Abstract
Two series of 2,5-dihalopyridine-Cu(I)A (A = I, Br) complexes based on 2-X-5-iodopyridine and 2-X-5-bromopyridine (X = F, Cl, Br and I) are characterized by using single-crystal X-ray diffraction analysis to examine the nature of C2−X2···A–Cu and C5−X5···A–Cu halogen bonds. The reaction of the 2,5-dihalopyridines and Cu(I) salts allows the synthesis of eight 1-D coordination polymers and a discrete structure. The resulting Cu(I)-complexes are linked by C−X···A–Cu halogen bonds forming 3-D supramolecular networks. The C−X···A–Cu halogen bonds formed between halopyridine ligands and copper(I)-bound halide ions are stronger than C−X···X’–C interactions between two 2,5-dihalopyridine ligands. The C5−I5···I–Cu and C5−Br5···Br–Cu halogens bonds are shorter for C2-fluorine than C2-chlorine due to the greater electron-withdrawing power of fluorine. In 2,5-diiodopyridine-Cu(I)Br complex, the shorter C2−I2···Br–Cu [3.473(5) Å] distances are due to the combined polarization of C2-iodine by C2−I2···Cu interactions and para-electronic effects offered by the C5-iodine, whilst the long halogen bond contacts for C5−I5···Br–Cu [3.537(5) Å] are indicative that C2-iodine has a less para-electronic influence on the C5-iodine. In 2-fluoro-5-X-pyridine-Cu(I) complexes, the C2-fluorine is halogen bond passive, while the other C2-halogens in 2,5-dihalopyridine-Cu(I), including C2-chlorine, participate in halogen bonding interactions.
1. Introduction
Supramolecular chemistry utilizes small molecules and non-covalent interactions to self-assembly molecular aggregates with properties that are different from their individual components [1]. The design and construction of materials by self-assembly are largely predestined based on hydrogen bonding interactions due to the small size, easily polarizable and ubiquitous nature of the hydrogen atom [2]. Hydrogen bonding is observed in organic, as well as coordination, compounds. Over the last few years, orthogonal and conceptually similar to hydrogen bonds, halogen bonding has been studied as an additional important non-covalent interaction [3]. The halogen bonding is highly directional and the contact distances between donor and acceptor molecules can be modulated, due to the polarization hierarchy of different halogen atoms, in co-crystals for applications in material science [4] and solid-state research [5].
The examination of halogen bonds in coordination compounds is still an underrepresented topic in halogen bond (XB) research [6,7,8,9,10,11,12,13,14,15]. mono-Halopyridines are common organic moieties used for exploring halogen bonds in coordination complexes [16,17,18,19]. The N–M (N = nitrogen, M = metal) coordination bond increases the σ-hole strength on the halogen atom that is covalently bound to the core aromatic ring [20,21]. The polarization also leads to the formation of anisotropically distributed electron density on the halogen atom orthogonal to the σ-hole facilitating XB acceptor properties. Two types of halogen bonds, therefore, are typically expected in coordination complexes, C–X···A–M [X = XB donor, A = XB acceptor] and C–X···X′–C [X = XB donor, X′ = XB acceptor] [22]. Square planar complexes of the type [M(mono-halopyridine)2A2] (M = Cu, Ni, Pt, Pd; A = Cl, Br, I) [23,24,25,26,27,28] and co-crystals of the type [(mono-halopyridine)H]+[MAn]− (for example, MAn = CoBr4, CoCl4, PtCl6) [29,30,31,32] have been studied as earlier contributions for halogen bonds in coordination complexes. In X-ray crystal structures, the short X···A distances and C–X···A linear angles are evidence for halogen bonds [33], the geometrical parameters typically utilized to characterize the hydrogen bonding interactions [34].
Our interest in the halogen bonding of coordination complexes led us to utilize 2,5-dihalopyridine to prepare 2:1 ligand:metal ratio [Cu(2,5-dihalopyridine)2A2] complexes, and to examine the electronic influence of C2-halogens on C5–X5···A–Cu halogen bonds and C5-halogens on C2–X2···A–Cu halogen bonds (A = Cl, Br) [35,36]. The electronic or substituent effects are monitored by measuring the C2–X2···A–Cu and C5–X5···A–Cu distances, and compared with the respective C2–X2···A–Cu and C3–X3···A–Cu distances in [Cu(2-halopyridine)2A2] and [Cu(3-halopyridine)2A2] complexes. This strategy also allowed us to rank the XB strengths for C5–X5···A–Cu (A = Cl, Br) halogen bonds, while a mixed order was observed for C2–X2···A–Cu halogen bonds. The X-ray crystal structures of [Cu(2,5-dihalopyridine)2A2] complexes are discrete 2:1 ligand:metal complexes. Therefore, the electrostatic attractions between externally directed Cu(II)-bound halide and electrophilic halogen of a 2,5-dihalopyridine for C–X···A–M halogen bonds are the major determinants when compared to the crystal packing forces. Consequently, the [Cu(2,5-dihalopyridine)2A2] complexes were the ideal candidates to understand the electronic effects in C–X···A–M halogen bonds. Although the C2– and C5–halogen polarizations seem to imply the strong dependence on the electronic structure of the pyridine ring, the C–X···A–M halogen bonds could be sensitive to the dimensionality of copper complexes. In this study, the Cu(I) salts provided a means for understanding the nature of C2–X2···A–Cu and C5–X5···A–Cu (A = Br, I) halogen bonds in 2,5-dihalopyridine-Cu(I) coordination polymeric structures.
