Optimizing the Microstructure and Corrosion Resistance of BDD Coating to Improve the Service Life of Ti/BDD Coated Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Characterization of Ti/BDD Samples
3. Experimental Results and Discussions
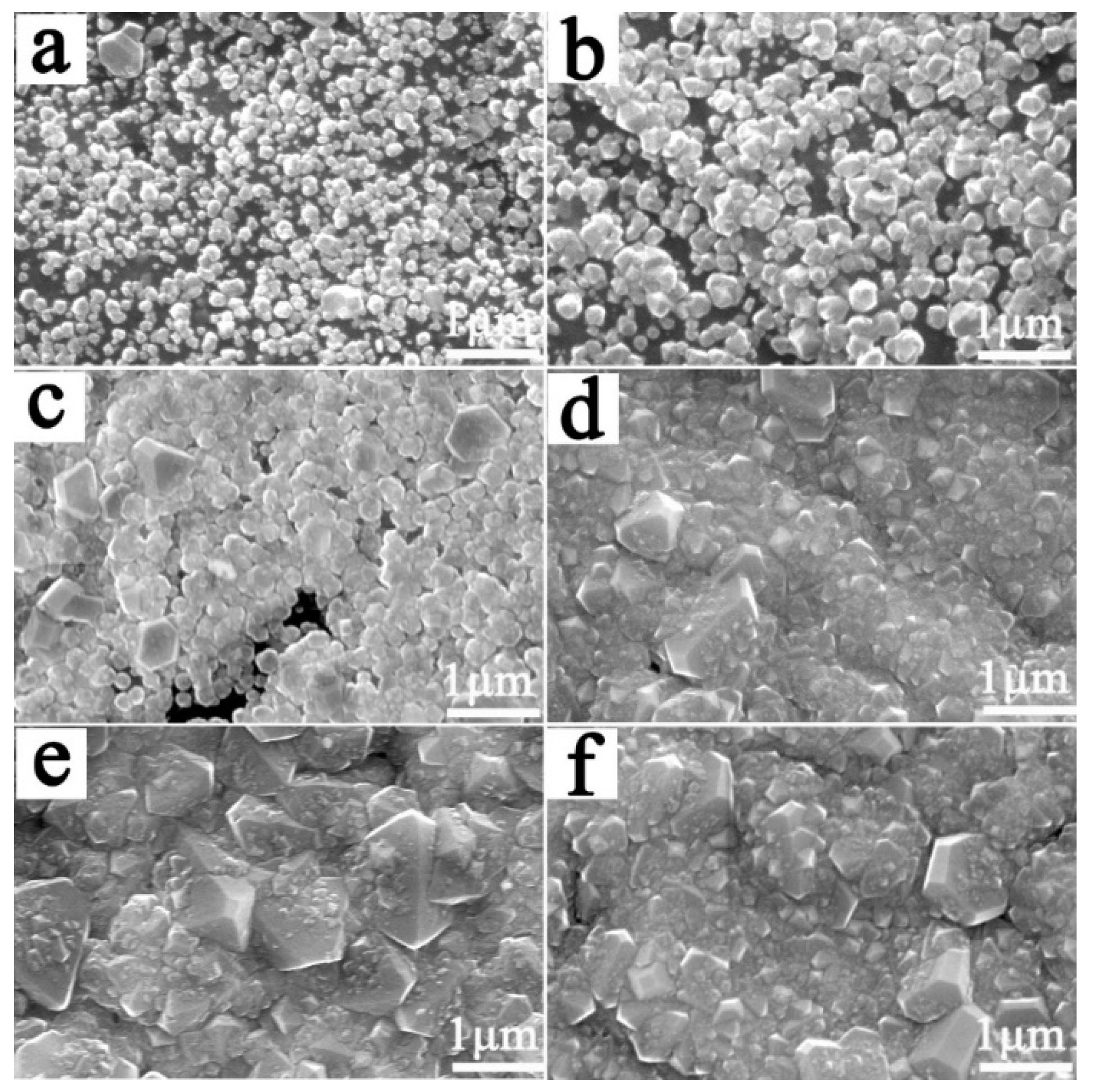
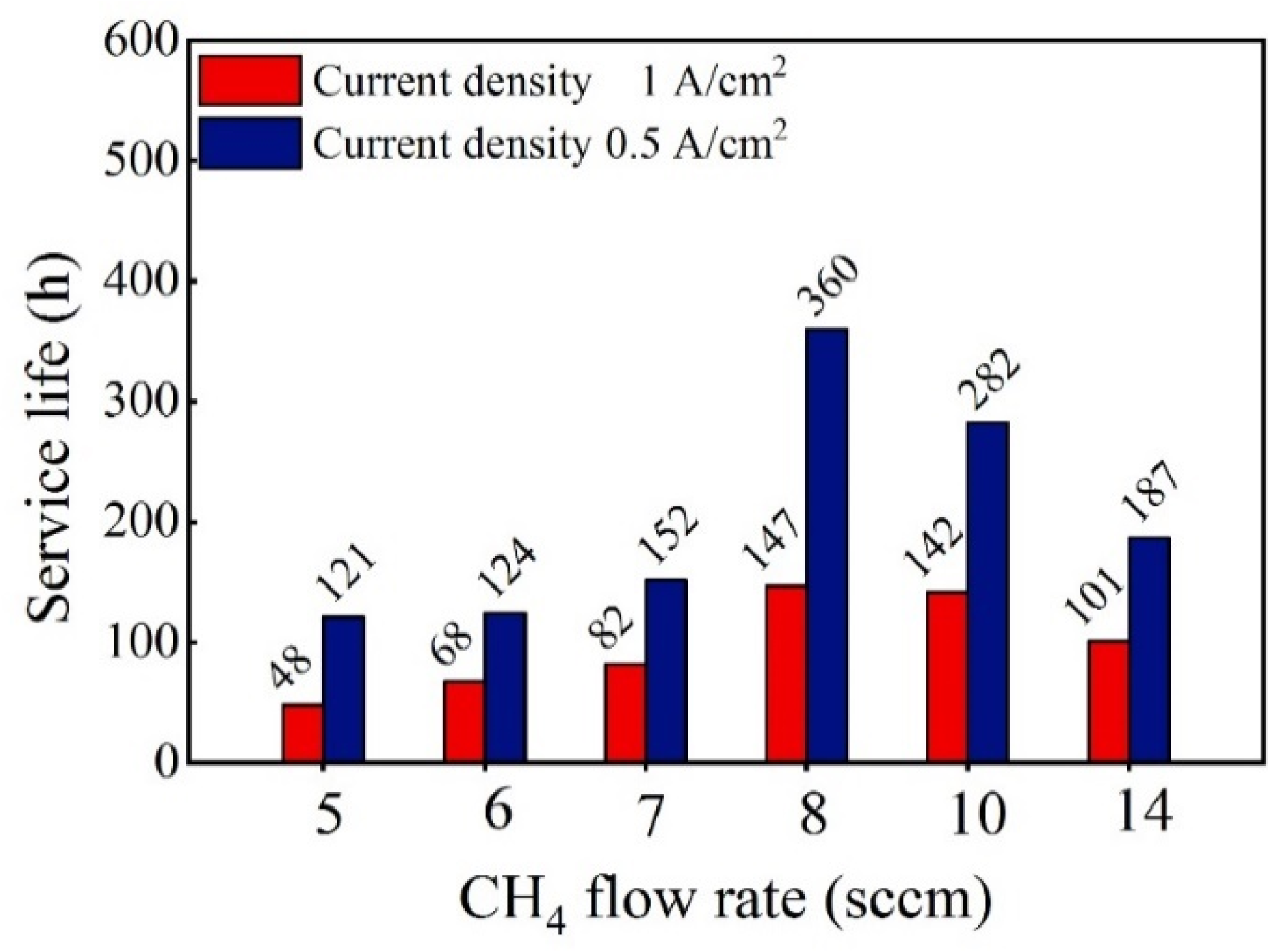
