A Combination of Keyhole GTAW with a Trapezoidal Interlayer: A New Insight into Armour Steel Welding
Abstract
1. Introduction
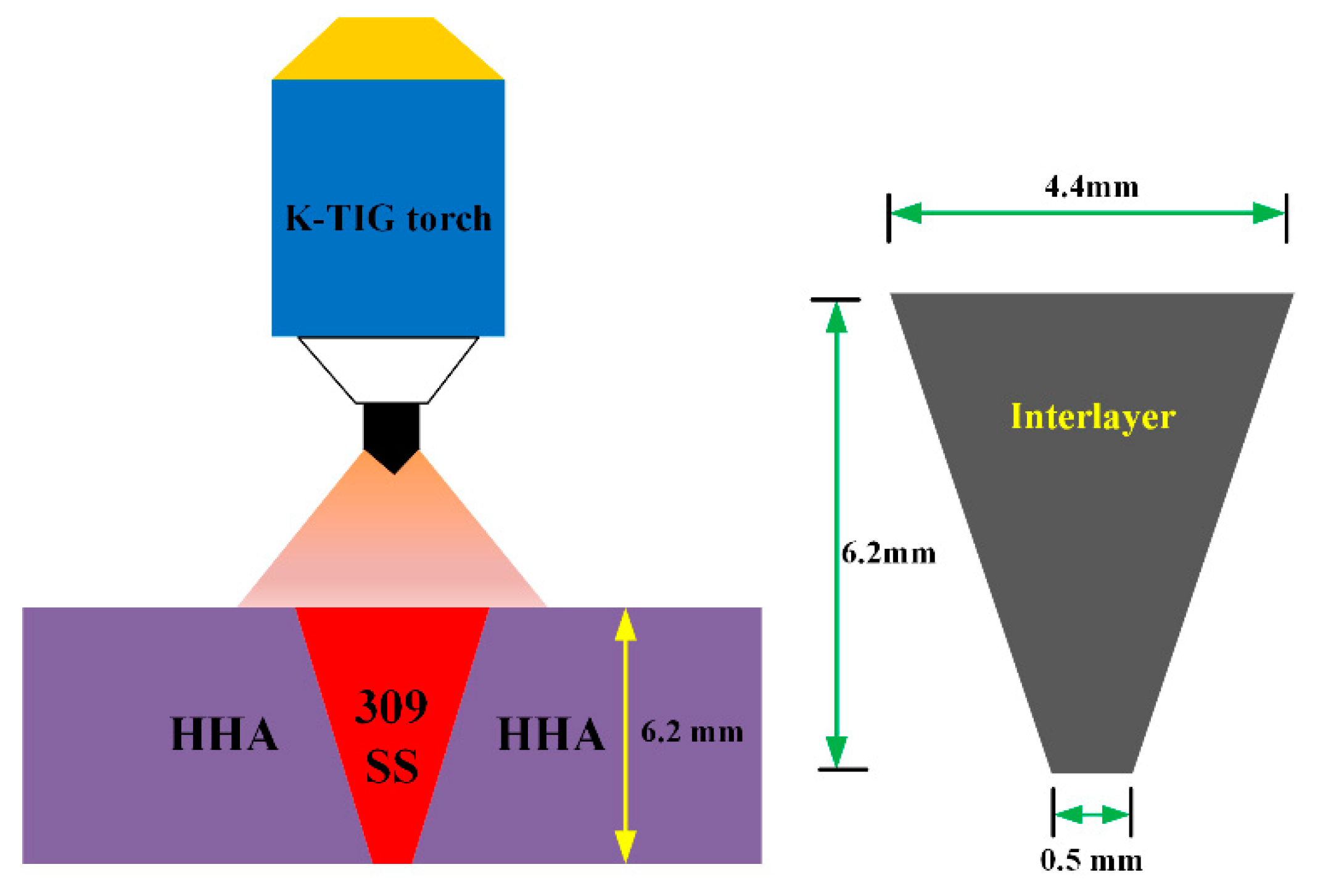
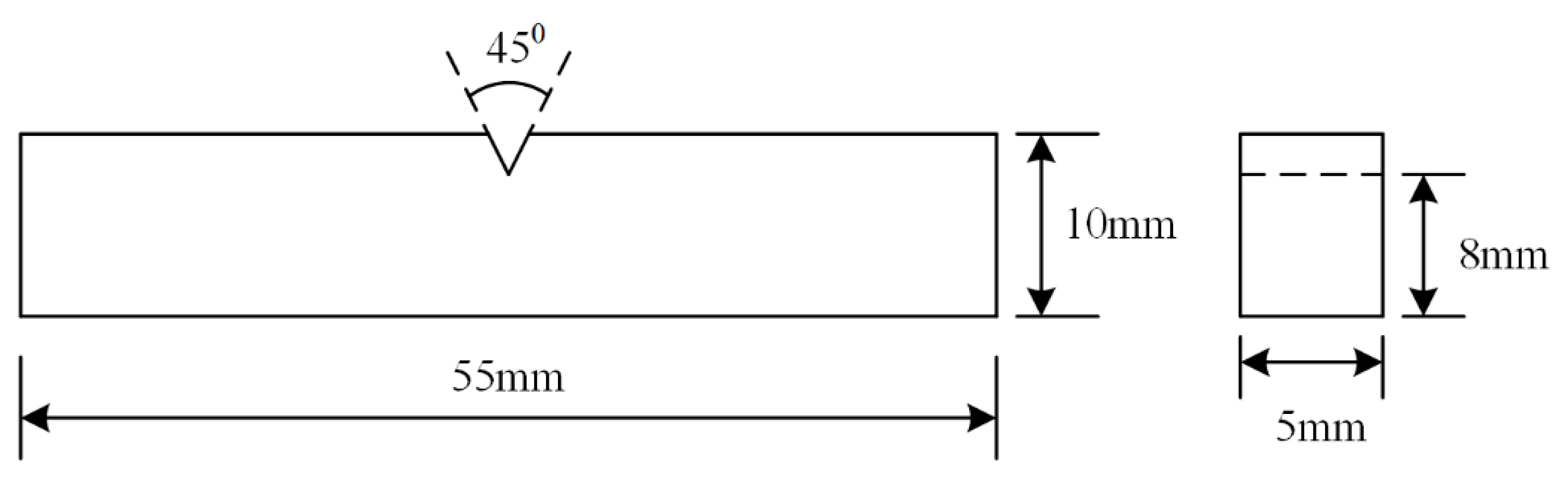
