Performance of Halloysite-Mg/Al LDH Materials for Aqueous As(V) and Cr(VI) Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of LDH and Halloysite-LDH Materials
2.3. Adsorption Experiments
2.4. Analytical Methods for Solid Samples
3. Results and Discussion
3.1. Characterization of Adsorbents
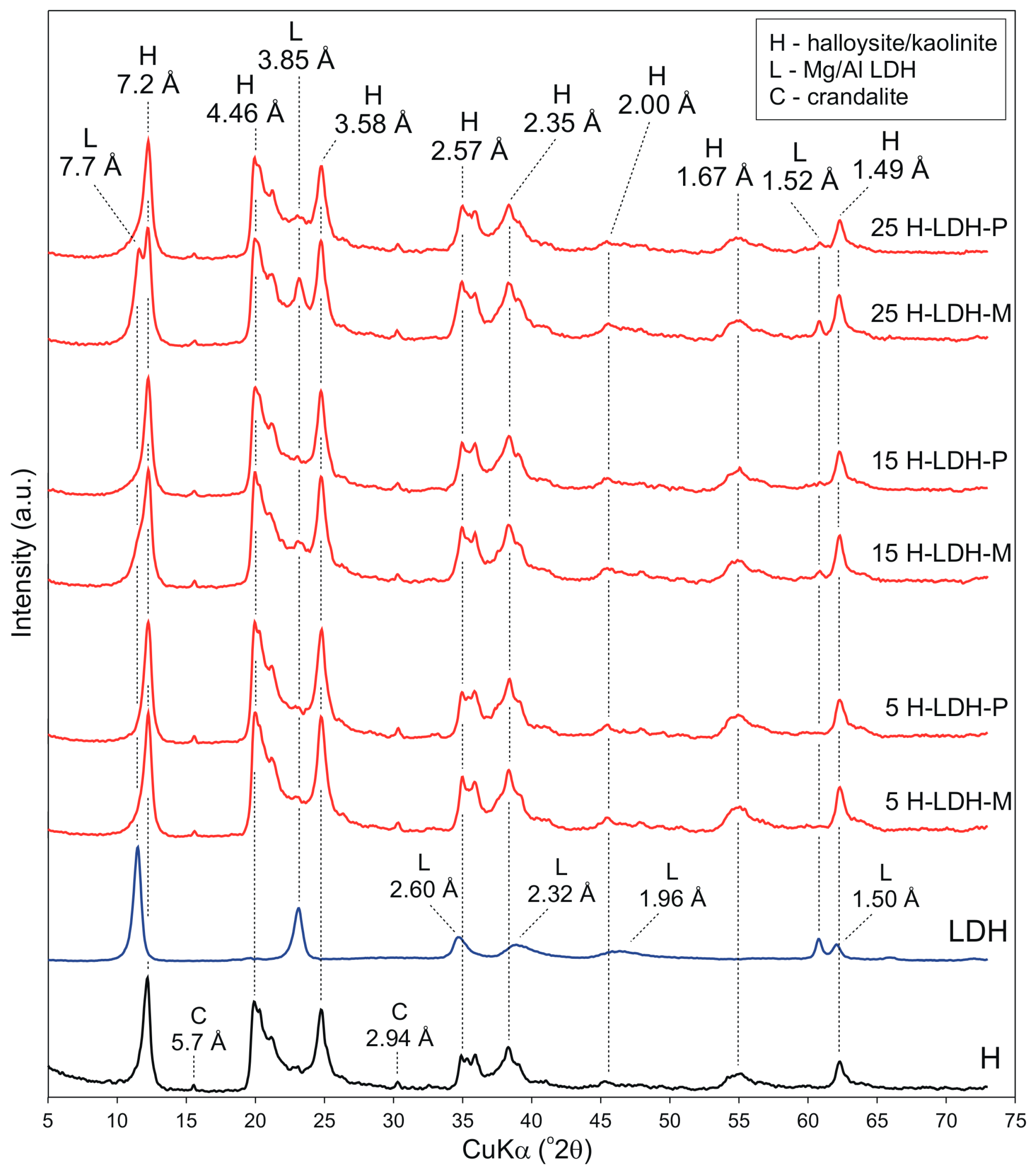
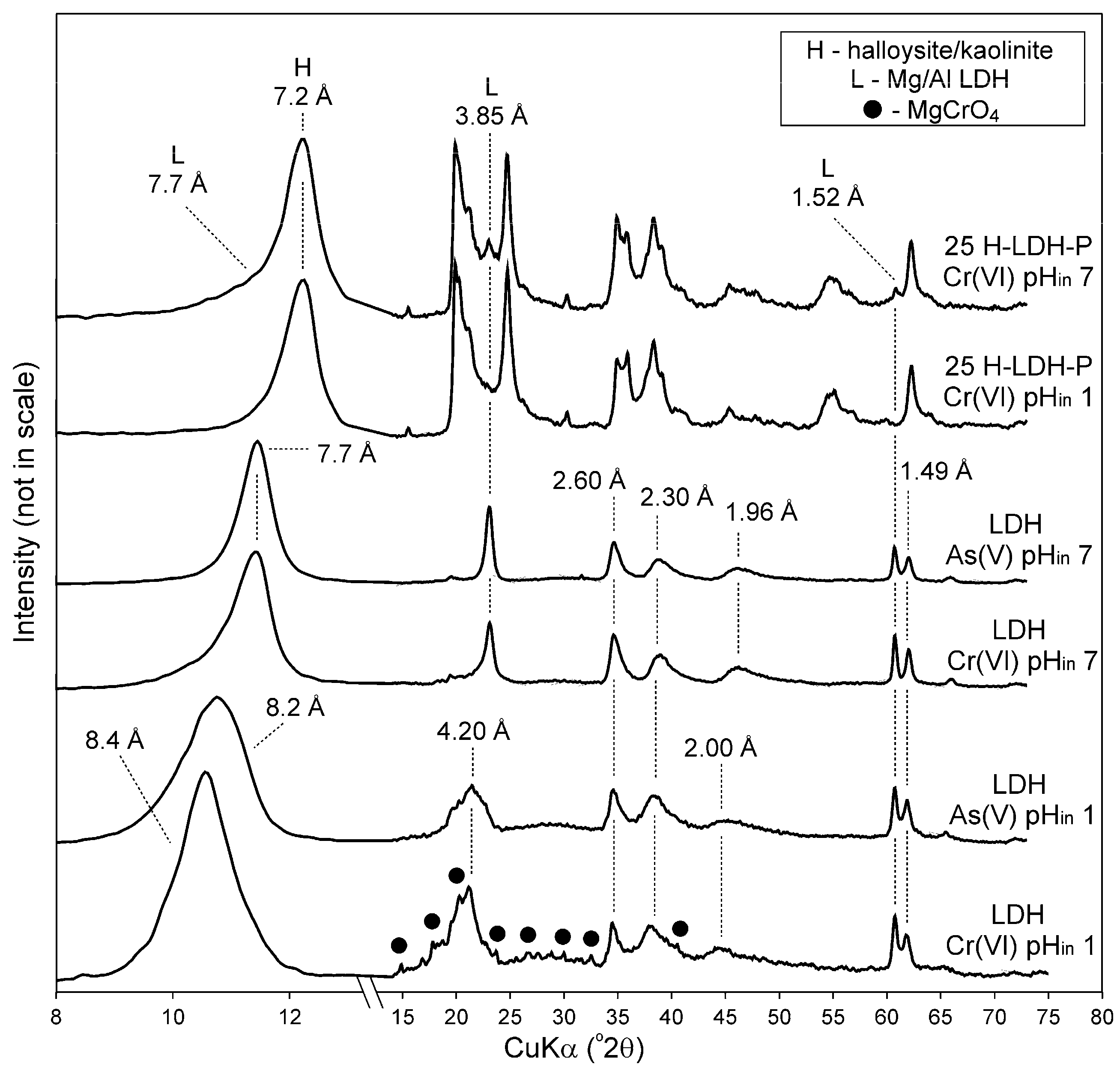
3.1.1. XRD Results
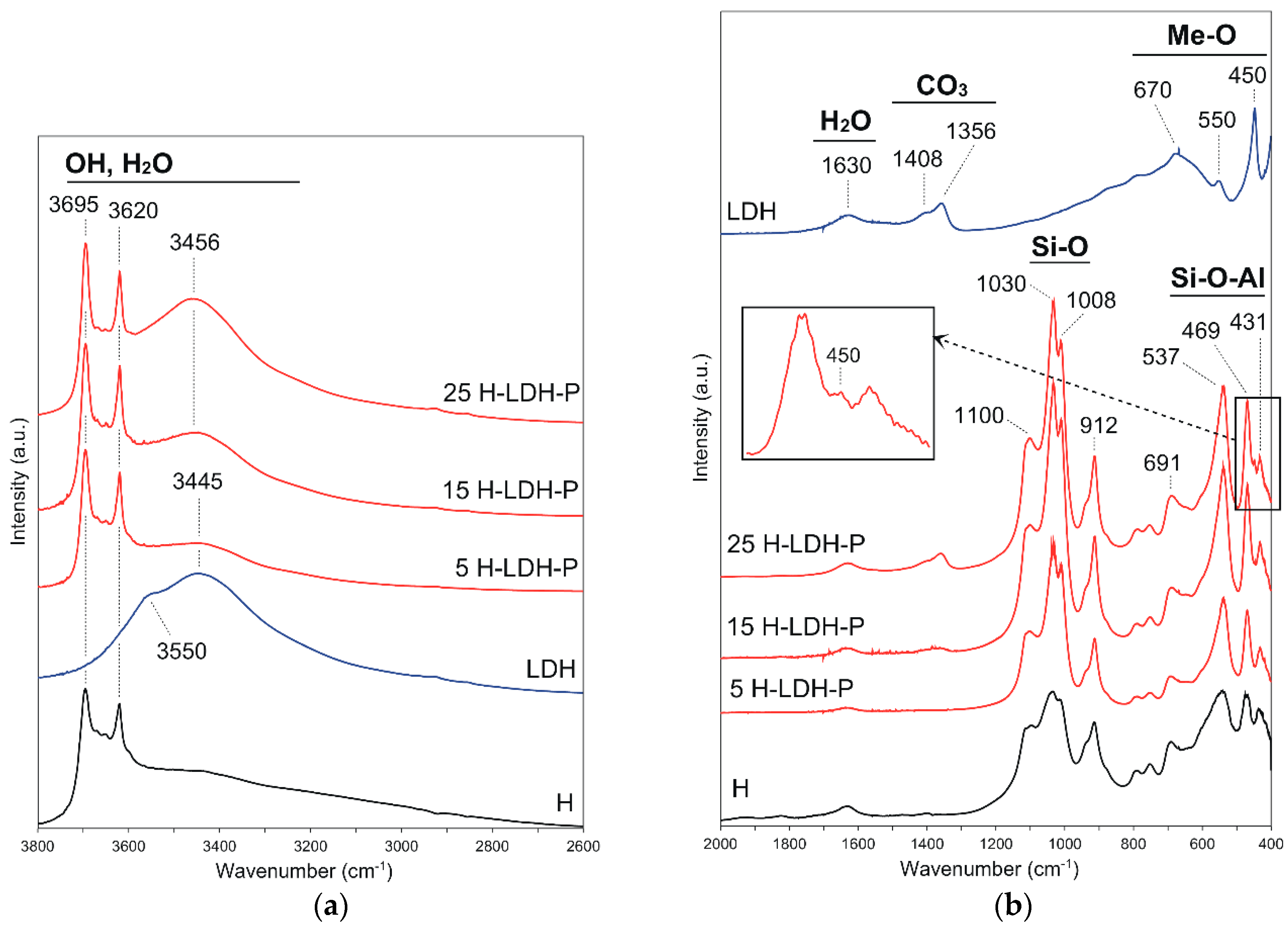
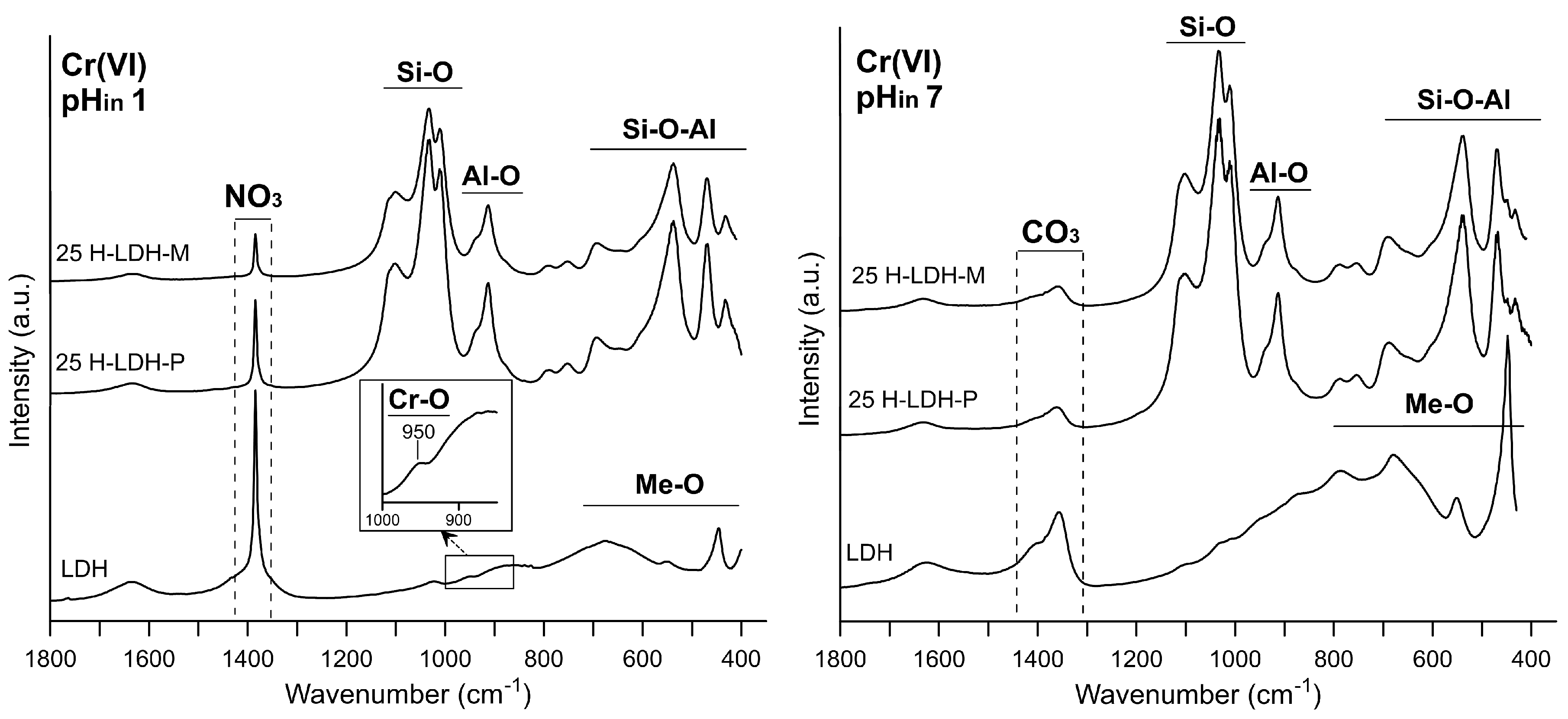
3.1.2. FTIR Results
3.1.3. SEM Results
3.1.4. DTA Results
3.2. Adsorption Experiments
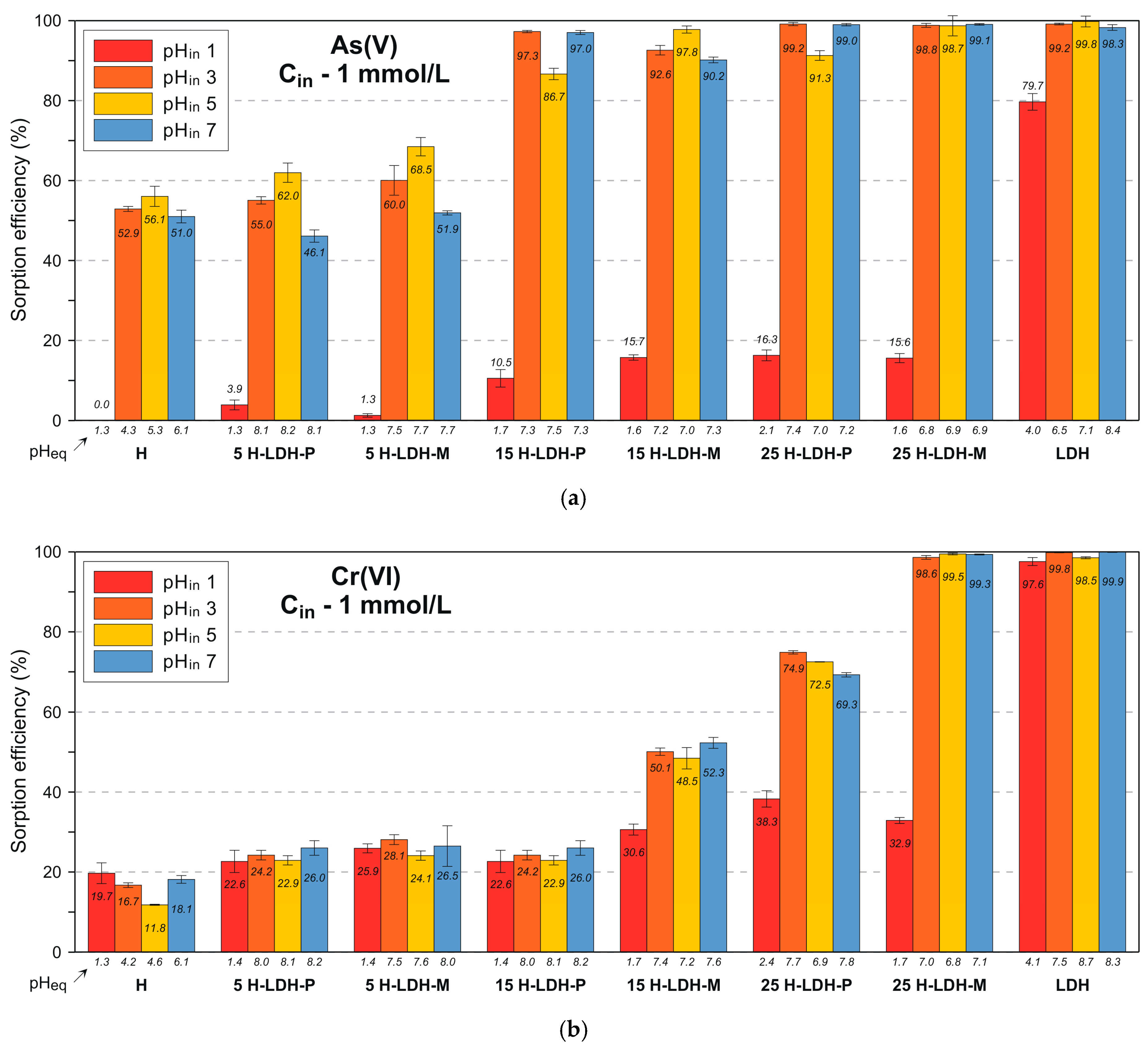
3.2.1. As(V) Removal
3.2.2. Cr(VI) Removal
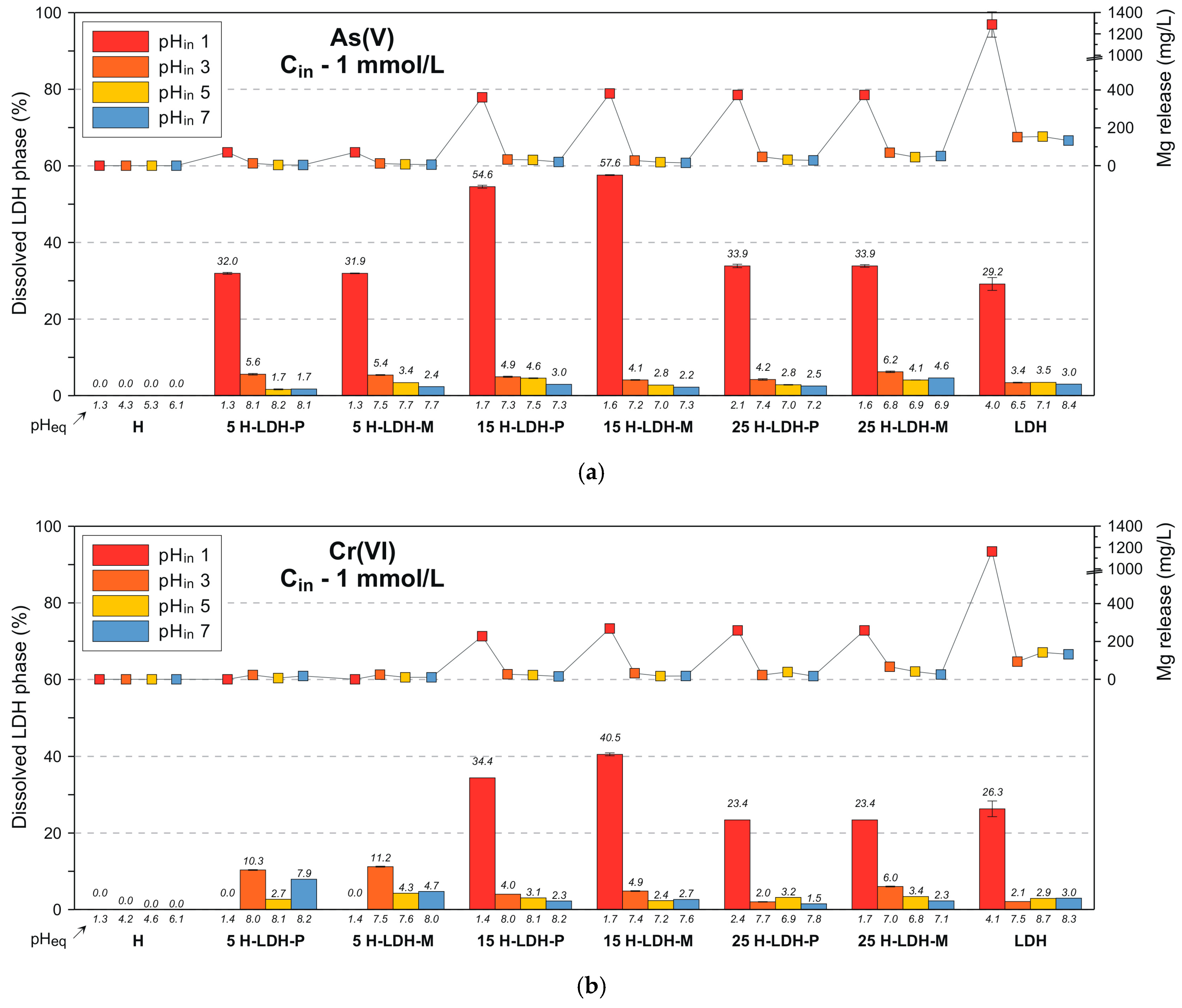
3.3. Solid State Analysis after Adsorption and Insight into Removal Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Maziarz, P.; Matusik, J.; Leiviskä, T.; Strączek, T.; Kapusta, C.; Marek Woch, W.; Tokarz, W.; Górniak, K. Toward highly effective and easily separable halloysite-containing adsorbents: The effect of iron oxide particles impregnation and new insight into As(V) removal mechanisms. Sep. Purif. Technol. 2019, 210, 390–401. [Google Scholar] [CrossRef]
- Schmidt, S.A.; Gukelberger, E.; Hermann, M.; Fiedler, F.; Grossmann, B.; Hoinkis, J.; Ghosh, A.; Chatterjee, D.; Bundschuh, J. Pilot study on arsenic removal from groundwater using a small-scale reverse osmosis system-Towards sustainable drinking water production. J. Hazard Mater. 2016, 318, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Wang, N.; Wang, C.; Li, G. Arsenic removal from water and river water by the combined adsorption - UF membrane process. Chemosphere 2018, 202, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Leiviskä, T.; Matusik, J.; Muir, B.; Tanskanen, J. Vanadium removal by organo-zeolites and iron-based products from contaminated natural water. J. Clean. Prod. 2017, 167, 589–600. [Google Scholar] [CrossRef]