2. Results
Nine Cu(I)-complexes were obtained by mixing a 1:1 molar ratio of six different 2,5-dihalopyridines (1–6, Figure 1) and two copper(I) halides (CuI and CuBr) in a 1:1 ratio of acetonitrile:ethanol mixture whilst heating gently. Slow evaporation of the resulting solutions provided single crystals suitable for X-ray diffraction analysis. Our attempts to grow single crystals of 5-bromo-2-iodopyridine-Cu(I) and 5-bromo-2-chloropyridine-Cu(I) complexes were unsuccessful. The Cu(I)-complexes of ligands 1–6 are labelled using the letter “a” for 2,5-dihalopyridine-CuI and “b” for 2,5-dihalopyridine-CuBr complexes. Five complexes (1a, 1b, 2a, 3a, 5a) form isostructural 1-D coordination polymers, whilst the other three complexes (2b, 4b, and 6b) show different structures and are described individually.
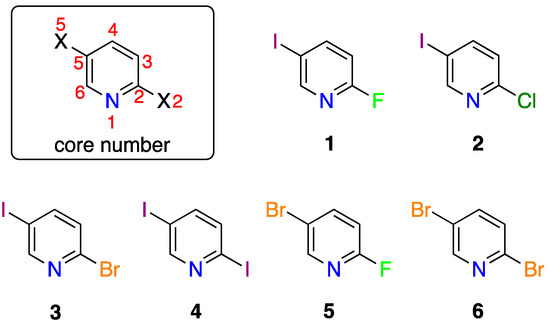
Figure 1.
Chemical structures of 2,5-dihalopyridines: 2-fluoro-5-iodopyridine (1), 2-chloro-5-iodopyridine (2), 2-bromo-5-iodopyridine (3), 2,5-diiodopyridine (4), 2-fluoro-5-bromopyridine (5), and 2,5-dibromopyridine (6).
In complexes 1a, 1b, 2a, 3a, and 5a, two Cu(I) ions and two halide atoms form Cu2A2 (A = I, Br) rhomboid-like units, where the two edges of each rhomboid are shared by two other adjacent rhomboids to yield 1-D coordination polymer ladders (Figure 2a,c,e,g). The Cu(I) ions are coordinated by one pyridine nitrogen and three halide atoms in a tetrahedral geometry with NA3 coordination spheres. The pyridine ligands in the ladder are parallel to each other, not forming any π–π interaction between the aromatic rings, indicated by adjacent aromatic centroid-to-centroid distances of 4.30 Å [1a], 4.0 Å [1b], 4.30 Å [2a], 4.20 Å [3a], and 4.20 Å [5a]. The small aromatic centroid-to-centroid distances further suggested that the interdigitation between 1-D polymers is not feasible. In the crystal lattice, C5-halogens and Cu(I)-bound halides of adjacent 1-D coordination polymer ladders manifest C5–X5∙∙∙A–Cu halogen bonds, as shown in Figure 2b,d,f,h. The higher the electronegativity of the C2-halogen, the shorter the C5–X5∙∙∙I–Cu halogen bonds. For example, the C5–I5∙∙∙I–Cu halogen bond (RXB = 0.92) in 1a is shorter in comparison to the C5–I5∙∙∙I–Cu halogen bonds (RXB = 0.93) in 2a due to the higher electron-withdrawing nature of the C2-fluorine when compared to C2-chlorine. The electron-withdrawing effect of C2-halogen is also observed in 5a and 6a complexes for C5–Br5∙∙∙I–Cu halogen bonds. The C2-halogens in 1a, 1b, 2a, and 5a are XB passive, meaning they did not function as XB-donor and acceptors.
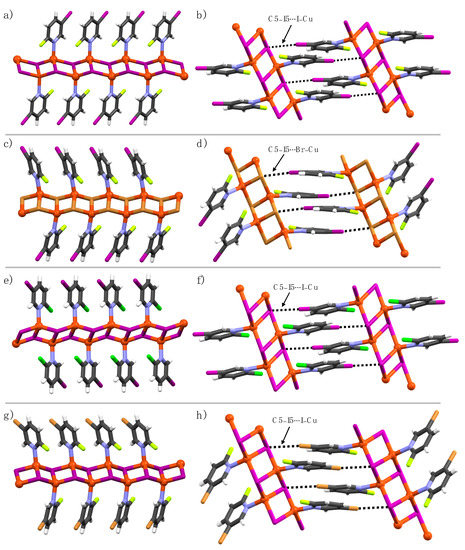
Figure 2.
1-D Polymeric view of (a) 1a, (b) 1b, (c) 2a, and (d) 5a, and (e–h) their respective section of crystal packing structures displaying halogen bonding interactions (black dotted lines).
