced manufactured titanium implants—A review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef]
- Costa, D.O.; Prowse, P.D.; Chrones, T.; Sims, S.M.; Hamilton, D.W.; Rizkalla, A.S.; Dixon, S.J. The differential regulation of osteoblast and osteoclast activity by surface topography of hydroxyapatite coatings. Biomaterials 2013, 34, 7215–7226. [Google Scholar] [CrossRef]
- Prochor, P. Finite element analysis of stresses generated in cortical bone during implantation of a novel Limb Prosthesis Osseointegrated Fixation System. Biocyber. Biomed. Eng. 2017, 37, 255–262. [Google Scholar] [CrossRef]
- Standard ISO 10993-5:2009. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Vörös, P.; Dobrindt, O.; Perka, C.; Windisch, C.; Matziolis, G.; Röhner, E. Human osteoblast damage after antiseptic treatment. Int. Orthop. 2014, 38, 177–182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Family, R.; Solati-Hashjin, M.; Namjoy Nik, S.; Nemati, A. Surface modification for titanium implants by hydroxyapatite nanocomposite. Casp. J. Intern. Med. 2012, 3, 460–465. [Google Scholar]
- Vanzillotta, P.; Soares, G.A.; Bastos, I.N.; Simão, R.A.; Kuromoto, N.K. Potentialities of some surface characterization techniques for the development of titanium biomedical alloys. Mater. Res. 2004, 7, 437–444. [Google Scholar] [CrossRef]
