Impact of Ag2O Content on the Optical and Spectroscopic Properties of Fluoro-Phosphate Glasses
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
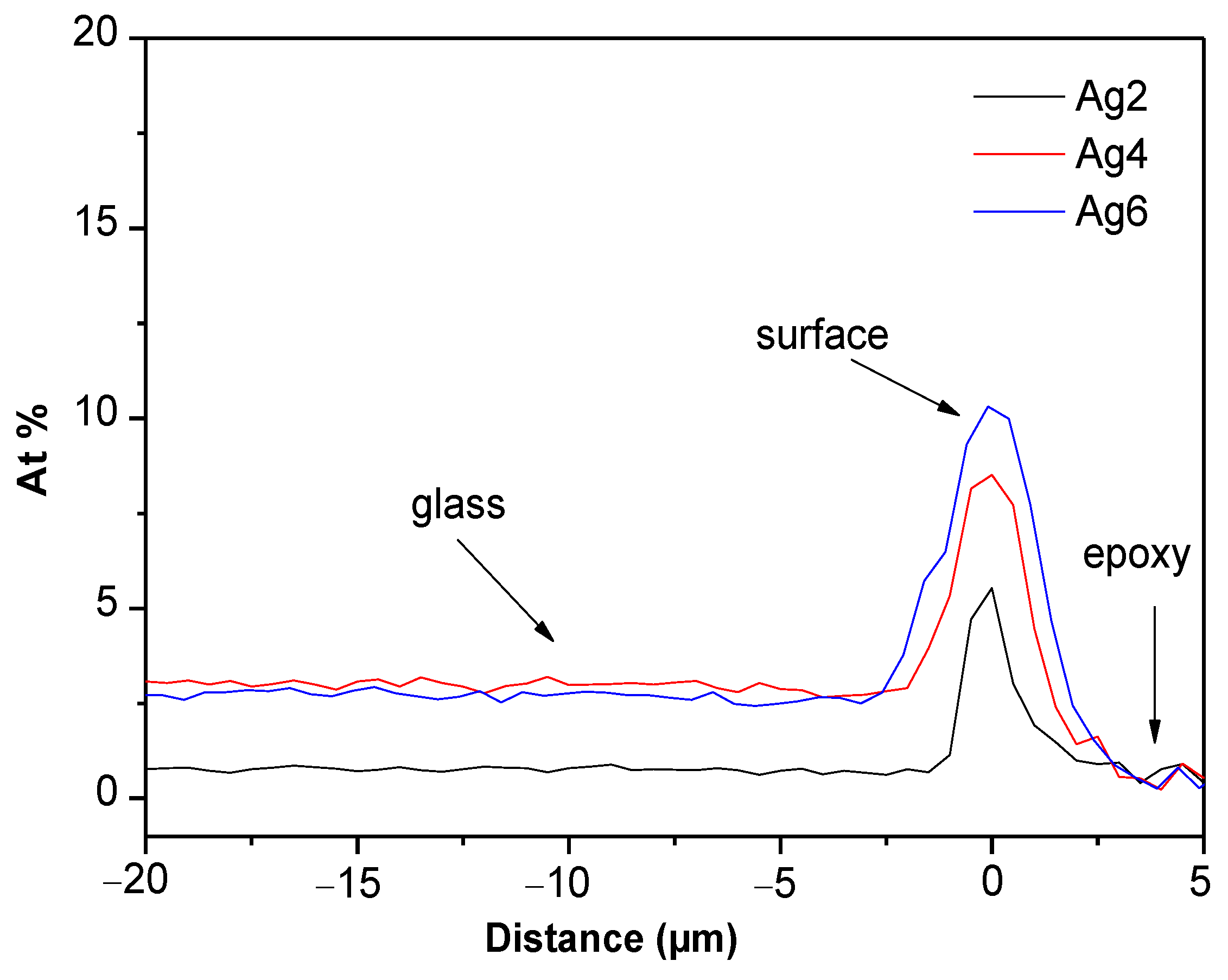
3.1. Thermal and Structural Properties of the Glasses
3.2. Optical and Spectroscopic Properties of the As-Prepared Glasses
3.3. Impact of Heat Treatment on the Optical and Spectroscopic Properties of the Glasses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Righini, G.C.; Chiappini, A. Glass optical waveguides: A review of fabrication techniques. Opt. Eng. 2014, 53, 071819–071832. [Google Scholar] [CrossRef]
- Fares, H.; Santos, S.N.C.; Santos, M.V.; Franco, D.F.; Souza, A.E.; Manzani, D.; Mendonça, C.R.; Nalin, M. Highly luminescent silver nanocluster-doped fluorophosphate glasses for microfabrication of 3D waveguides. RSC. Adv. 2017, 7, 55935–55944. [Google Scholar] [CrossRef]
- Jlassi, J.; Elhouichet, H.; Ferid, M.; Barthou, C. Judd-Ofelt analysis and improvement of thermal and optical properties of tellurite glasses by adding P2O5. J. Lumin. 2010, 130, 2394–2401. [Google Scholar] [CrossRef]
- Jlassi, I.; Elhouichet, H.; Hraiech, S.; Ferid, M. Effect of heat treatment on the structural and optical properties of tellurite glasses doped erbium. J. Lumin. 2012, 132, 832–840. [Google Scholar] [CrossRef]
- Thomas, S.; George, R.; Rasool, S.N.; Rathaiah, M.; Venkatramu, V.; Joseph, C.; Unnikrishnan, N.V. Optical properties of Sm3+ ions in zinc potassium fluorophosphate glasses. Opt Mat. 2013, 36, 242–250. [Google Scholar] [CrossRef]
- Saad, M.; Stambouli, W.; Mohamed, S.A.; Elhouichet, H. Ag nanoparticles induced luminescence enhancement of Eu3+ doped phosphate glasses. J. Alloys Compd. 2017, 705, 550–558. [Google Scholar] [CrossRef]
- Mechergui, I.; Fares, H.; Mohamed, S.A.; Nalin, M.; Elhouichet, H. Coupling between surface plasmon resonance and Sm3+ ions induced enhancement of luminescence properties in fluoro-tellurite glasses. J. Lumin. 2017, 190, 518–524. [Google Scholar] [CrossRef]
