Investigation and Characterization of Synthesis Conditions on Sucrose-ammonium Dihydrogen Phosphate (SADP) Adhesive: Bond Performance and Chemical Transformation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Adhesives
2.3. Bond Performance
2.3.1. Manufacture of Plywood
2.3.2. Shear Strength Measurement
2.4. Chemical Analysis of Synsized SADP Adhesives
2.4.1. High-Performance Liquid Chromatography (HPLC) Analysis
2.4.2. Attenuated Total Reflection-Fourier Transform Infrared Spectra (ATR-FTIR) Analysis
2.4.3. Pyrolysis Gas Chromatography and Mass Spectrometry (Py-GC/MS)
3. Results and Discussion
3.1. Effects of Synthesis Conditions on Viscosity, pH Values, and Crystallization of SADP Adhesives
3.2. Bond Performance
3.2.1. Effects of Mass Proportion between Sucrose and ADP
3.2.2. Effects of Synthesis Temperature
3.2.3. Effects of Synthesis Time
3.3. Chemical Analysis
3.3.1. HPLC
3.3.2. ATR FT-IR
3.3.3. Py-GC/MS
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ding, Q.Q.; Xu, X.W.; Yue, Y.Y.; Mei, C.T.; Huang, C.B.; Jiang, S.H.; Wu, Q.L.; Han, J.Q. Nanocellulose-Mediated Electroconductive Self-Healing Hydrogels with High Strength, Plasticity, Viscoelasticity, Stretchability, and Biocompatibility toward Multifunctional Applications. ACS Appl. Mater. Interfaces 2018, 10, 27987–28002. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, S.; Zhu, S.; Huang, C.; Yue, Y.; Mei, C.; Xu, X.; Xia, C. Electrospun Core-Shell Nanofibrous Membranes with Nanocellulose-Stabilized Carbon Nanotubes for Use as High-Performance Flexible Supercapacitor Electrodes with Enhanced Water Resistance, Thermal Stability and Mechanical Toughness. ACS Appl. Mater. Interfaces 2019, 11, 44624–44635. [Google Scholar] [CrossRef]
