Preparation and Properties of CaCO3-Supported Nano-TiO2 Composite with Improved Photocatalytic Performance
Abstract
:1. Introduction
2. Methods
2.1. Raw Materials and Reagents
2.2. Preparation of CaCO3–TiO2 Composite Photocatalyst
2.3. Photocatalytic Properties Test
2.4. Characterization
3. Results and Discussion
3.1. Photocatalytic Degradation of Methyl Orange by CaCO3–TiO2
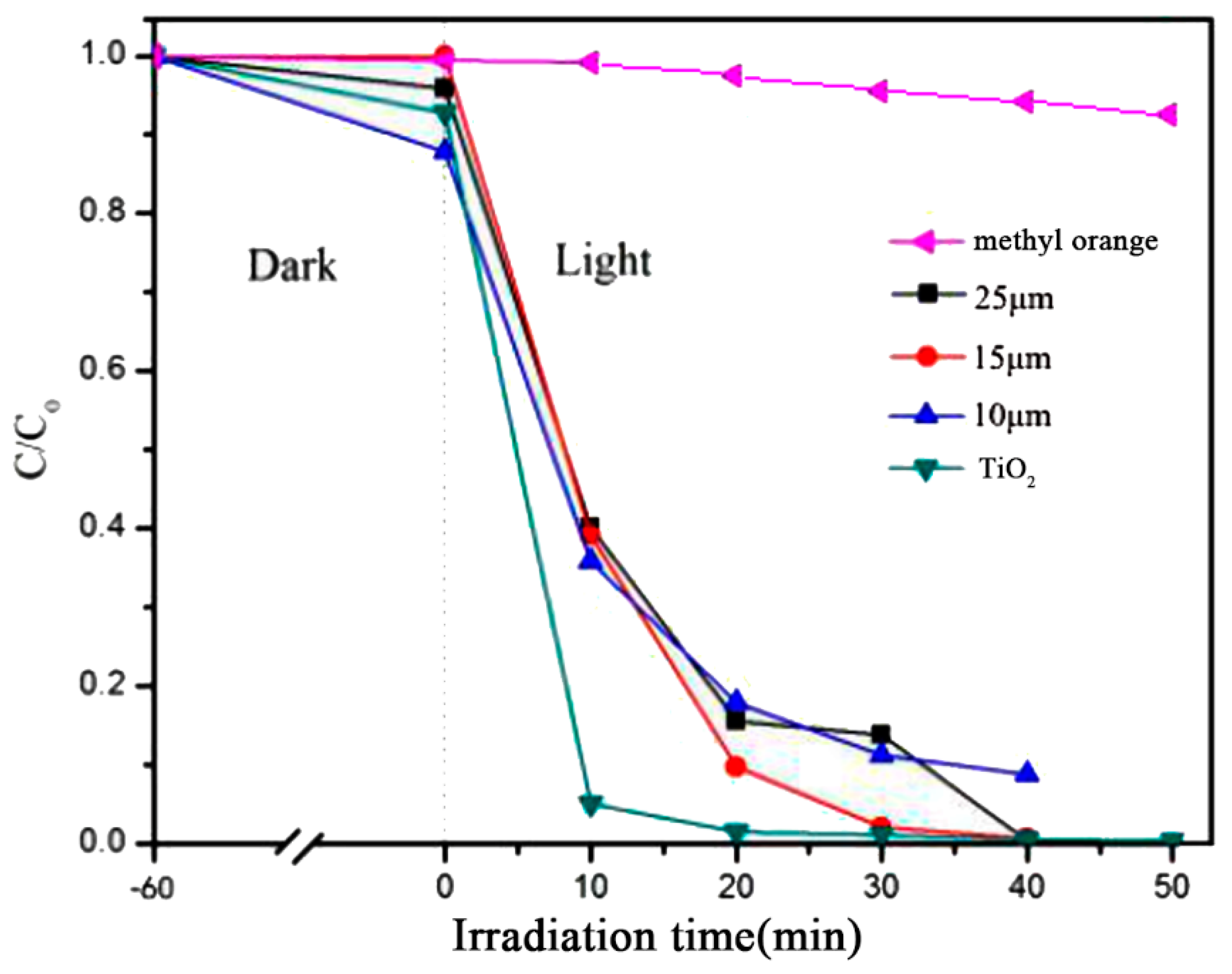
3.1.1. Effect of Particle Size of CaCO3
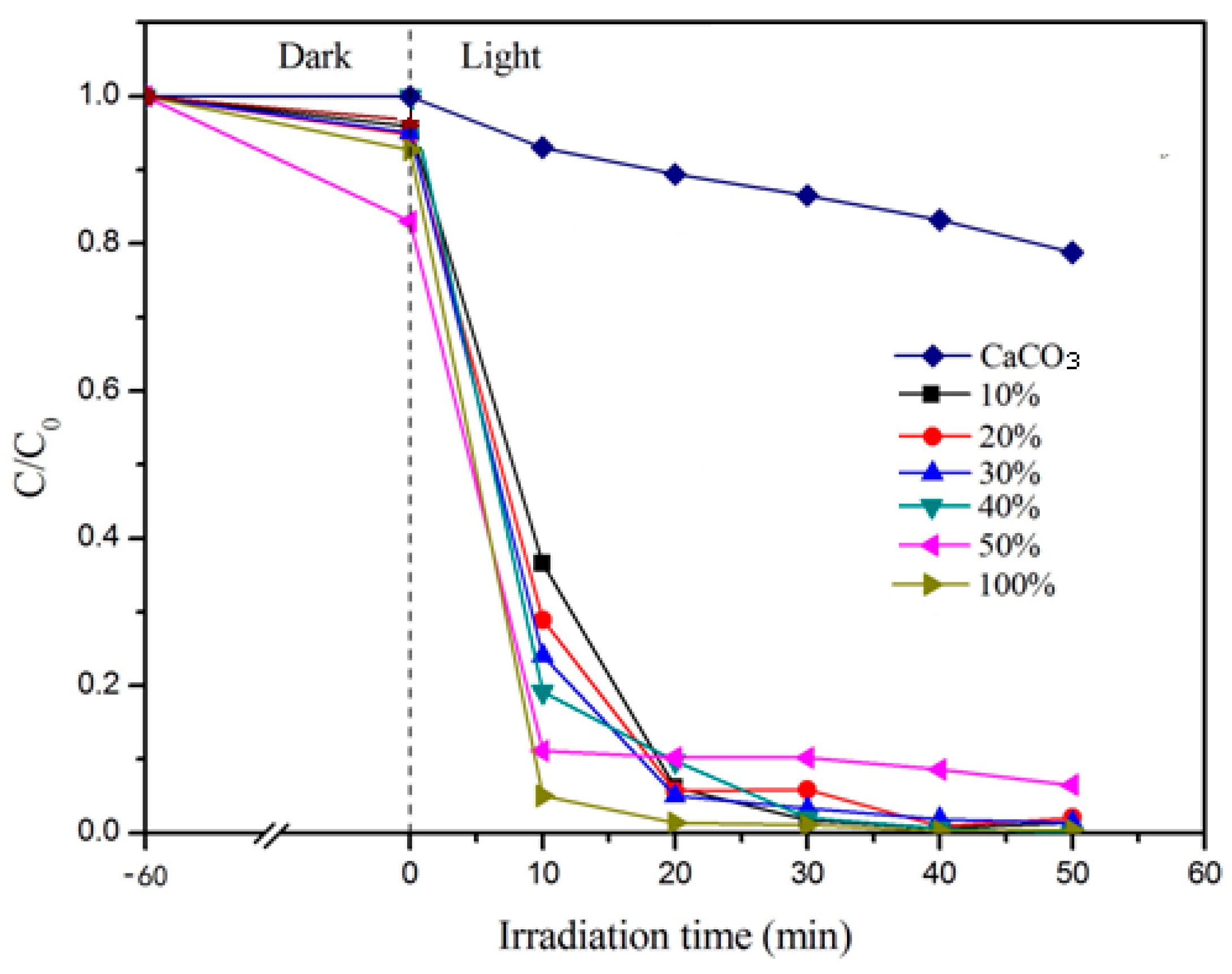
3.1.2. Effect of Loading Amount of Nano-TiO2
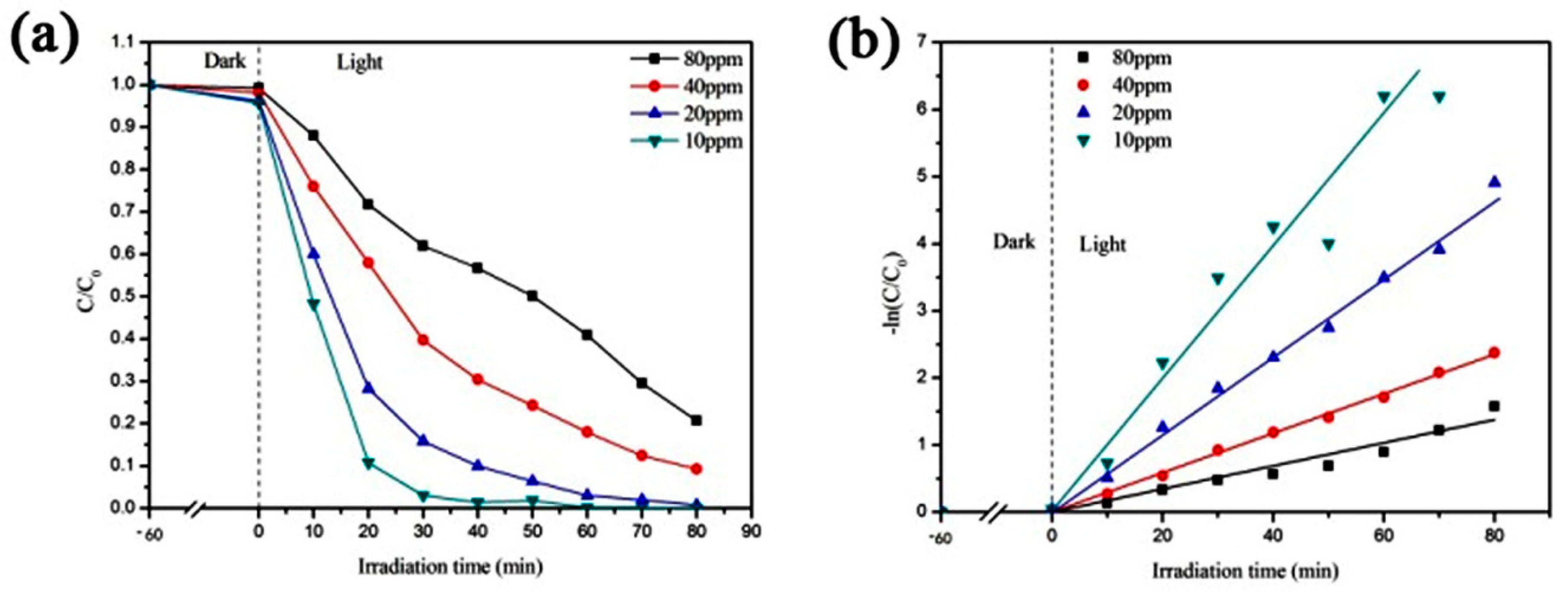
3.1.3. Photocatalytic Degradation of Different Concentrations of Methyl Orange Solution by CaCO3–TiO2
3.1.4. Recycling Performance of CaCO3–TiO2 Composite Photocatalyst
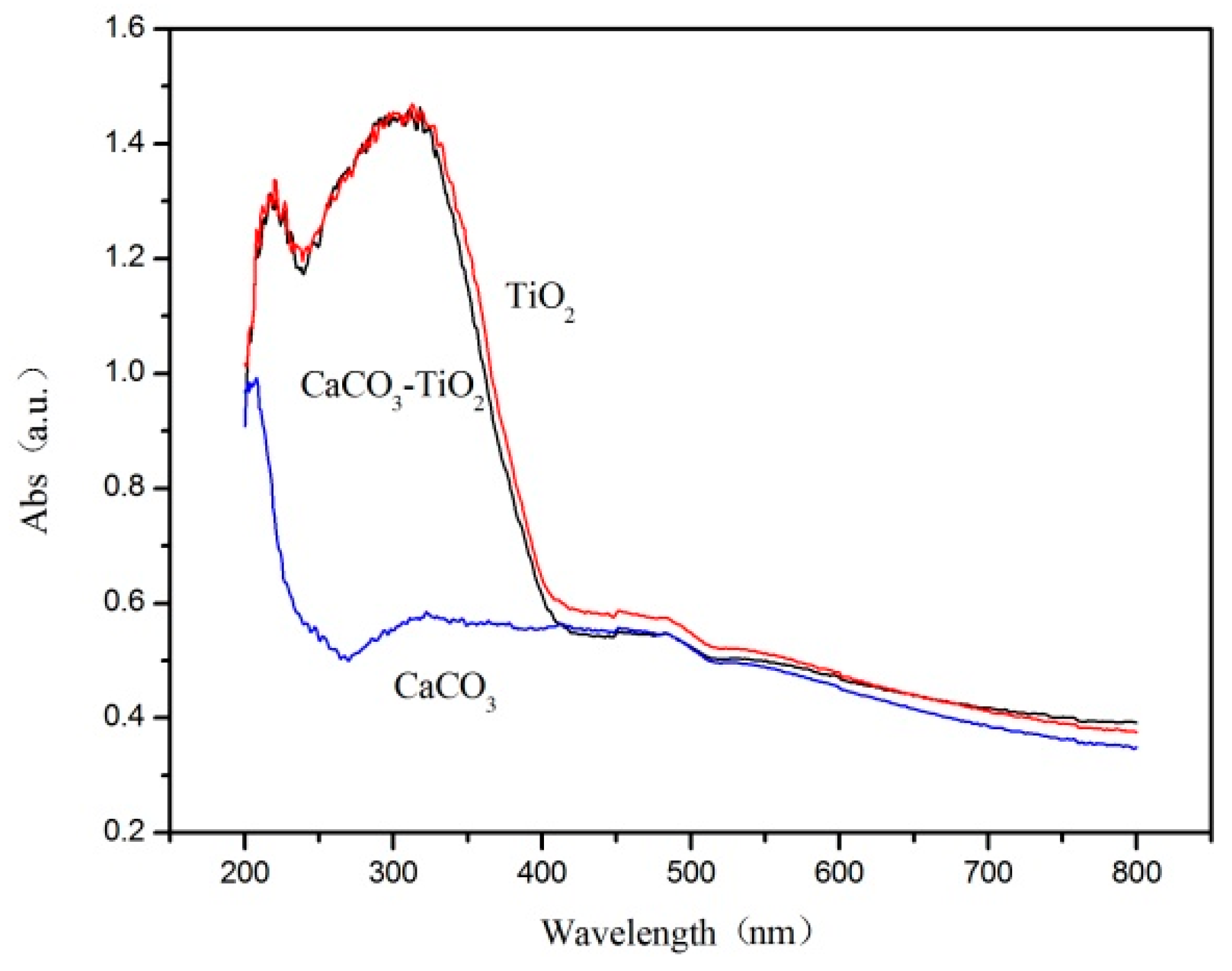
3.2. Optical Performance Test
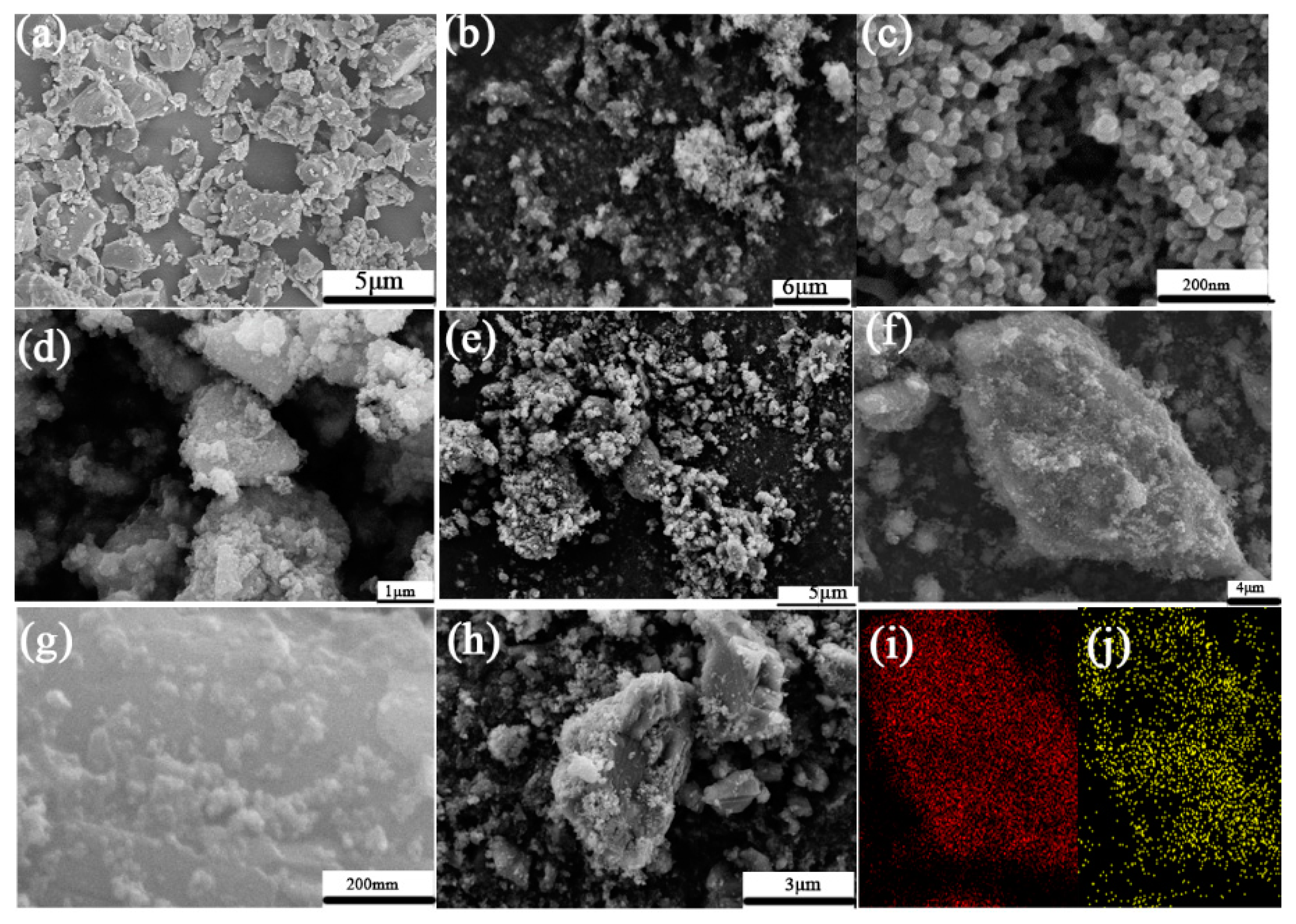
3.3. Morphology and Structure of CaCO3–TiO2 Composite Photocatalyst
3.3.1. SEM Analysis
3.3.2. XRD Analysis
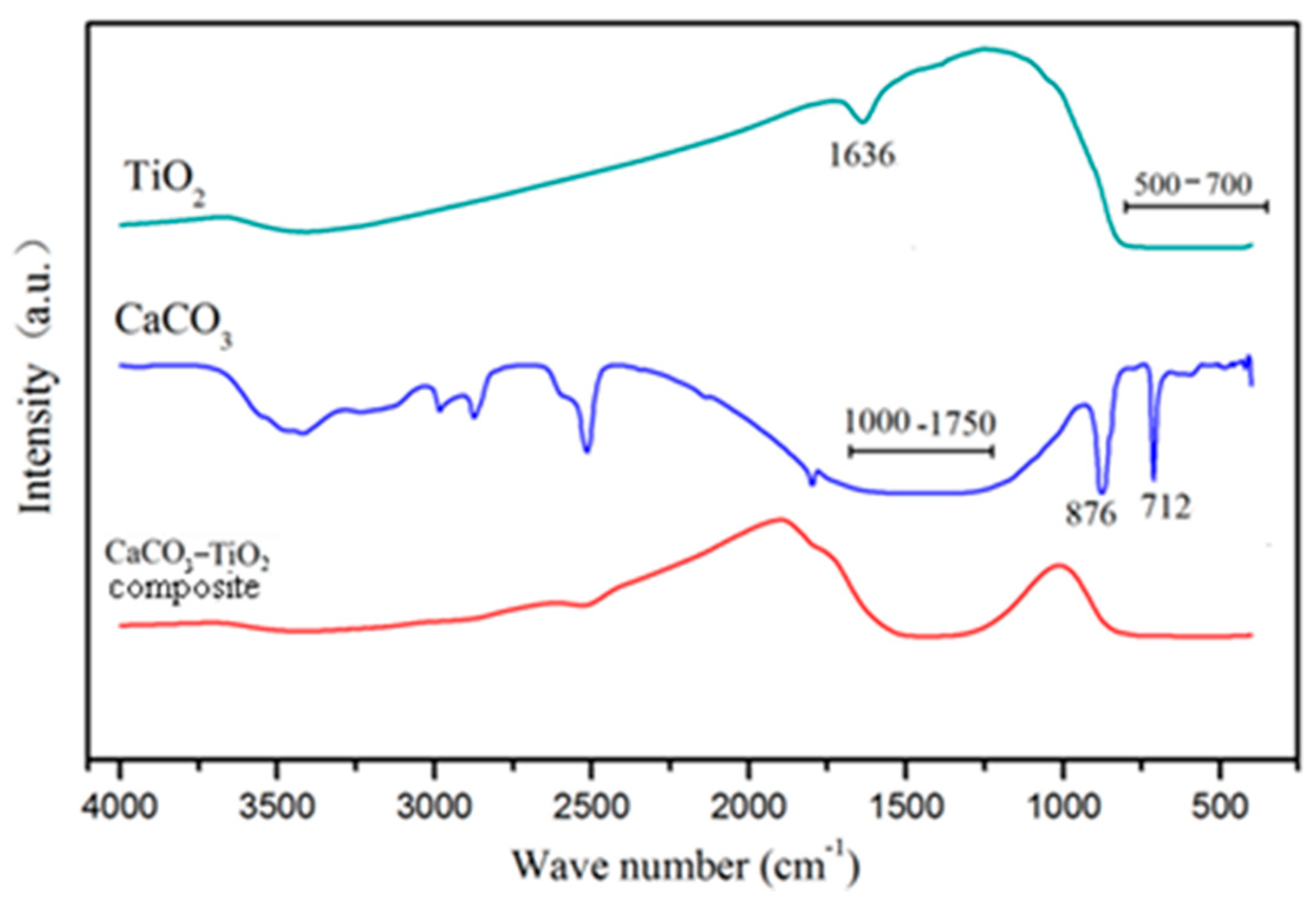
3.3.3. Infrared Spectrum Analysis
