A Comparative Study on Color Stability of Anthocyanin Hybrid Pigments Derived from 1D and 2D Clay Minerals
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of ACN/Clay Mineral Hybrid Pigments
2.3. Evaluation of Environmental Stability
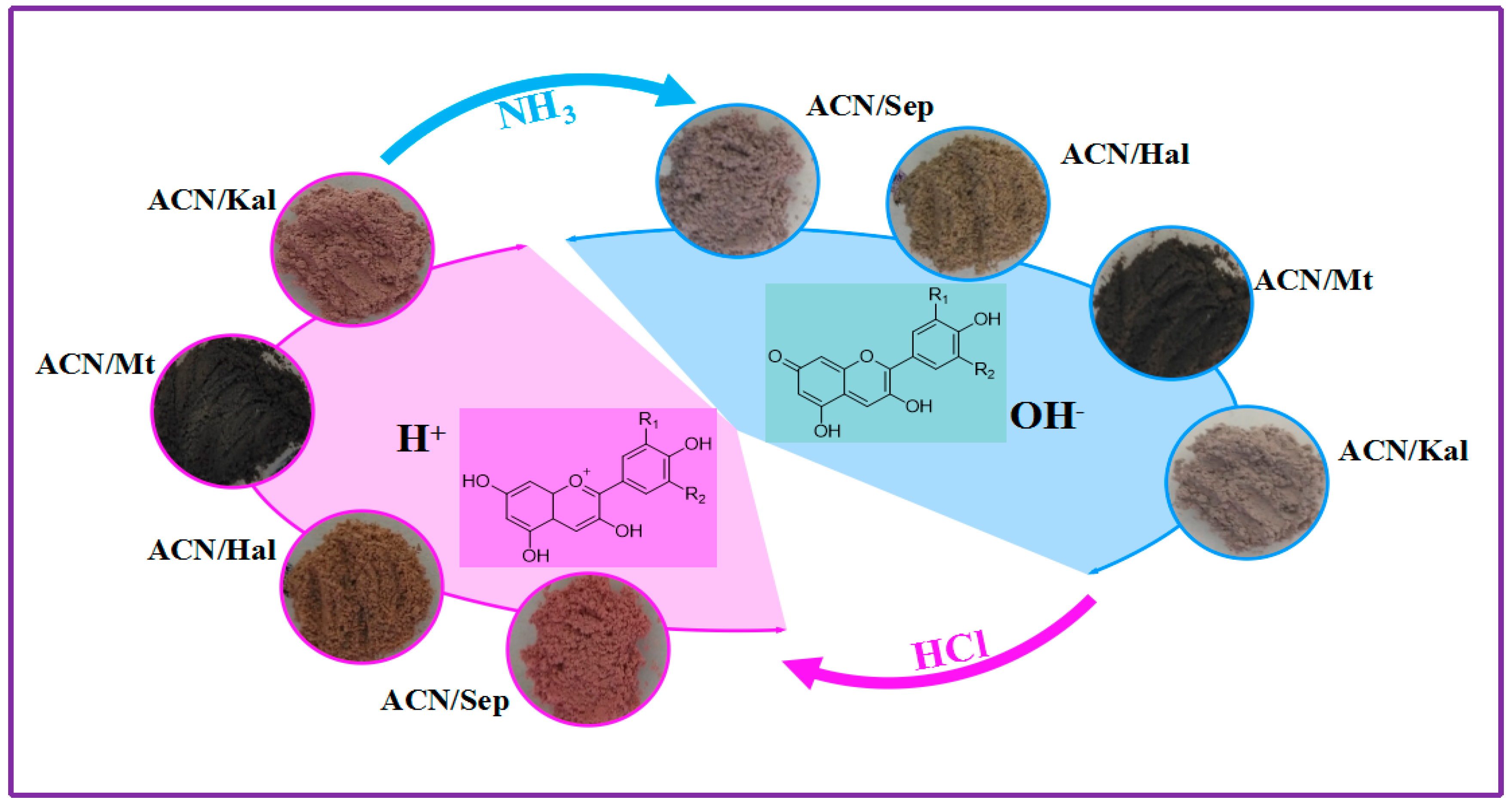
2.4. Reversible Acid/Base Allochroic Behavior
2.5. Color Response Analysis of pH Test Papers
2.6. Characterizations
3. Results and Discussion
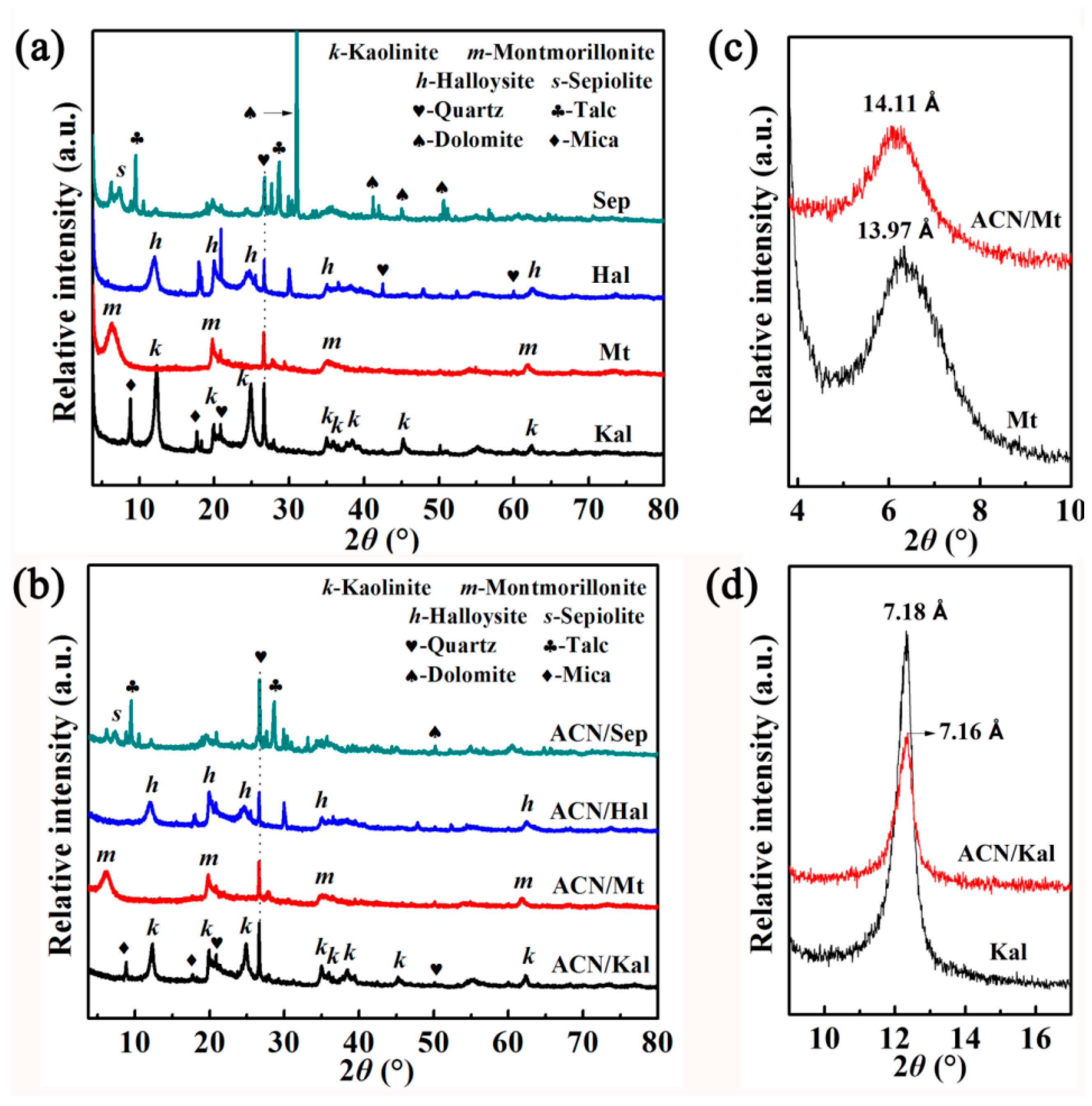
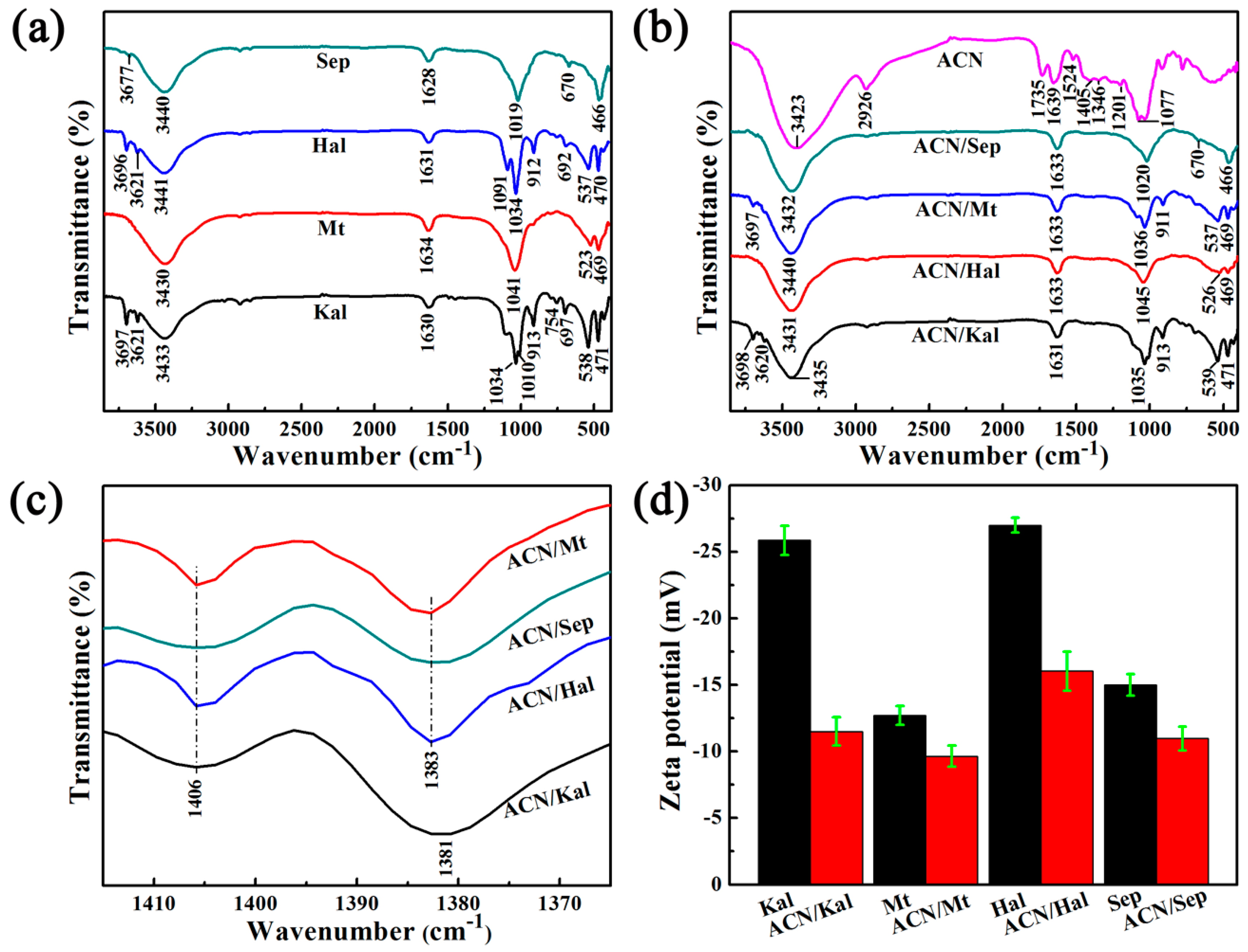
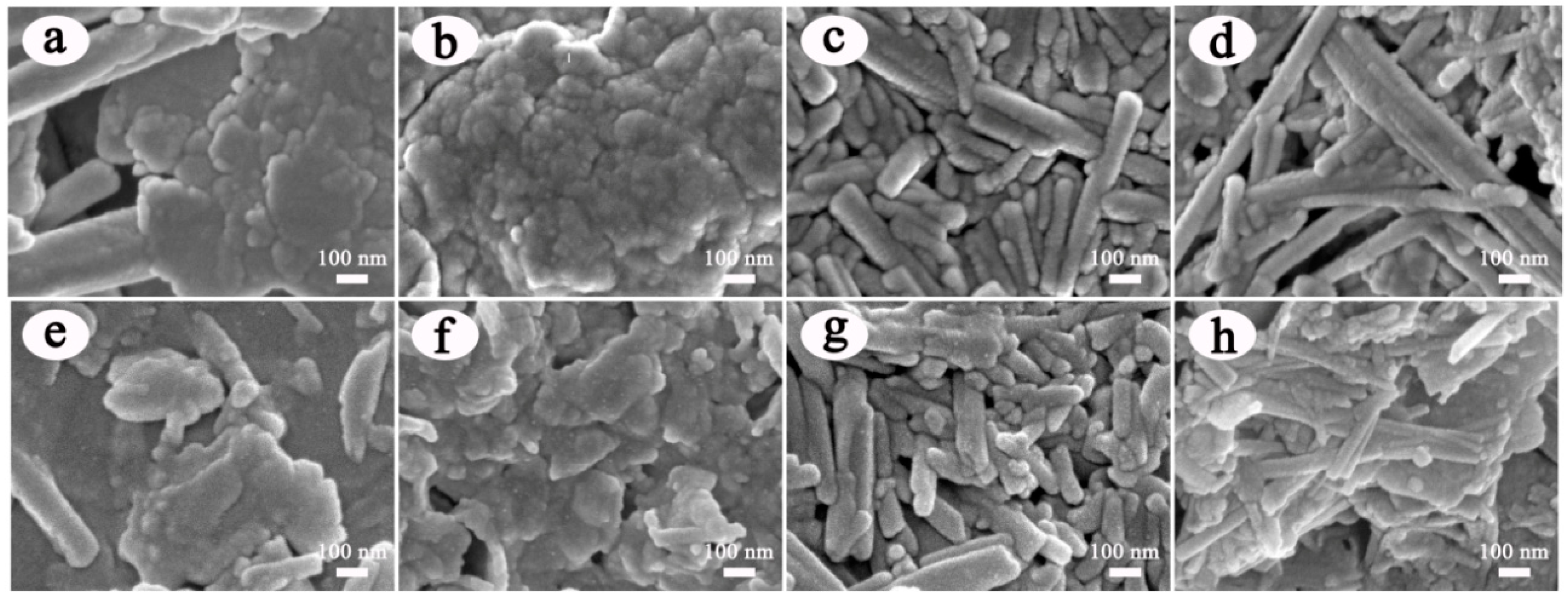
3.1. Characterization of ACN/clay Mineral Hybrid Pigments
3.2. Properties of ACN/Clay Mineral Hybrid Pigments
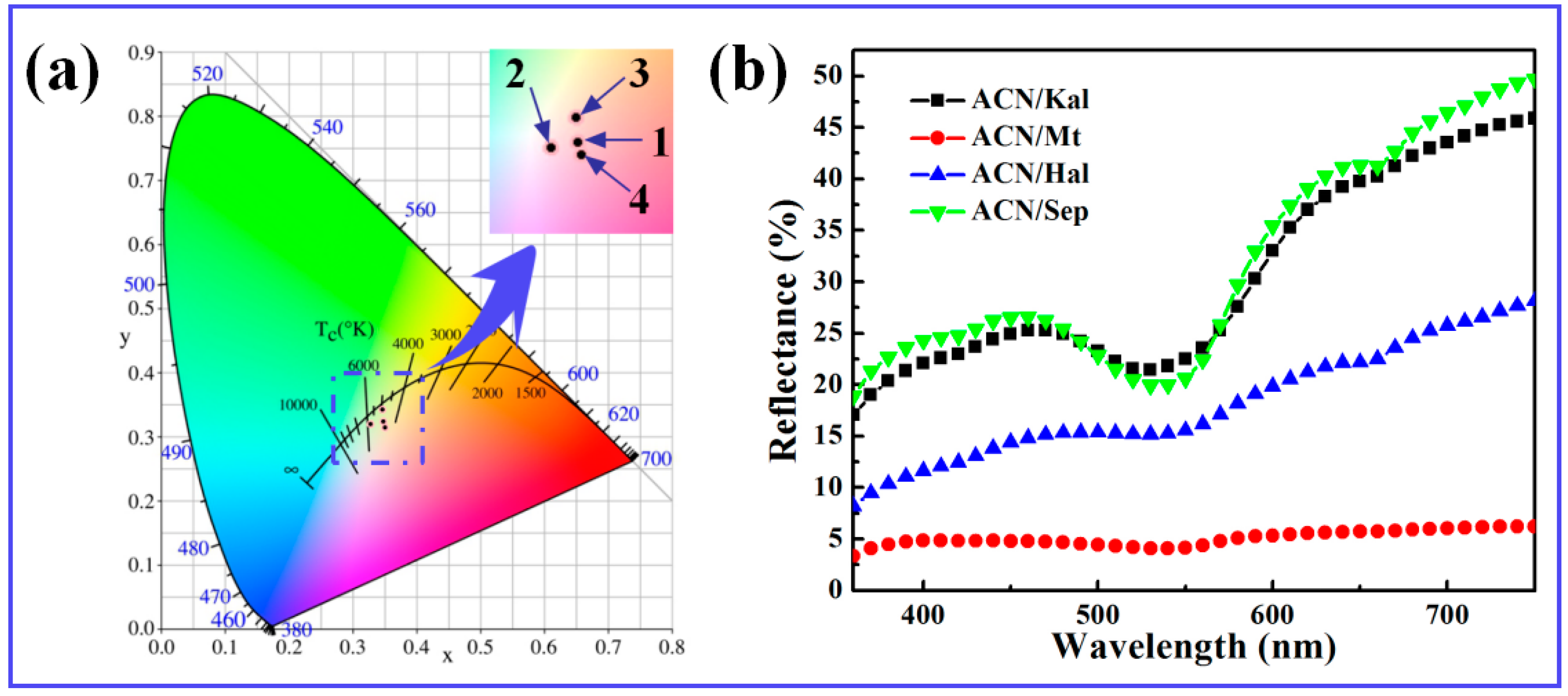
3.2.1. Color Properties
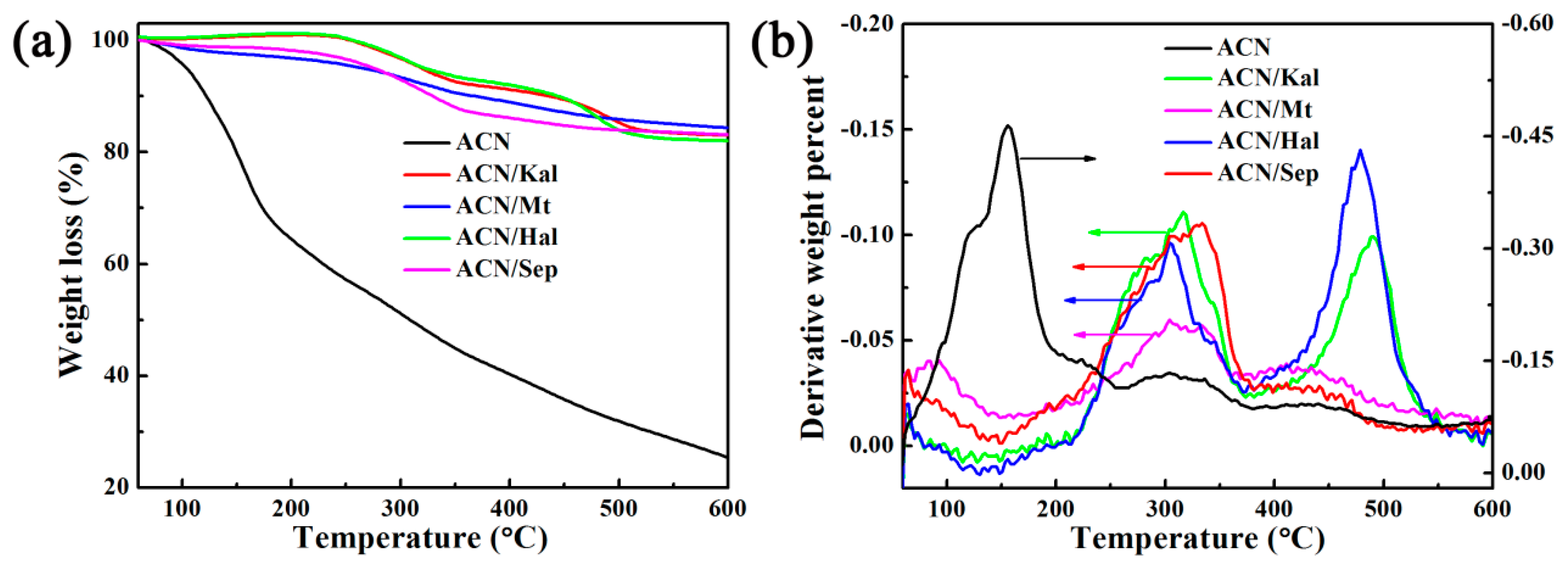
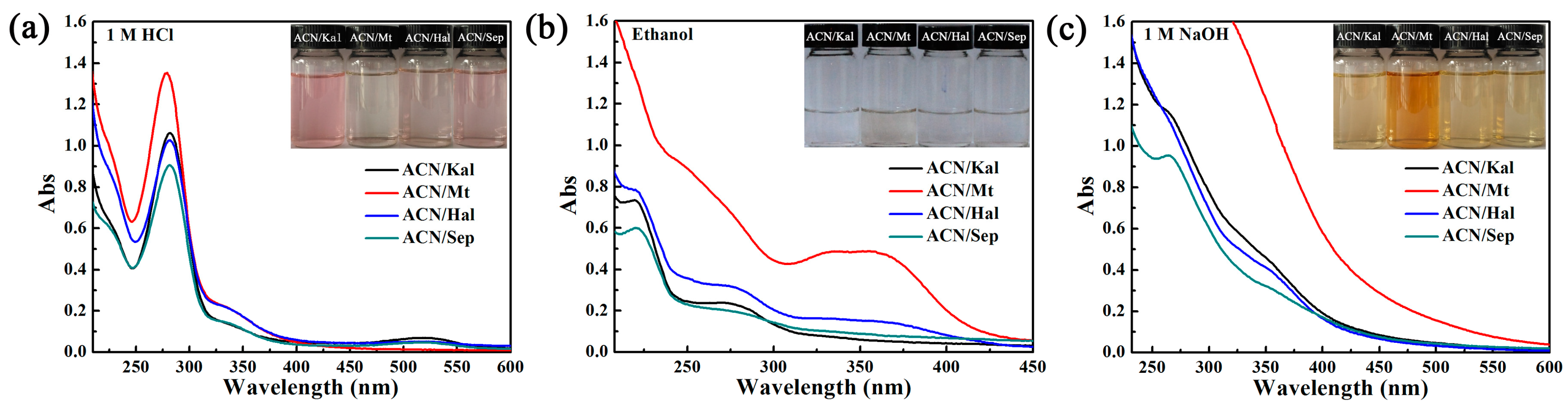
3.2.2. Environmental Stability of ACN/Clay Mineral Hybrid Pigments
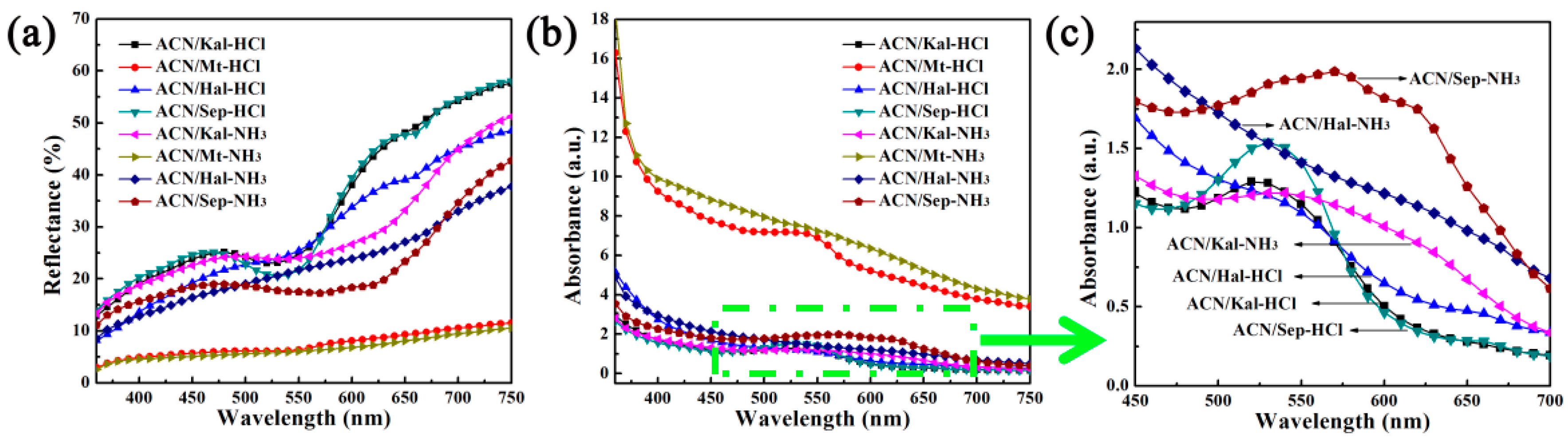
3.2.3. Reversible Acid/Base Allochroic Behavior in Different Atmospheres
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gutierrez, T.J.; Ponce, A.G.; Alvarez, V.A. Nano-clays from natural and modified montmorillonite with and without added blueberry extract for active and intelligent food nanopackaging materials. Mater. Chem. Phys. 2017, 194, 283–292. [Google Scholar] [CrossRef]
- Chorfa, N.; Savard, S.; Belkacem, K. An efficient method for high-purity anthocyanin isomers isolation from wild blueberries and their radical scavenging activity. Food Chem. 2016, 197, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Kohno, Y.; Kinoshita, R.; Ikoma, S.; Yoda, K.; Shibata, M.; Matsushima, R.; Tomita, Y.; Maeda, Y.; Kobayashi, K. Stabilization of natural anthocyanin by intercalation into montmorillonite. Appl. Clay Sci. 2009, 42, 519–523. [Google Scholar] [CrossRef]