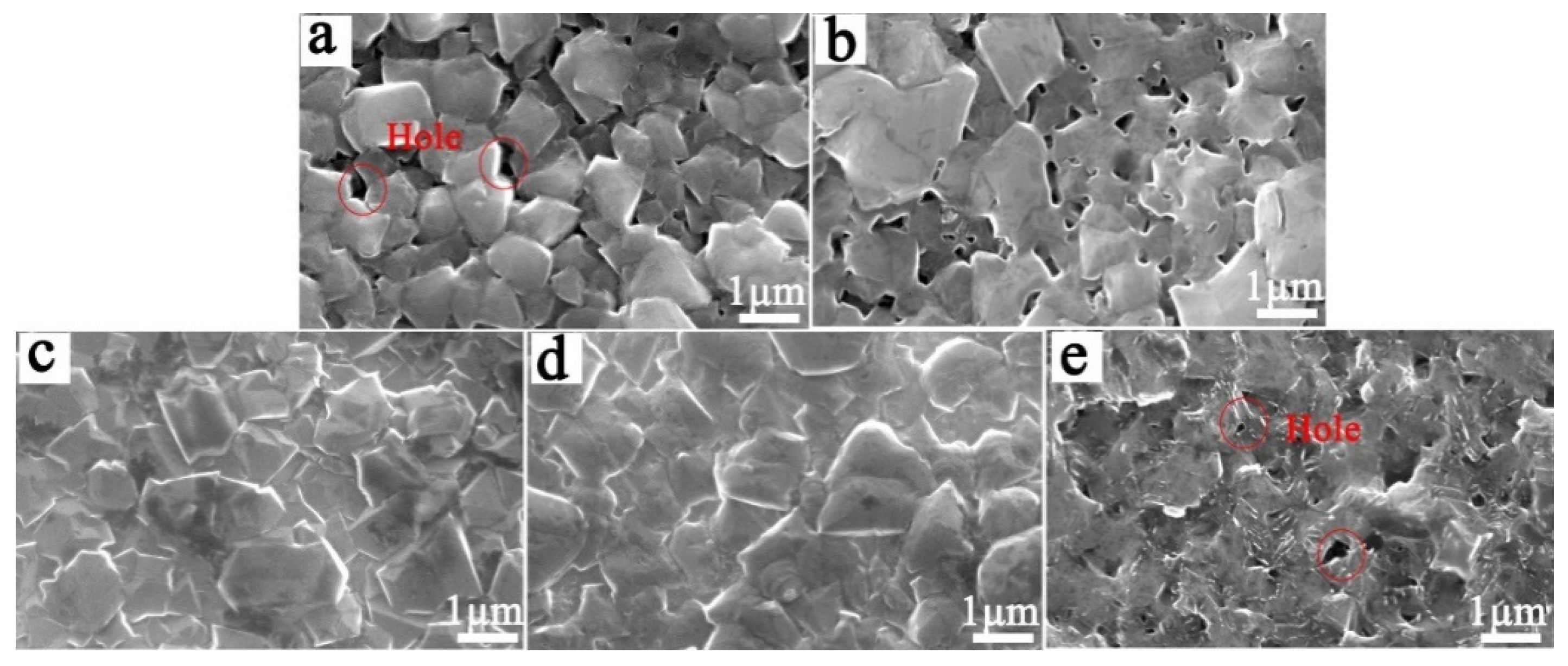
3.1. Effect of the CH4 Flow Rate during the Nucleation Stage on the Service Life of Ti/BDD Samples
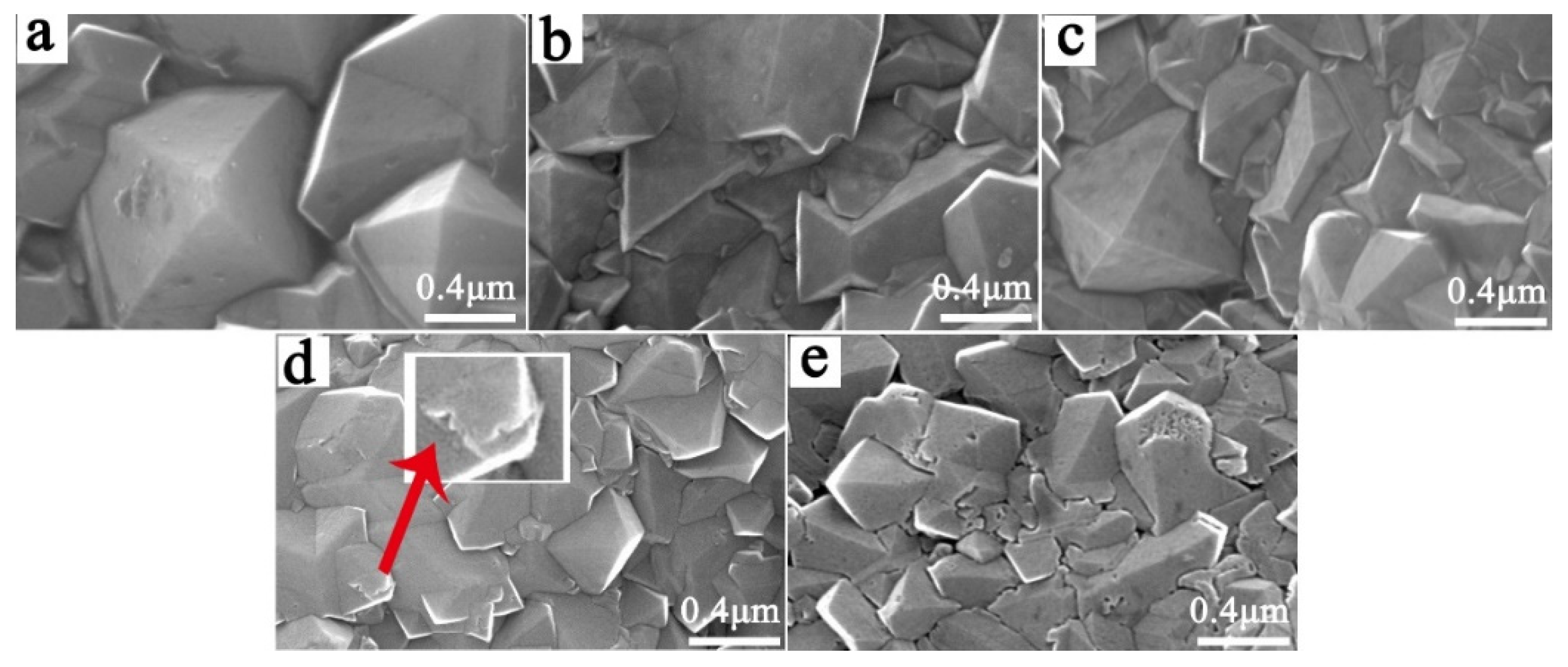
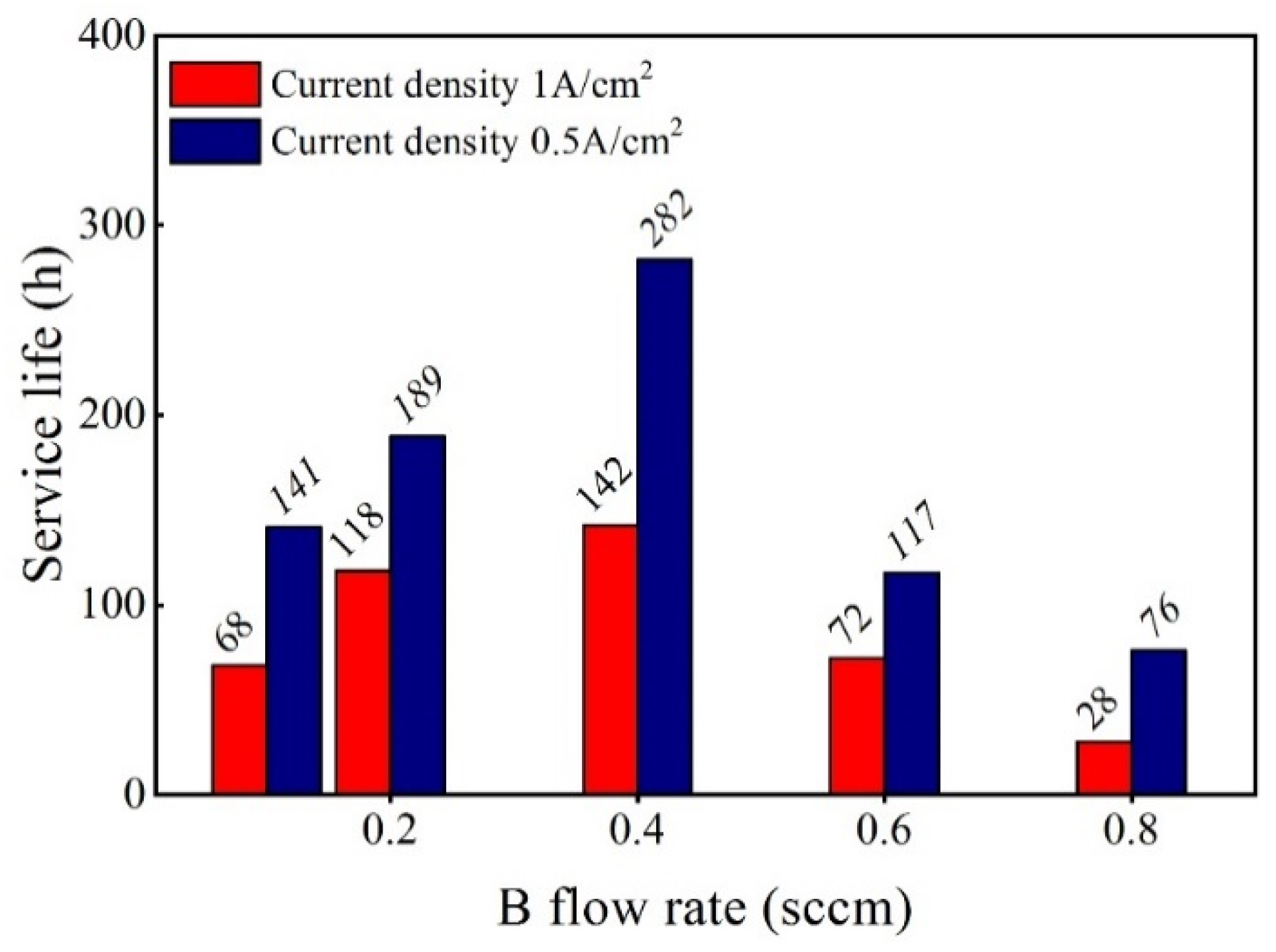
3.2. Effect of the B Source Flow Rate on the Service Life of Ti/BDD Samples
