1. Introduction
Commercially pure titanium (CpTi) and its alloys are the first-choice material for bone internal fixation in trauma or orthognathic surgery due to their biological and mechanical properties, including their excellent biocompatibility, corrosion resistance, and high mechanical strength [
1]. Even if they are well-tolerated, the long-term side effects within human tissues are still unclear and controversial [
2]. Plate removal is another debatable issue that has different approaches. Some approaches support immediate removal after bone healing [
3], whilst other studies have concluded that titanium and Ti-6Al-4V may be retained as permanent implants in maxillofacial fixation [
4,
5,
6].
The use of CpTi miniplates and screws (Ti-6Al-4V) in maxillofacial pathology helps avoid long-term intermaxillary immobilization and dental-retained splints, but, despite their undoubted advantages, their negative impact on the human body is still debated [
6,
7,
8]. Some papers have presented discolored gray tissues over the miniplates [
9,
10], and others have reported Ti toxicity due to oxidative stress [
11] and the occurrence of redox imbalance, as well as oxidative damage in the periosteum surrounding the Ti-6Al-4V alloy [
7].
Recent studies have reported chronic inflammation around titanium bone fixation devices due to the increased production of oxygen-free radicals and reactive nitrogen species [
9,
12]. Oxygen-free radicals can impair normal cell functioning as a result of the increased synthesis of proinflammatory mediators, which can affect cell growth, differentiation, and apoptosis processes [
13]. Therefore, the aim of our work was to evaluate the microscopic structure of soft tissue covering CpTi plates.
2. Materials and Methods
The experimental group in this study comprised 20 patients that had jaw surgery for a fracture (12 patients) or orthognathic treatment (8 patients), and underwent plate removal after one year from surgery. There were 13 male patients and 7 female patients, with an age range of 17–64 yrs. A total of 70 CpTi miniplates with 2.0 diameter screws made by Stryker Leibinger (GmbH & Co.KG Freiburg, Germany) were removed, and 20 samples of soft tissue overlying the titanium plates were harvested and analyzed by optic microscopy. Ethical approval was granted by the relevant Local Ethics Research Committee, and an informed consent form was signed by all the patients prior to surgery.
All the specimens excised were immediately fixed in 10% formaldehyde, embedded in paraffin wax, sectioned with a microtome, and stained with Mayer hematoxiline eozine.
Mayer hematoxiline eozine staining [
14].
Acidulated Mayer hemalaun substance: 75 g of alaun was dissolved in heat in 1000 mL of distilled water. Additionally, 75 mL of hematoxyline was filtrated in heat (from the base solution of 10%) and 0.30 g of potassium iodate in water. Then, it was all acidulated in 100 mL of solution, with 0.5 mL of acetic acid.
Eosin was boiled in a jar containing the following:
100 mL of alcohol 70°;
1 g of alcoholic eosine (Eosinblau siritus soluble);
1 g of watery eosine (Eosingelb aqua soluble);
0.25 g of orange G;
1 mL of officinalis HCl acid.
After cooling 20 mL of saturated liquid, lithium carbonate was added.
Lithium carbonate substance: 100 mL of distilled water + 15 g of lithium carbonate.
The technique was as follows:
- -
Mayer hemalaun stain, 10 min;
- -
Wash in water;
- -
Clorhydric alcohol differentiation, 1–2 s;
- -
Wash in water;
- -
Change in lithium carbonate, 2–4 min;
- -
Wash in water;
- -
Eozine coloration, 2–3 min;
- -
Wash in water;
- -
Ethanol dropped on slide until no more eozine;
- -
Dehydration with absolute and 95° alcohol;
- -
Clarification in fenicated xylene, xylene;
- -
Mounting on Canadian fabric.
Results:
Morphometric analysis
The samples were studied under light microscopy with a Leica DM750 microscope. In order to measure the dimensions of the metallic particles present in the soft tissue, ImageJ software used (
https://imagej.nih.gov/ij/download.html). Images of the samples under 400× magnification were used.