- Goda, Y.; Shimizu, T.; Kato, Y.; Nakamura, M.; Maitani, T.; Yamada, T.; Terahara, N.; Yamaguchi, M. Two acylated anthocyanins from purple sweet potato. Phytochemistry 1997, 1, 183–186. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent pH indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.L.; de Oliveira, A.V.; de Brito, E.S.; Ribeiro, P.R.V.; Souza, M.D.M.; Azeredo, H.M.C. Stabilizing effect of montmorillonite on acerola juice anthocyanins. Food Chem. 2018, 245, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.L.; Brito, E.S.; Souza, M.D.M.; Azeredo, H.M.C. Montmorillonite as a reinforcement and color stabilizer of gelatin films containing acerola juice. Appl. Clay Sci. 2018, 165, 1–7. [Google Scholar] [CrossRef]
- Pereira, V.A., Jr.; de Arruda, I.N.Q.; Stefani, R. Active chitosan/PVA films with anthocyanins from Brassica oleraceae (Red Cabbage) as time-temperature indicators for application in intelligent food packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci. Tech. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Hwang, S.; Chung, S.H.; Lee, J.H.; Kim, Y.T.; Kim, Y.J.; Oh, S.; Yeo, I.-S.L. Influence of acid, ethanol, and anthocyanin pigment on the optical and mechanical properties of a nanohybrid dental composite resin. Materials 2018, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Pina, F.; Melo, M.J.; Laia, C.A.J.; Parola, A.J.; Lima, J.C. Chemistry and applications of flavylium compounds: A handful of colours. Chem. Soc. Rev. 2012, 41, 869–908. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Zia-Ul-Haq, M.; Saad, B. Biosynthesis and stability of anthocyanins. In Anthocyanins and Human Health: Biomolecular and Therapeutic Aspects; Hartel, R.W., Ed.; Springer: Cham, Switzerland, 2016; pp. 50–150. [Google Scholar]
- Ogawa, M.; Takee, R.; Okabe, Y.; Seki, Y. Bio-geo hybrid pigment; clay-anthocyanin complex which changes color depending on the atmosphere. Dyes Pigments 2017, 139, 561–565. [Google Scholar] [CrossRef]
- Kohno, Y.; Haga, E.; Yoda, K.; Shibata, M.; Fukuhara, C.; Tomita, Y.; Maeda, Y.; Kobayashi, K. Adsorption behavior of natural anthocyanin dye on mesoporous silica. J. Phys. Chem. Solids 2014, 75, 48–51. [Google Scholar] [CrossRef]
