Properties of a Steel Slag–Permeable Asphalt Mixture and the Reaction of the Steel Slag–Asphalt Interface
Abstract
1. Introduction
2. Experiment
2.1. Materials and Reagents
2.2. Experimental Method
2.3. Performance Test and Characterization
3. Results and Discussion
3.1. Performance Analysis
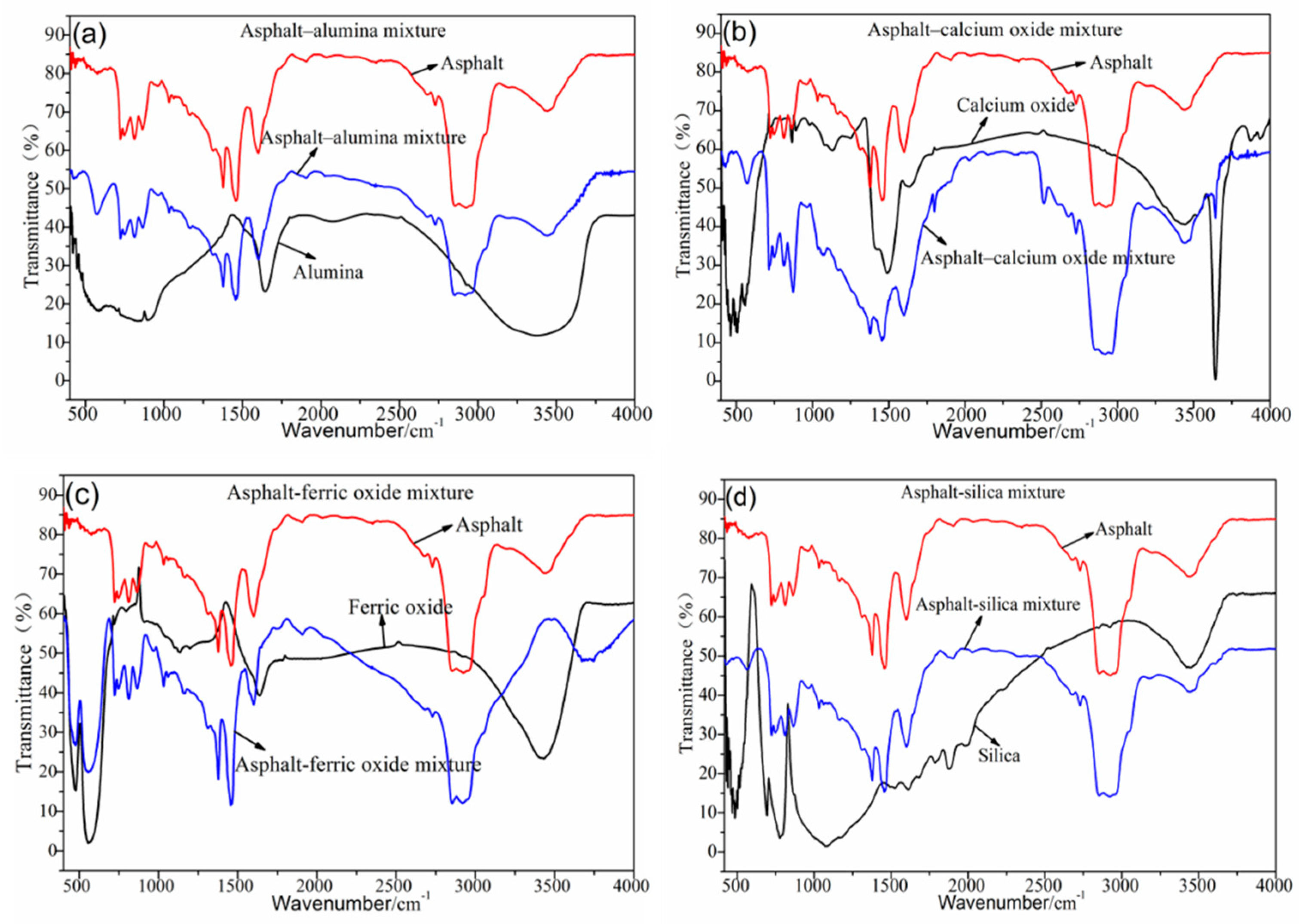
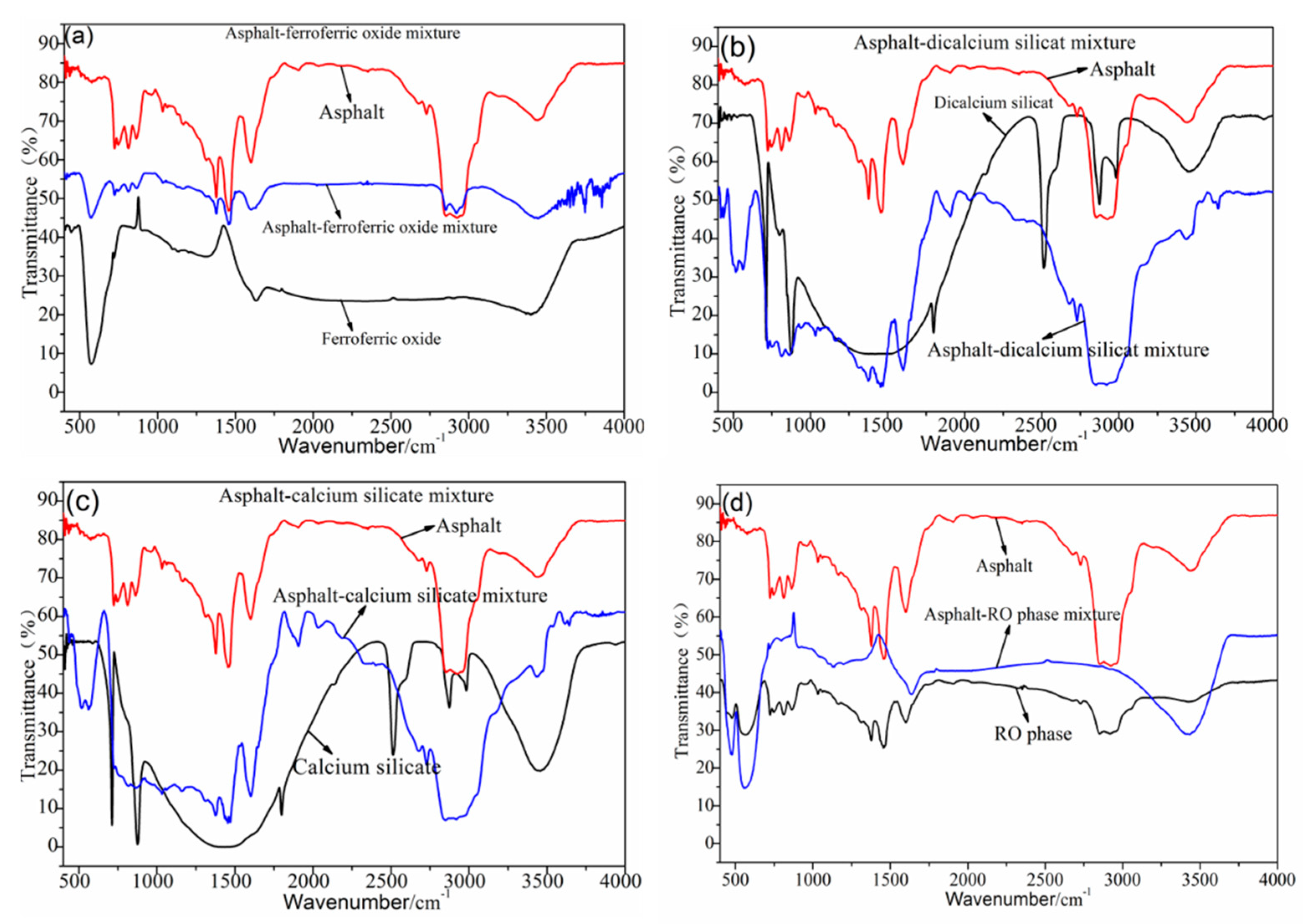
3.2. Analysis of the Chemical Composition of the Steel Slag–Asphalt Mixture
3.3. Analysis of the Mineral Composition of the Steel Slag–Asphalt Mixture
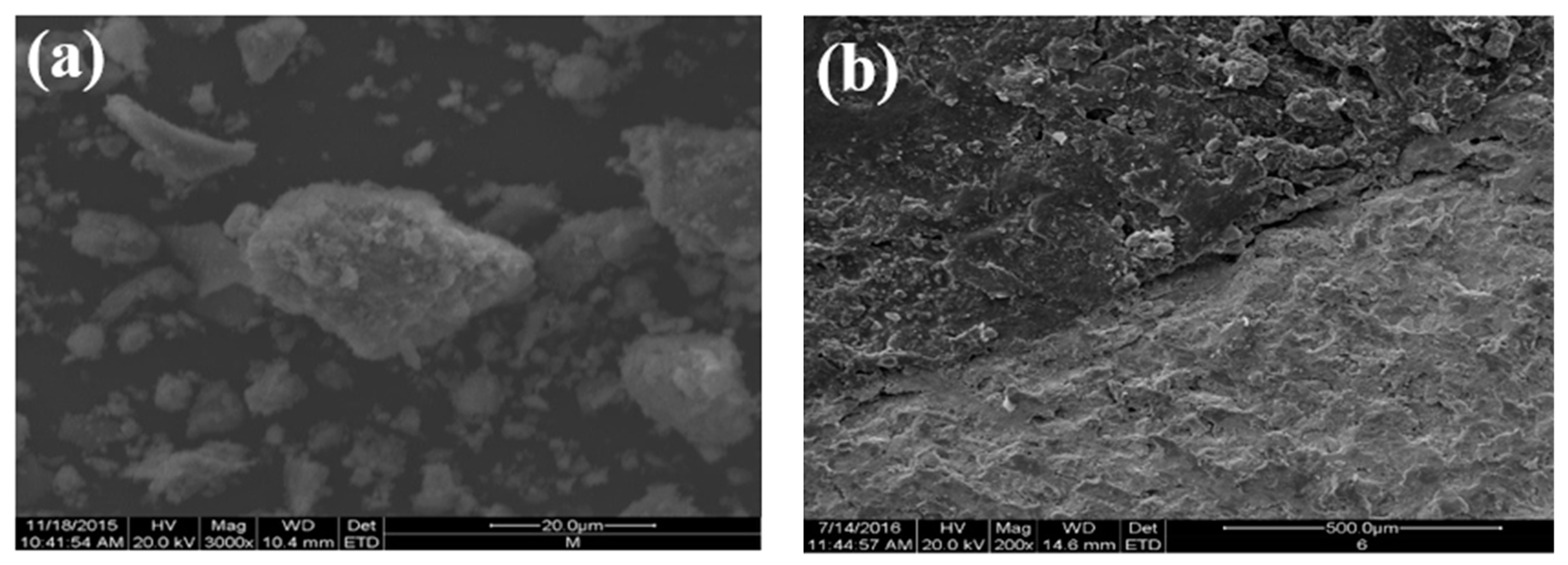
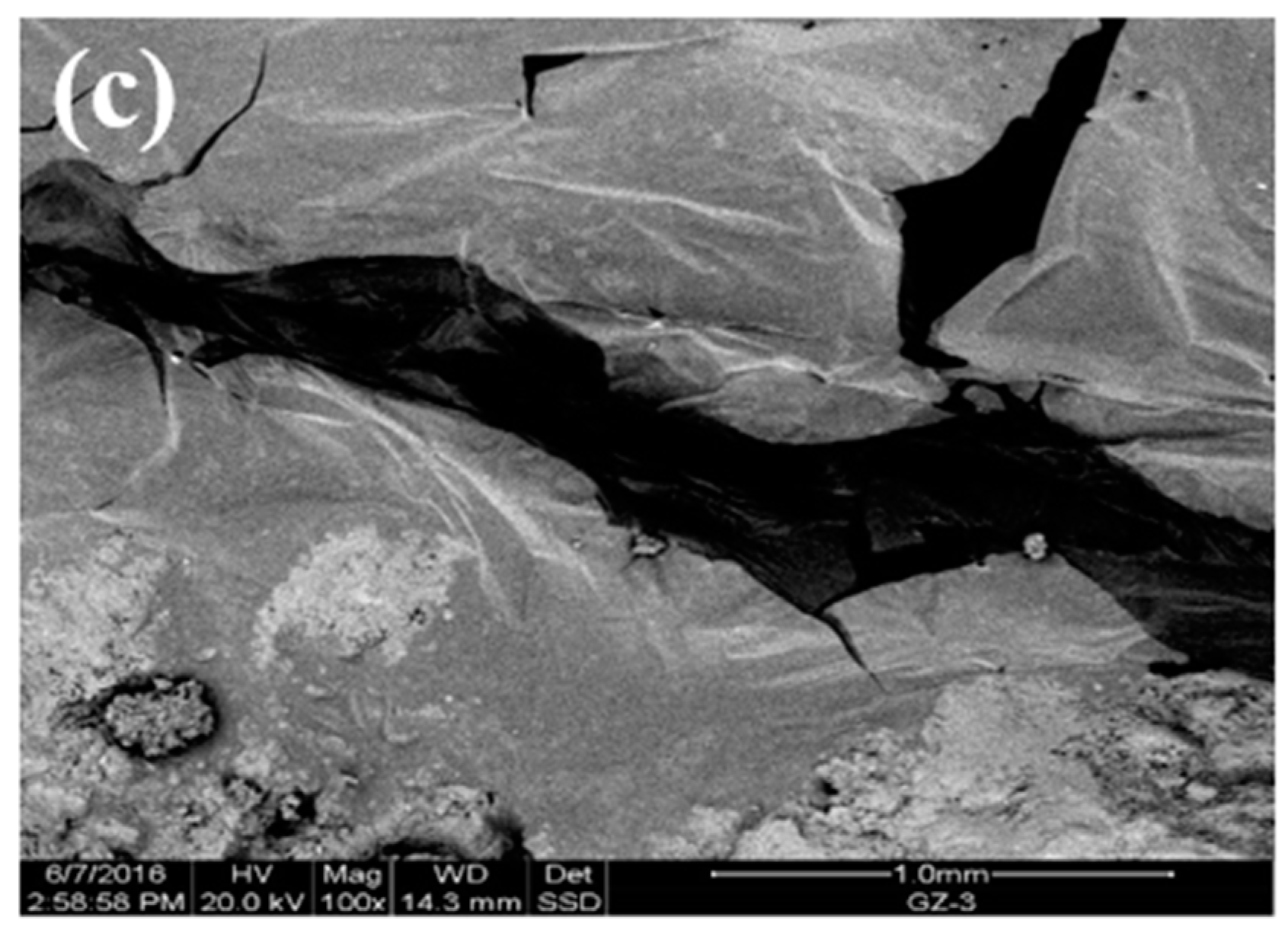
3.4. Analysis of the Reaction of the Steel Slag–Asphalt Interface
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ramezanianpour, A.A.; Kazemian, A.; Moghaddam, M.A.; Moodi, F.; Ramezanianpour, A.M. Studying effects of low-reactivity GGBFS on chloride resistance of conventional and high strength concretes. Mater. Struct. 2015, 49, 2597–2609. [Google Scholar] [CrossRef]
- Xie, J.; Chen, J.; Wu, S.; Lin, J.; Wei, W. Performance characteristics of asphalt mixture with basic oxygen furnace slag. Constr. Build. Mater. 2013, 38, 796–803. [Google Scholar] [CrossRef]