2. Materials and Methods
3. Results
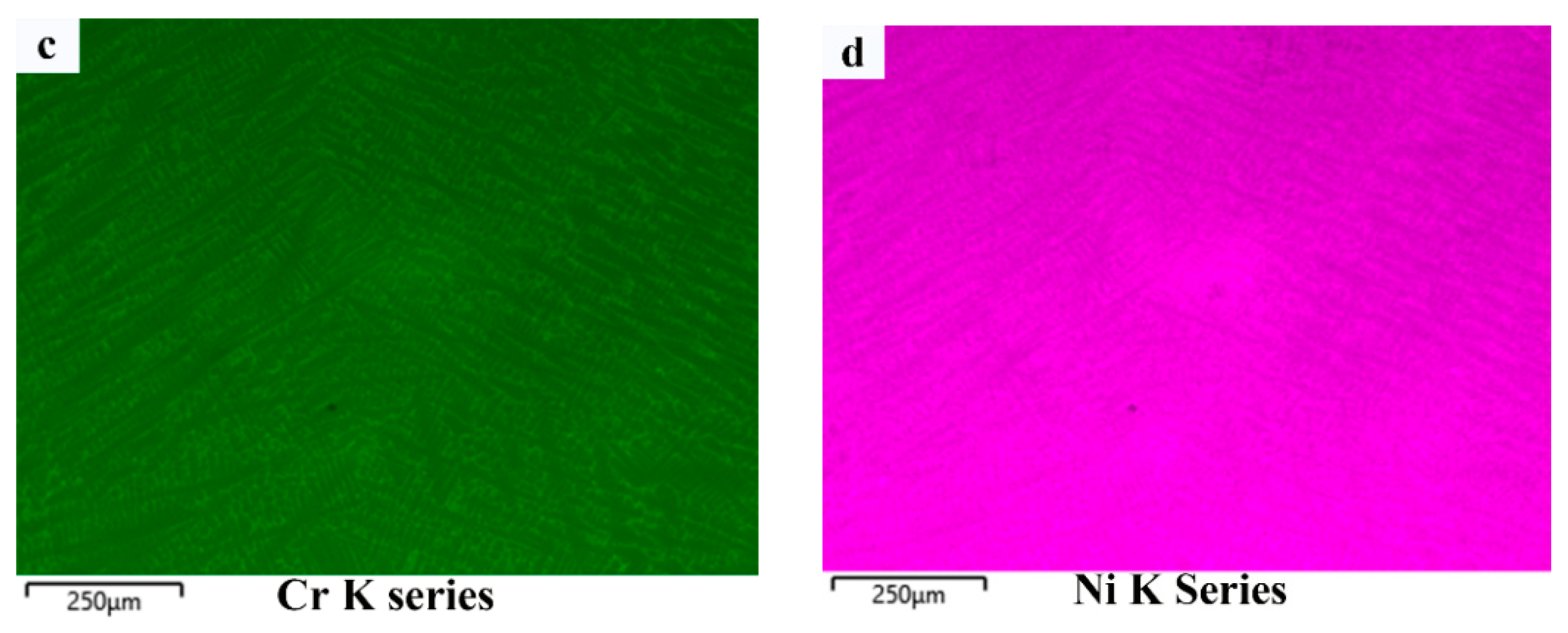
3.1. Macrostructure and Dilution
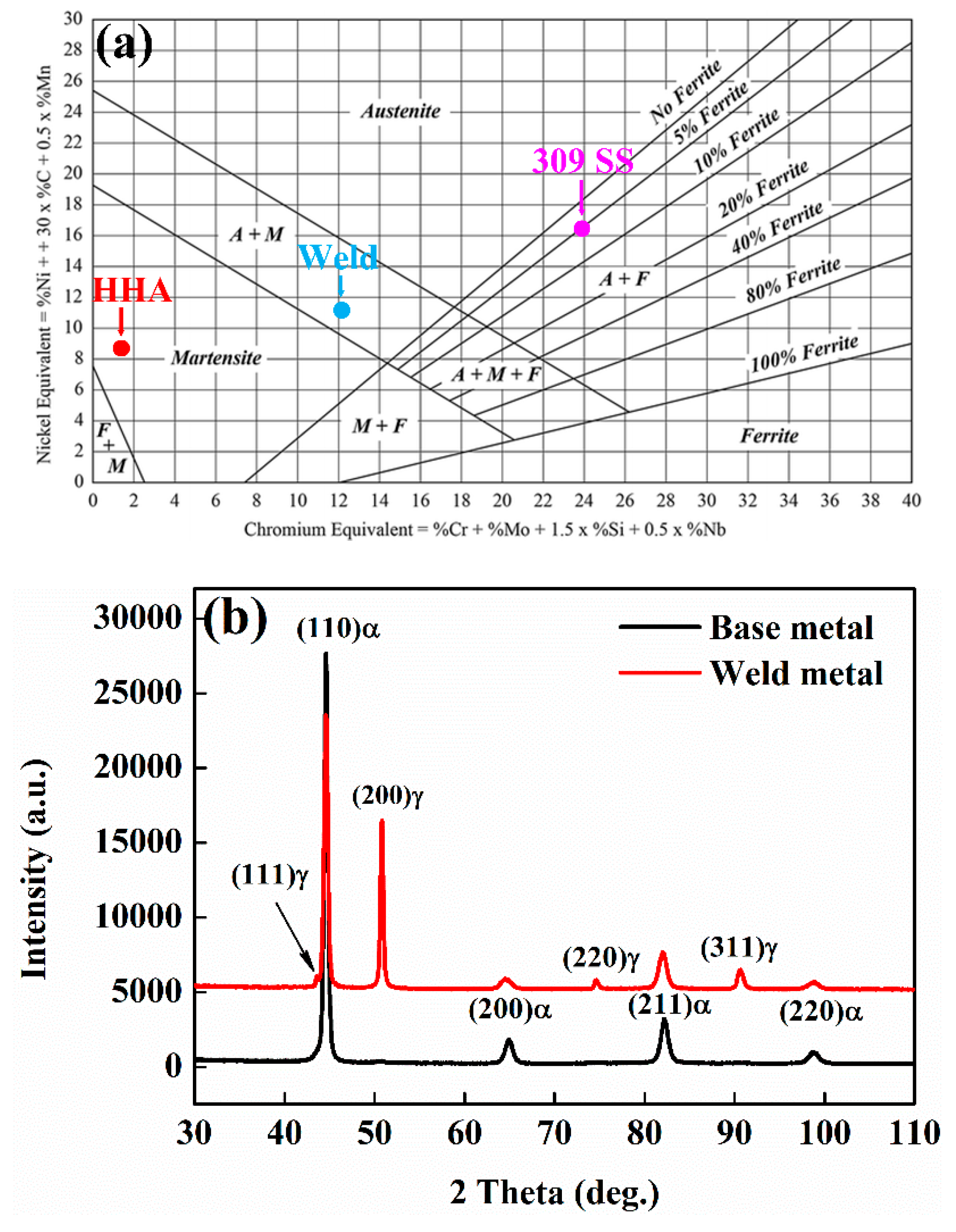
3.2. Phase Constituents