- Koosha, M.; Hamedi, S. Intelligent chitosan/PVA nanocomposite films containing black carrot anthocyanin and bentonite nanoclays with improved mechanical, thermal and antibacterial properties. Prog. Org. Coat. 2019, 127, 338–347. [Google Scholar] [CrossRef]
- Silva, G.T.M.; da Silva, K.M.; Silva, C.P.; Rodrigues, A.C.B.; Oake, J.; Gehlen, M.H.; Bohne, C.; Quina, F.H. Highly fluorescent hybrid pigments from anthocyanin- and red wine pyrano anthocyanin-analogs adsorbed on sepiolite clay. Quina Photochem. Photobiol. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Li, S.E.; Ding, J.J.; Mu, B.; Wang, X.W.; Kang, Y.R.; Wang, A.Q. Acid/base reversible allochroic anthocyanin/palygorskite hybrid pigments: Preparation, stability and potential applications. Dyes Pigments 2019, 171, 107738. [Google Scholar] [CrossRef]
- Micó-Vicent, B.; Martínez-Verdú, F.M.; Novikov, A.; Stavitskaya, A.; Vinokurov, V.; Rozhina, E.; Fakhrullin, R.; Yendluri, R.; Lvov, Y. Stabilized dye-pigment formulations with platy and tubular nanoclays. Adv. Funct. Mater. 2018, 28, 1703553. [Google Scholar] [CrossRef]
- Schoonheydt, R.A.; Johnston, C.T. Chapter 3 Surface and interface chemistry of clay minerals. Dev. Clay Sci. 2006, 1, 87–113. [Google Scholar] [CrossRef]
- Claverie, M.; Garcia, J.; Prevost, T.; Brendlé, J.; Limousy, L. Inorganic and hybrid (organic-inorganic) lamellar materials for heavy metals and radionuclides capture in energy wastes management-A Review. Materials 2019, 12, 1399. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Galan, E.; Theng, B.K.G. Chapter 2 Structures and mineralogy of clay minerals. Dev. Clay Sci. 2006, 1, 19–86. [Google Scholar] [CrossRef]
- Lu, Y.S.; Dong, W.K.; Wang, W.B.; Ding, J.J.; Wang, Q.; Hui, A.P.; Wang, A.Q. Optimal synthesis of environment-friendly iron red pigment from natural nanostructured clay minerals. Nanomaterials 2018, 8, 925. [Google Scholar] [CrossRef] [PubMed]
- Jose-Yacaman, M.; Rendon, L.; Arenas, J.; Serra Puche, M.C. Maya blue paint: An ancient nanostructured material. Science 1996, 273, 223–225. [Google Scholar] [CrossRef]