- Fang, N.J.; Guo, J.X.; Shu, S.; Li, J.J.; Chu, Y.H. Influence of textures, oxygen-containing functional groups and metal species on SO2, and NO removal over Ce-Mn/NAC. Fuel 2017, 202, 328–337. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, J.; Xiao, Y.; Chen, J.; Wu, S. Characteristics of bonding behavior between basic oxygen furnace slag and asphalt binder. Constr. Build. Mater. 2014, 64, 60–66. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, C.; Han, D.; Ma, S.; Guo, W.; Cai, H.; Wang, X. Effect of graphene on corrosion resistance of waterborne inorganic zinc-rich coatings. J. Alloy. Compd. 2019, 774, 255–264. [Google Scholar] [CrossRef]
- Zhao, L.H.; Wei, W.; Bai, H.; Zhang, X.; Cang, D.Q. Synthesis of steel slag ceramics: Chemical composition and crystalline phases of raw materials. Int. J. Miner. Metall. Mater. 2015, 22, 325–333. [Google Scholar] [CrossRef]
- Ziari, H.; Khabiri, M.M. Preventive maintenance of flexible pavement and mechanical properties of steel slag asphalt. J. Environ. Eng. Landsc. Manag. 2007, 15, 188–192. [Google Scholar] [CrossRef]