The complex formed by 2-bromo-5-iodopyridine (3) and CuI is the exotic structure of the 2-X-5-iodopyridine series (X = F, Cl, Br, and I). Typically, C2–X2∙∙∙I–Cu or C5–X5∙∙∙I–Cu XBs are observed but in this case, C2- and C5-halogens form C2–Br2∙∙∙I5–C5 halogen bonds. The C2-bromine is the XB donor and C5-iodine is the XB acceptor with ∠C2–Br2∙∙∙I5 = 172(1)° (Figure 3). The C2–Br2∙∙∙I5–C5 halogen bond contacts are just below the sum of the van der Waals radii of bromine and iodine atoms (3.83 Å). This could be either due to the less electron-withdrawing power of C2-bromine or the weak donating ability of C5-iodine caused by crystal-packing interactions. The copper(I)-bound iodine ion is XB passive, and only forms C4–H4∙∙∙I–Cu interactions with a distance of 3.10 Å (∠C4–H4∙∙∙I = 148°). Furthermore, the C5-iodines from the adjacent 1-D polymeric structures display weak C5–I5∙∙∙I5′–C5′ interactions at distances of 3.936(4) Å [RXB = 0.99; ∠C5–I5∙∙∙I5′ = ∠C5′–I5′∙∙∙I5 = 146.6(2)].
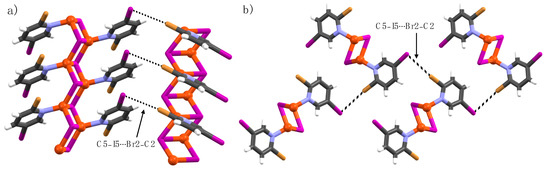
Figure 3.
(a,b) X-Ray crystal structure of 3a viewed from two different directions. Halogen bonds are depicted by using black dotted lines.
Single crystals of complex 1b・ACN were isolated from the bulk sample 1b. The asymmetric unit contains two crystallographically independent Cu(I) ions, a pyridine ligand and coordinating acetonitrile solvent molecule. Both Cu(I) ions have tetrahedral geometry with a NBr3 coordination sphere. However, one Cu(I)-centre is coordinated by pyridine nitrogen and three μ2-Br ions while the second Cu(I)-centre is coordinated by acetonitrile nitrogen and three μ2-Br ions, as shown in Figure 4a. The CuBr cluster in 1b・ACN extends into a 1-D polymeric structure, similar to complexes 1a, 1b, 2a, and 5a, with Cu(I) coordinated pyridine ligands and acetonitrile solvents decorated on the opposite side, as depicted in Figure 4a. The C5-iodine and Cu(I)-bound bromide from adjacent 1-D chains form C5–I5∙∙∙Br–Cu halogen bonds which are shorter in comparison to C5–I5∙∙∙Br–Cu XBs in 1b. In the crystal packing, the C2-fluorine and acetonitrile sp and sp3 carbon atoms are electrostatically driven closer for C2–F2∙∙∙C(sp) [d = 2.790(2) Å, ∠C2–F2∙∙∙C = 145.3(9)°] and C2–F2∙∙∙C(sp3) [d = 3.076 (2) Å, ∠C2–F2∙∙∙C = 171.2(8)°] short contacts, as shown in Figure 4b.
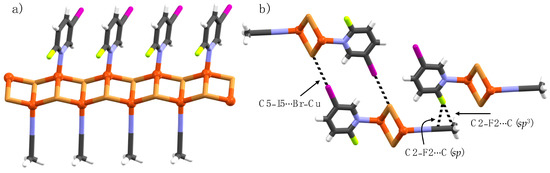
Figure 4.
(a) 1-D Polymer view of 1a・ACN, and (b) section of crystal packing viewing halogen bonds and C2–F2∙∙∙C interactions (black dotted lines).
Complex 4b crystallizes in the triclinic space group P-1 and is a 1-D polymeric chain with a CuBr cluster core different to 1b. The Cu(I) ions are coordinated in a trigonal planar fashion by two iodine ions and a pyridine nitrogen (Figure 5). Although the C2- and C5-positions in ligand 4 are iodine, which is typically known for its strong XB-donor ability, the C2–I2∙∙∙Br–Cu halogen bonds are shorter than the C5–I5∙∙∙Br–Cu XBs due to additional polarization caused by C2–I2∙∙∙Cu interactions [3.333(5) Å] and the electron-withdrawing C5-halogen. The short C2–I2∙∙∙Cu contacts are similar to C2–Br2∙∙∙Cu interactions in our previously reported [Cu(2,5-dihalopyridine)2Br2] complexes [31].
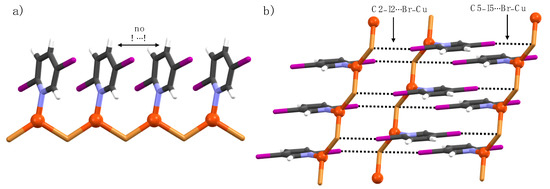
Figure 5.
(a) X-Ray crystal Structure of 4b, and (b) section of 1-D polymer chains in crystal packing displaying halogen bonds (black dotted lines).
Complex 6a crystallizes in the monoclinic space group P21/n. The asymmetric unit contains one pyridine ligand, two Cu(I) ions, and two iodide ions. The CuI and nitrogen in pyridine ligands of 6a form a 1-D honeycomb-like coordination polymer with Cu6I6N2 nodes, as shown in Figure 6. Both Cu(I) ions are tetrahedrally coordinated. One Cu(I) is coordinated by one pyridine nitrogen, one μ3- and two μ4-iodide ions, and the other Cu(I) is coordinated by two μ3- and two μ4-iodide ions, leading to Cu(I)-centers with NI3 and I4 coordination spheres, respectively. The pyridine ligands are decorated parallel to each other above and below the 1-D chain, with centroid-to-centroid distances of 4.20 Å. The C5-bromine σ-hole and nucleophilic μ3-iodide ion form C5–I5∙∙∙I–Cu halogen bonds whilst the C5-bromine anisotropic electron ′′belt′′ at the orthogonal directions and C2-bromide σ-hole form a C5–I5∙∙∙I2–C2 type halogen bond. The μ4-iodide ion is XB passive.