- Amendola, V.; Bakr, O.M.; Stellacci, F. A Study of the Surface Plasmon Resonance of Silver Nanoparticles by the Discrete Dipole Approximation Method: Effect of Shape, Size, Structure, and Assembly. Plasmonics 2010, 5, 85–97. [Google Scholar] [CrossRef]
- Fares, H.; Elhouichet, H.; Gelloz, B.; Ferid, M. Silver nanoparticles enhanced luminescence properties of Er3+ doped tellurite glasses: Effect of heat treatment. J. Appl. Phys. 2014, 116, 123504–123513. [Google Scholar] [CrossRef]
- Tikhomirov, V.K.; Rodríguez, V.D.; Kuznetsov, A.; Kirilenko, D.; Van Tendeloo, G.; Moshchalkov, V.V. Preparation and luminescence of bulk oxyfluoride glasses doped with Ag nanoclusters. Opt. Express. 2010, 18, 22032–22040. [Google Scholar] [CrossRef]
- Lin, H.; Chen, D.; Yu, Y.; Zhang, R.; Wang, Y. Molecular-like Ag clusters sensitized near-infrared down-conversion luminescence in oxyfluoride glasses for broadband spectral modification. Appl. Phys. Lett. 2013, 103, 091902. [Google Scholar] [CrossRef]
- Poudel, A.; Dmitrieva, I.; Gumenyuk, R.; Mihai, L.; Sporea, D.; Muresan, O.; Rusen, I.; Hakkarainen, T.; Boetti, N.G.; Niemi, T.; et al. Effect of ZnO Addition and of Alpha Particle Irradiation on Various Properties of Er3+, Yb3+ Doped Phosphate Glasses. J. Appl. Sci. 2017, 7, 1094. [Google Scholar] [CrossRef]
- Judd, B.R. Optical Absorption Intensities of Rare-Earth Ions. J. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Ofelt, G.S. Intensities of Crystal Spectra of Rare-Earth Ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Shanmugavelu, B.; Ravi Kanth Kumar, V.V.; Kuladeep, R.; Rao, D.N. Third order nonlinear optical properties of bismuth zinc borate glasses. J. Appl. Phys. 2013, 114, 243. [Google Scholar] [CrossRef]