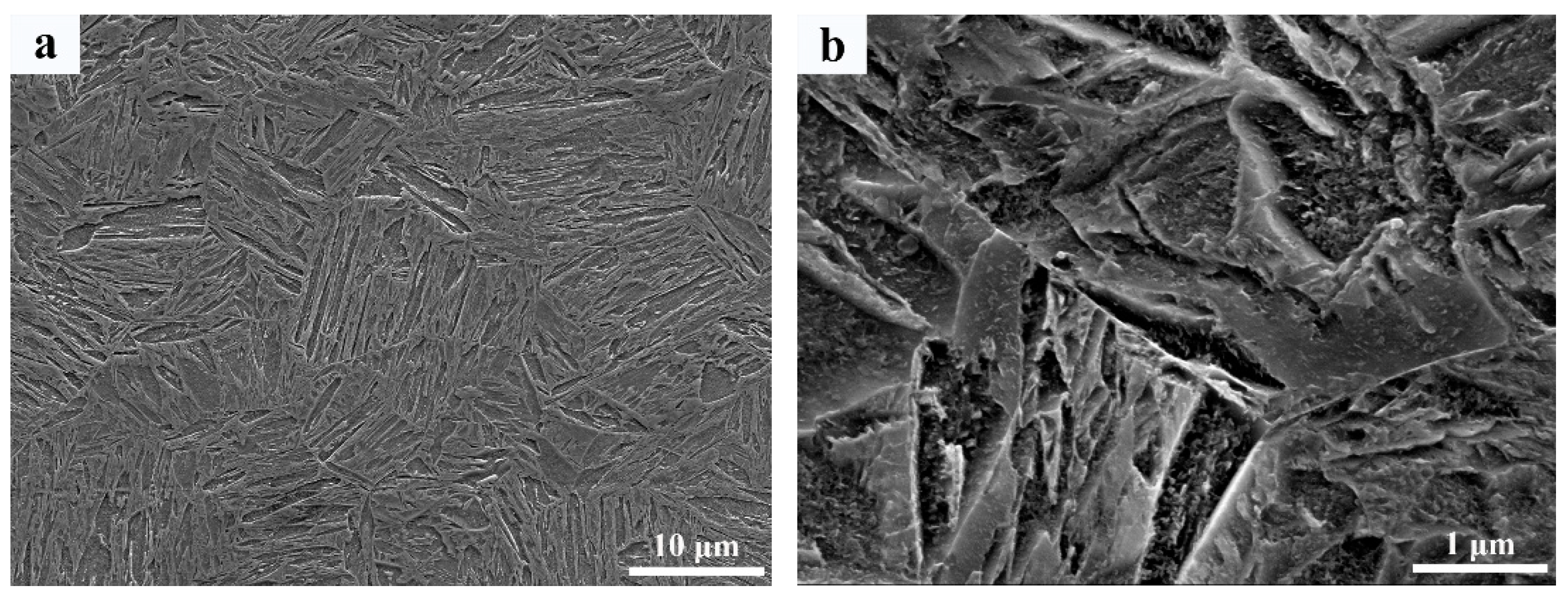
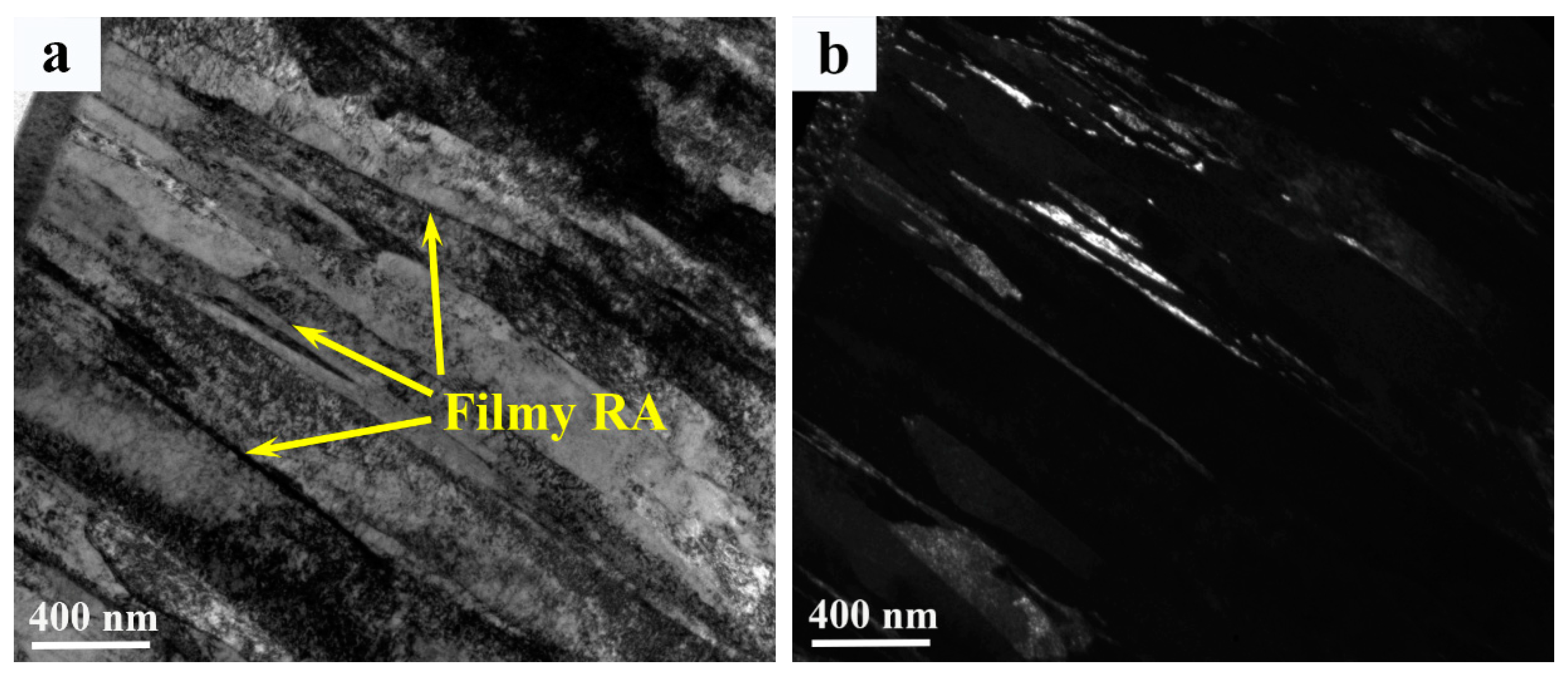
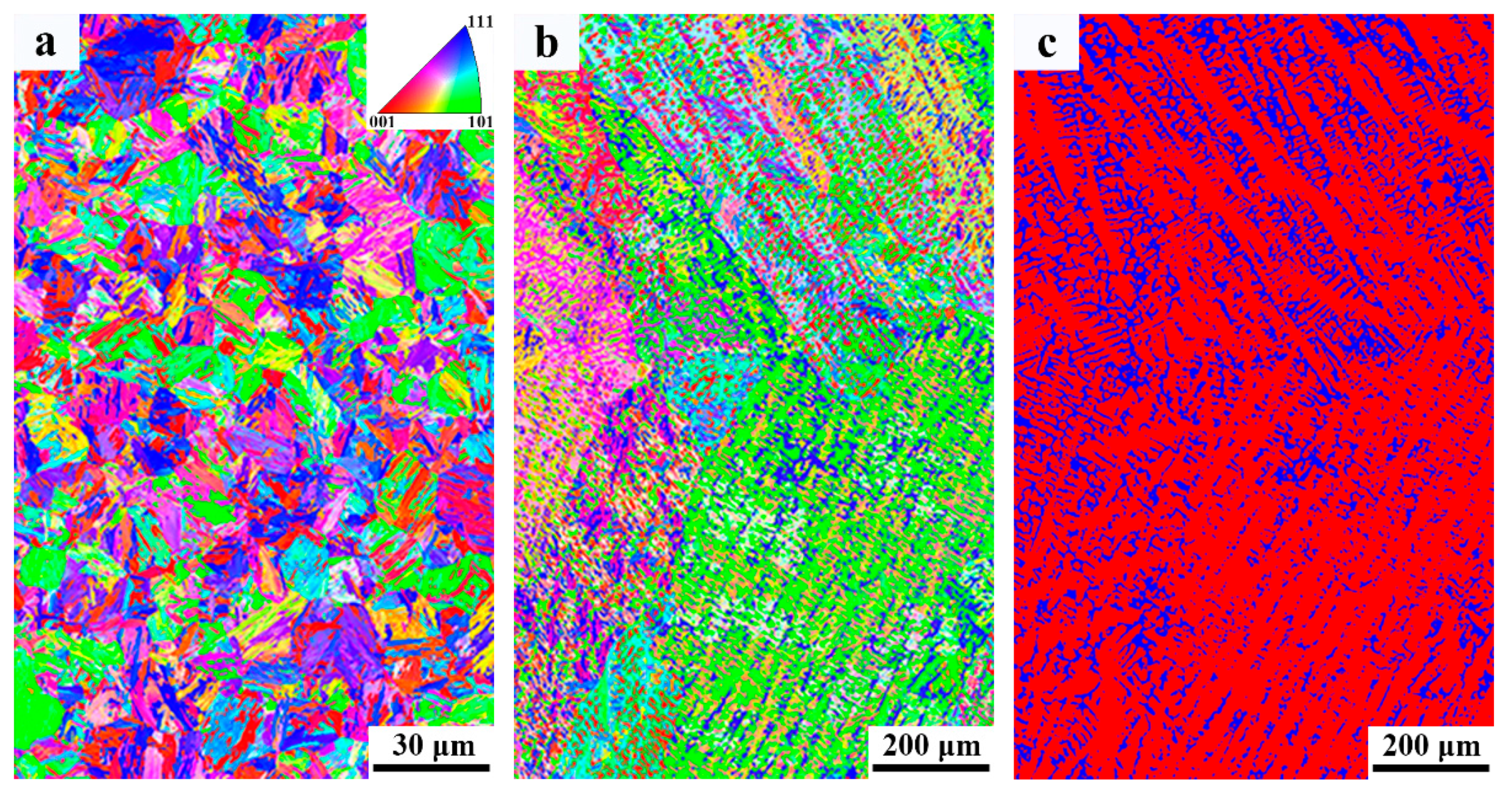
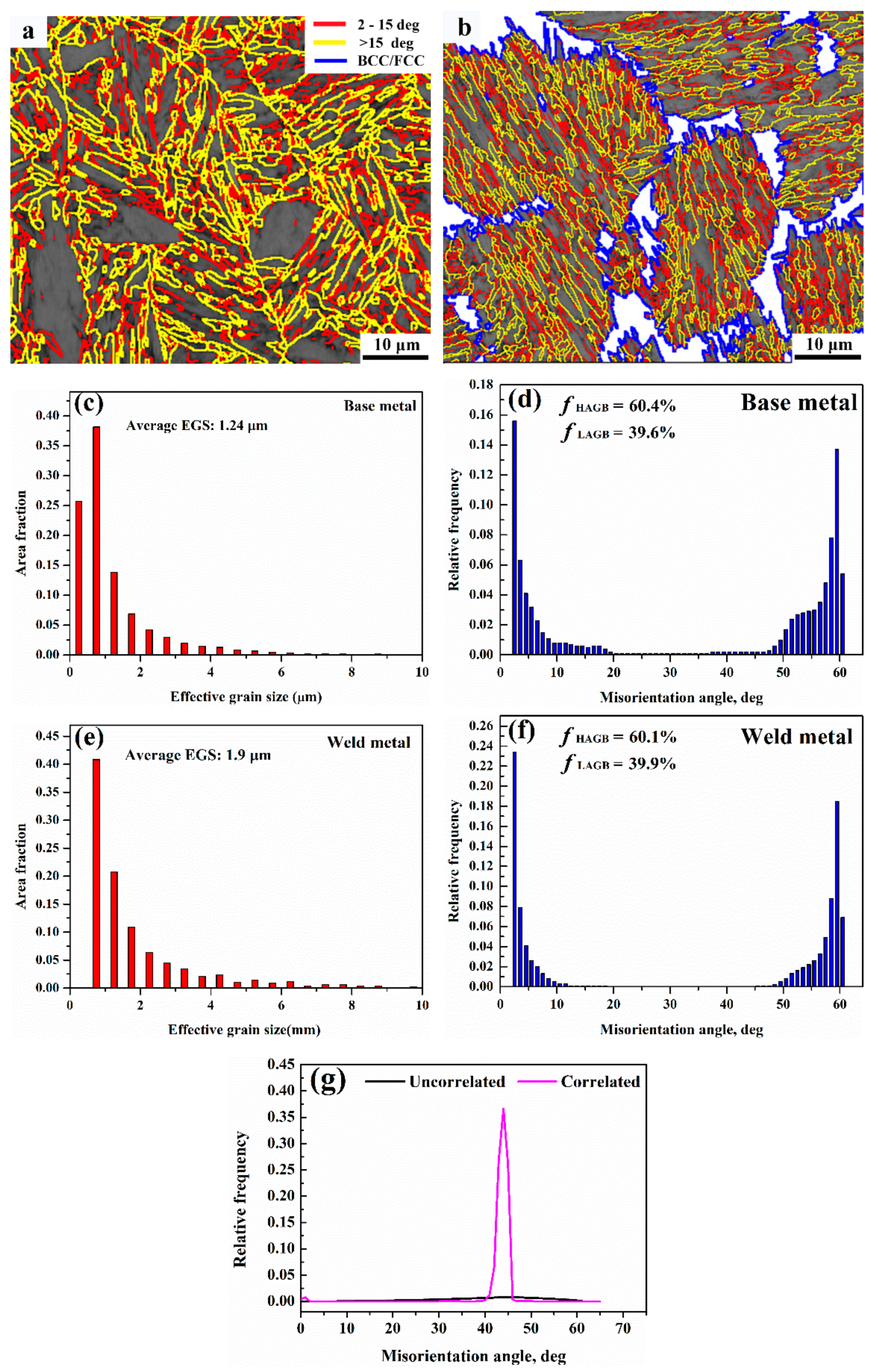
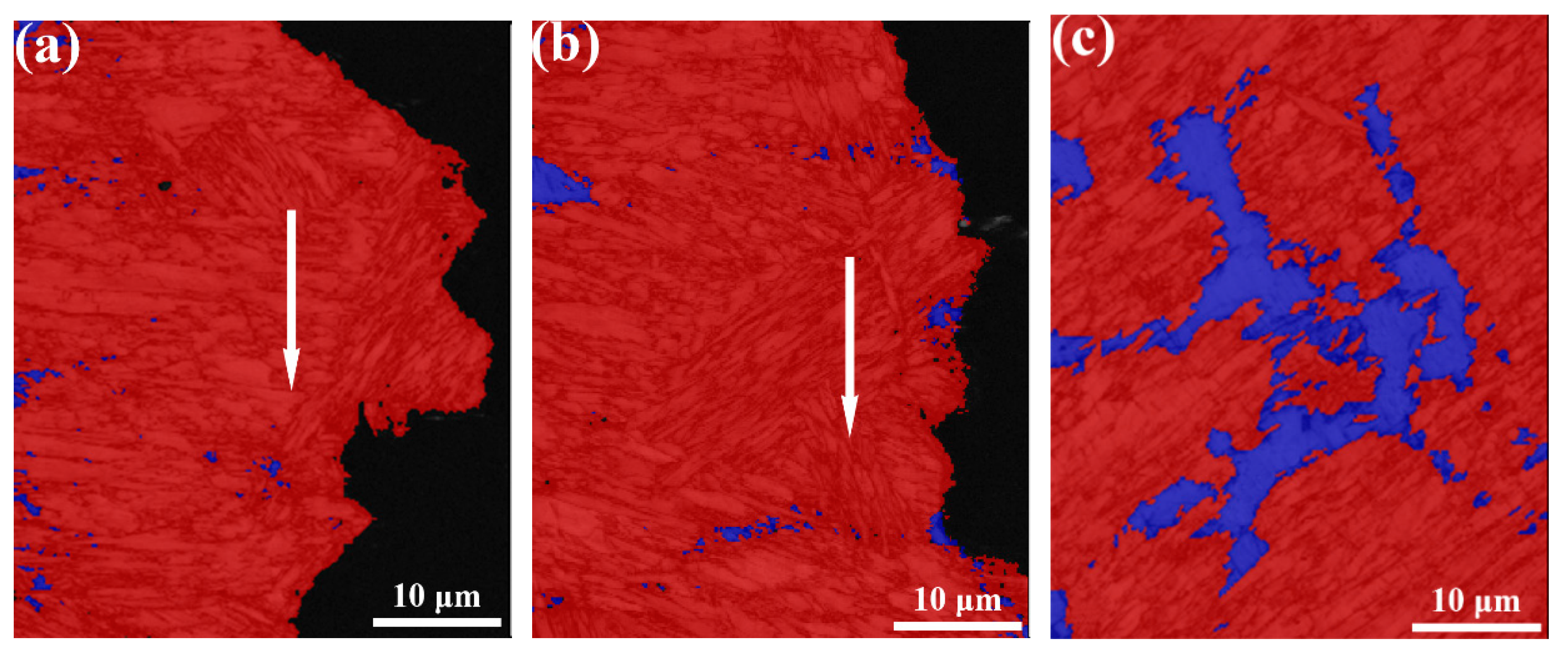