- Van Olphen, H. Maya blue: A clay-organic pigment? Science 1966, 154, 645–646. [Google Scholar] [CrossRef] [PubMed]
- Giustetto, R.; Seenivasan, K.; Bonino, F.; Ricchiardi, G.; Bordiga, S.; Chierotti, M.R.; Gobetto, R. Host/guest interactions in a sepiolite-based Maya blue pigment: A spectroscopic study. J. Phys. Chem. C 2011, 115, 16764–16776. [Google Scholar] [CrossRef]
- Ovarlez, S.; Giulieri, F.; Delamare, F.; Sbirrazzuoli, N.; Chaze, A.M. Indigo-sepiolite nanohybrids: Temperature-dependent synthesis of two complexes and comparison with indigo-palygorskite systems. Micropor. Mesopor. Mater. 2011, 142, 371–380. [Google Scholar] [CrossRef]
- Bernardino, N.D.; Brown-Xu, S.; Gustafson, T.L.; de Faria, D.L.A. Time-resolved spectroscopy of indigo and of a Maya blue simulant. J. Phys. Chem. C 2016, 120, 21905–21914. [Google Scholar] [CrossRef]
- Bernardino, N.D.; Constantino, V.R.L.D.; de Faria, L.A. Probing the indigo molecule in Maya blue simulants with resonance Raman spectroscopy. J. Phys. Chem. C 2018, 122, 11505–11515. [Google Scholar] [CrossRef]
- Ma, Y.M.; Lu, W.J. Study on the ethanol extraction of anthocyanins from the fruits ofblueberry. J. Anhui Agri. Sci. 2011, 39, 21768–21769. [Google Scholar] [CrossRef]
- Zhang, A.J.; Mu, B.; Luo, Z.H.; Wang, A.Q. Bright blue halloysite/CoAl2O4 hybrid pigments: Preparation, characterization and application in water-based painting. Dyes Pigments 2017, 139, 473–481. [Google Scholar] [CrossRef]
- Castillo, L.; López, O.; López, C.; Zaritzky, N.; García, M.A.; Barbosa, S.; Villar, M. Thermoplastic starch films reinforced with talc nanoparticles. Carbohyd. Polym. 2013, 95, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.J.; Mu, B.; Wang, X.W.; Wang, A.Q. CoAl2O4/Kaoline hybrid pigment prepared via solid-phase method for anticorrosion application. Front. Chem. 2018, 6, 586. [Google Scholar] [CrossRef] [PubMed]