- Carnall, W.T.; Fields, P.R.; Rajnak, R. Electronic Energy Levels in the Trivalent Lanthanide Aquo Ions. I. Pr3+, Nd3+, Pm3+, Sm3+, Dy3+, Ho3+, Er3+, and Tm3+. J. Chem. Phys. 1968, 49, 4424. [Google Scholar] [CrossRef]
- Stambouli, W.; Elhouichet, H.; Barthou, C.; Férid, M. Energy transfer induced photoluminescence improvement in Er3+/Ce3+/Yb3+ tri-doped tellurite glass. J. Alloys Compd. 2013, 580, 310–315. [Google Scholar] [CrossRef]
- Jorgensen, C.K.; Reisfeld, R. Judd-Ofelt parameters and chemical bonding. J. Less Common Met. 1983, 93, 107. [Google Scholar] [CrossRef]
- Weber, M.J. Probabilities for Radiative and Nonradiative Decay of Er3+ in LaF3. Phys. Rev. 1967, 157, 262. [Google Scholar] [CrossRef]
- Weber, M.J.; Myers, J.D.; Blackburn, D.H. Optical properties of Nd3+ in tellurite and phosphotellurite glasses. J. Appl. Phys. 1981, 52, 2944. [Google Scholar] [CrossRef]
- Jiang, C.; Deng, P.; Zhang, J.; Huang, G.; Gan, F. Yb: Tellurogermanate laser glass with high emission cross section. J. Lumin. 1999, 82, 321–326. [Google Scholar] [CrossRef]
- Soltani, I.; Hraiech, S.; Horchani-Naifer, K.; Elhouichet, H.; Gelloz, B.; Ferid, M. Thermal, structural and optical properties of Er3+ doped phosphate glasses containing silver nanoparticles. J. Alloys Compd. 2016, 686, 556–563. [Google Scholar] [CrossRef]
- Hu, Y.; Qiu, J.; Song, Z.; Yang, Z.; Yang, Y.; Zhou, D.; Jiao, Q.; Chenshuo, M. Spectroscopic properties of Tm3+/Er3+/Yb3+ co-doped oxyfluorogermanate glasses containing silver nanoparticles. J. Lumin. 2014, 145, 512–517. [Google Scholar] [CrossRef]
- Konidakis, I.; Varsamis, C.-P.E.; Kamitsos, E.I.; Möncke, D.; Ehrt, D. Structure and properties of mixed strontium-manganese metaphosphate glasses. J. Phys. Chem. 2010, 114, 9125–9138. [Google Scholar] [CrossRef]