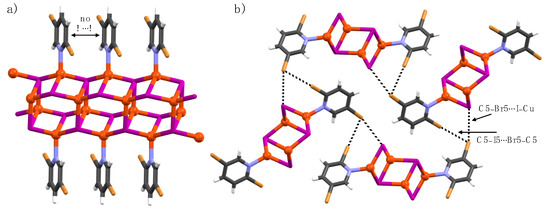
Figure 6.
(a) X-Ray crystal structure of 6a, and (b) section of crystal packing displaying halogen bonds. Halogen bonds are depicted by black dotted lines.
Complex 2b is the only example of a discrete Cu(I)-complex. The asymmetric unit consists of three crystallographically independent pyridine ligands (Py1-Py3) and two Cu(I) ions with a tetrahedral and a trigonal planar coordination geometry, respectively. In the molecular packing, the discrete structures exhibit several halogen bonds and halogen∙∙∙halogen interactions; the respective distances and bond parameters are given in Table 1. Within the asymmetric unit, the C2-chlorine (Py1) and C5-iodine (Py3) attract electrostatically to form weak (Py1)C2–Cl2∙∙∙I5–C5(Py3) halogen bonds with distances of 3.688(3) [∠C2–Cl2∙∙∙I5 = 176.8(4); RXB = 0.99]. The C2-chlorine (Py1) is an XB-donor to the C5-iodine (Py3) XB-acceptor (Figure 7b). These halogen-bond contacts are weak, just below the sum of the van der Waals radii of Cl- and I-atoms [3.73 Å]. The C5-iodine substituents of Py1 and Py2 form relatively strong halogen bonds between two crystallographically independent copper-bound bromines (Figure 7b,c). The C5-iodine of Py3 exhibits two other halogen∙∙∙halogen contacts to C2-chlorines of (Py3′’) [RXB = 0.95] and (Py2′) [RXB = 0.98] to neighboring complexes, as depicted in Figure 7c. The strongest interactions are observed between C5-iodine substituents and nucleophilic Cu(I)-bound bromine ions.

Table 1.
The solid-state X-ray crystal structure C–X∙∙∙A–Cu bond parameters of 2,5-dihalopyridine-Cu(I) complexes.
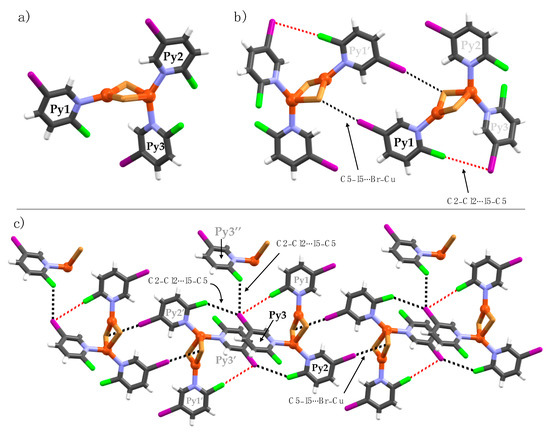
Figure 7.
Crystal structure of 2b. Halogen bonds are depicted as black and red dotted lines focusing on (a) Py1, (b) Py2 and (c) Py3.
3. Conclusions
The present study shows the application of rarely used 2,5-dihalopyrdine ligands to prepare copper(I)-coordination polymers that are stabilized by halogen bond interactions in the solid-state. The copper(I) complexes extend into 3-D supramolecular networks through C2–X2···A–Cu and C5–X5···A–Cu halogen bonds (X = Cl, Br, I; and A = Br, I) between the X2- or X5-halogen substituent (donor) and the copper(I)-bound halide anion (acceptor). With the exception of the C2-fluorine, all C2- and C5-halogen substituents, including the more electronegative C2-chlorine, participate in halogen bond interactions. Strong halogen bonds are formed between the C5-halogen substituent and the nucleophilic halide ions coordinated to copper(I), with C5-halogen always acting as XB donor and the halide anion as an XB acceptor. The flexible copper(I) coordination sphere allows the 2,5-dihalopyridine halogen substituents to function as a halogen bond acceptor for C–X···X’–C interactions, in addition to C2–X2···A–Cu, C5–X5···A–Cu halogen bonds. This feature is not promoted by copper(II) in our previously reported discrete structures of [Cu(2,5-dihalopyridine)2A2]. In 2-X-5-iodopyridine-Cu(I)A complexes (X = F, Cl, and A = Br, I), the C5–X5···A–Cu halogen bonds are shorter for C2-fluorine than C2-chlorine due to the higher electron-withdrawing effect of fluorine, similar to our previously reported [Cu(2,5-dihalopyridine)2A2] complexes. Of the four ligands, 2-chloro-5-iodopyridine, 2-bromo-5-iodopyridine, 2,5-diiodopyridine, and 2,5-dibromopyridine, only C2-iodine of 2,5-diiodopyridine displays C2–I2···Cu short contacts. This effect depends on the coordination geometry of the Cu(I)-center and is widely observed in square planar [Cu(2,5-dihalopyridine)2A2] complexes.