- Folens, K.; Huysman, S.; Van Hulle, S.; Du Laing, G. Chemical and economic optimization of the coagulation-flocculation process for silver removal and recovery from industrial wastewater. Sep. Purif. Technol. 2017, 179, 145–151. [Google Scholar] [CrossRef]
- Yuan, X.; Jing, Q.; Chen, J.; Li, L. Photocatalytic Cr(VI) reduction by mixed metal oxide derived from ZnAl layered double hydroxide. Appl. Clay Sci. 2017, 143, 168–174. [Google Scholar] [CrossRef]
- Cui, Y.; Ge, Q.; Liu, X.-Y.; Chung, T.-S. Novel forward osmosis process to effectively remove heavy metal ions. J. Membr. Sci. 2014, 467, 188–194. [Google Scholar] [CrossRef]
- Tran, H.N.; Lin, C.-C.; Woo, S.H.; Chao, H.-P. Efficient removal of copper and lead by Mg/Al layered double hydroxides intercalated with organic acid anions: Adsorption kinetics, isotherms, and thermodynamics. Appl. Clay Sci. 2018, 154, 17–27. [Google Scholar] [CrossRef]
- Sadyrbaeva, T.Z. Removal of chromium(VI) from aqueous solutions using a novel hybrid liquid membrane—electrodialysis process. Chem. Eng. Process. Process Intensif. 2016, 99, 183–191. [Google Scholar] [CrossRef]
- Novais, R.M.; Buruberri, L.H.; Seabra, M.P.; Labrincha, J.A. Novel porous fly-ash containing geopolymer monoliths for lead adsorption from wastewaters. J. Hazard Mater. 2016, 318, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.A.; Daida, P.; Kesmez, M.; Weir, M.; Moreno, H.; Parga, J.R.; Irwin, G.; McWhinney, H.; Grady, T.; Peterson, E.; et al. Arsenic removal by electrocoagulation using combined Al-Fe electrode system and characterization of products. J. Hazard Mater. 2007, 139, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, N.; Narazaki, Y.; Takemoto, K. Reusability of zero-valent iron particles for zinc ion separation. Sep. Purif. Technol. 2018, 193, 139–146. [Google Scholar] [CrossRef]