- Han, J.Q.; Lu, K.Y.; Yue, Y.Y.; Mei, C.T.; Huang, C.B.; Wu, Q.L.; Xu, X.W. Nanocellulose-templated assembly of polyaniline in natural rubber-based hybrid elastomers toward flexible electronic conductors. Ind. Crop. Prod. 2019, 128, 94–107. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.M.; Yang, F.; Tang, C.Y.; Huang, Q.T. Effect of H2O2 Bleaching Treatment on the Properties of Finished Transparent Wood. Polymers 2019, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Bian, Y.Q.; Yang, F.; Ding, Y.; Chen, K.X. Preparation and Properties of Chitosan/Graphene Modified Bamboo Fiber Fabrics. Polymers 2019, 11, 1540. [Google Scholar] [CrossRef] [PubMed]
- Kariz, M.; Kuzman, M.K.; Sernek, M. The effect of the heat treatment of spruce wood on the curing of melamine-urea-formaldehyde and polyurethane adhesives. J. Adhes. Sci. Technol. 2013, 27, 1911–1920. [Google Scholar] [CrossRef]
- Cuk, N.; Kunaver, M.; Poljansek, I.; Ugovsek, A.; Sernek, M.; Medved, S. Properties of liquefied wood modified melamine-formaldehyde (MF) resin adhesive and its application for bonding particleboards. J. Adhes. Sci. Technol. 2015, 29, 1553–1562. [Google Scholar] [CrossRef]
- Kariz, M.; Jost, M.; Sernek, M. Curing of Phenol-Formaldehyde Adhesive in Boards of Different Thicknesses. Wood Res.-Slovak 2009, 54, 41–48. [Google Scholar]
- Wang, X.Z.; Wang, S.Q.; Xie, X.Q.; Zhao, L.G.; Deng, Y.H.; Li, Y.J. Multi-scale evaluation of the effects of nanoclay on the mechanical properties of wood/phenol formaldehyde bondlines. Int. J. Adhes. Adhes. 2017, 74, 92–99. [Google Scholar] [CrossRef]