4. Experimental Section
General information: In the crystallization experiments, all used solvents were of reagent grade and were used as received from the supplier. The pyridine ligands, 2-fluoro-5-iodopyridine (1), 2-chloro-5-iodopyridine (2), 2-bromo-5-iodopyridine (3), 2,5-diiodopyridine (4), 5-bromo-2-fluoropyridine (5), and 2,5-dibromopyridine (6) are commercially available (TCI Chemicals Europe), the CuBr and CuI salts were purchased from Sigma Aldrich.
General synthesis of complexes 1a–6a: The solid 2,5-dihalopyridine (0.1046 mmol) was added to the solution of CuA (A = Br, I) (0.1046 mmol) in acetonitrile/ethanol (2.0 mL). If needed, the solutions were heated to dissolve the components. Single-crystals suitable for X-ray diffraction analysis were obtained by slow evaporation of the corresponding solutions.
Crystal structure determination: The X-ray data for 1b, 2a, 2b, 4b and 5a were obtained by using a Bruker-Nonius Kappa CCD diffractometer with an APEX-II CCD detector utilizing graphite-monochromated Mo-Kα (λ = 0.71073 Å) radiation. Those of 1b・ACN, 3a, and 6a data were collected with a Rigaku Oxford Diffraction SuperNova instrument with an EoS CCD detector. The used Mo-Kα (λ = 0.71073 Å) radiation was monochromatized using multi-layer optics. The data for 1a was collected using the Rigaku SuperNova dual-source Oxford diffractometer equipped with an Atlas detector using mirror-monochromated Cu-Kα (λ = 1.54184 Å) radiation. The CrysAlisPro program and Gaussian face-index absorption correction method were used for data collection and reduction for 1a, 1b・ACN, 3a, and 6a. For the data collection and reduction for 1b, 2a, 2b, 4b and 5a, the programs COLLECT and HKL DENZO AND SCALEPACK [38] were used. The intensities were multi-scan absorption-corrected using SADABS [39]. Direct methods (SHELXS) [40] and full-matrix least squares on F2 using the OLEX2 software [41] with SHELXL-2013 module [40] were used for all structures.
Crystal data for 1a: CCDC-1951451, C5H3CuFI2N, M = 413.42, colorless needle, 0.373 × 0.032 × 0.026 mm3, triclinic, space group P-1, a = 4.2414(9) Å, b = 8.9099(15) Å, c = 11.1529(17) Å, α = 77.445(14)°, β = 82.242(15)°, γ = 85.595(15)°, V = 407.14(13) Å3, Z = 2, Dc = 3.372 g/cm3, F000 = 368, μ= 62.874 mm−1, T = 120.01(10) K, θmax = 66.728°, 3438 total reflections, 1299 with Io > 2σ(Io), Rint = 0.0603, 1430 data, 79 parameters, 0 restraints, GooF = 1.055, R = 0.0613 and wR= 0.0644 [Io > 2σ(Io)], R = 0.1586 and wR= 0.1639 (all reflections), 3.472 < d∆ρ < −2.035 e/Å3.
Crystal data for 1b: CCDC-1951453, C5H3BrCuFIN, M = 366.43, colorless plate, 0.14 × 0.014 × 0.06 mm3, monoclinic, space group P21/n, a = 8.7766(18) Å, b = 4.0769(8) Å, c = 21.701(4) Å, α = 90°, β = 100.81(3)°, γ = 90°, V = 762.7(3) Å3, Z = 4, Dc = 3.191 g/cm3, F000 = 664, μ= 12.083 mm−1, T = 170.0(1) K, θmax = 24.994°, 5261 total reflections, 1154 with Io > 2σ(Io), Rint = 0.0603, 1329 data, 91 parameters, 12 restraints, GooF = 1.272, R = 0.0913 and wR = 0.2320 [Io > 2σ(Io)], R = 0.1006 and wR = 0.2352 (all reflections), 2.593 < d∆ρ < −2.201 e/Å3.
Crystal data for 1b・ACN: CCDC-1951452, C7H6Br2Cu2FIN2, M = 550.94, colorless needle, 0.07 × 0.03 × 0.021 mm3, monoclinic, space group P21/n, a = 3.9980(2) Å, b = 8.7053(5) Å, c = 35.319(3) Å, α = 90°, β = 91.118(6)°, γ = 90°, V = 1229.00(13) Å3, Z = 4, Dc = 2.978 g/cm3, F000 = 1008, μ= 12.454 mm−1, T = 120.0(1) K, θmax = 29.879°, 8335 total reflections, 2393 with Io > 2σ(Io), Rint = 0.0845, 3229 data, 138 parameters, 6 restraints, GooF = 1.202, R = 0.0999 and wR = 0.1436 [Io > 2σ(Io)], R = 0.1363 and wR = 0.1551 (all reflections), 1.996 < d∆ρ < −3.084 e/Å3.
Crystal data for 2a: CCDC-1951454, C5H3ClCuI2N, M = 429.87, colorless plate, 0.19 × 0.11 × 0.08 mm3, monoclinic, space group P21/c, a = 4.2871(9) Å, b = 18.208(4) Å, c = 11.464(2) Å, α = 90°, β = 99.46(3)°, γ = 90°, V = 882.7(3) Å3, Z = 4, Dc = 3.235 g/cm3, F000 = 768, μ= 9.696 mm−1, T = 170.0(1) K, θmax = 25.248°,5962 total reflections, 1343 with Io > 2σ(Io), Rint = 0.0406, 1592 data, 91 parameters, 0 restraints, GooF = 1.024, R = 0.0259 and wR = 0.0453 [Io > 2σ(Io)], R = 0.1006 and wR = 0.0478 (all reflections), 0.737 < d∆ρ < −0.610 e/Å3.