4. Conclusions
- (1)
- The CaCO3–TiO2 composite photocatalyst was prepared by the co-grinding of CaCO3 and TiO2. The optimal CaCO3–TiO2 composite photocatalyst, in which the particles size of CaCO3 is 15 μm and the mass ratio of TiO2 is 40%, shows excellent photocatalytic degradation performance towards methyl orange and good recovery performance. The degradation efficiency of optimal CaCO3–TiO2 composite photocatalyst was found to be 90% and 100% after 20 and 40 min of ultraviolet light illumination, respectively. The degradation effect is comparable to pure TiO2. Moreover, its degradation effect on methyl orange is not significantly reduced after five cycles.
- (2)
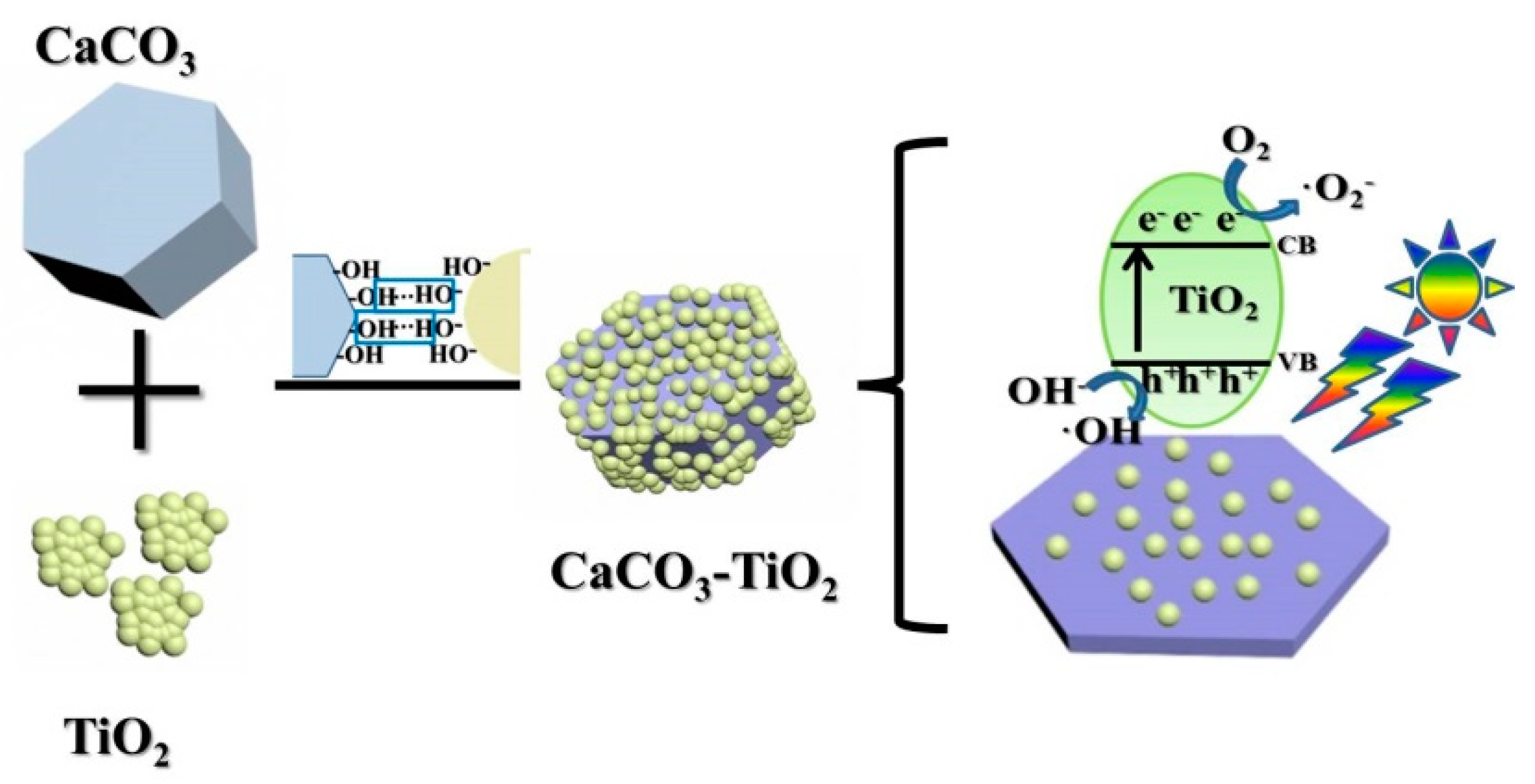
- CaCO3–TiO2 composite photocatalyst is characterized by CaCO3 loaded by nano-TiO2 uniformly and completely. The dispersibility of the loaded TiO2 is significantly enhanced compared to pure TiO2, and a strong chemical bond is formed between CaCO3 and TiO2 particle interfaces. These are important mechanisms for improving the photocatalytic efficiency of nano-TiO2 and reducing the its amount in CaCO3–TiO2.