- Sola-Ruiz, M.F.; Perez-Martinez, C.; Labaig-Rueda, C.; Carda, C.; Martín De Llano, J.J. Behavior of Human Osteoblast Cells Cultured on Titanium Discs in Relation to Surface Roughness and Presence of Melatonin. Int. J. Mol. Sci. 2017, 18, 823. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Baumann, M.J.; Case, E.D.; Irwin, R.K.; Meyer, S.E.; Pearson, C.S.; McCabe, L.R. Surface microcracks signal osteoblasts to regulate alignment and bone formation. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 191–200. [Google Scholar] [CrossRef] [PubMed]
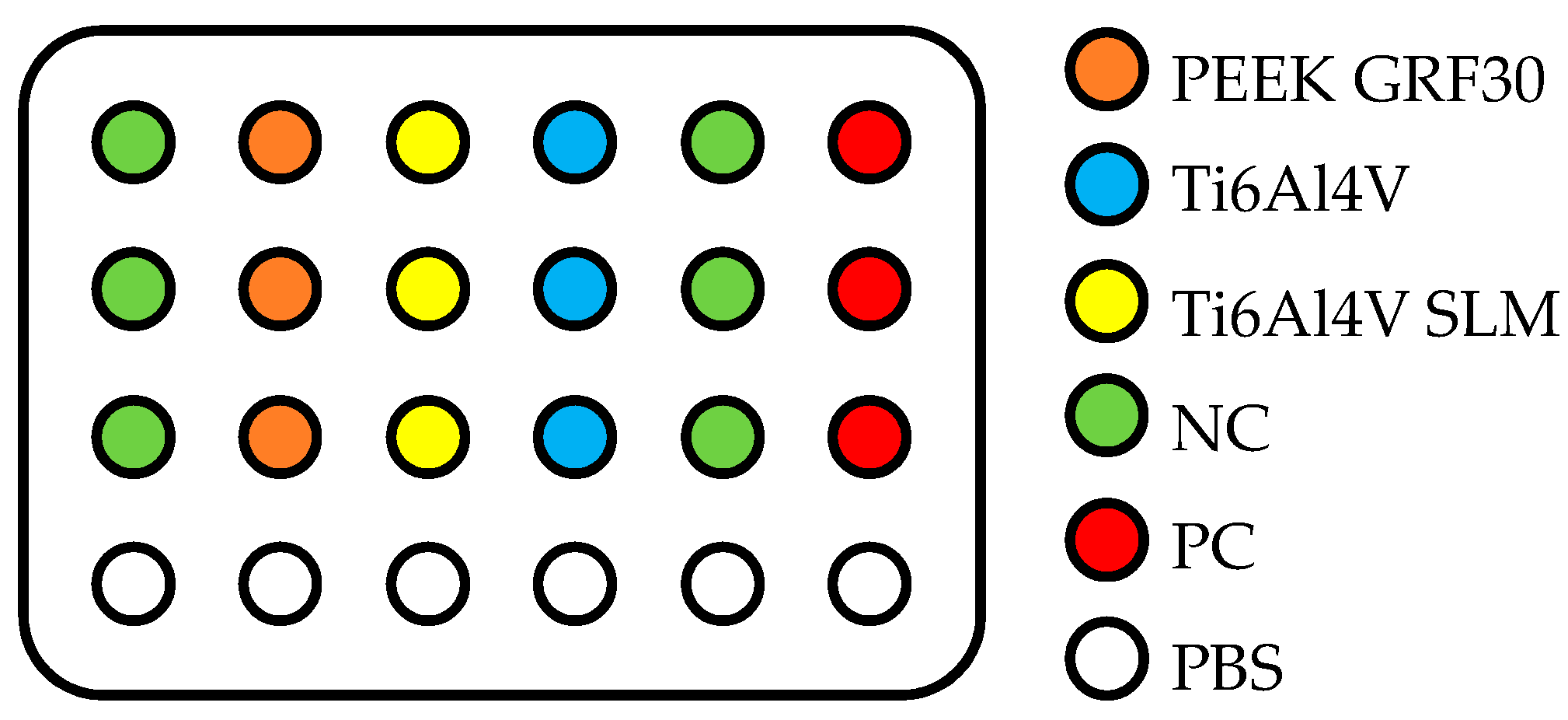


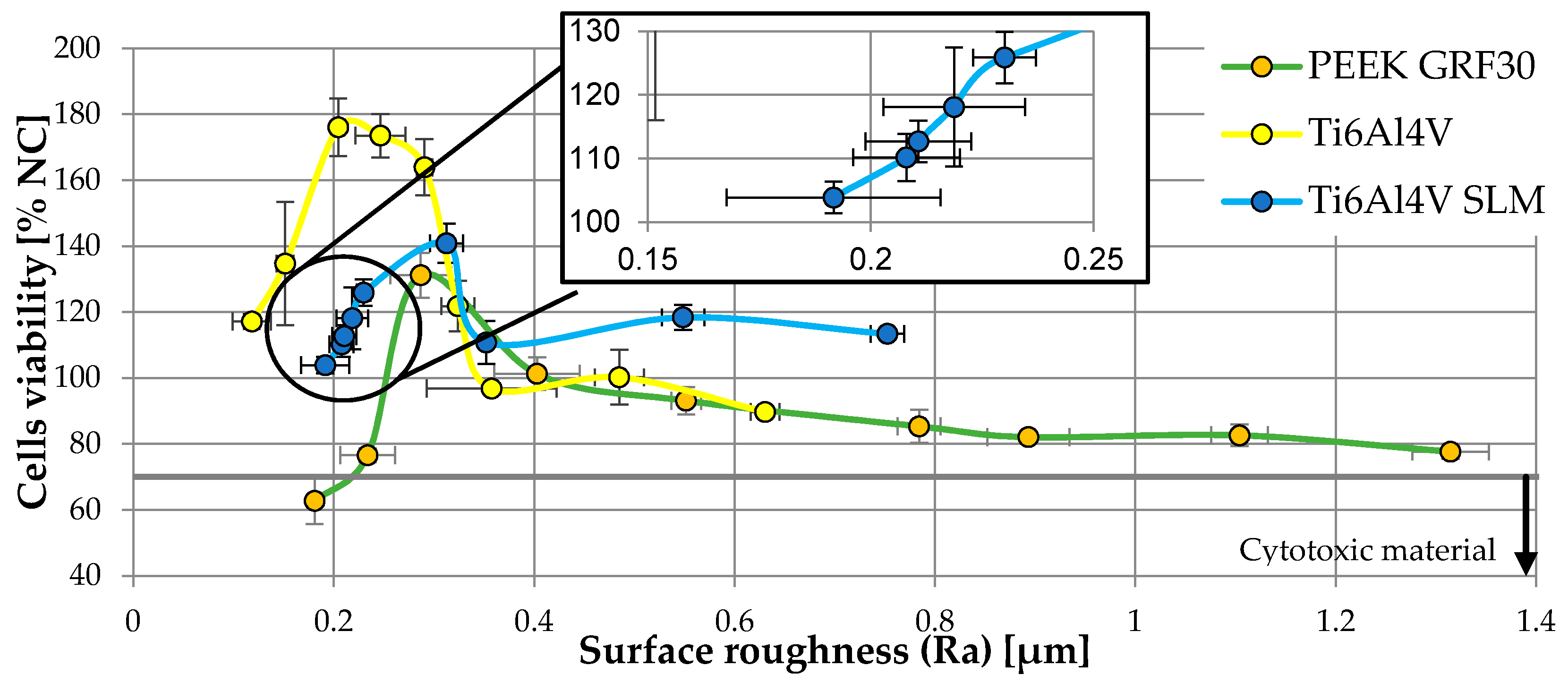
| Material | Surface Treatment | Surface Roughness Parameter | Cells Viability (% NC) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ra (µm) | Rp (µm) | Rv (µm) | Rz (µm) | ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| PEEK GRF30 | Polished | 0.181 | 0.004 | 0.630 | 0.055 | 1.040 | 0.170 | 1.669 | 0.124 | 62.72 | 7.07 |
| 2000 gradation | 0.234 | 0.027 | 0.899 | 0.084 | 1.737 | 0.272 | 2.736 | 0.419 | 76.54 | 2.07 | |
| 1500 gradation | 0.287 | 0.030 | 1.027 | 0.097 | 2.384 | 0.186 | 3.327 | 0.399 | 131.15 | 6.81 | |
| 1200 gradation | 0.403 | 0.043 | 1.198 | 0.099 | 2.765 | 0.329 | 4.132 | 0.688 | 101.26 | 4.91 | |
| 1000 gradation | 0.552 | 0.015 | 1.463 | 0.341 | 2.962 | 0.592 | 4.213 | 0.265 | 93.08 | 4.16 | |
| 800 gradation | 0.784 | 0.022 | 2.594 | 0.488 | 3.104 | 0.610 | 5.850 | 1.045 | 85.31 | 4.96 | |
| 600 gradation | 0.893 | 0.041 | 3.074 | 0.390 | 3.922 | 0.571 | 6.642 | 0.696 | 82.03 | 1.78 | |
| 400 gradation | 1.104 | 0.028 | 3.844 | 0.356 | 4.382 | 0.471 | 8.256 | 0.144 | 82.58 | 3.30 | |