- Hemmila, V.; Adamopoulos, S.; Karlsson, O.; Kumar, A. Development of sustainable bio-adhesives for engineered wood panels—A Review. RSC Adv. 2017, 7, 38604–38630. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhang, Y.; Li, J.Z.; Gao, Q. Development of a High-Performance Adhesive with a Microphase, Separation Crosslinking Structure Using Wheat Flour and a Hydroxymethyl Melamine Prepolymer. Polymers 2019, 11, 893. [Google Scholar] [CrossRef]
- Wang, Z.; Wen, Y.Y.; Zhao, S.J.; Zhang, W.; Ji, Y.; Zhang, S.F.; Li, J.Z. Soy protein as a sustainable surfactant to functionalize boron nitride nanosheets and its application for preparing thermally conductive biobased composites. Ind. Crop. Prod. 2019, 137, 239–247. [Google Scholar] [CrossRef]
- Pizzi, A. Tannin-Based Biofoams-A Review. J. Renew. Mater. 2019, 7, 477–492. [Google Scholar] [CrossRef]
- Pizzi, A. Biosourced Thermosets for Lignocellulosic Composites. Mater. Res. Found. 2018, 38, 81–111. [Google Scholar]
- Xiong, X.Q.; Bao, Y.L.; Guo, W.J.; Fang, L.; Wu, Z.H. Preparation and Application of High Performance Corn Starch Glue in Straw Decorative Panel. Wood Fiber Sci. 2018, 50, 88–95. [Google Scholar]
- Moreira, L.M.; Lyon, J.P.; Lima, P.; Santos, V.J.S.V.; Santos, F.V. Sucrose Chemistry. Food Nutr. Compon. Focus 2012, 3, 138–149. [Google Scholar] [CrossRef]
- Jarosz, S. The chemistry of sucrose. Pol. J. Chem. 1996, 70, 972–987. [Google Scholar]
- Vydra, T.; Capek, K. The Chemistry of Sucrose. Chem. Listy 1989, 83, 686–715. [Google Scholar]
- Umemura, K.; Sugihara, O.; Kawai, S. Investigation of a new natural adhesive composed of citric acid and sucrose for particleboard II: Effects of board density and pressing temperature. J. Wood Sci. 2015, 61, 40–44. [Google Scholar] [CrossRef]