3. Results
In all cases, it was noted that the miniplates were macroscopically covered by dense fibrotic tissue, and had no inflammatory signs. Visible dark-grey pigmentation was seen in 16 out of 20 samples taken.
Under light microscopy, varying degrees of fibrosis were seen, the samples displayed connective tissue without an inflammatory reaction of any kind, and pigmented debris were found in all samples, comprised of large or dust-like particles.
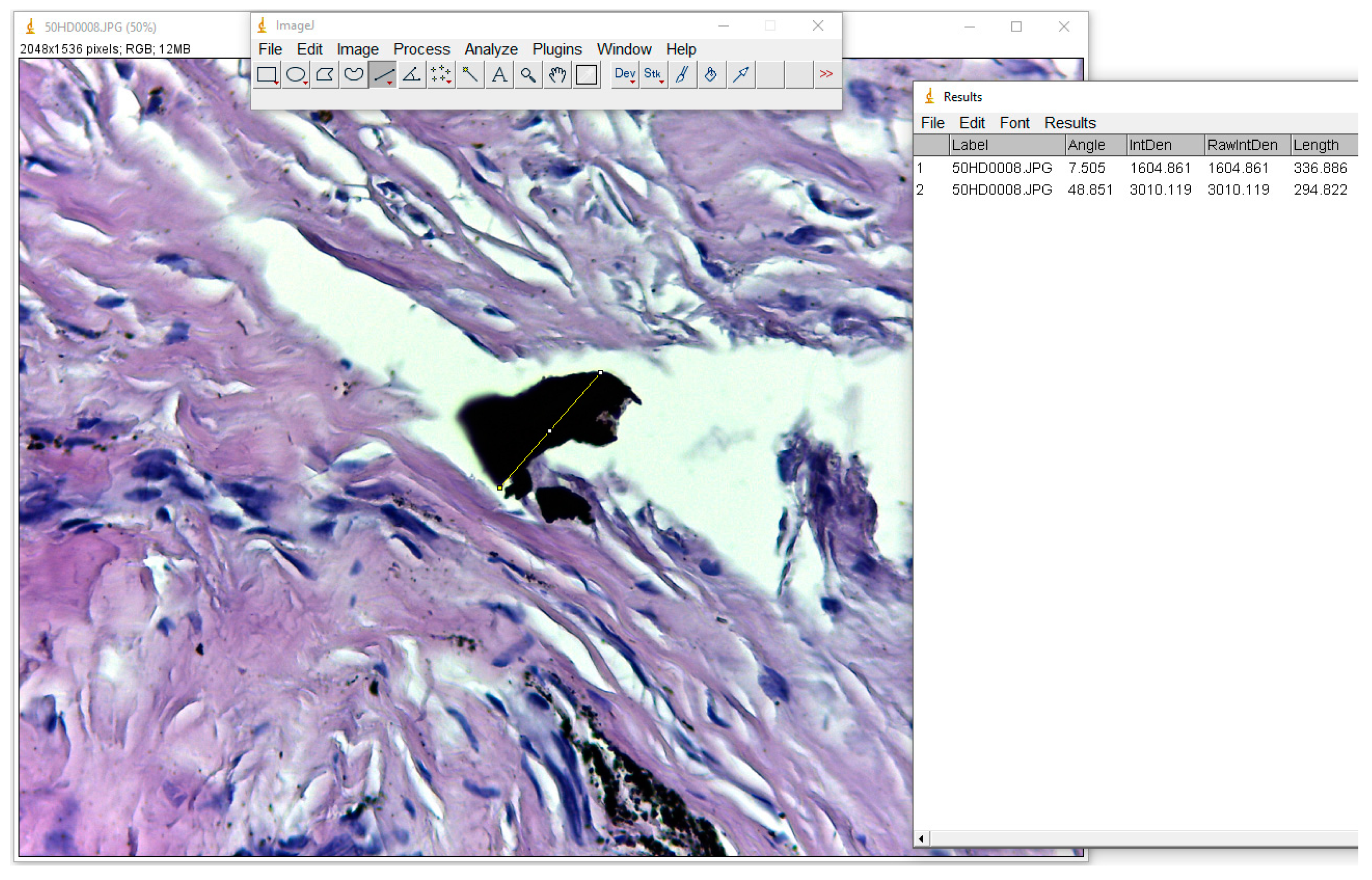
To produce an accurate measurement of these particles, we used the ”Straight” function in ImageJ software, which measures the distance between two points, and employing “CTR+M”, this value was passed into an Excel document, as seen in
Figure 1.