3.3. Adopting the Gradient B-Doping Method to Improve the Service Life of Ti/BDD Coated Electrode
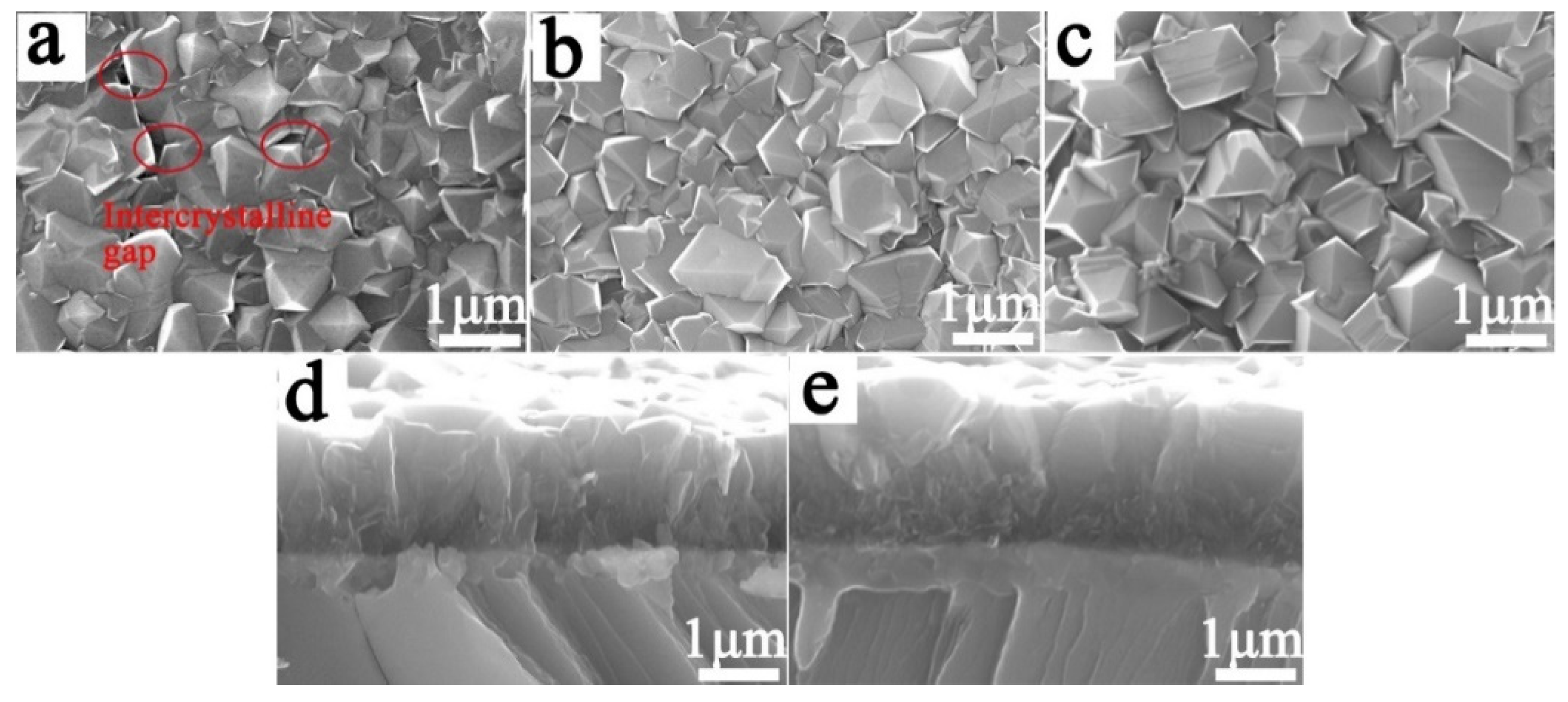
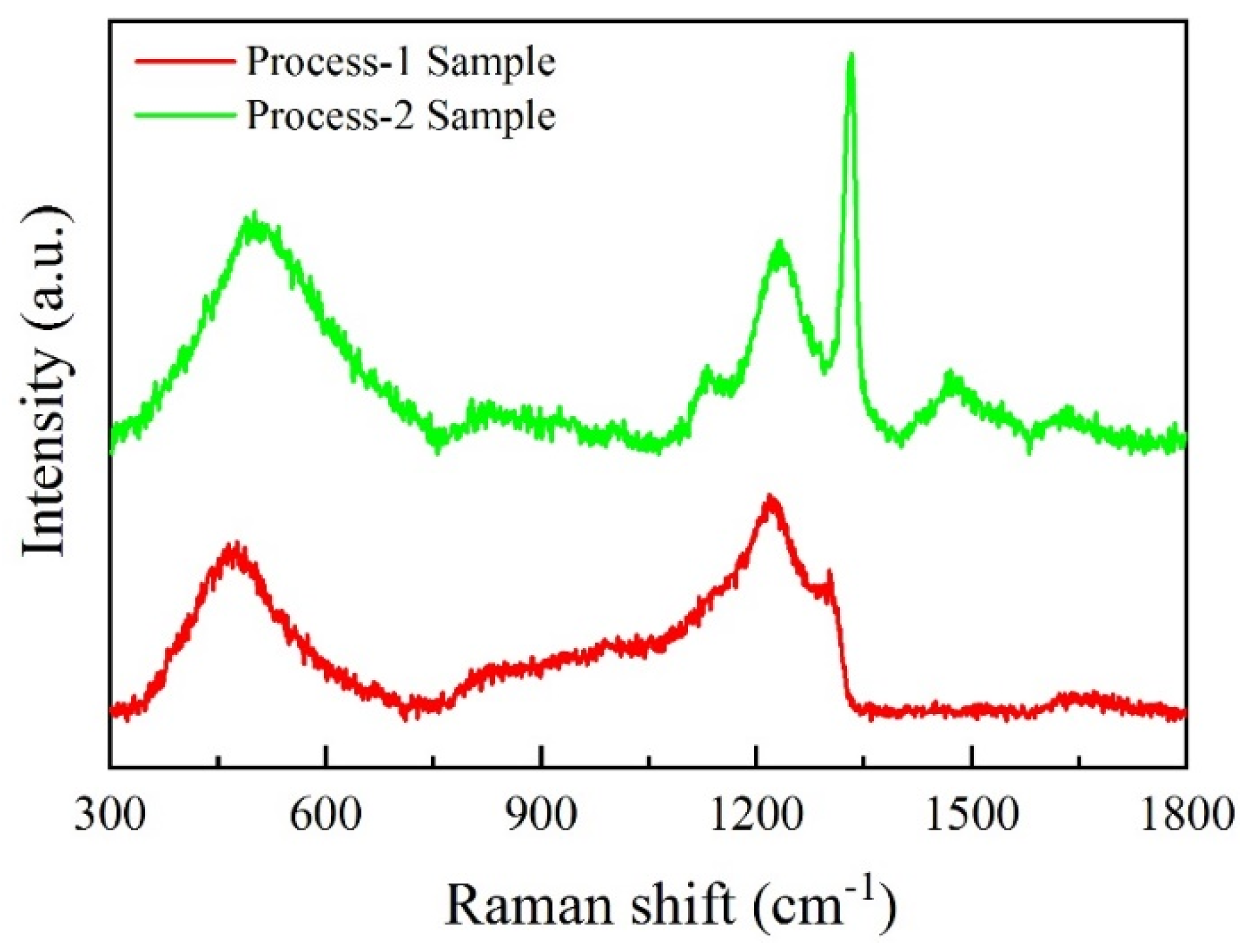
3.3.1. Characterization of Electrode Samples Prepared by Two Processes
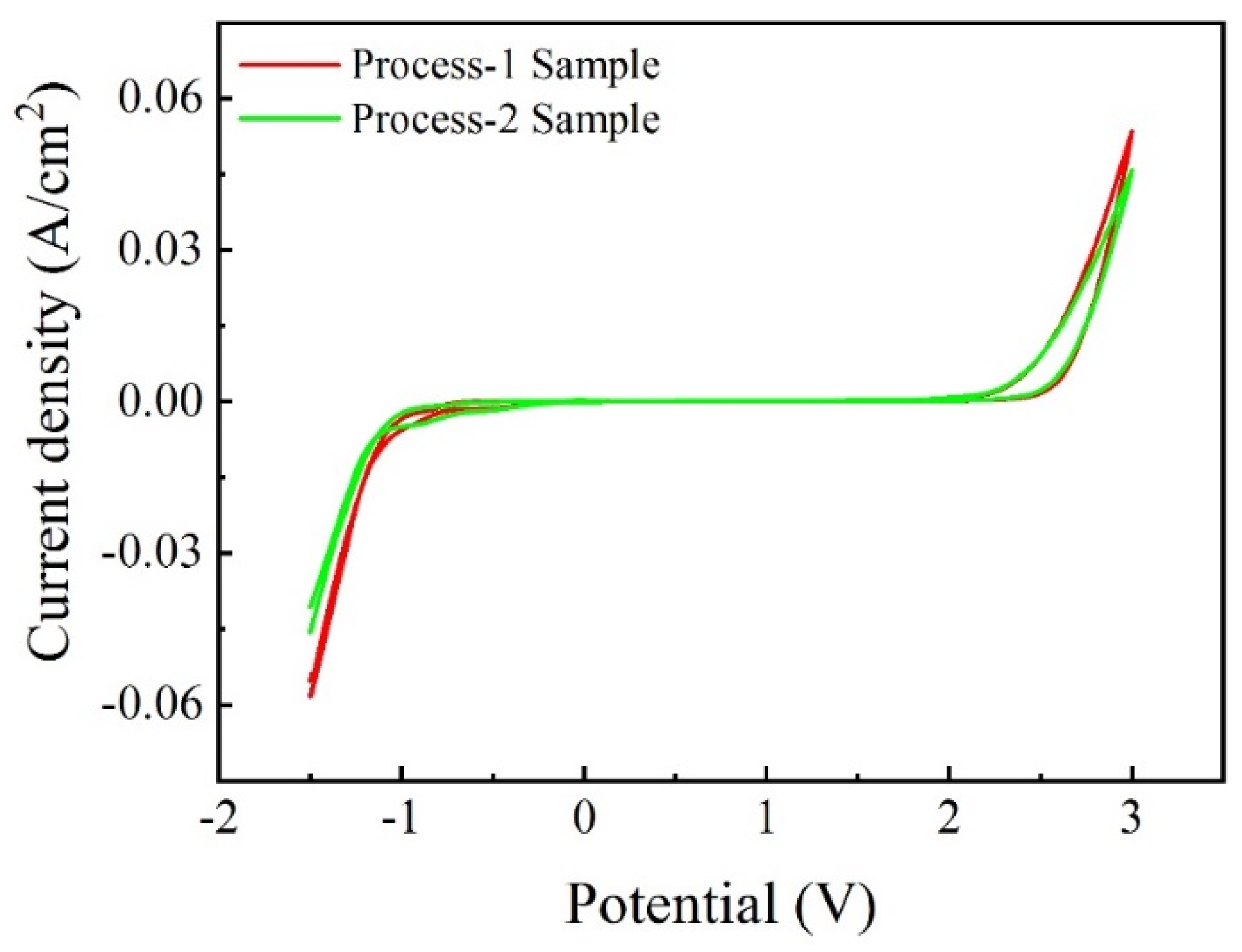