- Zubair, M.; Daud, M.; McKay, G.; Shehzad, F.; Al-Harthi, M.A. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci. 2017, 143, 279–292. [Google Scholar] [CrossRef]
- Tsoufis, T.; Katsaros, F.; Kooi, B.J.; Bletsa, E.; Papageorgiou, S.; Deligiannakis, Y.; Panagiotopoulos, I. Halloysite nanotube-magnetic iron oxide nanoparticle hybrids for the rapid catalytic decomposition of pentachlorophenol. Chem. Eng. J. 2017, 313, 466–474. [Google Scholar] [CrossRef]
- Lou, Z.; Cao, Z.; Xu, J.; Zhou, X.; Zhu, J.; Liu, X.; Ali Baig, S.; Zhou, J.; Xu, X. Enhanced removal of As(III)/(V) from water by simultaneously supported and stabilized Fe-Mn binary oxide nanohybrids. Chem. Eng. J. 2017, 322, 710–721. [Google Scholar] [CrossRef]
- Kumar, S.; Nair, R.R.; Pillai, P.B.; Gupta, S.N.; Iyengar, M.A.; Sood, A.K. Graphene oxide-MnFe2O4 magnetic nanohybrids for efficient removal of lead and arsenic from water. Acs Appl. Mater. Interfaces 2014, 6, 17426–17436. [Google Scholar] [CrossRef]
- Ouyang, K.; Zhu, C.; Zhao, Y.; Wang, L.; Xie, S.; Wang, Q. Adsorption mechanism of magnetically separable Fe3O4/graphene oxide hybrids. Appl. Surf. Sci. 2015, 355, 562–569. [Google Scholar] [CrossRef]
- Lu, L.; Li, J.; Ng, D.H.L.; Yang, P.; Song, P.; Zuo, M. Synthesis of novel hierarchically porous Fe3O4@MgAl–LDH magnetic microspheres and its superb adsorption properties of dye from water. J. Ind. Eng. Chem. 2017, 46, 315–323. [Google Scholar] [CrossRef]
- Wu, X.L.; Wang, L.; Chen, C.L.; Xu, A.W.; Wang, X.K. Water-dispersible magnetite-graphene-LDH composites for efficient arsenate removal. J. Mater. Chem. 2011, 21, 17353–17359. [Google Scholar] [CrossRef]