All the particles found were extracellular.
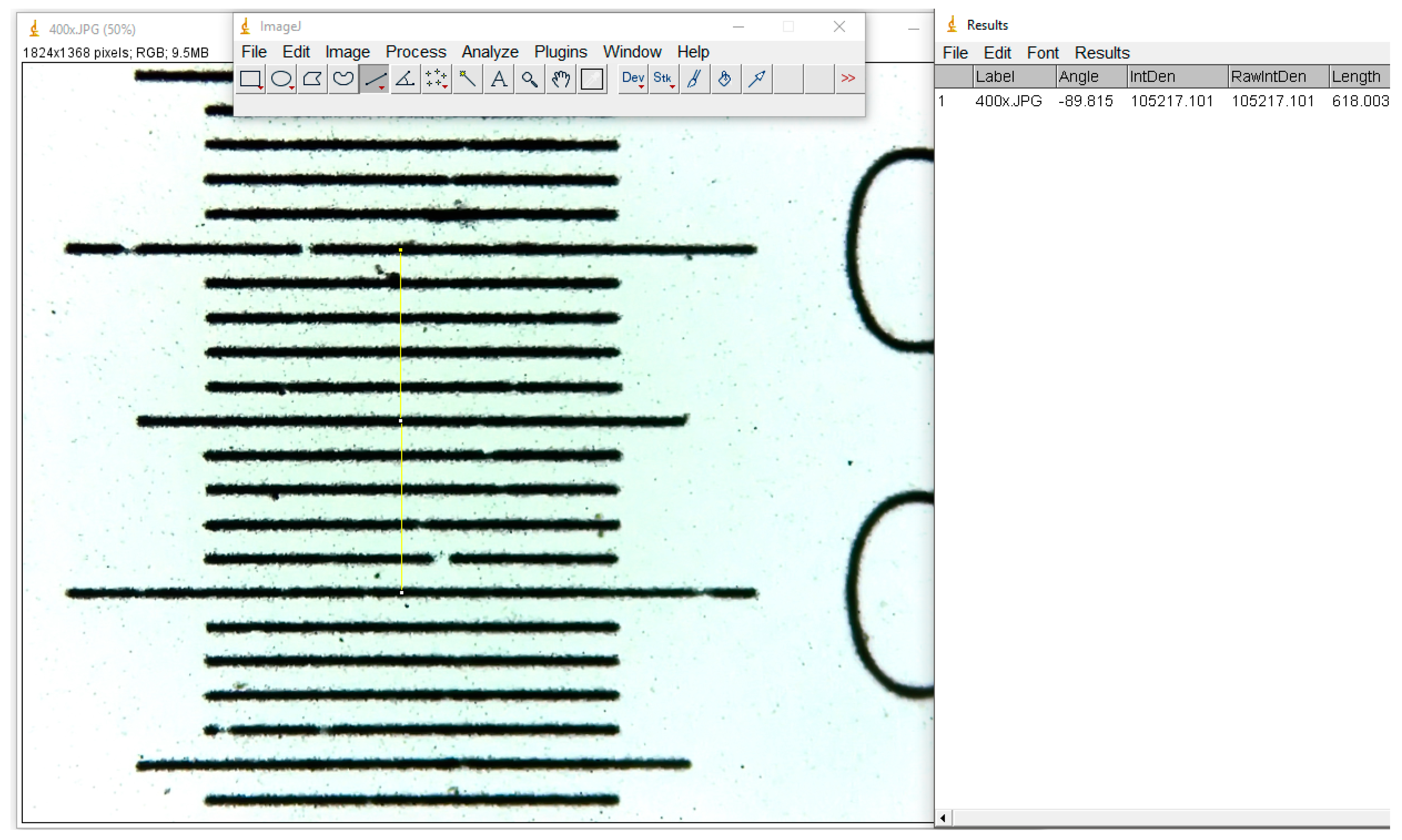
Three measurements were taken in all cases for a better accuracy, and the average value was calculated. The resulting number of these measurements represents the total number of pixels in the image, and for micrometrical conversion, we used an image photographed in identical conditions of a micrometric grid (
Figure 2).