3.3.2. The ALT Results of Ti/BDD Coated Electrodes Prepared by Two Processes
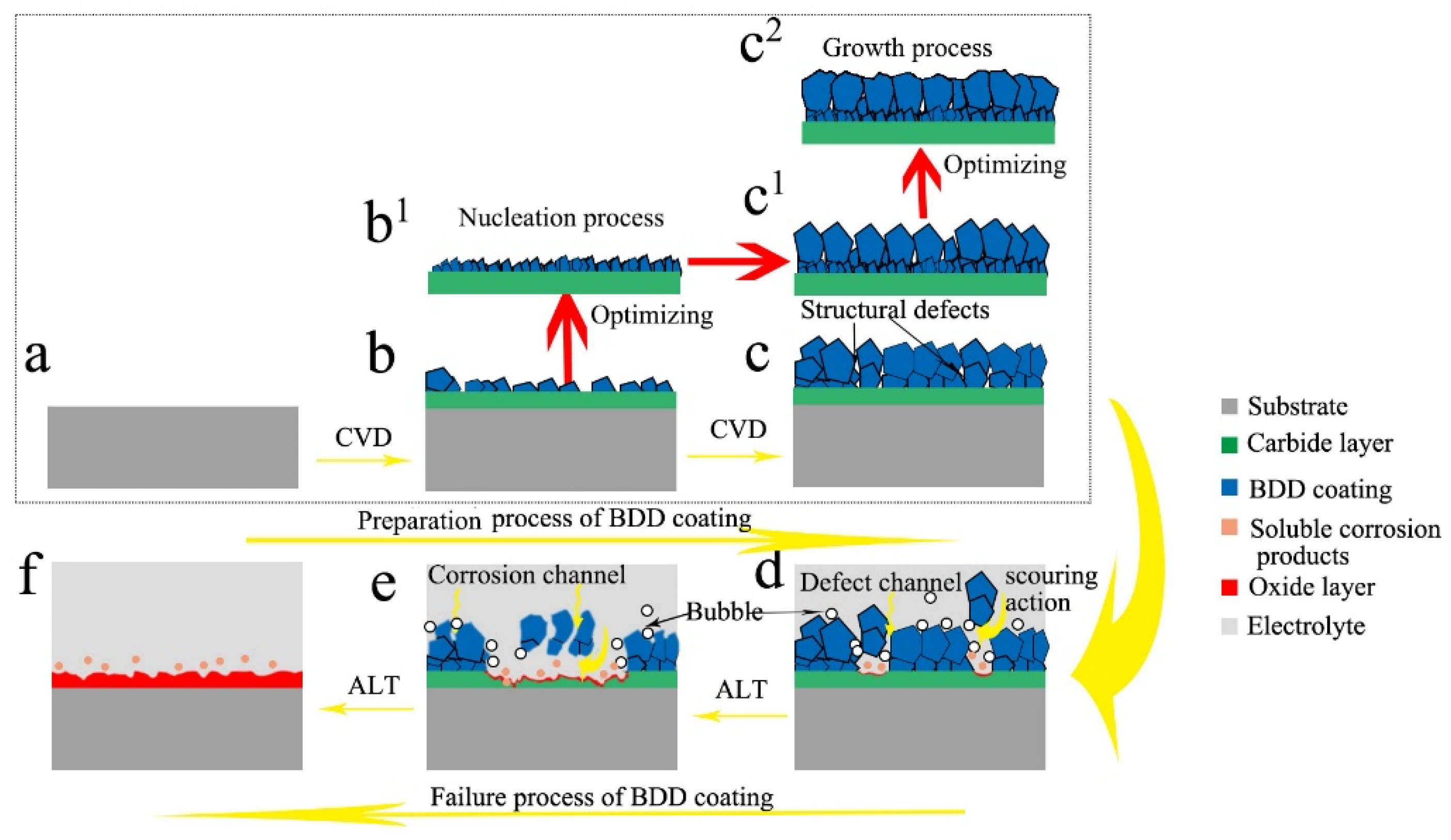
3.4. Mechanisms Analysis of Improving the Service Life of Ti/BDD Coated Electrode
4. Conclusions
- The nucleation process has an important effect on the coating/substrate interface microstructure of the Ti/BDD coated electrode. Optimizing the nucleation process and reducing the hole defects at the coating/substrate interface can significantly improve the service life of a Ti/BDD coated electrode.
- B-doping content has a significant effect on the structure and corrosion resistance of BDD coating. The low B-doping content made the structure of the BDD coating not compact and decreased the life of the electrode. The high B-doping content caused the grain boundary defects of the BDD coatings to increase and the corrosion resistance to decrease, which greatly reduced the service life of the Ti/BDD coated electrodes.
- By adopting the gradient B-doping method, the value of the growth parameter a of the BDD coating changed at the later deposition stage, which led to a change in the grain orientation, the structural defects in the coating to diminish, and significantly improved the service life of the electrode samples.