- Pasetto, M.; Baldo, N. Mix design and performance analysis of asphalt concretes with electric arc furnace slag. Constr. Build. Mater. 2011, 25, 3458–3468. [Google Scholar] [CrossRef]
- Ahmedzade, P.; Sengoz, B. Evaluation of steel slag coarse aggregate in hot mix asphalt concrete. J. Hazard. Mater. 2009, 165, 300–305. [Google Scholar] [CrossRef]
- Asi, I.M.; Qasrawi, H.Y.; Shalabi, F.I. Use of steel slag aggregate in asphalt concrete mixes. Can. J. Civ. Eng. 2007, 34, 902–911. [Google Scholar] [CrossRef]
- Behiry, E.M. Evaluation of steel slag and crushed limestone mixtures as subbase material in flexible pavement. Ain Shams Engin. J. 2013, 4, 43–53. [Google Scholar] [CrossRef]
- Amelian, S.; Manian, M.; Abtahi, S.M.; Goli, A. Moisture sensitivity and mechanical performance assessment of warm mix asphalt containing by-product steel slag. J. Clean. Prod. 2018, 176, 329–337. [Google Scholar] [CrossRef]
- Wan, J.; Wu, S.; Xiao, Y.; Chen, Z.; Zhang, D. Study on the effective composition of steel slag for asphalt mixture induction heating purpose. Constr. Build. Mater. 2018, 178, 542–550. [Google Scholar] [CrossRef]