Crystal data for 2b: CCDC-1951455, C15H9Br2Cl3Cu2I3N3, M = 1005.20, colorless needle, 0.06 × 0.028 × 0.027 mm3, triclinic, space group P-1, a = 8.4309(17) Å, b = 9.4460(19) Å, c = 16.953(3) Å, α = 100.81(3)°, β = 97.74(3)°, γ = 112.52(3)°, V = 1192.9(5) Å3, Z = 2, Dc = 2.799 g/cm3, F000 = 916, μ= 9.359 mm−1, T = 170.0(1) K, θmax = 25.250°, 7687 total reflections, 3498 with Io > 2σ(Io), Rint = 0.0531, 4270 data, 253 parameters, 0 restraints, GooF = 1.018, R = 0.0541 and wR = 0.1348 [Io > 2σ(Io)], R = 0.0674 and wR = 0.1432 (all reflections), 2.097 < d∆ρ < −1.399 e/Å3.
Crystal data for 3a: CCDC- 1951456, C5H3BrCuI2N, M = 474.33, colorless needle, 0.181 × 0.067 × 0.048 mm3, monoclinic, space group P21/c, a = 4.1815(3) Å, b = 14.7272(14) Å, c = 14.8718(12) Å, α = 90°, β = 91.173(7)°, γ = 90°, V = 915.64(13) Å3, Z = 4, Dc = 3.441 g/cm3, F000 = 840, μ= 13.420 mm−1, T = 170.0(1) K, θmax = 25.248°, 4840 total reflections, 1505 with Io > 2σ(Io), Rint = 0.0432, 1646 data, 49 parameters, 0 restraints, GooF = 1.248, R = 0.1279 and wR = 0.3369 [Io > 2σ(Io)], R = 0.1319 and wR = 0.3384 (all reflections), 5.704 < d∆ρ < −2.773 e/Å3.
Crystal data for 4b: CCDC-1951457, C5H3BrCuI2N, M = 474.33, colorless needle, 0.12 × 0.11 × 0.09 mm3, triclinic, space group P-1, a = 4.0630(8) Å, b = 8.9680(18) Å, c = 12.931(3) Å, α = 74.44(3)°, β = 85.36(3)°, γ = 84.90(3)°, V = 451.31(17) Å3, Z = 2, Dc = 3.491 g/cm3, F000 = 420, μ= 13.613 mm−1, T = 170.0(1) K, θmax = 24.998°, 2593 total reflections, 1381 with Io > 2σ(Io), Rint = 0.0400, 1513 data, 80 parameters, 36 restraints, GooF = 1.220, R = 0.0930 and wR = 0.2613 [Io > 2σ(Io)], R = 0.1002 and wR = 0.2652 (all reflections), 4.442 < d∆ρ < −2.380 e/Å3.
Crystal data for 5a: CCDC-1951458, C5H3BrCuFIN, M = 366.43, colorless plate, 0.409 × 0.071 × 0.043 mm3, monoclinic, space group P21/n, a = 8.9290(18) Å, b = 4.1740(8) Å, c = 21.620(4) Å, α = 90°, β = 101.57(3)°, γ = 90°, V = 789.4(3) Å3, Z = 4, Dc = 3.083 g/cm3, F000 = 664, μ= 11.675 mm−1, T = 170.0(1) K, θmax = 28.363°, 6126 total reflections, 1630 with Io > 2σ(Io), Rint = 0.0343, 1932 data, 91 parameters, 0 restraints, GooF = 1.164, R = 0.0354 and wR = 0.0795 [Io > 2σ(Io)], R = 0.0464 and wR = 0.0834 (all reflections), 1.100 < d∆ρ < −0.862 e/Å3.
Crystal data for 6a: CCDC-1951459, C5H3Br2Cu2I2N, M = 617.78, colorless needle, 0.14 × 0.09 × 0.08 mm3, monoclinic, space group P21/n, a = 4.19570(10) Å, b = 13.8695(7) Å, c = 18.9218(7) Å, α = 90°, β = 90.509(3)°, γ = 90°, V = 1101.06(7) Å3, Z = 4, Dc = 3.727 g/cm3, F000 = 1096, μ= 16.674 mm−1, T = 170.03(10) K, θmax = 25.240°, 6988 total reflections, 1655 with Io > 2σ(Io), Rint = 0.0457, 1982 data, 109 parameters, 0 restraints, GooF = 1.019, R = 0.0294 and wR = 0.0615 [Io > 2σ(Io)], R = 0.0390 and wR = 0.0676 (all reflections), 0.927 <d∆ρ < −0.888 e/Å3.
Author Contributions
The project was designed by R.P., and the single-crystal X-ray diffraction analysis for copper (I) complexes was performed by C.v.E and R.P. The manuscript was written by R.P., and edited from contributions of all co-authors C.v.E., R.P., and K.R.