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photoch. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Fagan, R.; Mccormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mat. Sci. Semicon. Proc. 2016, 42, 2–14. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Sun, B.; Reddy, E.P.; Smirniotis, P.G. Effect of the Cr6+ concentration in Cr-incorporated TiO2-loaded MCM-41 catalysts for visible light photocatalysis. Appl. Catal. B Environ. 2005, 57, 139–149. [Google Scholar] [CrossRef]
- Wei, Z.; Liang, F.; Liu, Y.; Luo, W.; Wang, J.; Yao, W.; Zhu, Y. Photoelectrocatalytic degradation of phenol-containing wastewater by TiO2/g-C3N4 hybrid heterostructure thin film. Appl. Catal. B Environ. 2017, 201, 600–606. [Google Scholar] [CrossRef]
- Su, Y.F.; Wang, G.B.; Kuo, D.T.F.; Chang, M.L.; Shih, Y.H. Photoelectrocatalytic degradation of the antibiotic sulfamethoxazole using TiO2/Ti photoanode. Appl. Catal. B Environ. 2016, 186, 184–192. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, Z.; Duan, Y.; Ma, R.; Zheng, S. Synthesis of nano-TiO2/diatomite composite and its photocatalytic degradation of gaseous formaldehyde. Appl. Sur. Sci. 2017, 412, 105–112. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, G.; Sun, Z.; Zheng, S. Synthesis of natural porous minerals supported TiO2 nanoparticles and their photocatalytic performance towards Rhodamine B degradation. Powder Technol. 2014, 262, 1–8. [Google Scholar] [CrossRef]
- Zelekew, O.A.; Kuo, D.H.; Yassin, J.M.; Ahmed, K.E.; Abdullah, H. Synthesis of efficient silica supported TiO2/Ag2O heterostructured catalyst with enhanced photocatalytic performance. Appl. Sur. Sci. 2017, 410, 454–463. [Google Scholar] [CrossRef]
- Kibanova, D.; Trejo, M.; Destaillats, H.; Cervini-Silva, J. Synthesis of hectorite–TiO2 and kaolinite–TiO2 nanocomposites with photocatalytic activity for the degradation of model air pollutants. Appl. Clay Sci. 2009, 42, 563–568. [Google Scholar] [CrossRef]