3.3. Microstructure
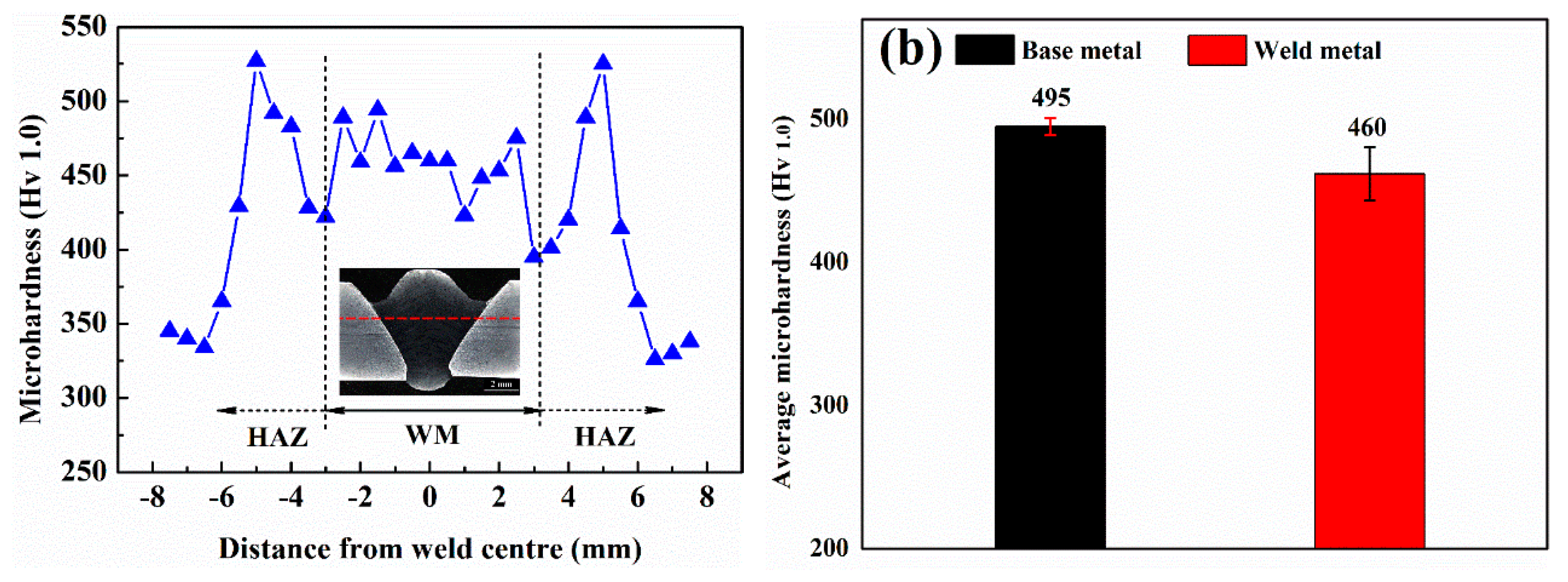
3.4. Hardness
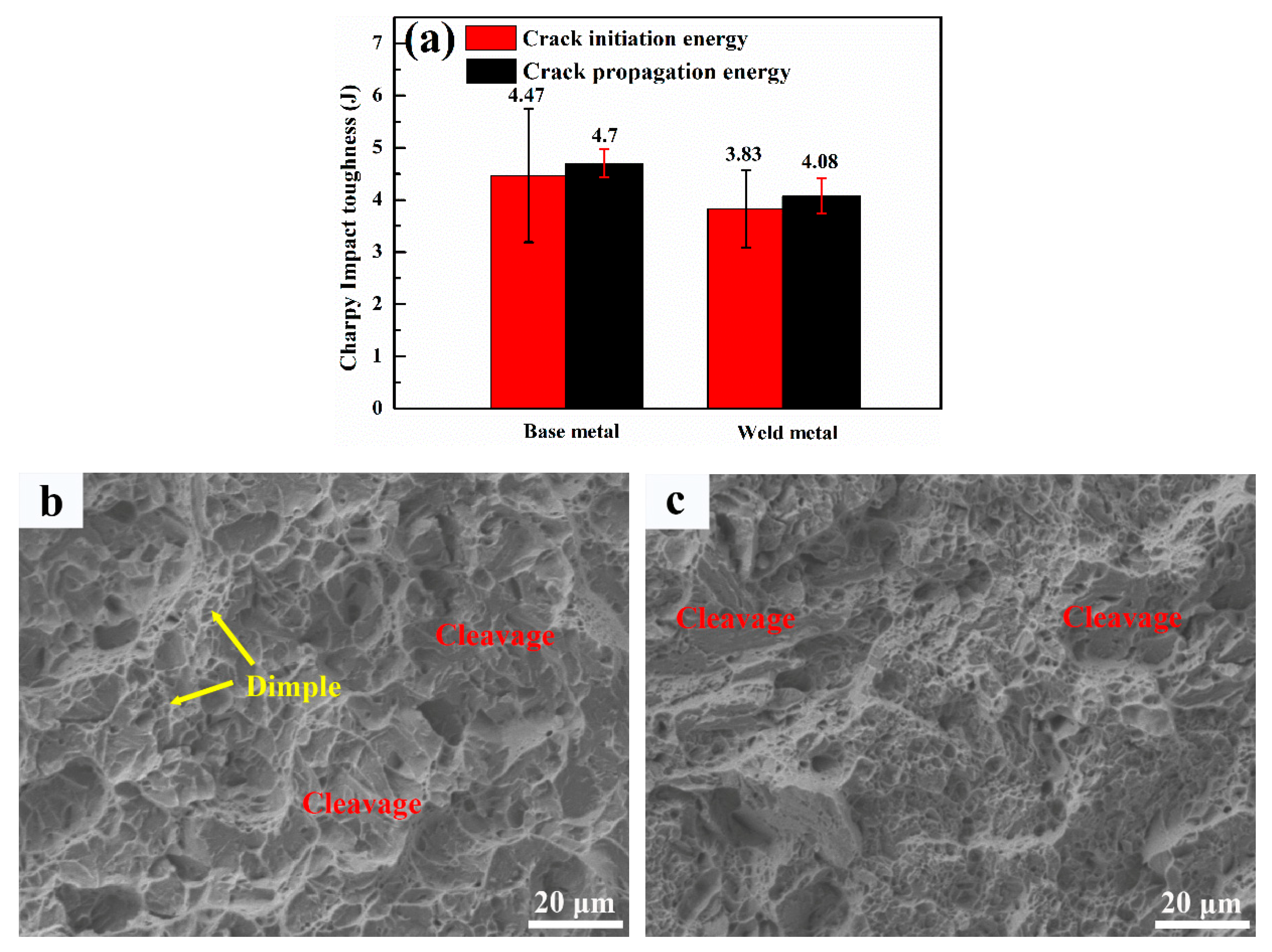
3.5. Impact Toughness
4. Discussion
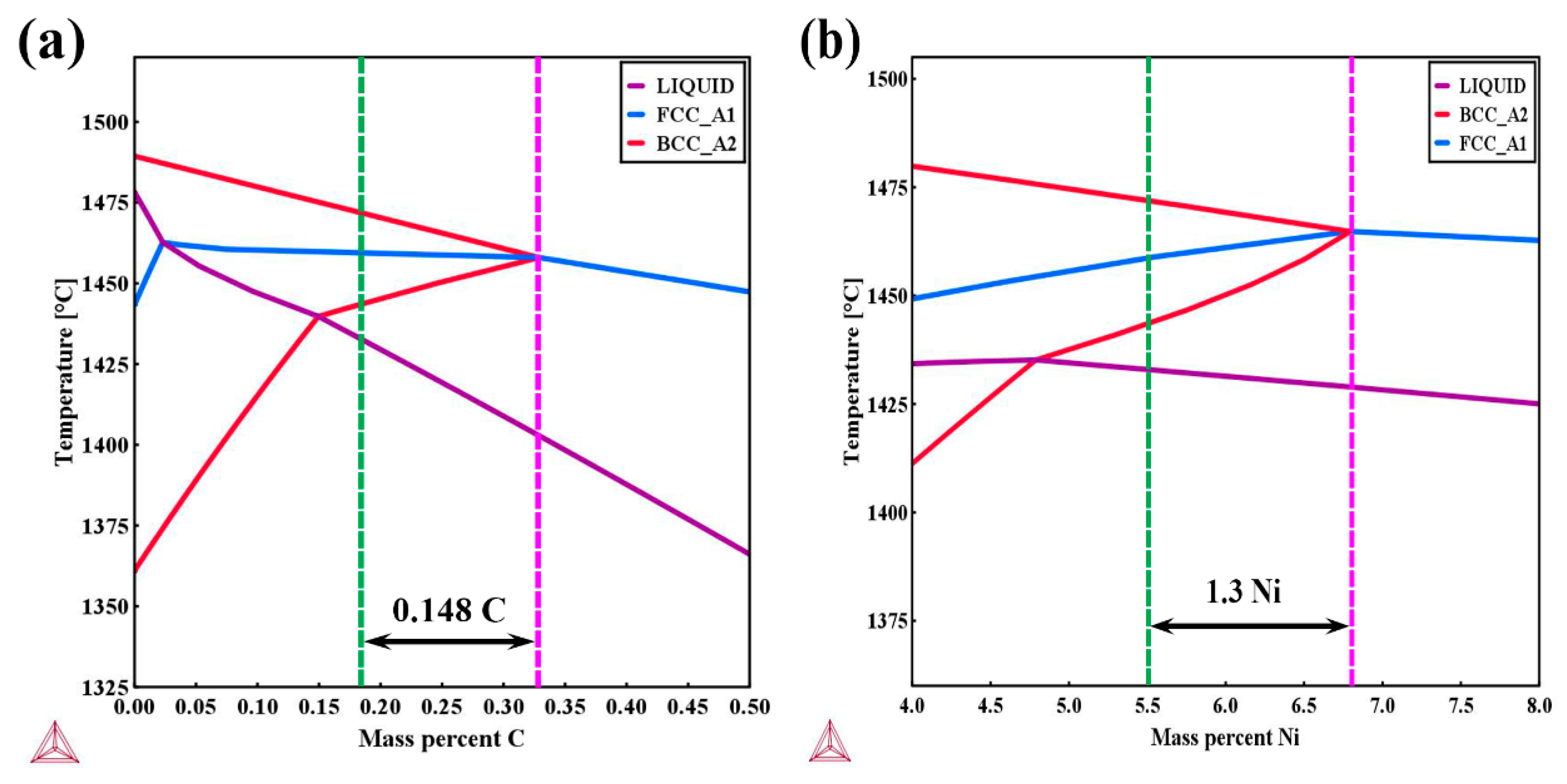