- Gao, H.; Tan, T.; Wang, D. Effect of composition on the release kinetics of phosphate controlled release glasses in aqueous medium. J. Controlled Release 2004, 96, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Abou, E.N.; Chrzanowski, W.; Pickup, D.M.; O’Dell, L.A.; Mordan, N.J.; Newport, R.J.; Smith, M.E.; Knowles, J.C. Structure and properties of strontium-doped phosphate-based glasses. J. R. Soc. Interface 2009, 6, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Massera, J.; Lastusaari, M.; Hupa, L.; Petit, L. Novel oxyfluorophosphate glasses and glass-ceramics. J. Non-Cryst. Solids 2016, 445–446, 40–44. [Google Scholar] [CrossRef]
- Murthy, M.K. Phosphate-Halide Systems: II, Infrared Spectra of Glasses in the System NaPO3-Na. J. Am. Ceram. Soc. 1963, 46, 558–559. [Google Scholar] [CrossRef]
- Wilder, J.A.; Shelby, J.E. Property Variation in Alkali Alkaline-Earth Metaphosphate Glasses. J. Am. Ceram. Soc. 1984, 67, 438–444. [Google Scholar] [CrossRef]
- Mishra, A.; Rocherullé, J.; Massera, J. Ag-doped phosphate bioactive glasses: Thermal, structural and in-vitro dissolution properties. Biomed. Glasses 2016, 2, 38–48. [Google Scholar] [CrossRef]
- Righini, G.C.; Pelli, S.; Fossi, M.; Brenci, M.; Lipovskii, A.A.; Kolobkova, E.V.; Bettinelli, M. Characterization of Er-doped sodium-niobium phosphate glasses. Int. Soc. Opt. Photonics 2001, 4282, 210–215. [Google Scholar]
- Babu, S.; Seshadri, M.; Reddy Prasad, V.; Ratnakaram, Y.C. Spectroscopic and laser properties of Er3+ doped fluoro-phosphate glasses as promising candidates for broadband optical fiber lasers and amplifiers. Mater. Res. Bull. 2015, 70, 935–944. [Google Scholar] [CrossRef]
- Langar, A.; Bouzidi, C.; Elhouichet, H.; Ferid, M. Er–Yb codoped phosphate glasses with improved gain characteristics for an efficient 1.55 µm broadband optical amplifiers. J. Lumin. 2014, 148, 249–255. [Google Scholar] [CrossRef]