- Reddy, E.P.; Sun, B.; Smirniotis, P.G. Transition metal modified TiO2-loaded MCM-41 catalysts for visible-and UV-Light driven photodegradation of aqueous organic pollutants. J. Phys. Chem. B 2004, 108, 17198–17205. [Google Scholar] [CrossRef]
- Xia, Y.; Li, F.; Jiang, Y.; Xia, M.; Xue, B.; Li, Y. Interface actions between TiO2 and porous diatomite on the structure and photocatalytic activity of TiO2-diatomite. Appl. Sur. Sci. 2014, 303, 290–296. [Google Scholar] [CrossRef]
- Anandan, S.; Yoon, M. Photocatalytic activities of the nano-sized TiO2-supported Y-zeolites. J. Photochem. Photobiol. C 2003, 4, 5–18. [Google Scholar] [CrossRef]
- Mamulová, K.K.; Tokarský, J.; Kovář, P.; Vojtěšková, S.; Kovářová, A.; Smetana, B.; Kukutschová, J.; Čapková, P.; Matějka, V. Preparation and characterization of photoactive composite kaolinite/TiO2. J. Hazard. Mater. 2011, 188, 212–220. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, Q.; Zhou, F.; Deng, X.; Li, F. Synthesis and photocatalytic performances of the TiO2 pillared montmorillonite. J. Hazard. Mater. 2012, 235, 186–193. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The role of calcium carbonate in cement hydration. Cem. Concr. Res. 2007, 37, 551–558. [Google Scholar] [CrossRef]