4.1. Solidification Sequence
4.2. Microstructure Evolution
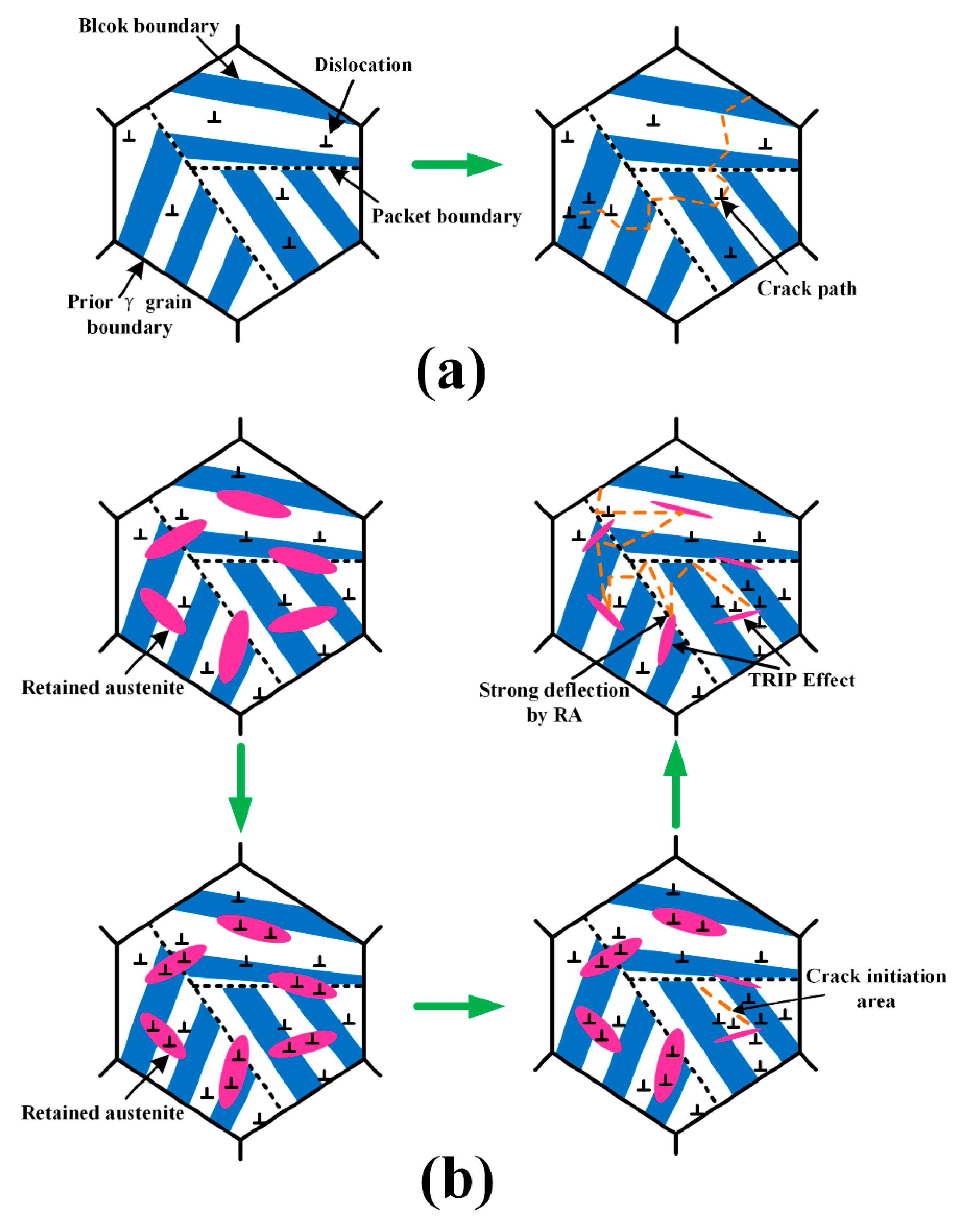
4.3. Hardening Mechanism
4.4. Toughening Mechanism
4.5. Application Potential
5. Conclusions
- Through the introduction of specially designed trapezoid interlayer in between HHA, up to a 43.5% dilution rate can be achieved, providing a very great scope for microstructure modification in the K-GTAW welding.