- Vaiana, R.; Balzano, F.; Iuele, T.; Gallelli, V. Microtexture performance of EAF Slag used as aggregates in asphalt mixes: A comparative study with surface properties of natural stones. Appl. Sci. 2019, 9, 3197. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.Y.; Long, H.M. Spectroscopic analysis of weak acid modified steel slag powder. Spectrosc. Spectr. Anal. 2018, 38, 3502–3506. [Google Scholar]
- Noureldin, A.S.; Mcdaniel, R.S. Performance evaluation of steel furnace slag-natural sand asphalt surface mixtures (with discussion and closure). J. Assoc. Asph. Paving Technol. 1990, 59, 276–303. [Google Scholar]
- Airey, G.D.; Collop, A.C.; Thom, N.H.; Thom, N.H.; Zoroob, S.E.; Shiratori, A. Laboratory evaluation of secondary aggregates in bituminous mixtures (with discussion). J. Assoc. Asph. Paving Technol. 2004, 73, 731–769. [Google Scholar]
- Chen, J.S.; Wei, S.H. Engineering properties and performance of asphalt mixtures incorporating steel slag. Constr. Build. Mater. 2016, 128, 148–153. [Google Scholar] [CrossRef]
- Ding, J.; Zhong, Q.; Zhang, S. Catalytic efficiency of iron oxides in decomposition of H2O2 for simultaneous NOX and SO2 removal: Effect of calcination temperature. J. Mol. Catal. A Chem. 2014, 393, 222–231. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, Y. Temperature dependent photoluminescence of surfactant assisted electrochemically synthesized ZnSe nanostructures. J. Alloy. Compd. 2019, 781, 201–208. [Google Scholar] [CrossRef]
- Morel, F.; Bounor-Legaré, V.; Espuche, E.; Persyn, O.; Lacroix, M. Surface modification of calcium carbonate nanofillers by fluoro-and alkyl-alkoxysilane: Consequences on the morphology, thermal stability and gas barrier properties of polyvinylidene fluoride nanocomposites. Eur. Polym. J. 2012, 48, 919–929. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Long, H.M. Study on composite activating mechanism of alkali steel slag cementations materials by XRD and FTIR. Spectrosc. Spectr. Anal. 2018, 38, 2302–2306. [Google Scholar]
- Zhang, H.; Liu, X.Y.; Liu, Y. Study on physical excitation mechanism of steel slag tailings by XRD and SEM. Spectrosc. Spectr. Anal. 2019, 39, 937–941. [Google Scholar]
- Chinese Standard Service Net Home Page. Available online: http://www.cssn.net.cn/cssn/cssn/MandStandard/gyDetail.jsp?bz_code=NjU1OTgzMw==&db_info=VF9OX1RSU19TVEFOREFSRF9XRUI=&A100=Q0pKL1QgMTkwLTIwMTI=&A101=MjAxMi0wOC0yMw==&price= (accessed on 23 August 2012).