- Poh, C.L.; Mariatti, M.; Fauzi, M.A.; Ng, C.H.; Chee, C.K.; Chuah, T.P. Tensile, dielectric, and thermal properties of epoxy composites filled with silica, mica, and calcium carbonate. J. Mater. Sci. Mater. Electroc. 2014, 25, 2111–2119. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, Z.; Yan, Y.; Zheng, S. Effect of preparation conditions on the characteristics and photocatalytic activity of TiO2/purified diatomite composite photocatalysts. Appl. Surf. Sci. 2014, 314, 251–259. [Google Scholar] [CrossRef]
- Kun, R.; Mogyorósi, K.; Dékány, I. Synthesis and structural and photocatalytic properties of TiO2/montmorillonite nanocomposites. Appl. Clay Sci. 2006, 32, 99–110. [Google Scholar] [CrossRef]
- Tanabe, K.; Anabe, K.; Minitsuhashi, K.; Yoshida, T. Titanium Dioxide–Calcium Carbonate Composite Particles. U.S. Patent 6,991,677, 31 January 2006. [Google Scholar]
- Sun, S.; Ding, H.; Hou, X.; Chen, D.; Yu, S.; Zhou, H.; Chen, Y. Effects of organic modifiers on the properties of TiO2-coated CaCO3 composite pigments prepared by the hydrophobic aggregation of particles. Appl. Surf. Sci. 2018, 456, 923–931. [Google Scholar] [CrossRef]
- Sun, S.; Ding, H.; Hou, X. Preparation of CaCO3-TiO2 composite particles and their pigment properties. Materials 2018, 11, 1131. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, Z.; Zhang, W.; Yu, C.; Zheng, S. Highly efficient g-C3N4/TiO2/kaolinite composite with novel three-dimensional structure and enhanced visible light responding ability towards ciprofloxacin and S. aureus. Appl. Catal. B Environ. 2017, 220, 272–282. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, Y.; You, Y.; Zeng, C.; Xiao, X.; Ma, T.; Huang, H. Bifunctional Hydrogen Production and Storage on 0D–1D Heterojunction of Cd0.5Zn0.5S@Halloysites. Adv. Funct. Mater. 2019, 1903825. [Google Scholar] [CrossRef]