- By carefully tailoring the ratio of Creq to Nieq in the weld metal, favourable solidification sequence was obtained. The weld solidifies from liquid to delta ferrite first, followed by peritectic reaction from liquid plus delta ferrite to austenite, which has a very low risk of solidification cracking compared to the single austenitic solidification mode due to the reduction of enrichment of S and P impurities in the final liquid suppressing crack initiation and the formation of more irregular austenite/delta ferrite boundaries hindering crack propagation.
- The microstructure in the weld metal region contains martensite and retained a austenite dual phase because of the addition of large amounts of alloy elements, which lowers martensite transformation finish temperature to a level much lower than room temperature. No precipitates were detected in the weld metal due to the fast cooling rate and low rates of diffusion of alloying elements in the austenitic phase.
- The hardness of the weld metal reaches up to 460 Hv. Toughness of the weld is comparable to the base metal. The high dislocation density and solid solution provide sufficient strengthening in the weld, while DARA, TRIP effects, and crack deflection by retained austenite are the main reason for the toughness improvement in the weld metal.
- Due to the mitigation of the three main factors affecting HICC, this kind of chemical composition and microstructure combination shows great potential to produce high performance armour steel joints, while the K-GTAW welding technology provides an advanced solution for armour steel welding at the same time.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reddy, G.M.; Mohandas, T. Ballistic performance of high-strengh low-alloy steel weldments. J. Mater. Process. Technol. 1996, 57, 23–30. [Google Scholar] [CrossRef]
- Jena, P.; Mishra, B.; Rameshbabu, M.; Babu, A.; Singh, A.; Sivakumar, K.; Bhat, T.B. Effect of heat treatment on mechanical and ballistic properties of a high strength armour steel. Int. J. Impact Eng. 2010, 37, 242–249. [Google Scholar] [CrossRef]
- Woodward, R. Materials for projectile disruption. Mater. Forum 1998, 12, 26–30. [Google Scholar]
- Magudeeswaran, G.; Balasubramanian, V.; Madhusudhan Reddy, G. Effect of welding consumables on hydrogen induced cracking of armour grade quenched and tempered steel welds. Ironmak. Steelmak. 2008, 35, 549–560. [Google Scholar] [CrossRef]
- Magudeeswaran, G.; Balasubramanian, V.; Reddy, G.M. Cold cracking of flux cored arc welded armour grade high strength steel weldments. J. Mater. Sci. Technol. 2009, 25, 516–526. [Google Scholar]
- Reddy, G.M.; Mohandas, T.; Papukutty, K. Effect of welding process on the ballistic performance of high-strength low-alloy steel weldments. J. Mater. Process. Technol. 1998, 74, 27–35. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Balasubramanian, V.; Reddy, G.M. Effect of joint design on ballistic performance of quenched and tempered steel welded joints. Mater. Des. 2014, 54, 616–623. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Balasubramanian, V.; Reddy, G.M. Effect of hardfaced interlayer thickness on ballistic performance of armour steel welds. Mater. Des. 2013, 44, 59–68. [Google Scholar] [CrossRef]
- Maweja, K.; Stumpf, W. The design of advanced performance high strength low-carbon martensitic armour steels: Microstructural considerations. Mater. Sci. Eng. A 2008, 480, 160–166. [Google Scholar] [CrossRef]
- Murthy, N.K.; Ram, G.D.J.; Murty, B.S.; Reddy, G.M.; Rao, T.J.P. Carbide-Free Bainitic Weld Metal: A New Concept in Welding of Armor Steels. Metall. Mater. Trans. B 2014, 45, 2327–2337. [Google Scholar] [CrossRef]
- Pramanick, A.; Das, H.; Reddy, G.; Ghosh, M.; Nandy, S.; Pal, T. Effect of Bainitic Microstructure on Ballistic Performance of Armour Steel Weld Metal Using Developed High Ni-Coated Electrode. J. Mater. Eng. Perform. 2018, 27, 2110–2123. [Google Scholar] [CrossRef]
- Luo, C.; Cao, Y.; Zhao, Y.; Zhao, L.; Shan, J. Fiber Laser Welding of 1700-MPa, Ultrahigh-Strength Steel. Weld. J. 2018, 97, 214s–228s. [Google Scholar]
- Fei, Z.Y.; Pan, Z.X.; Cuiuri, D.; Li, H.J.; Wu, B.T.; Ding, D.H.; Su, L.H.; Gazder, A.A. Investigation into the viability of K-TIG for joining armour grade quenched and tempered steel. J. Manuf. Process. 2018, 32, 482–493. [Google Scholar] [CrossRef]
- Lathabai, S.; Jarvis, B.L.; Barton, K.J. Comparison of keyhole and conventional gas tungsten arc welds in commercially pure titanium. Mater. Sci. Eng. A 2001, 299, 81–93. [Google Scholar] [CrossRef]
- Fei, Z.Y.; Pan, Z.X.; Cuiuri, D.; Li, H.J.; Wu, B.T.; Su, L.H. Improving the weld microstructure and material properties of K-TIG welded armour steel joint using filler material. Int. J. Adv. Manuf. Technol. 2019, 100, 1931–1944. [Google Scholar] [CrossRef]
- Kuzmikova, L. An Investigation of the Weldability of High Hardness Armour Steels. Ph.D. Thesis, University of Wollongong, Wollongong, Australia, 2013. [Google Scholar]
- Cui, S.L.; Liu, Z.M.; Fang, Y.Q.; Luo, Z.; Manladan, S.M.; Yi, S. Keyhole process in K-TIG welding on 4 mm thick 304 stainless steel. J. Mater. Process. Technol. 2017, 243, 217–228. [Google Scholar] [CrossRef]
- Long, S.-L.; Liang, Y.-L.; Jiang, Y.; Liang, Y.; Yang, M.; Yi, Y.-L. Effect of quenching temperature on martensite multi-level microstructures and properties of strength and toughness in 20CrNi2Mo steel. Mater. Sci. Eng. A 2016, 676, 38–47. [Google Scholar] [CrossRef]