- Tian, Y.; Tian, B.; Chen, B.; Cui, C.; Huang, P.; Wang, L.; Hua, R. Morphological tuning and enhanced luminescence of NaEuF4 nano-/submicro-crystals. J. Appl. Surf. Sci. 2014, 313, 504–511. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Marimuthu, K.; Sudarsan, V. Concentration dependent spectroscopic behavior of Sm3+ doped leadfluoro-borophosphate glasses for laser and LED applications. J. Alloys Compd. 2015, 647, 209–220. [Google Scholar] [CrossRef]
- Jlassi, I.; Elhouichet, H.; Ferid, M.; Chtourou, R.; Oueslati, M. Study of photoluminescence quenching in Er3+-doped tellurite glasses. Opt. Mater. 2010, 32, 743–747. [Google Scholar] [CrossRef]
- Kaminskii, A. Crystalline Lasers: Physical Processes and Operating Schemes; CRC: Boca Raton, FL, USA, 1996. [Google Scholar]
- Reddy, B.S.; Buddhudu, S.; Rao, K.S.R.K.; Babu, P.N.; Annapurna, K. Optical Analysis of Er3+: Boro-Fluoro-Phosphate Glasses. Spectrosc. Lett. 2008, 41, 376–384. [Google Scholar] [CrossRef]
- Sreedhara, V.B.; Vijayac, N.; Ramacharia, D.; Jayasankar, C.K. Luminescence studies on Er3+-doped zincfluorophosphate glasses for 1.53 μm laser applications. J. Mol. Struct. 2017, 1130, 1001–1008. [Google Scholar] [CrossRef]
- Moustafa, S.Y.; Sahar, M.R.; Ghoshal, S.K. Spectroscopic attributes of Er3+ ions in antimony phosphate glass incorporated with Ag nanoparticles: Judd-Ofelt analysis. J. Alloys Compd. 2017, 712, 781–794. [Google Scholar] [CrossRef]
- Hasim, N.; Rohani, M.S. The Effect of Nd3+ Composition on Judd-Ofelt Analysis of Lithium Niobate Tellurite Glasses Codoped with Er3+. Solid State Phenom. 2017, 268, 191–197. [Google Scholar] [CrossRef]
- Belharouak, I.; Weill, F.; Parent, C.; Le Flem, G.; Moine, B. Silver particles in glasses of the ‘Ag2O–ZnO–P2O5’ system. J. Non-Cryst. Solids 2001, 293, 649–656. [Google Scholar] [CrossRef]
- Wei, R.F.; Li, J.J.; Gao, J.Y.; Guo, H. Enhancement of Eu3+ Luminescence by Ag Species (Ag NPs, ML-Ag, Ag+) in Oxyfluoride Glasses. J. Am. Ceram. Soc. 2012, 95, 3380–3382. [Google Scholar] [CrossRef]
- Miniscalco, W.J.; Quimby, R.S. General procedure for the analysis of Er3+ cross sections. Opt. Lett. 1991, 16, 258. [Google Scholar] [CrossRef] [PubMed]