| 240 gradation | 1.315 | 0.038 | 9.353 | 0.400 | 7.750 | 0.543 | 16.569 | 1.319 | 77.64 | 2.19 | |
| Ti6Al4V SLM | Polished | 0.192 | 0.024 | 0.558 | 0.091 | 0.789 | 0.101 | 1.347 | 0.166 | 103.86 | 2.49 |
| 2000 gradation | 0.208 | 0.012 | 0.629 | 0.070 | 0.793 | 0.184 | 1.423 | 0.234 | 110.16 | 3.71 | |
| 1500 gradation | 0.211 | 0.012 | 0.704 | 0.057 | 0.831 | 0.088 | 1.502 | 0.155 | 112.67 | 3.25 | |
| 1200 gradation | 0.219 | 0.016 | 0.953 | 0.200 | 0.906 | 0.083 | 1.825 | 0.208 | 118.07 | 9.35 | |
| 1000 gradation | 0.230 | 0.007 | 1.116 | 0.225 | 1.217 | 0.140 | 2.437 | 0.215 | 125.87 | 4.05 | |
| 800 gradation | 0.313 | 0.017 | 1.221 | 0.076 | 1.541 | 0.163 | 2.820 | 0.362 | 140.85 | 5.92 | |
| 600 gradation | 0.352 | 0.006 | 1.579 | 0.252 | 2.225 | 0.107 | 3.464 | 0.528 | 110.76 | 6.48 | |
| 400 gradation | 0.549 | 0.021 | 2.232 | 0.603 | 2.681 | 0.479 | 4.656 | 0.851 | 118.36 | 3.84 | |
| 240 gradation | 0.753 | 0.017 | 2.469 | 0.701 | 3.238 | 0.630 | 5.707 | 0.361 | 113.35 | 1.50 | |
| Ti6Al4V | Polished | 0.118 | 0.019 | 0.512 | 0.107 | 0.531 | 0.031 | 1.105 | 0.034 | 117.11 | 2.28 |
| 2000 gradation | 0.152 | 0.008 | 0.575 | 0.018 | 0.651 | 0.156 | 1.162 | 0.191 | 134.70 | 18.65 | |
| 1500 gradation | 0.205 | 0.006 | 0.582 | 0.073 | 0.749 | 0.078 | 1.330 | 0.022 | 176.03 | 8.76 | |
| 1200 gradation | 0.247 | 0.025 | 0.755 | 0.083 | 1.046 | 0.070 | 1.801 | 0.126 | 173.51 | 6.62 | |
| 1000 gradation | 0.291 | 0.008 | 0.972 | 0.281 | 1.071 | 0.201 | 2.163 | 0.057 | 163.87 | 8.53 | |