- Kujanpää, V.; David, S.; White, C. Formation of hot cracks in austenitic stainless steel welds–solidification cracking. Weld. J. 1986, 65, 203s–212s. [Google Scholar]
- Sourmail, T.; Smanio, V. Low temperature kinetics of bainite formation in high carbon steels. Acta Mater. 2013, 61, 2639–2648. [Google Scholar] [CrossRef]
- Song, Y.; Ping, D.; Yin, F.; Li, X.; Li, Y. Microstructural evolution and low temperature impact toughness of a Fe–13% Cr–4% Ni–Mo martensitic stainless steel. Mater. Sci. Eng. A 2010, 527, 614–618. [Google Scholar] [CrossRef]
- Michiuchi, M.; Nambu, S.; Ishimoto, Y.; Inoue, J.; Koseki, T. Relationship between local deformation behavior and crystallographic features of as-quenched lath martensite during uniaxial tensile deformation. Acta Mater. 2009, 57, 5283–5291. [Google Scholar] [CrossRef]
- Takebayashi, S.; Kunieda, T.; Yoshinaga, N.; Ushioda, K.; Ogata, S. Comparison of the dislocation density in martensitic steels evaluated by some X-ray diffraction methods. ISIJ Int. 2010, 50, 875–882. [Google Scholar] [CrossRef]
- Hutchinson, B.; Hagström, J.; Karlsson, O.; Lindell, D.; Tornberg, M.; Lindberg, F.; Thuvander, M. Microstructures and hardness of as-quenched martensites (0.1–0.5% C). Acta Mater. 2011, 59, 5845–5858. [Google Scholar] [CrossRef]
- Macek, K.; Lukáš, P.; Janovec, J.; Mikula, P.; Strunz, P.; Vrána, M.; Zaffagnini, M. Austenite content and dislocation density in electron-beam welds of a stainless maraging steel. Mater. Sci. Eng. A 1996, 208, 131–138. [Google Scholar] [CrossRef]
- Zhou, T.; Yu, H.; Wang, S. Effect of microstructural types on toughness and microstructural optimization of ultra-heavy steel plate: EBSD analysis and microscopic fracture mechanism. Mater. Sci. Eng. A 2016, 658, 150–158. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Shi, J.; Hui, W.; Dong, H. Effect of microstructural refinement on the toughness of low carbon martensitic steel. Scr. Mater. 2008, 58, 492–495. [Google Scholar] [CrossRef]
- Ma, X.; Wang, L.; Liu, C.; Subramanian, S. Microstructure and properties of 13Cr5Ni1Mo0. 025Nb0. 09V0. 06N super martensitic stainless steel. Mater. Sci. Eng. A 2012, 539, 271–279. [Google Scholar] [CrossRef]
- Morsdorf, L.; Jeannin, O.; Barbier, D.; Mitsuhara, M.; Raabe, D.; Tasan, C.C. Multiple mechanisms of lath martensite plasticity. Acta Mater. 2016, 121, 202–214. [Google Scholar] [CrossRef]
- Krauss, G. Martensite in steel: Strength and structure. Mater. Sci. Eng. A 1999, 273, 40–57. [Google Scholar] [CrossRef]
- Lambert-Perlade, A.; Gourgues, A.-F.; Besson, J.; Sturel, T.; Pineau, A. Mechanisms and modeling of cleavage fracture in simulated heat-affected zone microstructures of a high-strength low alloy steel. Metall. Mater. Trans. A 2004, 35, 1039–1053. [Google Scholar] [CrossRef]
- Hu, J.; Du, L.-X.; Wang, J.-J.; Gao, C.-R. Effect of welding heat input on microstructures and toughness in simulated CGHAZ of V–N high strength steel. Mater. Sci. Eng. A 2013, 577, 161–168. [Google Scholar] [CrossRef]
- Wu, S.; Wang, D.; Zhang, Z.; Li, C.; Liu, X.; Meng, X.; Feng, Z.; Di, X. Mechanical properties of low-transformation-temperature weld metals after low-temperature postweld heat treatment. Sci. Technol. Weld. Join. 2019, 24, 112–120. [Google Scholar] [CrossRef]
- Ooi, S.; Garnham, J.; Ramjaun, T. Low transformation temperature weld filler for tensile residual stress reduction. Mater. Des. 2014, 56, 773–781. [Google Scholar] [CrossRef]





| Materials | C | Si | Mn | P | S | Ni | Cr | Mo | Fe |
|---|---|---|---|---|---|---|---|---|---|
| HHA | 0.27 | 0.3 | 0.3 | 0.014 | 0.0025 | 0.19 | 1.05 | 0.25 | Bal. |
| 3 09 | 0.07 | 0.8 | 1.4 | 0.02 | 0.005 | 13.4 | 22.7 | N/A | Bal. |
| Process Parameters | Details |
|---|---|
| Peak current, A | 550 |
| Base current, A | 230 |
| Duty cycle | 50% |
| Pulse frequency, Hz | 5 |
| Travel speed, mm/min | 350 |
| Shielding gas | Pure argon |
| Shielding gas flow rate, L/min | 25 |
| Back purging gas | Pure argon |
| Purging gas flow rate, L/min | 5 |
| Arc length, mm | 1 |
| Operation mode | DCEN |
| Element | C | Si | Mn | P | S | Ni | Cr | Mo | Fe | Dilution (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| wt% | 0.183 | 0.52 | 0.78 | 0.0166 | 0.0036 | 5.5 | 11.4 | 0.141 | Bal. | 43.54 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fei, Z.; Pan, Z.; Cuiuri, D.; Li, H.; Gazder, A.A. A Combination of Keyhole GTAW with a Trapezoidal Interlayer: A New Insight into Armour Steel Welding. Materials 2019, 12, 3571. https://doi.org/10.3390/ma12213571
Fei Z, Pan Z, Cuiuri D, Li H, Gazder AA. A Combination of Keyhole GTAW with a Trapezoidal Interlayer: A New Insight into Armour Steel Welding. Materials. 2019; 12(21):3571. https://doi.org/10.3390/ma12213571
Chicago/Turabian StyleFei, Zhenyu, Zengxi Pan, Dominic Cuiuri, Huijun Li, and Azdiar A. Gazder. 2019. "A Combination of Keyhole GTAW with a Trapezoidal Interlayer: A New Insight into Armour Steel Welding" Materials 12, no. 21: 3571. https://doi.org/10.3390/ma12213571
APA StyleFei, Z., Pan, Z., Cuiuri, D., Li, H., & Gazder, A. A. (2019). A Combination of Keyhole GTAW with a Trapezoidal Interlayer: A New Insight into Armour Steel Welding. Materials, 12(21), 3571. https://doi.org/10.3390/ma12213571