- Chinese Standard Service Net Home Page. Available online: http://www.cssn.net.cn/cssn/cssn/MandStandard/gyDetail.jsp?bz_code=NjI0NjQyMg==&db_info=VF9OX1RSU19TVEFOREFSRF9XRUI=&A100=SlRHIEUyMC0yMDEx&A101=MjAxMS0wOS0xMw==&price= (accessed on 13 September 2011).


| Water Permeability | Water Stability | Marshall Stability | ||||||
|---|---|---|---|---|---|---|---|---|
| Volume /mL | Time /s | coefficient of permeability /(mL/s) | Stability of 0.5h /kN | 48 h stability /kN | Residual degree of immersion /% | Marshall stability /kN | Flow value /mm | Void ratio /% |
| 400 | 6.6 | 60.61 | 9.12 | 8.27 | 90.68 | 9.12 | 23.3 | 19.64 |
| High Temperature Stability | Expansibility | ||
|---|---|---|---|
| Dynamic stability (60 °C, 1 h, Times /mm) | Initial volume /mm3 | Final volume /mm3 | Expansion rate /% |
| 6350 | 509235 | 511721 | 0.49 |
| CaO | Fe2O3 | SiO2 | Al2O3 | MgO | MnO | P2O5 | TiO2 | SO3 | Na2O | K2O | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 44.83 | 21.65 | 14.38 | 5.48 | 3.42 | 1.94 | 0.83 | 0.57 | 0.23 | 0.05 | 0.04 | 6.58 |
| Test Item | Measured Value | Technical Indicators | Normative References of the Tests |
|---|---|---|---|
| Apparent relative density (g/cm3) | 3.39 | ≥2.90 | JTG E20-2011 |
| Water absorption (%) | 2.4 | ≤3.0 | JTG E20-2011 |
| Needle particle content (%) | 4.56 | ≤12 | JTG E20-2011 |
| Aggregate crushing value (%) | 13.9 | ≤26 | JTG E20-2011 |
| Water washing method <0.075 mm (%) | 0.2 | ≤1.0 | JTG E20-2011 |
| Los Angeles abrasion loss (%) | 13.2 | ≤26 | JTG E20-2011 |
| Incorruptibility (%) | 2.6 | ≤12 | JTG E20-2011 |
| Soaking expansion rate (%) | 1.2 | ≤2.0 | JTG E20-2011 |
| Adhesion to Asphalt (%) | 5 | ≥4 | JTG E20-2011 |
| f-CaO (%) | 1.7 | ≤3.0 | JTG E20-2011 |
| Sample | FTIR Peak Wavenumbers (cm−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Asphalt | 3200 and 3700 | 2500~3200 | 2920 and 2850 | 2950 | 1560 and 1640 | 1460 and 1375 | 1400~1420 | 1030 and 1280 | 650~900 | 800 and 860 |
| Steel slag powder | 3200 and 3700 | 1300 and 1600 | 750~1200 | 750 and 500 | 400~500 | 3407 | 1430 | 921 | 710 | 500 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Li, H.; Zhu, H.; Xu, P. Properties of a Steel Slag–Permeable Asphalt Mixture and the Reaction of the Steel Slag–Asphalt Interface. Materials 2019, 12, 3603. https://doi.org/10.3390/ma12213603
Liu W, Li H, Zhu H, Xu P. Properties of a Steel Slag–Permeable Asphalt Mixture and the Reaction of the Steel Slag–Asphalt Interface. Materials. 2019; 12(21):3603. https://doi.org/10.3390/ma12213603
Chicago/Turabian StyleLiu, Wenhuan, Hui Li, Huimei Zhu, and Pinjing Xu. 2019. "Properties of a Steel Slag–Permeable Asphalt Mixture and the Reaction of the Steel Slag–Asphalt Interface" Materials 12, no. 21: 3603. https://doi.org/10.3390/ma12213603
APA StyleLiu, W., Li, H., Zhu, H., & Xu, P. (2019). Properties of a Steel Slag–Permeable Asphalt Mixture and the Reaction of the Steel Slag–Asphalt Interface. Materials, 12(21), 3603. https://doi.org/10.3390/ma12213603