Funding
The authors kindly acknowledge the Academy of Finland (Project numbers RP: 298817) and the University of Jyväskylä for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lehn, J.-M. Toward complex matter: Supramolecular chemistry and self-organization. Proc. Natl. Acad. Sci. USA 2002, 99, 4763–4768. [Google Scholar] [CrossRef] [PubMed]
- Gilli, G.; Gilli, P. The Nature of the Hydrogen Bond: Outline of a Comprehensive Hydrogen Bond Theory; International Union of Crystallography Monographs on Crystallography; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef]
- Metrangolo, P.; Resnati, G. Halogen Bonding I: Impact on Materials Chemistry and Life Sciences; Topics in Current Chemistry; Springer International Publishing: Berlin, Germany, 2015. [Google Scholar]
- Ding, X.; Tuikka, M.; Haukka, M. Halogen Bonding in Crystal Engineering; Recent Advances in Crystallography; Benedict, J.B., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Li, B.; Zang, S.-Q.; Wang, L.-Y.; Mak, T.C.W. Halogen bonding: A powerful, emerging tool for constructing high-dimensional metal-containing supramolecular networks. Coord. Chem. Rev. 2016, 308, 1–21. [Google Scholar] [CrossRef]
- Bertani, R.; Sgarbossa, P.; Venzo, A.; Lelj, F.; Amati, M.; Resnati, G.; Pilati, T.; Metrangolo, P.; Terraneo, G. Halogen bonding in metal–organic–supramolecular networks. Coord. Chem. Rev. 2010, 254, 677–695. [Google Scholar] [CrossRef]
- Sivchik, V.; Sarker, R.K.; Liu, Z.-Y.; Chung, K.-Y.; Grachova, E.V.; Karttunen, A.J.; Chou, P.-T.; Koshevoy, I.O. Improvement of the Photophysical Performance of Platinum-Cyclometalated Complexes in Halogen-Bonded Adducts. Chem. A Eur. J. 2018, 24, 11475–11484. [Google Scholar] [CrossRef]
- Derossi, S.; Brammer, L.; Hunter, C.A.; Ward, M.D. Halogen Bonded Supramolecular Assemblies of [Ru(bipy)(CN)4]2− Anions and N-Methyl-Halopyridinium Cations in the Solid State and in Solution. Inorg. Chem. 2009, 48, 1666–1677. [Google Scholar] [CrossRef] [PubMed]
- Kalaj, M.; Carter, K.P.; Cahill, C.L. Isolating Equatorial and Oxo Based Influences on Uranyl Vibrational Spectroscopy in a Family of Hybrid Materials Featuring Halogen Bonding Interactions with Uranyl Oxo Atoms. Eur. J. Inorg. Chem. 2017, 2017, 4702–4713. [Google Scholar] [CrossRef]
- Carter, K.P.; Kalaj, M.; Cahill, C.L. Harnessing uranyl oxo atoms via halogen bonding interactions in molecular uranyl materials featuring 2,5-diiodobenzoic acid and N-donor capping ligands. Inorg. Chem. Front. 2017, 4, 65–78. [Google Scholar] [CrossRef]
- Carter, K.P.; Kalaj, M.; Surbella, R.G., III; Ducati, L.C.; Autschbach, J.; Cahill, C.L. Cover Feature: Engaging the Terminal: Promoting Halogen Bonding Interactions with Uranyl Oxo Atoms. Chem. A Eur. J. 2017, 23, 15355–15369. [Google Scholar] [CrossRef]
- Ormond-Prout, J.E.; Smart, P.; Brammer, L. Cyanometallates as Halogen Bond Acceptors. Cryst. Growth Des. 2012, 12, 205–216. [Google Scholar] [CrossRef]
- Oliveira, V.; Cremer, D. Transition from metal-ligand bonding to halogen bonding involving a metal as halogen acceptor a study of Cu, Ag, Au, Pt, and Hg complexes. Chem. Phys. Lett. 2017, 681, 56–63. [Google Scholar] [CrossRef]
- Puttreddy, R.; Peuronen, A.; Lahtinen, M.; Rissanen, K. Metal-Bound Nitrate Anion as an Acceptor for Halogen Bonds in Mono-Halopyridine-Copper(II) Nitrate Complexes. Cryst. Growth Des. 2019, 19, 3815–3824. [Google Scholar] [CrossRef]
- Brammer, L.; Minguez Espallargas, G.; Libri, S. Combining metals with halogen bonds. CrystEngComm 2008, 10, 1712–1727. [Google Scholar] [CrossRef]
- Gorokh, I.D.; Adonin, S.A.; Novikov, A.S.; Usoltsev, A.N.; Plyusnin, P.E.; Korolkov, I.V.; Sokolov, M.N.; Fedin, V.P. Halobismuthates with 3-iodopyridinium cations: Halogen bonding-assisted crystal packing. Polyhedron 2019, 166, 137–140. [Google Scholar] [CrossRef]
- Adonin, S.A.; Gorokh, I.D.; Novikov, A.S.; Samsonenko, D.G.; Yushina, I.V.; Sokolov, M.N.; Fedin, V.P. Halobismuthates with halopyridinium cations: appearance or non-appearance of unusual colouring. CrystEngComm 2018, 20, 7766–7772. [Google Scholar] [CrossRef]