- Trigueiro, P.; Pereira, F.A.R.; Guillermin, D.; Rigaud, B.; Balme, S.; Janot, J.M.; dos Santos, I.M.G.; Fonseca, M.G.; Walter, P.; Jabe, M. When anthraquinone dyes meet pillared montmorillonite: Stability or fading upon exposure to light? Dyes Pigments 2018, 159, 384–394. [Google Scholar] [CrossRef]
- Panda, A.K.; Mishra, B.G.; Mishra, D.K.; Singh, R.K. Effect of sulphuric acid treatment on the physico-chemical characteristics of kaolin clay. Colloids Surf. A Physicochem. Eng. Aspects 2010, 363, 98–104. [Google Scholar] [CrossRef]
- Tian, G.Y.; Wang, W.B.; Mu, B.; Wang, Q.; Wang, A.Q. Cost-efficient, vivid and stable red hybrid pigments derived from naturally available sepiolite and halloysite. Ceram. Int. 2017, 43, 1862–1869. [Google Scholar] [CrossRef]
- Moreira, M.A.; Ciuffi, K.J.; Rives, V.; Vicente, M.A.; Trujillano, R.; Gil, A.; Korili, S.A.; de Faria, E.H. Effect of chemical modification of palygorskite and sepiolite by 3-aminopropyltriethoxisilane on adsorption of cationic and anionic dyes. Appl. Clay Sci. 2017, 135, 394–404. [Google Scholar] [CrossRef]
- Lv, G.C.; Liu, S.Y.; Liu, M.; Liao, L.B.; Wu, L.M.; Mei, L.F.; Li, Z.H.; Pan, C.F. Detection and quantification of phenol in liquid and gas phases using a clay/dye composite. J. Ind. Eng. Chem. 2018, 62, 284–290. [Google Scholar] [CrossRef]
- Dellisanti, F.; Valdré, G. Study of structural properties of ion treated and mechanically deformed commercial bentonite. Appl. Clay Sci. 2005, 28, 233–244. [Google Scholar] [CrossRef]
- Bekri-Abbes, I.; Srasra, E. Effect of mechanochemical treatment on structure and electrical properties of montmorillonite. J. Alloy. Compd. 2016, 671, 34–42. [Google Scholar] [CrossRef]
- Perraki, T.; Orfanoudaki, A. Study of raw and thermally treated sepiolite from the Mantoudi area, Euboea, Greece. J. Therm. Anal. Calorim. 2008, 91, 589–593. [Google Scholar] [CrossRef]
- Mishra, G.; Mukhopadhyay, M. Enhanced antifouling performance of halloysite nanotubes (HNTs) blended poly(vinyl chloride) (PVC/HNTs) ultrafiltration membranes: For water treatment. J. Ind. Eng. Chem. 2018, 63, 366–379. [Google Scholar] [CrossRef]