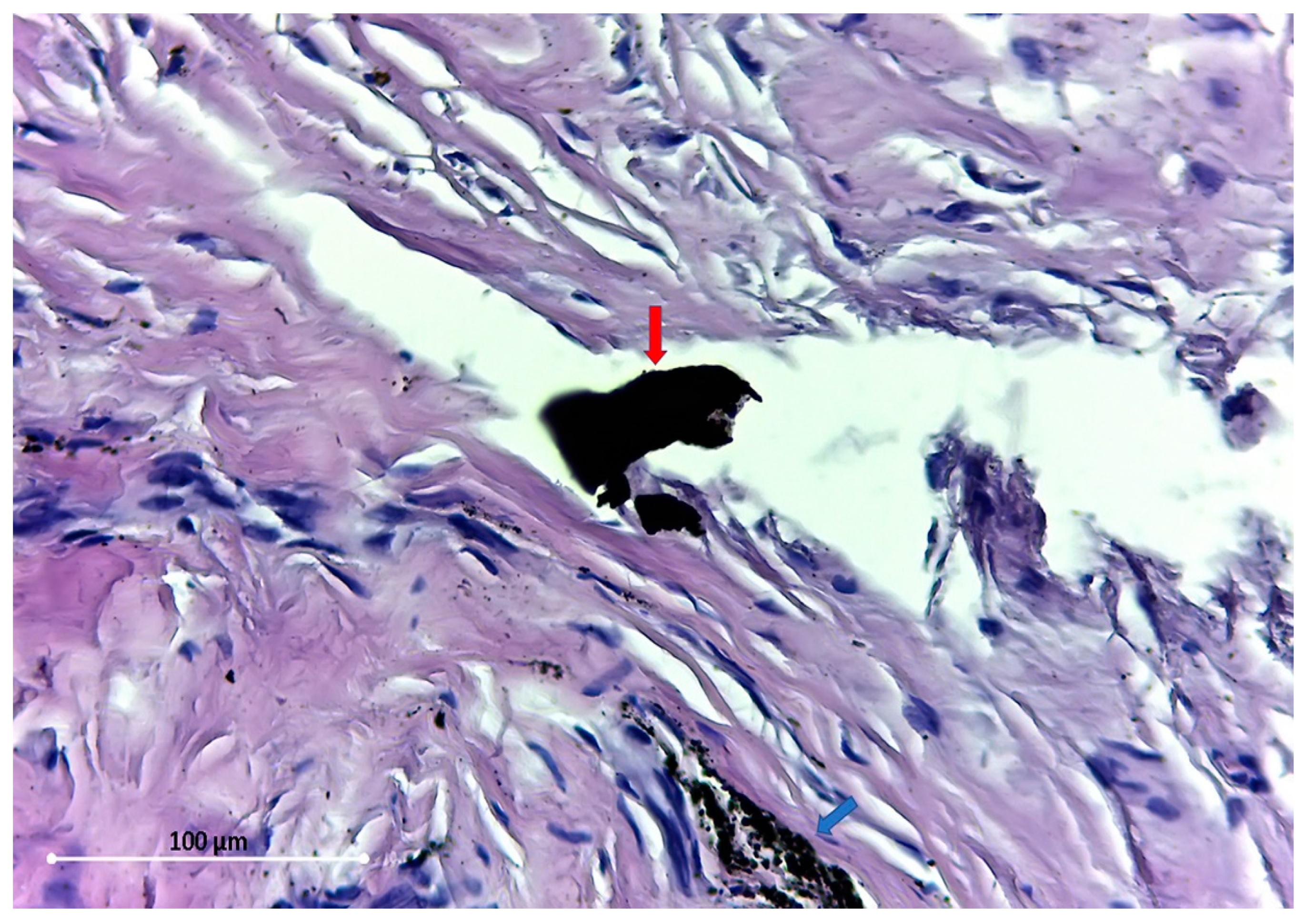
The particles found are of different sizes, varying from 54.5 Micron (