- Fares, H.; Elhouichet, H.; Gelloz, B.; Férid, M. Surface plasmon resonance induced Er3+ photoluminescence enhancement in tellurite glass. J. Appl. Phys. 2015, 117, 193102. [Google Scholar] [CrossRef]
- Soltani, I.; Hraiech, S.; Horchani-Naifer, K.; Elhouichet, H.; Férid, M. Effect of silver nanoparticles on spectroscopic properties of Er3+ doped phosphate glass. Opt. Mater. 2015, 46, 454–460. [Google Scholar] [CrossRef]
- Qi, J.; Xu, T.; Wu, Y.; Shen, X.; Dai, S.; Xu, Y. Ag nanoparticles enhanced near-IR emission from Er3+ ions doped glasses. Opt. Mater. 2013, 35, 2502–2506. [Google Scholar] [CrossRef]
- Jimenez, J.A.; Sendova, M.; Liu, H.; Fernandez, F.E. Supersaturation-driven optical tuning of Ag nanocomposite glasses for photonics: An in situ optical microspectroscopy study. Plasmonics 2011, 6, 399–405. [Google Scholar] [CrossRef]
- Malta, O.L.; Santa-Cruz, P.A.; De Sa, G.F.; Auzel, F. Fluorescence enhancement induced by the presence of small silver particles in Eu3+ doped materials. J. Lumin. 1985, 33, 261–272. [Google Scholar] [CrossRef]
- Silva, G.; Holgado, D.; Anjos, V. Effect of Ag nanoparticles on the radiative properties of tellurite glasses doped with Er3+, Yb3+ and Tm3+ ions. J. Opt. Mat. 2014, 37, 281–286. [Google Scholar] [CrossRef]
- Sazali, E.S.; Sahar, M.R.; Ghoshal, S.K.; Arifin, R.; Rohani, M.S.; Amjad, R.J. Efficient optical enhancement of Er3+ doped lead–tellurite glass embedded with gold nanoparticles: Role of heat-treatment. J. Non-Cryst. Solids 2015, 410, 174–179. [Google Scholar] [CrossRef]
- Mahraz, Z.A.S.; Sahar, M.R.; Ghoshal, S.K.; Dousti, M.R.; Amjad, R.J. Silver nanoparticles enhanced luminescence of Er3+ ions in boro-tellurite glasses. Mater. Lett. 2013, 112, 136–138. [Google Scholar] [CrossRef]
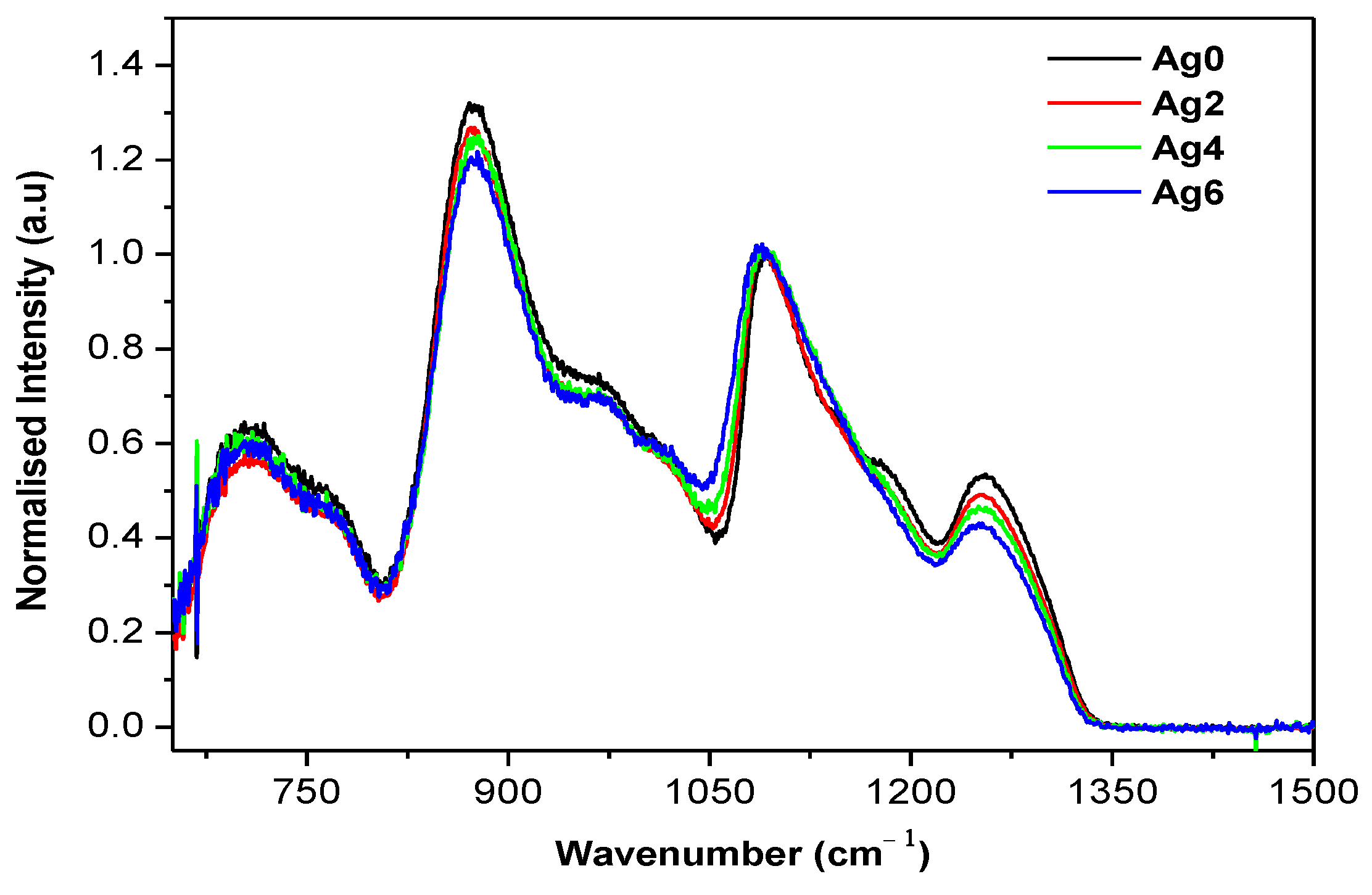
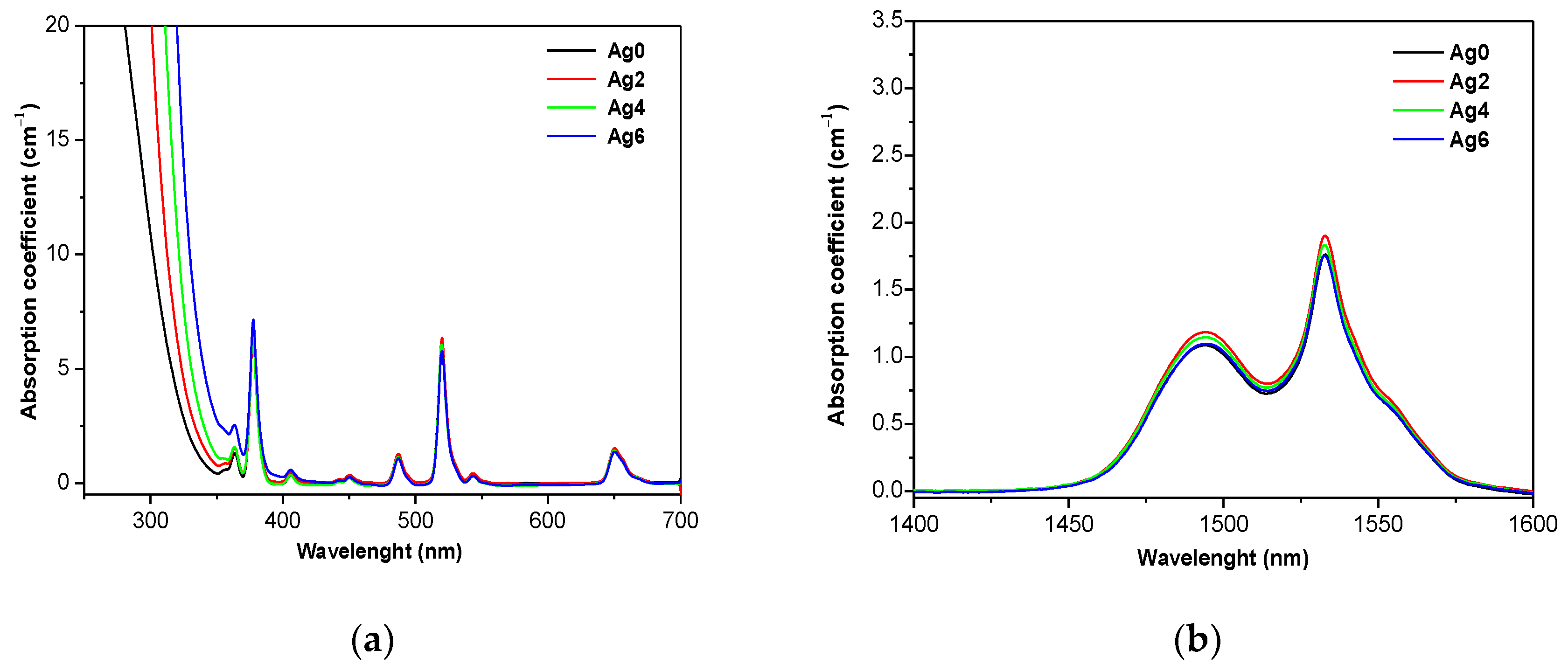