- Li, G.; Park, S.; Rittmann, B.E. Developing an efficient TiO2-coated biofilm carrier for intimate coupling of photocatalysis and biodegradation. Water. Res. 2012, 46, 6489–6496. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Sun, S.; Pan, L.; Xu, Z.; Ding, H.; Li, W. Preparation and Properties of CaCO3-Supported Nano-TiO2 Composite with Improved Photocatalytic Performance. Materials 2019, 12, 3369. https://doi.org/10.3390/ma12203369
Wang J, Sun S, Pan L, Xu Z, Ding H, Li W. Preparation and Properties of CaCO3-Supported Nano-TiO2 Composite with Improved Photocatalytic Performance. Materials. 2019; 12(20):3369. https://doi.org/10.3390/ma12203369
Chicago/Turabian StyleWang, Jie, Sijia Sun, Lei Pan, Zhuoqun Xu, Hao Ding, and Wei Li. 2019. "Preparation and Properties of CaCO3-Supported Nano-TiO2 Composite with Improved Photocatalytic Performance" Materials 12, no. 20: 3369. https://doi.org/10.3390/ma12203369
APA StyleWang, J., Sun, S., Pan, L., Xu, Z., Ding, H., & Li, W. (2019). Preparation and Properties of CaCO3-Supported Nano-TiO2 Composite with Improved Photocatalytic Performance. Materials, 12(20), 3369. https://doi.org/10.3390/ma12203369