- Dong, W.X.; Pu, X.L.; Ren, Y.J.; Zhai, Y.F.; Gao, F.; Xie, W. Thermoresponsive bentonite for water-based drilling fluids. Materials 2019, 12, 2115. [Google Scholar] [CrossRef] [PubMed]
- Guillermin, D.; Debroise, T.; Trigueiro, P.; de Viguerie, L.; Rigaud, B.; Morlet-Savary, F.; Balme, S.; Janot, J.M.; Tielens, F.; Michot, L.; et al. New pigments based on carminic acid and smectites: A molecular investigation. Dyes Pigments 2019, 160, 971–982. [Google Scholar] [CrossRef]
- Wu, S.T.; Cui, H.S.; Wang, C.H.; Hao, F.; Liu, P.L.; Xiong, W. In situ self-assembled preparation of the hybrid nanopigment from raw sepiolite with excellent stability and optical performance. Appl. Clay Sci. 2018, 163, 1–9. [Google Scholar] [CrossRef]
- Azha, S.F.; Shamsudin, M.S.; Shahadat, M.; Ismail, S. Low cost zwitterionic adsorbent coating for treatment of anionic and cationic dyes. J. Ind. Eng. Chem. 2018, 67, 187–198. [Google Scholar] [CrossRef]
- Matusik, J.; Rybka, K. Removal of chromates and sulphates by Mg/Fe LDH and heterostructured LDH/halloysite materials: Efficiency, selectivity, and stability of adsorbents in single- and multi-element systems. Materials 2019, 12, 1373. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Eggleton, R.A. Cation exchange capacity of kaolinite. Clays ClayMiner. 1999, 47, 174–180. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Evaluating the role of metal ions in the bathochromic and hyperchromic responses of cyanidin derivatives in acidic and alkaline pH. Food Chem. 2016, 208, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Ratanapoompinyo, J.; Nguyen, L.T.; Devkota, L.; Shrestha, P. The effects of selected metal ions on the stability of red cabbage anthocyanins and total phenolic compounds subjected to encapsulation process. J. Food Process. Preserv. 2017, 41, 1–8. [Google Scholar] [CrossRef]
- Fedenko, V.S.; Shemet, S.A.; Landi, M. UV-vis spectroscopy and colorimetric models for detecting anthocyanin-metal complexes in plants: An overview of in vitro and in vivo techniques. J. Plant. Physiol. 2017, 212, 13–28. [Google Scholar] [CrossRef]