- Adonin, S.A.; Gorokh, I.D.; Novikov, A.S.; Abramov, P.A.; Sokolov, M.N.; Fedin, V.P. Halogen Contacts-Induced Unusual Coloring in BiIII Bromide Complex: Anion-to-Cation Charge Transfer via Br⋅⋅⋅Br Interactions. Chem. A Eur. J. 2017, 23, 15612–15616. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding and other sigma-hole interactions: a perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Halogen Bonding: An Interim Discussion. Chem. Phys. Chem. 2013, 14, 278–294. [Google Scholar] [CrossRef]
- Bui, T.T.T.; Dahaoui, S.; Lecomte, C.; Desiraju, G.R.; Espinosa, E. The Nature of Halogen⋅⋅⋅Halogen Interactions: A Model Derived from Experimental Charge-Density Analysis. Angew. Chem. Int. Ed. 2009, 48, 3838–3841. [Google Scholar] [CrossRef]
- Awwadi, F.F.; Willett, R.D.; Haddad, S.F.; Twamley, B. The Electrostatic Nature of Aryl–Bromine–Halide Synthons: The Role of Aryl–Bromine–Halide Synthons in the Crystal Structures of the trans-Bis(2-bromopyridine)dihalocopper(II) and trans-Bis(3-bromopyridine)dihalocopper(II) Complexes. Cryst. Growth Des. 2006, 6, 1833–1838. [Google Scholar] [CrossRef]
- Awwadi, F.F.; Willett, R.D.; Twamley, B.; Turnbull, M.M.; Landee, C.P. Dual Behavior of Bromine Atoms in Supramolecular Chemistry: The Crystal Structure and Magnetic Properties of Two Copper(II) Coordination Polymers. Cryst. Growth Des. 2015, 15, 3746–3754. [Google Scholar] [CrossRef]
- Awwadi, F.F.; Haddad, S.F.; Turnbull, M.M.; Landee, C.P.; Willett, R.D. Copper-halide bonds as magnetic tunnels; structural, magnetic and theoretical studies of trans-bis(2,5-dibromopyridine)dihalo copper(II) and trans-bis(2-bromopyridine)dibromo copper(II). Cryst. Eng. Comm. 2013, 15, 3111–3118. [Google Scholar] [CrossRef]
- Awwadi, F.; Willett, R.D.; Twamley, B. Tuning Molecular Structures Using Weak Noncovalent Interactions: Theoretical Study and Structure of trans-Bis(2-chloropyridine)dihalocopper(II) and trans-Bis(3-chloropyridine)dibromocopper(II). Cryst. Growth Des. 2011, 11, 5316–5323. [Google Scholar] [CrossRef]
- Awwadi, F.F.; Turnbull, M.M.; Alwahsh, M.I.; Haddad, S.F. May halogen bonding interactions compete with Cu⋯Cl semi-coordinate bonds? Structural, magnetic and theoretical studies of two polymorphs of trans-bis(5-bromo-2-chloro pyridine)dichlorocopper(II) and trans-bis(2,5-dichloropyridine)dichlorocopper(II). New J. Chem. 2018, 42, 10642–10650. [Google Scholar] [CrossRef]
- Awwadi, F.F.; Willett, R.D.; Peterson, K.A.; Twamley, B. The Nature of Halogen⋅⋅⋅Halogen Synthons: Crystallographic and Theoretical Studies. Chem. A Eur. J. 2006, 12, 8952–8960. [Google Scholar] [CrossRef] [PubMed]
- Zordan, F.; Purver, S.L.; Adams, H.; Brammer, L. Halometallate and halide ions: nucleophiles in competition for hydrogen bond and halogen bond formation in halopyridinium salts of mixed halide-halometallate anions. Cryst. Eng. Comm. 2005, 7, 350–354. [Google Scholar] [CrossRef]
- Brammer, L.; Espallargas, G.M.; Adams, H. Involving metals in halogen-halogen interactions: Second-sphere Lewis acid ligands for perhalometallate ions (M–X⋅⋅⋅X–C). Cryst. Eng. Comm. 2003, 5, 343–345. [Google Scholar] [CrossRef]
- Zordan, F.; Espallargas, G.M.; Brammer, L. Unexpected structural homologies involving hydrogen-bonded and halogen-bonded networks in halopyridinium halometallate salts. Cryst. Eng. Comm. 2006, 8, 425–431. [Google Scholar] [CrossRef]
- Mínguez Espallargas, G.; Brammer, L.; Sherwood, P. Designing Intermolecular Interactions between Halogenated Peripheries of Inorganic and Organic Molecules: Electrostatically Directed M–X⋅⋅⋅X′–C Halogen Bonds. Angew. Chem. Int. Ed. 2006, 45, 435–440. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef]
- Puttreddy, R.; von Essen, C.; Rissanen, K. Halogen Bonds in Square Planar 2,5-Dihalopyridine–Copper(II) Bromide Complexes. Eur. J. Inorg. Chem. 2018, 2018, 2393–2398. [Google Scholar] [CrossRef]
- Puttreddy, R.; von Essen, C.; Peuronen, A.; Lahtinen, M.; Rissanen, K. Halogen bonds in 2,5-dihalopyridine-copper(II) chloride complexes. CrystEngComm 2018, 20, 1954–1959. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Otwinowski, Z.; Minor, W. Processing of X-ray diffraction data collected in oscillation mode. In Macromolecular Crystallography Part A. Methods Enzymol; Carter, C.W., Jr., Ed.; Academic Press: Chapel Hill, NC, USA, 1997; Volume 276, pp. 307–326. [Google Scholar]
- Blessing, R.H. Outlier Treatment in Data Merging. J. Appl. Crystallogr. 1997, 30, 421–426. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).