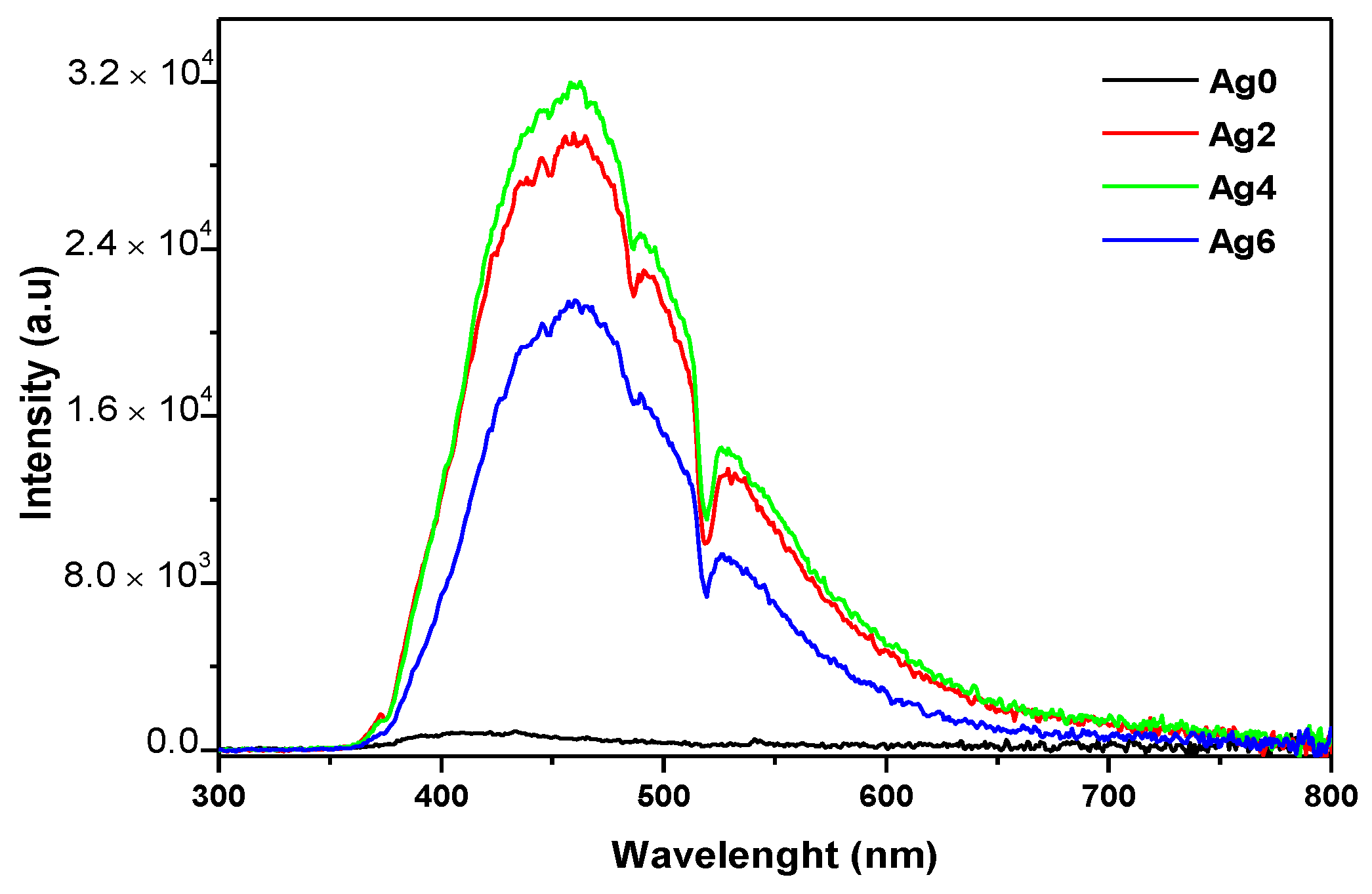
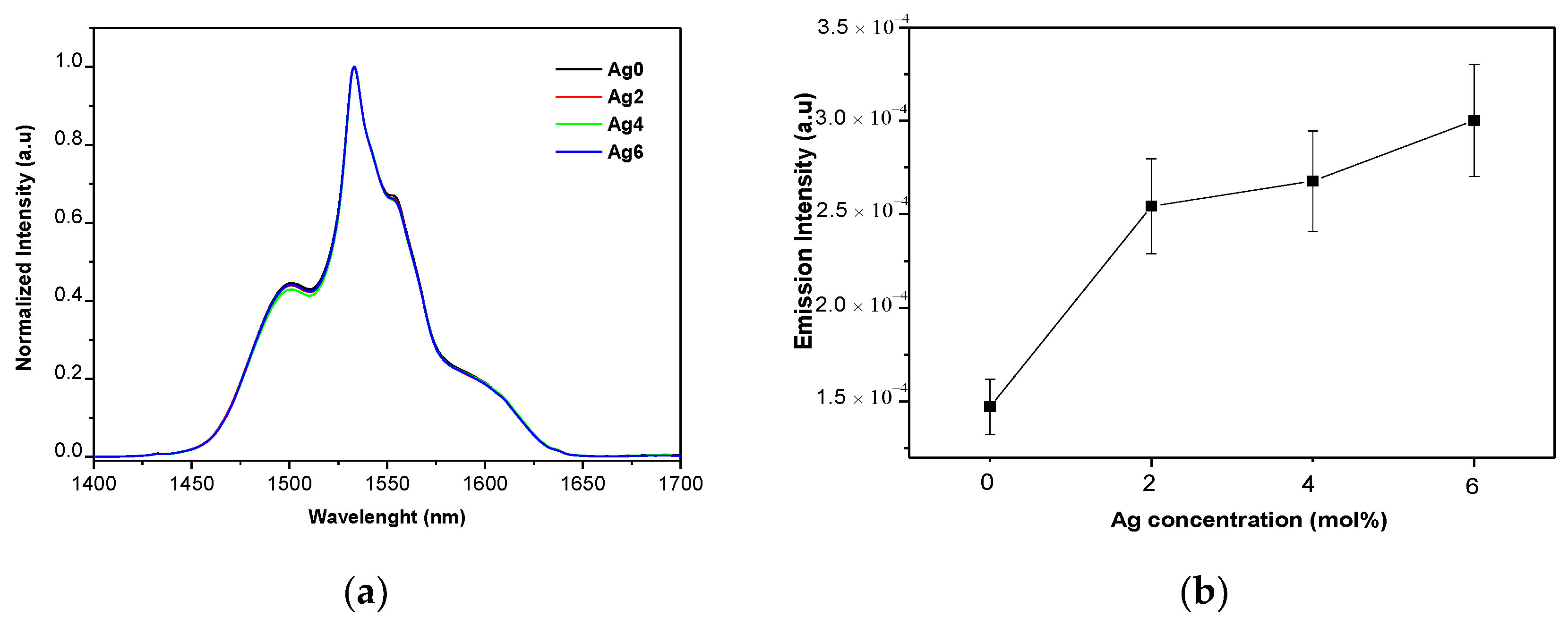




| Sample | Tg ± 3 °C | Tx ± 3 °C | Tp ± 3 °C | ΔT = Tx − Tg ± 6 °C | ρ (±0.02 g/cm3) | Vm (mol·cm3) |
|---|---|---|---|---|---|---|
| Ag0 | 297 | 390 | 457 | 95 | 2.67 | 36.75 |
| Ag2 | 290 | 343 | 375 | 53 | 2.75 | 36.67 |
| Ag4 | 285 | 345 | 384 | 60 | 2.84 | 36.46 |
| Ag6 | 280 | 340 | 392 | 60 | 2.93 | 36.27 |
| Wavenumber (cm−1) | Attribution | Reference |
|---|---|---|
| 770 | νs(P-O-P) of Q2 units | [24] |
| 875 | νas(P-O-P) of Q2 units in chain | [24] |
| 960 | νas(P-O-P) of Q2 units in small rings | [25,26] |
| 980 | νs(PO32−) in Q1 units | [27] |
| 1015 | νs(PO3F) bonds | [28] |
| 1020 | νas(P-O-P) of Q2 units in large rings | [25,26] |
| 1085 | νas(P-O-P) of Q1 units vs(P-O-P) of Q2 units | [29] |
| 1183 | νas(P-O-P) in Q2 units | [27] |
| 1255 | νas(O-P-O) of Q2 units | [25] |
| Sample | Ω2 (10−20 cm2) | Ω4 (10−20 cm2) | Ω6 (10−20 cm2) | χ | ΔSrms (10−22) |
|---|---|---|---|---|---|
| Ag0 | 3.41 | 0.81 | 1.08 | 0.75 | 0.09 |
| Ag2 | 3.50 | 0.72 | 1.02 | 0.70 | 0.08 |
| Ag4 | 3.62 | 0.66 | 0.97 | 0.68 | 0.08 |
| Ag6 | 3.65 | 0.60 | 0.91 | 0.65 | 0.19 |
| Phosphate [31] | 3.89 | 1.01 | 0.55 | 1.83 | - |
| Fluoro-phosphate [32] | 4.90 | 1.37 | 1.27 | 1.08 | - |
| Fluoride [33] | 2.91 | 1.27 | 1.11 | 1.14 | - |
| Sample Code | Ag0 | Ag2 | Ag4 | Ag6 | ZnO-AlF3 [38] | PKAZFEr10 [39] | SAMEA0.9 [40] | |
|---|---|---|---|---|---|---|---|---|
| 4I13/2 → 4I15/2 | A (s−1) | 209.04 | 220.25 | 220.11 | 219.73 | 148 | 177.18 | 485 |
| βJJ’ (%) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| τ (ms) | 2.09 | 2.20 | 2.20 | 2.19 | 6.79 | 5.64 | 2.61 | |
| 4I11/2 → 4I15/2 | A (s−1) | 210.87 | 226.79 | 220.89 | 219.21 | 174.25 | 206.09 | 137 |
| βJJ’ (%) | 0.83 | 0.84 | 0.83 | 0.83 | 0.89 | 0.86 | 0.84 | |
| τ (ms) | 2.51 | 2.69 | 2.63 | 2.61 | 5.08 | 4.16 | 6.14 | |
| 4I9/2 → 4I15/2 | A (s−1) | 154.01 | 142.55 | 145.86 | 143.06 | - | 83.46 | 183 |