- Hu, B.; Ye, F.; Jin, C.; Ma, X.; Huang, C.; Sheng, G.; Ma, J.; Wang, X.; Huang, Y. The enhancement roles of layered double hydroxide on the reductive immobilization of selenate by nanoscale zero valent iron: Macroscopic and microscopic approaches. Chemosphere 2017, 184, 408–416. [Google Scholar] [CrossRef]
- Deng, L.; Shi, Z.; Li, B.; Yang, L.; Luo, L.; Yang, X. Adsorption of Cr(VI) and Phosphate on Mg–Al Hydrotalcite Supported Kaolin Clay Prepared by Ultrasound-Assisted Coprecipitation Method Using Batch and Fixed-Bed Systems. Ind. Eng. Chem. Res. 2014, 53, 7746–7757. [Google Scholar] [CrossRef]
- Gómez-Avilés, A.; Aranda, P.; Ruiz-Hitzky, E. Layered double hydroxide/sepiolite heterostructured materials. Appl. Clay Sci. 2016, 130, 83–92. [Google Scholar] [CrossRef]
- Gong, J.; Liu, T.; Wang, X.; Hu, X.; Zhang, L. Efficient removal of heavy metal ions from aqueous systems with the assembly of anisotropic layered double hydroxide nanocrystals@carbon nanosphere. Environ. Sci. Technol. 2011, 45, 6181–6187. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.H.; Lim, T.T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef]
- Forano, C.; Costantino, U.; Prévot, V.; Gueho, C.T. Layered Double Hydroxides (LDH). In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 745–782. [Google Scholar]
- Teixeira, M.A.; Mageste, A.B.; Dias, A.; Virtuoso, L.S.; Siqueira, K.P.F. Layered double hydroxides for remediation of industrial wastewater containing manganese and fluoride. J. Clean. Prod. 2018, 171, 275–284. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J. Mater. Chem. A 2016, 4, 10744–10766. [Google Scholar] [CrossRef]
- Ding, P.; Zhang, M.; Gai, J.; Qu, B. Homogeneous dispersion and enhanced thermal properties of polystyrene-layered double hydroxide nanocomposites prepared by in situ reversible addition–fragmentation chain transfer (RAFT) polymerization. J. Mater. Chem. 2007, 17, 1117–1122. [Google Scholar] [CrossRef]