- Cheng, H.F.; Liu, Q.H.; Xu, P.J.; Hao, R.W. A comparison of molecular structure and de-intercalation kinetics of kaolinite/quaternary ammonium salt and alkylamine intercalation compounds. J. Solid State Chem. 2018, 268, 36–44. [Google Scholar] [CrossRef]
- Martinez-Martinez, V.; Corcostegui, C.; Prieto, J.B.; Gartzia, L.; Salleres, S.; Arbeloa, I.L. Distribution and orientation study of dyes intercalated into single sepiolite fibers. A confocal fluorescence microscopy approach. J. Mater. Chem. 2011, 21, 269–276. [Google Scholar] [CrossRef]
- Hubbard, B.; Kuang, W.X.; Moser, A.; Facey, G.A. Structural study of Maya blue: Textural, thermal and solidstate multinuclear magnetic resonance characterization of the palygorskite-indigo and sepiolite-indigo adducts. Clay Clay Miner. 2003, 51, 318–326. [Google Scholar] [CrossRef]
- DePascual, T.S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. 2003, 14, 217–225. [Google Scholar] [CrossRef]
| Samples | SBET (m2/g) | Smicro (m2/g) | Sext (m2/g) | Vtotal (cm3/g) |
|---|---|---|---|---|
| Kal | 20.71 | - | 23.83 | 0.0408 |
| ACN/Kal | 12.66 | - | 19.36 | 0.0232 |
| Mt | 100.10 | 14.91 | 85.20 | 0.1191 |
| ACN/Mt | 29.41 | - | 47.76 | 0.0667 |
| Hal | 65.67 | - | 80.73 | 0.1731 |
| ACN/Hal | 30.41 | - | 34.14 | 0.1261 |
| Sep | 61.29 | - | 72.97 | 0.0857 |
| ACN/Sep | 25.89 | - | 41.08 | 0.0537 |
| Color Parameters | Kal | ACN/Kal | Mt | ACN/Mt | Hal | ACN/Hal | Sep | ACN/Sep |
|---|---|---|---|---|---|---|---|---|
| L* | 88.85 | 58.02 | 78.64 | 25.72 | 79.74 | 48.09 | 84.20 | 58.12 |
| a* | 0.17 | 13.21 | 0.55 | 4.57 | 4.55 | 5.89 | 0.25 | 17.37 |
| b* | 4.59 | 2.50 | 10.60 | -0.56 | 11.51 | 6.11 | 6.30 | 0.47 |
| C* | 4.59 | 13.44 | 10.61 | 4.60 | 12.38 | 8.49 | 6.30 | 17.38 |
| h° | 87.85 | 78.77 | 87.75 | 81.08 | 85.31 | 83.40 | 87.84 | 75.63 |
| Samples | After Being Treated with HCl | After Being Treated with Ethanol | After Being Treated with NaOH | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | L* | a* | b* | |