Figure 3) to dust-like 1 Micron particles (
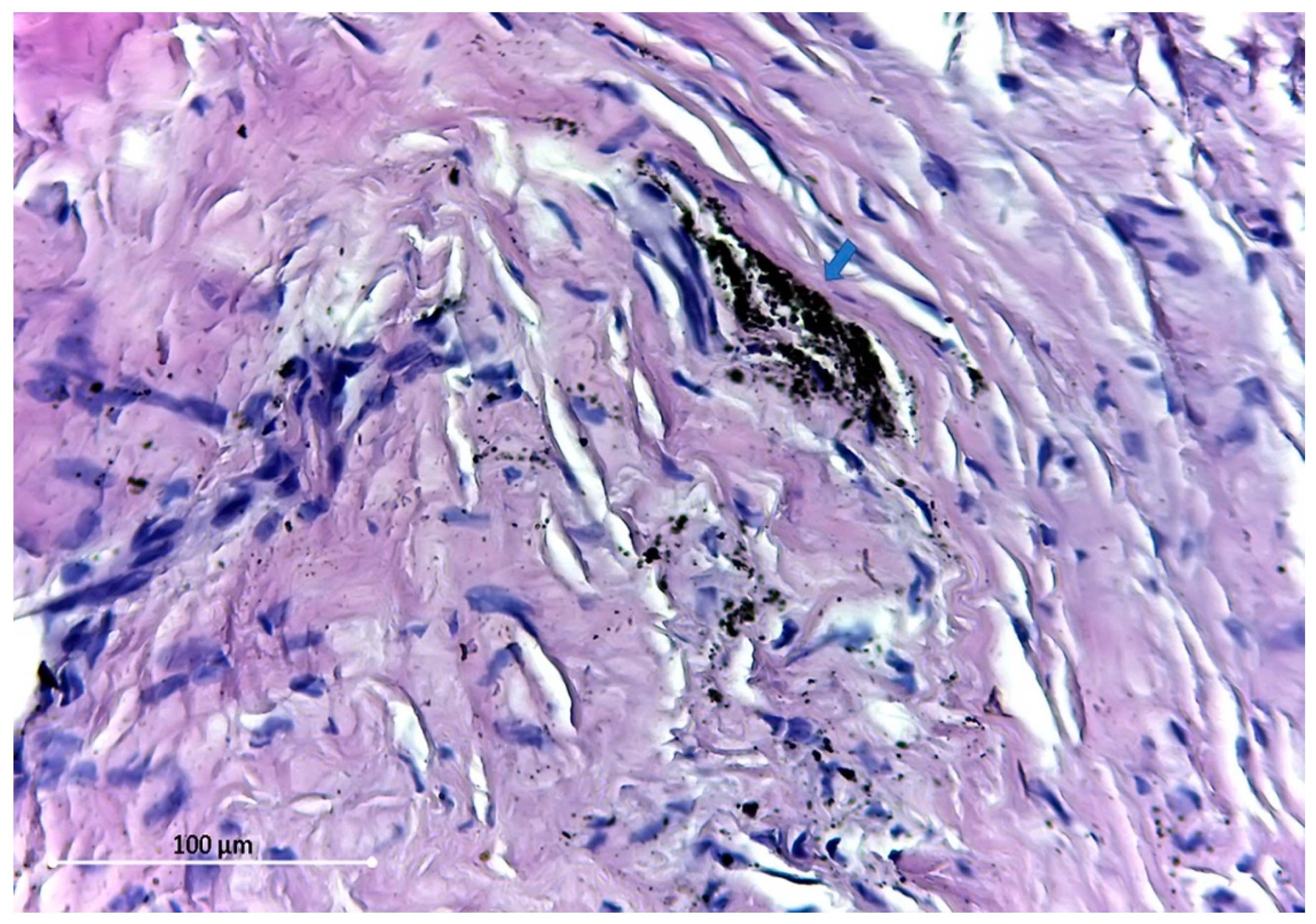
Figure 4).