- Umemura, K.; Sugihara, O.; Kawai, S. Investigation of a new natural adhesive composed of citric acid and sucrose for particleboard. J. Wood Sci. 2013, 59, 203–208. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Umemura, K. Investigation of a new natural particleboard adhesive composed of tannin and sucrose. J. Wood Sci. 2014, 60, 269–277. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Umemura, K. Investigation of a New Natural Particleboard Adhesive Composed of Tannin and Sucrose. 2. Effect of Pressing Temperature and Time on Board Properties, and Characterization of Adhesive. Bioresources 2015, 10, 2444–2460. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Miao, Y.F.; Yang, Z.Q.; Wang, H.; Sang, R.J.; Fu, Y.C.; Huang, C.X.; Wu, Z.H.; Zhang, M.; Sun, S.J.; et al. Effects of Sulfuric Acid on the Curing Behavior and Bonding Performance of Tannin-Sucrose Adhesive. Polymers 2018, 10, 651. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Y.; Hayashi, S.; Xu, W.; Wu, Z.H.; Tanaka, S.; Sun, S.J.; Zhang, M.; Kanayama, K.; Umemura, K. A Novel Eco-Friendly Wood Adhesive Composed by Sucrose and Ammonium Dihydrogen Phosphate. Polymers 2018, 10, 1251. [Google Scholar] [CrossRef]
- Queneau, Y.; Jarosz, S.; Lewandowski, B.; Fitremann, J. Sucrose chemistry and applications of sucrochemicals. Adv. Carbohydr. Chem. Biochem. 2008, 61, 217–292. [Google Scholar] [CrossRef]
- Khan, R. Sucrose Chemistry—Its Position as a Raw-Material for the Chemical-Industry. Abstr. Pap. Am. Chem. Soc. 1987, 194, 138-Agfd. [Google Scholar]