- Gu, Z.; Rolfe, B.E.; Xu, Z.P.; Thomas, A.C.; Campbell, J.H.; Lu, G.Q. Enhanced effects of low molecular weight heparin intercalated with layered double hydroxide nanoparticles on rat vascular smooth muscle cells. Biomaterials 2010, 31, 5455–5462. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Huang, X.; Li, G.; Li, Z. Layered Double Hydroxide Nano- and Microstructures Grown Directly on Metal Substrates and Their Calcined Products for Application as Li-Ion Battery Electrodes. Adv. Funct. Mater. 2008, 18, 1448–1458. [Google Scholar] [CrossRef]
- Manzi-Nshuti, C.; Wang, D.; Hossenlopp, J.M.; Wilkie, C.A. Aluminum-containing layered double hydroxides: The thermal, mechanical, and fire properties of (nano)composites of poly(methyl methacrylate). J. Mater. Chem. 2008, 18, 3091. [Google Scholar] [CrossRef]
- Baig, N.; Sajid, M. Applications of layered double hydroxides based electrochemical sensors for determination of environmental pollutants: A review. Trends Environ. Anal. Chem. 2017, 16, 1–15. [Google Scholar] [CrossRef]
- Maziarz, P.; Matusik, J.; Leiviska, T. Mg/Al LDH Enhances Sulfate removal and Clarification of AMD Wastewater in Precipitation Processes. Mater. (Basel) 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Cavani, F.; Trifirò, A.V.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and application. Catal. Today 1991, 11, 171–301. [Google Scholar] [CrossRef]
- Chubar, N.; Gilmour, R.; Gerda, V.; Micusik, M.; Omastova, M.; Heister, K.; Man, P.; Fraissard, J.; Zaitsev, V. Layered double hydroxides as the next generation inorganic anion exchangers: Synthetic methods versus applicability. Adv. Colloid Interface Sci. 2017, 245, 62–80. [Google Scholar] [CrossRef]