The distribution pattern of these tiny dust-like particles suggests the disintegration of larger particles. All the particles found were extracellular.
4. Discussion
Our study shows that there are dark-colored particles in the soft tissues after at least one year post-insertion, which vary in their dimensions between 1 micron and 54.5 microns. It is debatable whether these particles are detached when the Ti material is inserted, whether they are the result of a metabolic process of fagocytosis [
2], or whether they are caused by the Ti oxides at the plate surface [
3,
5]. The miniplates used in cranio-facial surgery are highly resistant to corrosion and mechanical wear [
15,
16]; some studies suggest that the titanium release into the surrounding tissue is caused by plate damage through manipulation or movement while “in situ” [
3,
16]. Our patients did not present any screw loosening or plate mobility at removal. It is believed that if wear damages the surface TiO
2 layer, the surrounding tissue turns black [
17].
All the particles found were extracellular, but other studies have proved that they can be intracellular [
2,
18], and some small extracellular particles could be the result of phagocytic cell apoptosis, which could explain the powder-like particles present in our study. Other paper presents the fact that the transport of titanium particles can occur via lymphatics to regional lymph nodes [
19], which would sustain the mandatory plate removal protocol postulated by Kim et al. [
3], who found tissue degeneration around the titanium particles. Our study found various degrees of fibrosis next to the implant site, without signs of inflammation or tissue degeneration. Titanium particles present at a distance from the implant site were found by Bessho et al.—lungs, spleen, kidneys, and liver [
20]. Onodera et al. reported a submandibular lymph node with titanium particles 2 years after a mandibular titanium plate implant in a 41-year-old man [
21].
Other papers have described that large implant-derived particles are produced exclusively by the mechanical wear process in the articular coupling of prostheses and are not observed with osteosynthesis implants [
22]. In contrast with this, our findings show the presence of Ti particles at the osteosynthesis site. A possible explanation for this could be related to the mechanical “damage” of the plate-screw system at the insertion time, but the particles were also found on top of the plates, and not only on the screws.
Adverse reactions to titanium have been seen over time through titanium hypersensitivity reactions, pacemaker case reports, dental implant case reports, orthopedic implant case reports, and neurosurgical implants [
23]. A case of granulomatous pulmonary disease with the associated pulmonary deposition of titanium was reported in a 45-year-old man who worked as a furnace feeder for an aluminum smelting company [
22]. All these adverse reactions, even if few in number, prove that Ti implants can cause local and general problems.
It is believed that the passive layer of TiO
2 effectively protects the surface of the implant from the effects of corrosive agents present in the environment of body fluids. The TiO2 layer may be damaged, resulting in the release of metal ions (such as titanium) into the surrounding tissues. Mandibular movements can cause micromovements of the fixed bone fragments, which increases the friction between the miniplates and the screws, and this phenomenon (tribocorrosion) could be responsible for the corrosion of titanium fixations and the release of titanium ions into the tissues/organs surrounding the implants, promoting inflammatory reactions [
7,
24].
Metal debris may be released into the tissues as a result of manufacturing defects, corrosion, surface contamination, or mechanical damage which may occur during insertion or removal, or may be due to wear whilst in situ [
5,
25]. Tissue trauma, hematoma, tissue healing, and mechanical instability between the implant and the surrounding tissues may be at least partly responsible for the histological changes seen in the tissues [
1].
It is generally suggested that the particles detached from titanium plates are tolerated relatively well, even at high concentrations. However, we must consider the long-term tissue reaction to debris from titanium. The concentration of metals that could cause adverse effects is not currently known, and the clinical significance and long-term effects of titanium particles in the tissues, both locally and systemically, are unknown.