- Manleyharris, M.; Richards, G.N. A Novel Fructoglucan from the Thermal Polymerization of Sucrose. Carbohydr. Res. 1993, 240, 183–196. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Hu, X.; Li, C.Z. Levulinic esters from the acid-catalysed reactions of sugars and alcohols as part of a bio-refinery. Green Chem. 2011, 13, 1676–1679. [Google Scholar] [CrossRef]
- Agyei-Aye, K.; Chian, M.X.; Lauterbach, J.H.; Moldoveanu, S.C. The role of the anion in the reaction of reducing sugars with ammonium salts. Carbohydr. Res. 2002, 337, 2273–2277. [Google Scholar] [CrossRef]
- Whitfield, M. The Hydrolysis of Ammonium Ions in Sea Water-a Theoretical Study. J. Mar. Biol. Assoc. UK 2009, 54, 565–580. [Google Scholar] [CrossRef]
- Quintas, M.; Brandao, T.R.S.; Silva, C.L.M.; Cunha, R.L. Rheology of supersaturated sucrose solutions. J. Food Eng. 2006, 77, 844–852. [Google Scholar] [CrossRef]
- Vaz, P.D.; Ribeiro-Claro, P.J.A. C-H O hydrogen bonds in liquid cyclohexanone revealed by the vC = O splitting and the vC-H blue shift. J. Raman Spectrosc. 2003, 34, 863–867. [Google Scholar] [CrossRef]
- Surendra, B.S.; Veerabhadraswamy, M. Microwave assisted synthesis of Schiff base via bioplatform chemical intermediate (HMF) derived from Jatropha deoiled seed cake catalyzed by modified Bentonite clay. Mater. Today-Proc. 2017, 4, 11968–11976. [Google Scholar] [CrossRef]
- Nikolic, G.; Zlatkovic, S.; Cakic, M.; Cakic, S.; Lacnjevac, C.; Rajic, Z. Fast Fourier Transform IR Characterization of Epoxy GY Systems Crosslinked with Aliphatic and Cycloaliphatic EH Polyamine Adducts. Sensors 2010, 10, 684–696. [Google Scholar] [CrossRef]