- Matusik, J.; Rybka, K. Removal of Chromates and Sulphates by Mg/Fe LDH and Heterostructured LDH/Halloysite Materials: Efficiency, Selectivity, and Stability of Adsorbents in Single- and Multi-Element Systems. Materials 2019, 12, 1373. [Google Scholar] [CrossRef]
- Matusik, J.; Gaweł, A.; Bielańska, E.; Osuch, W.; Bahranowski, K. The effect of structural order on nanotubes derived from kaolin-group minerals. Clays Clay Min. 2009, 57, 452–464. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 18th ed.; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
- Lenoble, V. Arsenite oxidation and arsenate determination by the molybdene blue method. Talanta 2003, 61, 267–276. [Google Scholar] [CrossRef]
- Balan, E.; Saitta, A.M.; Mauri, F.; Calas, G. First-principles modeling of the infrared spectrum of kaolinite. Am. Miner. 2001, 86, 1321–1330. [Google Scholar] [CrossRef]
- Di Cosimo, J.I.; Díez, V.K.; Xu, M.; Iglesia, E.; Apesteguía, C.R. Structure and Surface and Catalytic Properties of Mg-Al Basic Oxides. J. Catal. 1998, 178, 499–510. [Google Scholar] [CrossRef]
- Olszówka, J.E.; Karcz, R.; Bielańska, E.; Kryściak-Czerwenka, J.; Napruszewska, B.D.; Sulikowski, B.; Socha, R.P.; Gaweł, A.; Bahranowski, K.; Olejniczak, Z.; et al. New insight into the preferred valency of interlayer anions in hydrotalcite-like compounds: The effect of Mg/Al ratio. Appl. Clay Sci. 2018, 155, 84–94. [Google Scholar] [CrossRef]
- Farmer, V.C.; Russell, J.D. Infrared Absorption Spectrometry in Clay Studies. Clays Clay Min. 1967, 15, 121–142. [Google Scholar] [CrossRef]
- Matusik, J. Arsenate, orthophosphate, sulfate, and nitrate sorption equilibria and kinetics for halloysite and kaolinites with an induced positive charge. Chem. Eng. J. 2014, 246, 244–253. [Google Scholar] [CrossRef]
- Lazaridis, N.K.; Hourzemanoglou, A.; Matis, K.A. Flotation of metal-loaded clay anion exchangers. Part II: The case of arsenates. Chemosphere 2002, 47, 319–324. [Google Scholar] [CrossRef]