5. Conclusions
The microscopic evaluation of the soft tissues covering the cpTi plates does not present any kind of inflammatory signs, but presents numerous dark-colored particles, of various dimensions.
Tissue irritation, hardware loosening, and other adverse reactions can occur over time. Therefore, future studies are needed in order to make a proper assessment of the soft tissue changes on CpTi plates.
Author Contributions
Conceptualization, G.A., F.O., A.M. and S.B.; Data curation, D.G. and I.M.; Formal analysis, D.G.; Investigation, M.L.; Methodology, F.O., I.M., A.M., M.L. and M.B.; Project administration, G.A. and S.B.; Supervision, G.B.; Validation, F.O.; Visualization, V.T.; Writing–original draft, G.A. and A.M.; Writing–review & editing, V.T., G.B., M.B. and S.B.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Souza, J.C.M.; Tajiri, H.A.; Morsch, C.S.; Buciumeanu, M.; Mathew, M.T.; Silva, F.S.; Henriques, B. Tribocorrosion Behavior of Ti6Al4V Coated with a Bio-absorbable Polymer for Biomedical Applications. J. Bio- Tribo-Corros. 2015, 1, 27. [Google Scholar] [CrossRef]
- Langford, R.J.; Frame, J.W. Tissue changes adjacent to titanium plates in patients. J. Cranio-Maxillofac. Surg. 2002, 30, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; HoYeo, H.; Lim, S.C. Tissue response to titanium plates: A transmitted electron microscopic study. J. Oral Maxillofac. Surg. 1997, 55, 322–326. [Google Scholar] [CrossRef]
- Haug, R.H. Retention of asymptomatic bone plates used for orthognathic surgery and facial fractures. J. Oral Maxillofac. Surg. 1996, 54, 611–617. [Google Scholar] [CrossRef]
- Langford, R.J.; Frame, J.W. Surface analysis of titanium maxillofacial plates and screws retrieved from patients. Int. J. Oral Maxillofac. Surg. 2002, 31, 511–518. [Google Scholar] [CrossRef]
- O’Connell, J.; Murphy, C.; Ikeagwuani, O.; Adley, C.; Kearns, G. The fate of titanium miniplates and screws used in maxillofacial surgery: A 10 year retrospective study. Int. J. Oral Maxillofac. Surg. 2009, 38, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Krętowski, A.; Waszkiel, D.; Bortnik, P.; Czarniecka-Bargłowska, K.; Kocisz, M.; Szulimowska, J.; Czajkowski, M.; et al. Exposure to Ti4Al4V Titanium Alloy Leads to Redox Abnormalities, Oxidative Stress, and Oxidative Damage in Patients Treated for Mandible Fractures. Oxidative Med. Cell. Longev. 2018, 2018, 3714725. [Google Scholar] [CrossRef]
- Kuriakose, M.A.; Fardy, M.; Sirikumara, M.; Patton, D.W.; Sugar, A.W. A comparative review of 266 mandibular fractures with internal fixation using rigid (AO/ASIF) plates or mini-plates. Br. J. Oral Maxillofac. Surg. 1996, 34, 315–321. [Google Scholar] [CrossRef]
- Borys, J.; Maciejczyk, M.; Krętowski, A.J.; Antonowicz, B.; Ratajczak-Wrona, W.; Jabłońska, E.; Załęski, P.; Waszkiel, D.; Ładny, J.R.; Żukowski, P.; et al. The redox balance in erythrocytes, plasma, and periosteum of patients with titanium fixation of the jaw. Front. Physiol. 2017, 8, 386. [Google Scholar] [CrossRef]
- Voggenreiter, G.; Leiting, S.; Brauer, H.; Leiting, P.; Majetschak, M.; Bardenheuer, M.; Obertacke, U. Immuno-Inflam-Matory tissue reaction to Stainless-Steel and titanium plates used for internal fixation of long bones. Biomaterials 2003, 24, 247–254. [Google Scholar] [CrossRef]