- Lu, Q.; Liao, H.-t.; Zhang, Y.; Zhang, J.-J.; Dong, C.-Q. Reaction mechanism of low-temperature fast pyrolysis of fructose to produce 5-hydroxymethyl furfural. J. Fuel Chem. Technol. 2013, 41, 1070–1076. [Google Scholar] [CrossRef]
- Locas, C.P.; Yaylayan, V.A. Isotope labeling studies on the formation of 5-(hydroxymethyl)-2-furaldehyde (HMF) from sucrose by pyrolysis-GC/MS. J. Agric. Food Chem. 2008, 56, 6717–6723. [Google Scholar] [CrossRef]
- Nishibori, S.; Kawakishi, S. Formation of 2,3-Dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one from Fructose and beta.-Alanine under Conditions Used for Baking. J. Agric. Food Chem. 1994, 42, 1080–1084. [Google Scholar] [CrossRef]
- Gardiner, D. The pyrolysis of some hexoses and derived di-, tri-, and poly-saccharides. J. Chem. Soc. C Org. 1966, 1473–1476. [Google Scholar] [CrossRef]
- Fagerson, I.S. Thermal degradation of carbohydrates; a review. J. Agric. Food Chem. 1969, 17, 747–750. [Google Scholar] [CrossRef]
- Zhu, Y.; Zajicek, J.; Serianni, A.S. Acyclic Forms of [1-13C] Aldohexoses in Aqueous Solution: Quantitation by 13C NMR and Deuterium Isotope Effects on Tautomeric Equilibria. J. Org. Chem. 2001, 66, 6244–6251. [Google Scholar] [CrossRef] [PubMed]
- Sanders, E.B.; Goldsmith, A.I.; Seeman, J.I. A model that distinguishes the pyrolysis of d-glucose, d-fructose, and sucrose from that of cellulose. Application to the understanding of cigarette smoke formation. J. Anal. Appl. Pyrolysis 2003, 66, 29–50. [Google Scholar] [CrossRef]
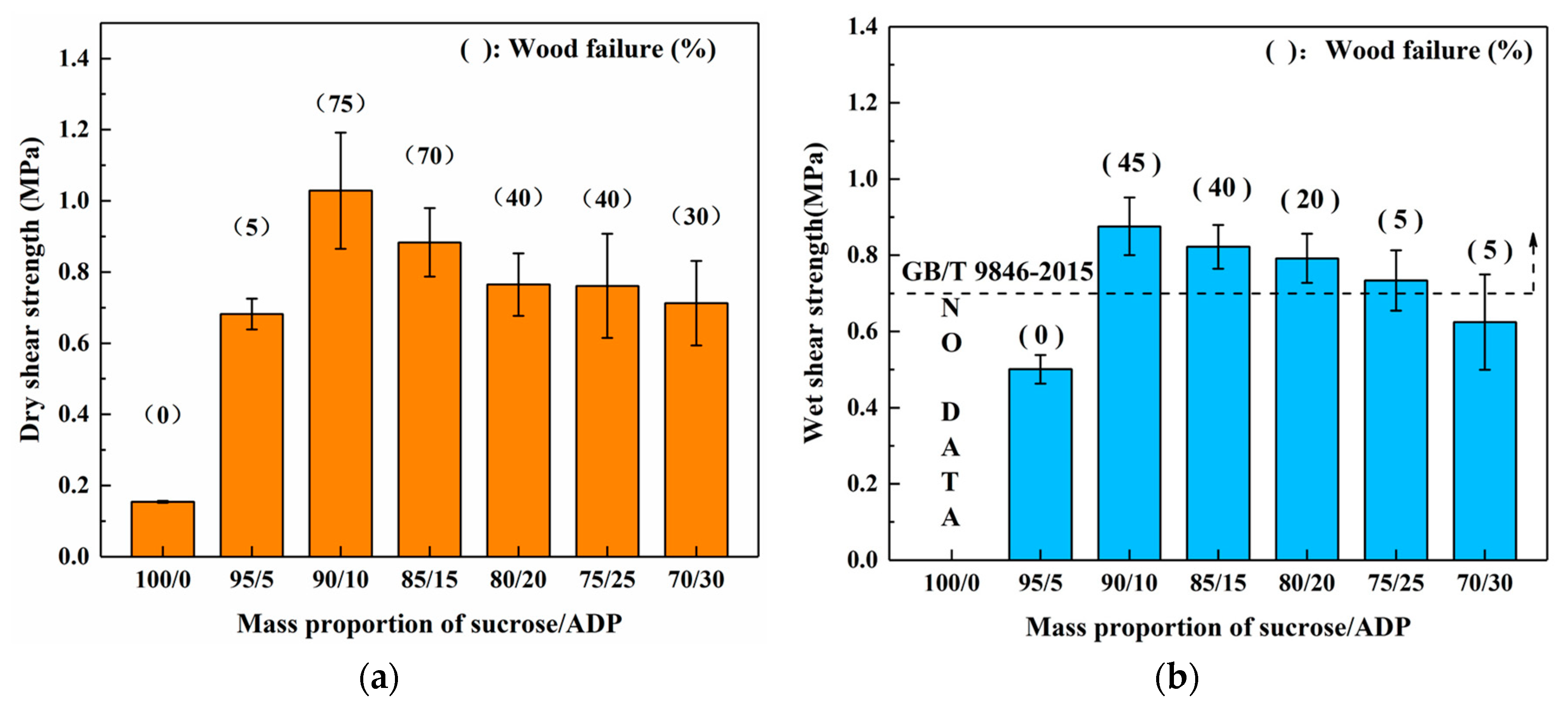

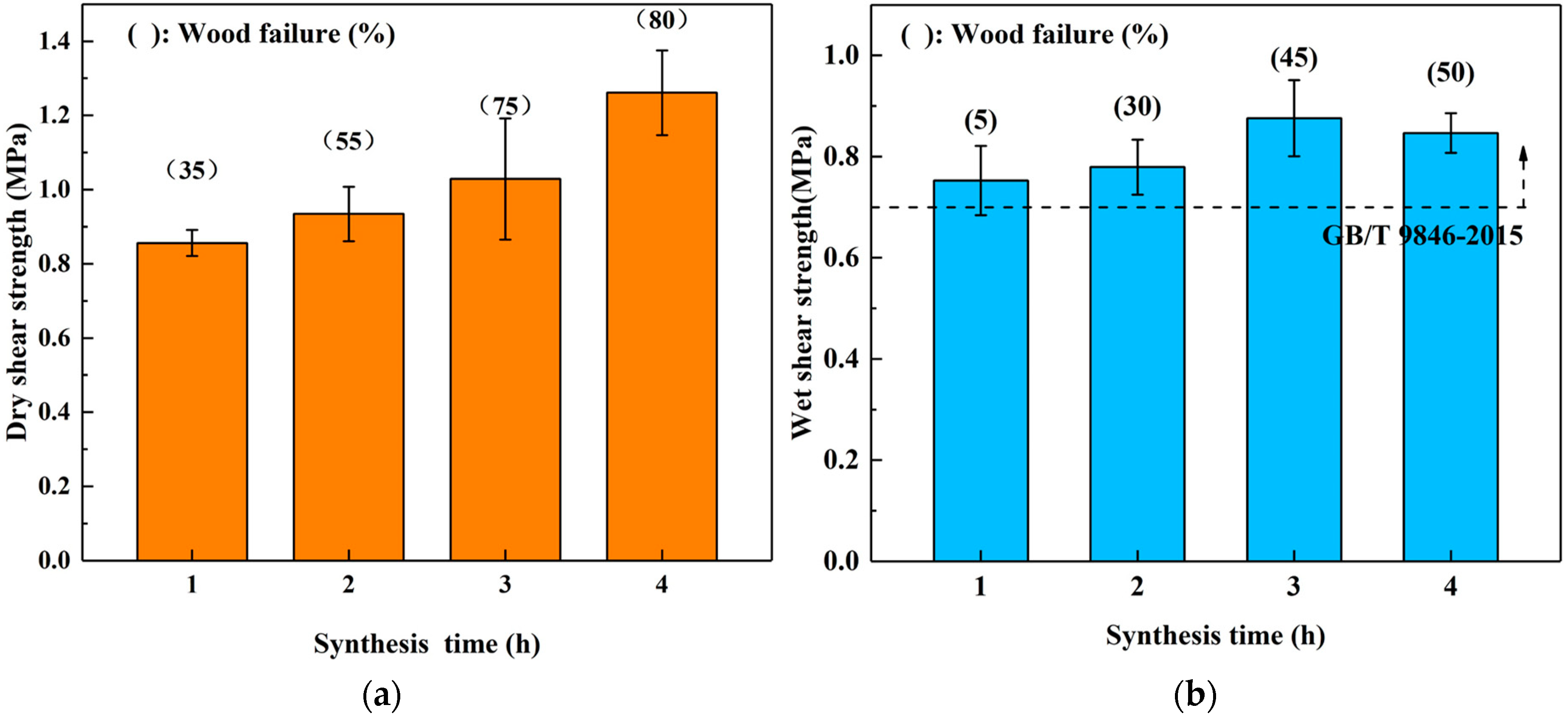
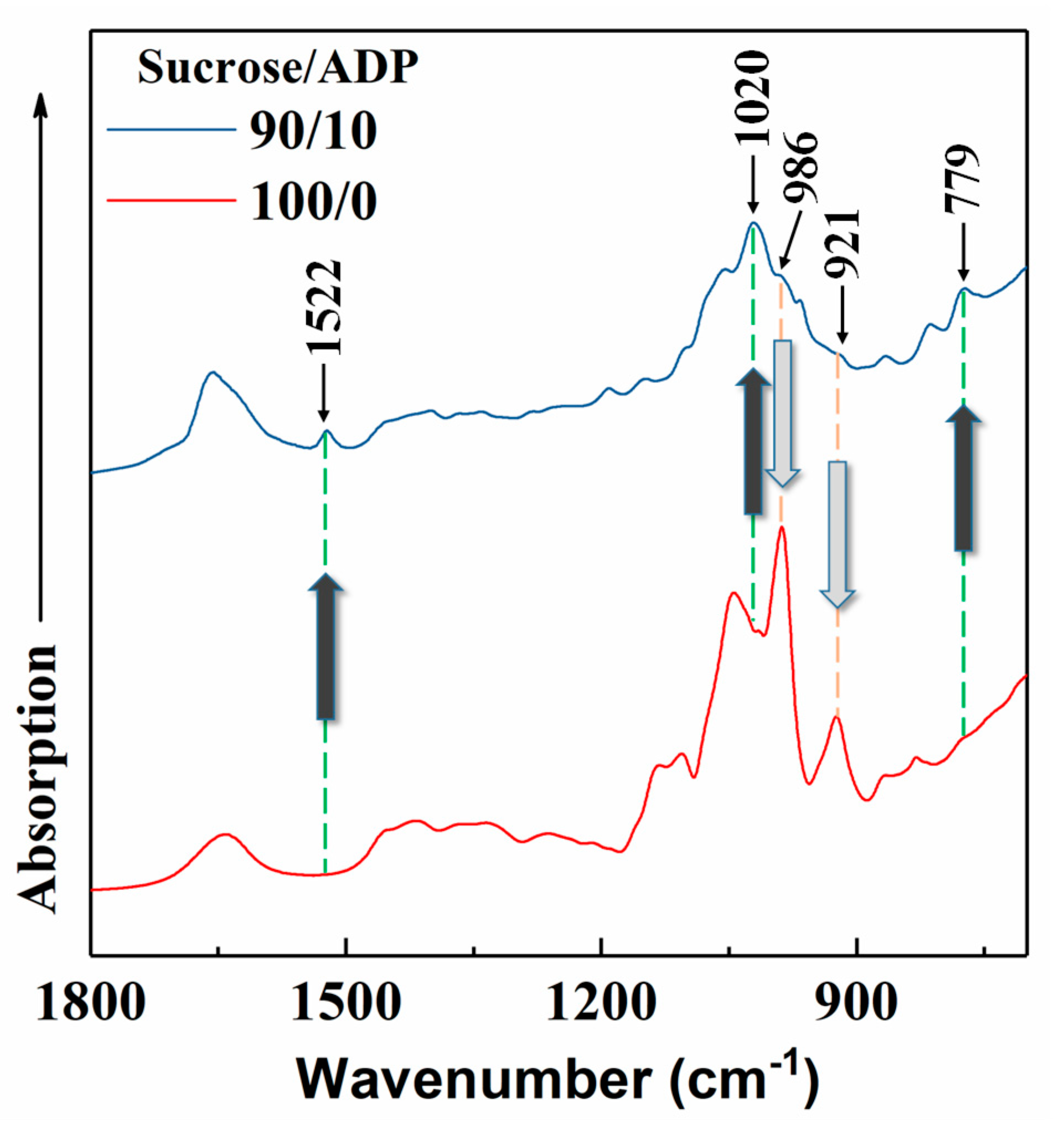
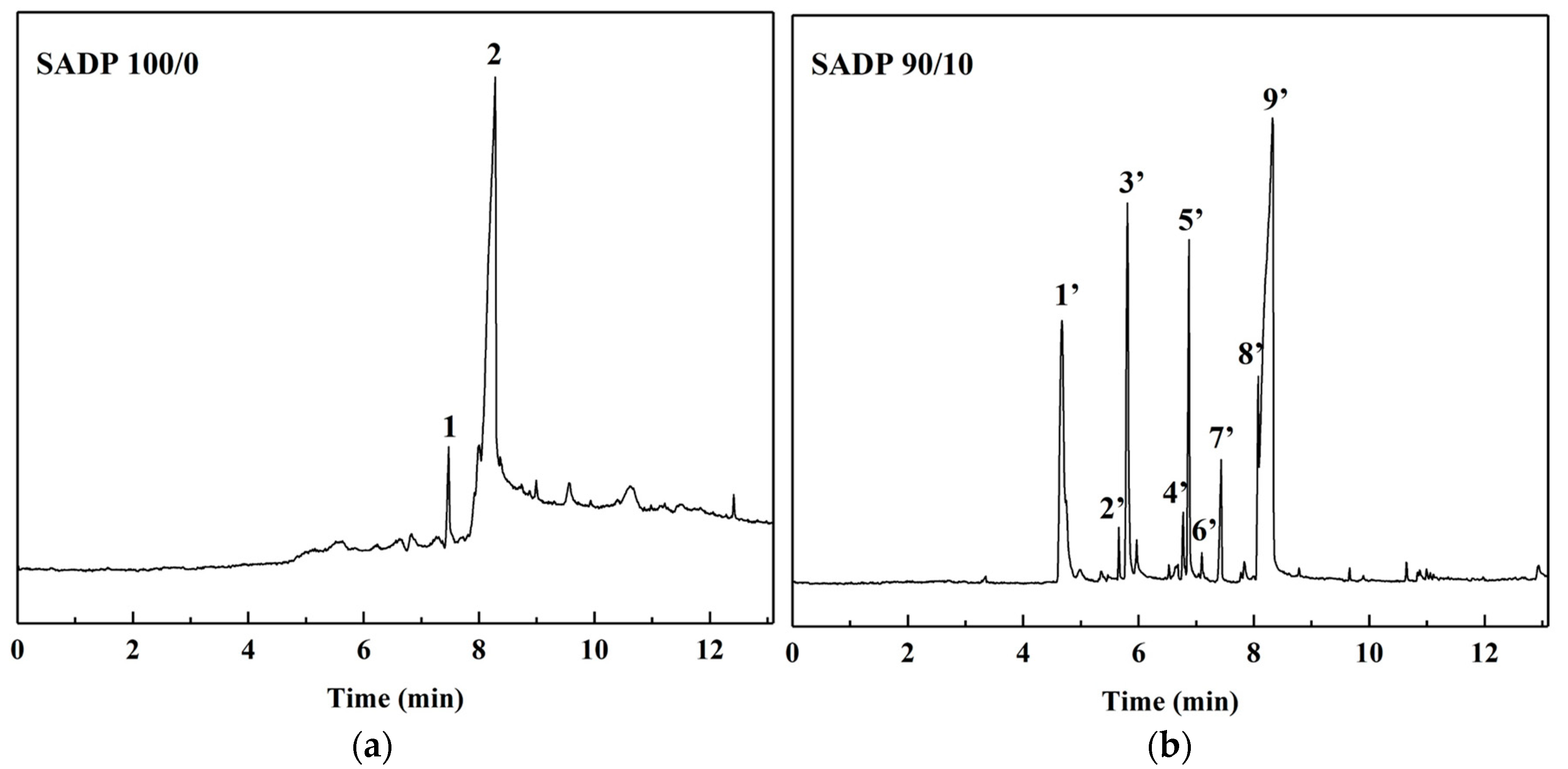