- Bujdosó, T.; Patzkó, Á.; Galbács, Z.; Dékány, I. Structural characterization of arsenate ion exchanged MgAl-layered double hydroxide. Appl. Clay Sci. 2009, 44, 75–82. [Google Scholar] [CrossRef]
- Lazaridis, N.K.; Pandi, T.A.; Matis, K.A. Chromium(VI) Removal from Aqueous Solutions by Mg-Al-CO3 Hydrotalcite: Sorption-Desorption Kinetic and Equilibrium Studies. Ind. Eng. Chem. Res. 2004, 43, 2209–2215. [Google Scholar] [CrossRef]
- Alvarez-Ayuso, E.; Nugteren, H.W. Purification of chromium(VI) finishing wastewaters using calcined and uncalcined Mg-Al-CO3-hydrotalcite. Water Res. 2005, 39, 2535–2542. [Google Scholar] [CrossRef]
- Terry, P.A. Characterization of Cr ion exchange with hydrotalcite. Chemosphere 2004, 57, 541–546. [Google Scholar] [CrossRef]
- Palin, L.; Milanesio, M.; van Beek, W.; Conterosito, E. Understanding the ion exchange process in LDH nanomaterials by fast in situ XRPD and PCA-assisted kinetic analysis. J. Nanomater. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Muller, O.; White, W.B.; Roy, R. Infrared spectra of the chromates of magnesium, nickel and cadmium. Spectrochim. Acta 1969, 25A, 1491–1499. [Google Scholar] [CrossRef]
- Maziarz, P.; Matusik, J.; Strączek, T.; Kapusta, C.; Woch, W.M.; Tokarz, W.; Radziszewska, A.; Leiviskä, T. Highly effective magnet-responsive LDH-Fe oxide composite adsorbents for As(V) removal. Chem. Eng. J. 2019, 362, 207–216. [Google Scholar] [CrossRef]
- Huang, P.-P.; Cao, C.-Y.; Wei, F.; Sun, Y.-B.; Song, W.-G. MgAl layered double hydroxides with chloride and carbonate ions as interlayer anions for removal of arsenic and fluoride ions in water. Rsc. Adv. 2015, 5, 10412–10417. [Google Scholar] [CrossRef]