- Tsaryk, R.; Kalbacova, M.; Hempel, U.; Scharnweber, D.; Unger, R.E.; Dieter, P.; Kirkpatrick, C.J.; Peters, K. Response of human endothelial cells to oxidative stress on Ti6Al4V alloy. Biomaterials 2007, 28, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.; Unger, R.E.; Gatti, A.M.; Sabbioni, E.; Tsaryk, R.; Kirkpatrick, C.J. Metallic nanoparticles exhibit paradoxical effects on oxidative stress and Pro-Inflammatory response in endothelial cells in vitro. Int. J. Immunopa-Thology Pharmacol. 2007, 20, 685–695. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal Phys-Iological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.L.; Kiernan, J.A. Education Guide Special Stains and H&E, 2nd ed.; Dako North America: Carpinteria, CA, USA, 2010. [Google Scholar]
- Moberg, L.E.; Nordenram, A.; Kjellman, O. Metal release from plates used in jaw fracture treatment. Int. J. Oral Maxillofac. Surg. 1989, 18, 311–314. [Google Scholar] [CrossRef]
- Schliephake, H.; Lehmann, H.; Kunz, U.; Schmelzeisen, R. Ultrastructural findings in soft tissues adjacent to titanium miniplates used in jaw fracture treatment. Int. J. Oral Maxillofac. Surg. 1993, 22, 20–25. [Google Scholar] [CrossRef]
- Onodera, K.; Ooya, K.O.; Kawamura, H. Titanium lymph node pigmentation in the reconstruction plate system of a mandibular defect. Oral Surg. Oral Med. Oral Pathol. 1993, 75, 495–497. [Google Scholar] [CrossRef]
- Witt, J.D.; Swann, M. Metal wear and tissue response in failed titanium alloy total hip replacements. J. Bone Jt. Surg. Br. 1991, 73, 559–563. [Google Scholar] [CrossRef]
- Bessho, K.; Fujimura, K.; Iizuka, T. Experimental longterm study of titanium ions eluted from pure titanium miniplates. J. Biomed. Mater. Res. 1995, 29, 901–904. [Google Scholar] [CrossRef]
- Konttinen, Y.T.; Zhao, D.; Beklen, A.; Ma, G.; Takagi, M.; Kivelä-Rajamäki, M.; Ashammakhi, N.; Santavirta, S. The microenvironment around total hip replacement prostheses. Clin. Orthop. Relat. Res. 2005, 430, 28–38. [Google Scholar] [CrossRef]
- Weingart, D.; Steinemann, S.; Schilli, W.; Strub, J.R.; Hellerich, U.; Assenmacher, J.; Simpson, J. Titanium deposition in regional lymph nodes after insertion of titanium screw implants in maxillofacial region. Int. J. Oral Maxillofac. Surg. 1994, 23, 450–452. [Google Scholar] [CrossRef]
- Wood, M.M.; Warshaw, E.M. Hypersensitivity reactions to titanium: Diagnosis and management. Dermat. Contact Atopic Occup. Drug. 2015, 26, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, E.N.; Catalfamo, L.; Allegra, A.; Musolino, C.; De Ponte, F.S. Titanium miniplates: A new risk factor for the development of the bisphosphonate-related osteonecrosis of the jaw. J. Craniofac. Surg. 2013, 24, e1–e2. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Goschorska, M.; Baranowska-Bosiacka, I.; Chlubek, D. In Vitro Effect of 3D Plates Used for Surgical Treatment of Condylar Fractures on Prostaglandin E2 (PGE2) and Thromboxane B2 (TXB2) Concentration in THP-1 Macrophages. Int. J. Mol. Sci. 2017, 18, 2638. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, G.; Li, Z.; Zeng, X.; Xu, Y.; Zhao, S.; Hu, H.; Zhang, Y.; Ren, T. Tribological behavior of Ti-6Al-4V against cortical bone in different biolubricants. J. Mech. Behav. Biomed. Mater. 2019, 90, 460–471. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).