| 800 gradation | 0.324 | 0.017 | 1.092 | 0.148 | 1.510 | 0.200 | 2.482 | 0.441 | 121.79 | 7.72 | |
| 600 gradation | 0.358 | 0.065 | 1.275 | 0.179 | 1.629 | 0.111 | 3.028 | 0.678 | 96.76 | 1.63 | |
| 400 gradation | 0.485 | 0.025 | 1.659 | 0.343 | 1.952 | 0.398 | 3.288 | 0.409 | 100.19 | 8.30 | |
| 240 gradation | 0.631 | 0.014 | 2.582 | 0.237 | 3.390 | 0.419 | 5.972 | 0.318 | 89.67 | 1.59 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prochor, P.; Mierzejewska, Ż.A. Influence of the Surface Roughness of PEEK GRF30 and Ti6Al4V SLM on the Viability of Primary Human Osteoblasts Determined by the MTT Test. Materials 2019, 12, 4189. https://doi.org/10.3390/ma12244189
Prochor P, Mierzejewska ŻA. Influence of the Surface Roughness of PEEK GRF30 and Ti6Al4V SLM on the Viability of Primary Human Osteoblasts Determined by the MTT Test. Materials. 2019; 12(24):4189. https://doi.org/10.3390/ma12244189
Chicago/Turabian StyleProchor, Piotr, and Żaneta Anna Mierzejewska. 2019. "Influence of the Surface Roughness of PEEK GRF30 and Ti6Al4V SLM on the Viability of Primary Human Osteoblasts Determined by the MTT Test" Materials 12, no. 24: 4189. https://doi.org/10.3390/ma12244189
APA StyleProchor, P., & Mierzejewska, Ż. A. (2019). Influence of the Surface Roughness of PEEK GRF30 and Ti6Al4V SLM on the Viability of Primary Human Osteoblasts Determined by the MTT Test. Materials, 12(24), 4189. https://doi.org/10.3390/ma12244189