| Groups | Mass Proportion (Sucrose/ADP) | Synthesis Temperature (°C) | Synthesis Time (h) | Solid Content (%) | Viscosity (mPa·s) | pH | Whether Contain the Precipitation after 3 Days Storing |
|---|---|---|---|---|---|---|---|
| Group 1 | 100/0 | 90 | 3 | 80 | 1770 | 5.1 | Yes |
| 90/10 | 826.7 | 3.7 | NO | ||||
| 85/15 | 784.9 | 3.3 | NO | ||||
| 80/20 | 557.6 | 3.1 | NO | ||||
| 75/25 | 333.8 | 2.9 | NO | ||||
| 70/30 | 252.5 | 2.5 | NO | ||||
| Group 2 | 90/10 | 80 | 3 | 80 | 911.5 | 4.0 | NO |
| 90 | 826.7 | 3.7 | NO | ||||
| 100 | 472.9 | 3.1 | NO | ||||
| 110 | 393.2 | 2.5 | NO | ||||
| Group 3 | 90/10 | 90 | 1 | 80 | 1020.4 | 4.6 | NO |
| 2 | 877.0 | 4.1 | NO | ||||
| 3 | 826.7 | 3.7 | NO | ||||
| 4 | 427.3 | 2.5 | NO |
| Groups | Sucrose-ADP | Synthesis Temperature (°C) | Synthesis Time (h) | 5-HMF (g/L) |
|---|---|---|---|---|
| Group 2 | 90/10 | 80 | 3 | 7.3 |
| 90 | 31.1 | |||
| 100 | 35.6 | |||
| 110 | 42.8 | |||
| Group 3 | 90/10 | 90 | 1 | 6.7 |
| 2 | 22.3 | |||
| 3 | 31.1 | |||
| 4 | 44.5 |
| Samples | Peak Number | RT (min) | SI | Compound | CAS | MW | Formula |
|---|---|---|---|---|---|---|---|
| SADP (100/0) | 1 | 7.47 | 89 | 4H-Pyran-4-one,2,3-dihydro-3,5-dihydroxy-6-methyl- | 28564-83-2 | 144 | C6H8O4 |
| 2 | 8.27 | 95 | 5-Hydrxoymethylfurfura | 67-47-0 | 126 | C6H6O3 | |
| SADP (90/10) | 1′ | 4.67 | 98 | Furfural | 1998-1-1 | 96 | C5H4O2 |
| 96 | 3-Furaldehyde | 498-60-2 | 96 | C5H4O2 | |||
| 2′ | 5.66 | 95 | 2(5H)-Furanone, 5-methyl- | 591-11-7 | 98 | C5H6O2 | |
| 3′ | 5.81 | 98 | 2-Furancarboxaldehyde, 5-methyl- | 620-02-0 | 110 | C6H6O2 | |
| 4′ | 6.78 | 96 | 2,5-Furandicarboxaldehyde | 823-82-5 | 124 | C6H4O3 | |
| 5′ | 6.88 | 95 | Furyl hydroxymethyl ketone | 17678-19-2 | 126 | C6H6O3 | |