- Maziarz, P.; Matusik, J. The effect of acid activation and calcination of halloysite on the efficiency and selectivity of Pb(II), Cd(II), Zn(II) and As(V) uptake. Clay Min. 2016, 51, 385–394. [Google Scholar] [CrossRef]
- Jobbagy, M.; Regazzoni, A.E. Complexation at the edges of hydrotalcite: The cases of arsenate and chromate. J. Colloid Interface Sci. 2013, 393, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mulligan, C.N. Speciation and surface structure of inorganic arsenic in solid phases: A review. Environ. Int. 2008, 34, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Tran, H. Comment on “simultaneous and efficient removal of Cr(VI) and methyl orange on LDHs decorated porous carbons”. Chem. Eng. J. 2019, 359, 810–812. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Brown, C.; Mycroft, J.R.; Davidson, R.D.; McIntyre, N.S. X-ray photoelectron spectroscopy studies of chromium compounds. Surf. Interface Anal. 2004, 36, 1550–1563. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matusik, J.; Hyla, J.; Maziarz, P.; Rybka, K.; Leiviskä, T. Performance of Halloysite-Mg/Al LDH Materials for Aqueous As(V) and Cr(VI) Removal. Materials 2019, 12, 3569. https://doi.org/10.3390/ma12213569
Matusik J, Hyla J, Maziarz P, Rybka K, Leiviskä T. Performance of Halloysite-Mg/Al LDH Materials for Aqueous As(V) and Cr(VI) Removal. Materials. 2019; 12(21):3569. https://doi.org/10.3390/ma12213569
Chicago/Turabian StyleMatusik, Jakub, Jakub Hyla, Paulina Maziarz, Karolina Rybka, and Tiina Leiviskä. 2019. "Performance of Halloysite-Mg/Al LDH Materials for Aqueous As(V) and Cr(VI) Removal" Materials 12, no. 21: 3569. https://doi.org/10.3390/ma12213569
APA StyleMatusik, J., Hyla, J., Maziarz, P., Rybka, K., & Leiviskä, T. (2019). Performance of Halloysite-Mg/Al LDH Materials for Aqueous As(V) and Cr(VI) Removal. Materials, 12(21), 3569. https://doi.org/10.3390/ma12213569