| βJJ’ (%) | 0.66 | 0.63 | 0.64 | 0.64 | - | 0.53 | 0.89 | |
| τ (ms) | 2.30 | 2.24 | 2.23 | 2.25 | - | 6.40 | 4.83 | |
| Measured Lifetimes | |||
|---|---|---|---|
| Sample | As-Prepared | Heat-Treated | Heat-Treated After Polishing |
| Ag0 | 1.3 | - | - |
| Ag2 | 1.7 | 1.9 | 1.7 |
| Ag4 | 1.7 | 1.8 | 1.6 |
| Ag6 | 1.5 | 1.2 | 1.5 |
| Glass Sample | Er3+ (1020 ions cm−3) ±5% | α at 1.5 μm (cm−1) | Δλeff (nm) | σa at 1.5 μm (10−21 cm2) ±10% | σe at 1.5 μm (10−21 cm2) ±10% | FWHM (nm) | σe*FWHM (10−21·nm) | G (P = 1) (cm−1) |
|---|---|---|---|---|---|---|---|---|
| Ag0 | 3.277 | 1.759 | 37 | 5.57 | 4.61 | 36 | 165.97 | 1.51 |
| Ag2 | 3.284 | 1.902 | 57 | 5.97 | 5.02 | 41 | 205.82 | 1.66 |
| Ag4 | 3.303 | 1.829 | 57 | 5.74 | 4.86 | 37 | 179.82 | 1.61 |
| Ag6 | 3.320 | 1.750 | 56 | 5.46 | 4.69 | 37 | 173.53 | 1.56 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ennouri, M.; Kuusela, L.; Jlassi, I.; Gelloz, B.; Petit, L.; Elhouichet, H. Impact of Ag2O Content on the Optical and Spectroscopic Properties of Fluoro-Phosphate Glasses. Materials 2019, 12, 3516. https://doi.org/10.3390/ma12213516
Ennouri M, Kuusela L, Jlassi I, Gelloz B, Petit L, Elhouichet H. Impact of Ag2O Content on the Optical and Spectroscopic Properties of Fluoro-Phosphate Glasses. Materials. 2019; 12(21):3516. https://doi.org/10.3390/ma12213516
Chicago/Turabian StyleEnnouri, Marwa, Luukas Kuusela, Ifa Jlassi, Bernard Gelloz, Laeticia Petit, and Habib Elhouichet. 2019. "Impact of Ag2O Content on the Optical and Spectroscopic Properties of Fluoro-Phosphate Glasses" Materials 12, no. 21: 3516. https://doi.org/10.3390/ma12213516
APA StyleEnnouri, M., Kuusela, L., Jlassi, I., Gelloz, B., Petit, L., & Elhouichet, H. (2019). Impact of Ag2O Content on the Optical and Spectroscopic Properties of Fluoro-Phosphate Glasses. Materials, 12(21), 3516. https://doi.org/10.3390/ma12213516