| 94 | Furan-2-carbohydrazide | 3326-71-4 | 126 | C5H6N2O2 | |||
| 94 | Methyl 2-furoate | 611-13-2 | 126 | C6H6O3 | |||
| 94 | 3-Furancarboxylic acid, methyl ester | 13129-23-2 | 126 | C6H6O3 | |||
| 6′ | 7.10 | 94 | Levoglucosenone | 37112-31-5 | 126 | C6H6O3 | |
| 7′ | 7.43 | 94 | 4H-Pyran-4-one,2,3-dihydro-3,5-dihydroxy-6-methyl- | 28564-83-2 | 144 | C6H8O4 | |
| 8′ | 8.08 | 90 | 5-Acetoxymethyl-2-furaldehyde | 10551-58-3 | 168 | C8H8O4 | |
| 9′ | 8.29 | 96 | 5-Hydrxoymethylfurfura | 67-47-0 | 126 | C6H6O3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Zhang, M.; Umemura, K.; Zhao, Z. Investigation and Characterization of Synthesis Conditions on Sucrose-ammonium Dihydrogen Phosphate (SADP) Adhesive: Bond Performance and Chemical Transformation. Materials 2019, 12, 4078. https://doi.org/10.3390/ma12244078
Sun S, Zhang M, Umemura K, Zhao Z. Investigation and Characterization of Synthesis Conditions on Sucrose-ammonium Dihydrogen Phosphate (SADP) Adhesive: Bond Performance and Chemical Transformation. Materials. 2019; 12(24):4078. https://doi.org/10.3390/ma12244078
Chicago/Turabian StyleSun, Shijing, Min Zhang, Kenji Umemura, and Zhongyuan Zhao. 2019. "Investigation and Characterization of Synthesis Conditions on Sucrose-ammonium Dihydrogen Phosphate (SADP) Adhesive: Bond Performance and Chemical Transformation" Materials 12, no. 24: 4078. https://doi.org/10.3390/ma12244078
APA StyleSun, S., Zhang, M., Umemura, K., & Zhao, Z. (2019). Investigation and Characterization of Synthesis Conditions on Sucrose-ammonium Dihydrogen Phosphate (SADP) Adhesive: Bond Performance and Chemical Transformation. Materials, 12(24), 4078. https://doi.org/10.3390/ma12244078