| ACN/Kal | 58.18 ± 1.14 | 11.15 ± 0.08 | 4.06 ± 0.27 | 57.89 ± 0.04 | 13.74 ± 0.04 | 1.60 ± 0.01 | 74.45 ± 0.60 | 6.07 ± 0.20 | 19.76 ± 0.33 |
| ACN/Mt | 19.64 ± 0.50 | 4.58 ± 0.38 | −0.81 ± 0.10 | 25.9 ± 0.54 | 4.16 ± 0.22 | −1.10 ± 0.24 | 41.84 ± 1.13 | 6.07 ± 0.34 | 12.35 ± 0.79 |
| ACN/Hal | 48.96 ± 0.81 | 5.49 ± 0.10 | 7.87 ± 0.10 | 49.06 ± 0.66 | 4.16 ± 0.22 | 4.91 ± 0.07 | 69.86 ± 0.09 | 6.01 ± 0.04 | 19.53 ± 0.03 |
| ACN/Sep | 52.24 ± 0.29 | 16.45 ± 0.03 | 1.85 ± 0.04 | 58.02 ± 0.10 | 16.42 ± 0.41 | −1.25 ± 0.45 | 67.53 ± 0.29 | 6.39 ± 0.06 | 20.68 ± 0.04 |
| Samples | Color Parameters in HCl | Color Parameters in NH3 | ||||
|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | |
| ACN/Kal | 60.54 ± 0.25 | 15.08 ± 0.49 | 8.50 ± 0.19 | 57.09 ± 0.16 | 3.34 ± 0.10 | 4.35 ± 0.18 |
| ACN/Mt | 31.54 ± 0.65 | 4.52 ± 0.14 | 4.98 ± 0.21 | 29.92 ± 0.16 | 2.46 ± 0.18 | 4.93 ± 0.35 |
| ACN/Hal | 59.20 ± 0.17 | 8.30 ± 0.20 | 14.17 ± 0.13 | 53.63 ± 0.03 | 2.48 ± 0.08 | 10.58 ± 0.12 |
| ACN/Sep | 59.62 ± 0.22 | 19.36 ± 0.15 | 6.00 ± 0.01 | 49.67 ± 0.46 | 1.70 ± 0.04 | −0.22 ± 0.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Mu, B.; Wang, X.; Kang, Y.; Wang, A. A Comparative Study on Color Stability of Anthocyanin Hybrid Pigments Derived from 1D and 2D Clay Minerals. Materials 2019, 12, 3287. https://doi.org/10.3390/ma12203287
Li S, Mu B, Wang X, Kang Y, Wang A. A Comparative Study on Color Stability of Anthocyanin Hybrid Pigments Derived from 1D and 2D Clay Minerals. Materials. 2019; 12(20):3287. https://doi.org/10.3390/ma12203287
Chicago/Turabian StyleLi, Shue, Bin Mu, Xiaowen Wang, Yuru Kang, and Aiqin Wang. 2019. "A Comparative Study on Color Stability of Anthocyanin Hybrid Pigments Derived from 1D and 2D Clay Minerals" Materials 12, no. 20: 3287. https://doi.org/10.3390/ma12203287
APA StyleLi, S., Mu, B., Wang, X., Kang, Y., & Wang, A. (2019). A Comparative Study on Color Stability of Anthocyanin Hybrid Pigments Derived from 1D and 2D Clay Minerals. Materials, 12(20), 3287. https://doi.org/10.3390/ma12203287