Author Contributions
Funding
Conflicts of Interest
References
- He, Y.; Lin, H.; Guo, Z.; Zhang, W.; Li, H.; Huang, W. Recent developments and advances in boron-doped diamond electrodes for electrochemical oxidation of organic pollutants. Sep. Purif. Technol. 2019, 212, 802–821. [Google Scholar] [CrossRef]
- Loos, G.; Scheers, T.; Van Eyck, K.; Van Schepdael, A.; Adams, E.; Van der Bruggen, B.; Cabooter, D.; Dewil, R. Electrochemical oxidation of key pharmaceuticals using a boron doped diamond electrode. Sep. Purif. Technol. 2018, 195, 184–191. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Li, M.; Li, C.; Qian, L.; Su, L.; Yang, B. Preparation of polycrystalline bdd/ta electrodes for electrochemical oxidation of organic matter. Electrochim. Acta 2018, 290, 109–117. [Google Scholar] [CrossRef]
- Alexander, K. Doped diamond: A compact review on a new, versatile electrode material. Int. J. Electrochem. Sci. 2007, 2, 355–385. [Google Scholar]
- He, Y.; Huang, W.; Chen, R.; Zhang, W.; Lin, H. Improved electrochemical performance of boron-doped diamond electrode depending on the structure of titanium substrate. J. Electroanal. Chem. 2015, 758, 170–177. [Google Scholar] [CrossRef]
- Migliorini, F.L.; Alegre, M.D.; Baldan, M.R.; Lanza, M.R.V.; Ferreira, N.G. Doped diamond electrodes on titanium substrates with controlled sp2/sp3 hybridization at different boron levels. Thin Solid Films 2014, 564, 97–103. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Application of diamond electrodes to electrochemical processes. Electrochim. Acta 2005, 51, 191–199. [Google Scholar] [CrossRef]
- Honda, Y.; Ivandini, T.A.; Watanabe, T.; Murata, K.; Einaga, Y. An electrolyte-free system for ozone generation using heavily boron-doped diamond electrodes. Diam. Relat. Mater. 2013, 40, 7–11. [Google Scholar] [CrossRef]
- Nie, M.; Neodo, S.; Wharton, J.A.; Cranny, A.; Harris, N.R.; Wood, R.J.K.; Stokes, K.R. Electrochemical detection of cupric ions with boron-doped diamond electrode for marine corrosion monitoring. Electrochim. Acta 2016, 202, 345–356. [Google Scholar] [CrossRef]
- Chaplin, B.P.; Wyle, I.; Zeng, H.; Carlisle, J.A.; Farrell, J. Characterization of the performance and failure mechanisms of boron-doped ultrananocrystalline diamond electrodes. J. Appl. Electrochem. 2011, 41, 1329–1340. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G. Proper hot filament cvd conditions for fabrication of ti-boron doped diamond electrodes. J. Electrochem. Soc. 2004, 151, B214–B219. [Google Scholar] [CrossRef]
- Gerger, I.; Haubner, R.; Kronberger, H.; Fafilek, G. Investigation of diamond coatings on titanium substrates for electrochemical applications. Diam. Relat. Mater. 2004, 13, 1062–1069. [Google Scholar] [CrossRef]
- Guo, L.; Chen, G. Long-term stable ti/bdd electrode fabricated with hfcvd method using two-stage substrate temperature. J. Electrochem. Soc. 2007, 154, D657–D661. [Google Scholar] [CrossRef]
- Wei, J.J.; Li, C.M.; Gao, X.H.; Hei, L.F.; Lvun, F.X. The influence of boron doping level on quality and stability of diamond film on ti substrate. Appl. Surf. Sci. 2012, 258, 6909–6913. [Google Scholar] [CrossRef]
- Xueming, C.; Guohua, C.; Furong, G.; Po Lock, Y. High-performance ti/bdd electrodes for pollutant oxidation. Environ. Sci. Technol. 2003, 37, 5021–5026. [Google Scholar]
- Tian, Y.; Chen, X.; Shang, C.; Chen, G. Active and stable Ti/Si/bdd anodes for electrooxidation. J. Electrochem. Soc. 2006, 153, J80–J85. [Google Scholar] [CrossRef]
- Sun, J.; Lu, H.; Lin, H.; Huang, W.; Li, H.; Lu, J.; Cui, T. Boron doped diamond electrodes based on porous ti substrates. Mater. Lett. 2012, 83, 112–114. [Google Scholar] [CrossRef]
- Lu, X.; Ding, M.; Zhang, C.; Tang, W. Investigation on microstructure evolution and failure mechanism of boron doped diamond coated titanium electrode during accelerated life test. Thin Solid Films 2018, 660, 306–313. [Google Scholar] [CrossRef]
- Lu, X.; Ding, M.; Zhang, C.; Tang, W. Comparative study on stability of boron doped diamond coated titanium and niobium electrodes. Diam. Relat. Mater. 2019, 93, 26–33. [Google Scholar] [CrossRef]
- Miao, J.; Song, J.; Xue, Y.; Tong, Y.; Tang, W. Effect of a two-step pretreatment method on adhesion of cvd diamond coatings on cemented carbide substrates. Surf. Coat. Tech. 2004, 187, 33–36. [Google Scholar] [CrossRef]
- Joffreau, P.O.; Haubner, R.; Lux, B. Low-pressure diamond growth on refractory metals. Int. J. Refract. Hard Met. 1988, 7, 186–194. [Google Scholar]
- Fu, Y.; Yan, B.; Loh, N.L.; Sun, C.Q.; Hing, P. Characterization and tribological evaluation of mw-pacvd diamond coatings deposited on pure titanium. Mater. Sci. Eng. A 2000, 282, 38–48. [Google Scholar] [CrossRef]
- Liao, X.Z.; Zhang, R.J.; Lee, C.S.; Lee, S.T.; Lam, Y.W. The influence of boron doping on the structure and characteristics of diamond thin films. Diam. Relat. Mater. 1997, 6, 521–525. [Google Scholar] [CrossRef]
- Schwarzová-Pecková, K.; Vosáhlová, J.; Barek, J.; Šloufová, I.; Pavlova, E.; Petrák, V.; Zavázalová, J. Influence of boron content on the morphological, spectral, and electroanalytical characteristics of anodically oxidized boron-doped diamond electrodes. Electrochim. Acta 2017, 243, 170–182. [Google Scholar] [CrossRef]
- Gao, X.; Li, W.; Mei, R.; Zhu, C.; Zhou, B.; Ma, L.; Wei, Q.; Liu, T. Effect of the b2h6/ch4/h2 ratios on the structure and electrochemical properties of boron-doped diamond electrode in the electrochemical oxidation process of azo dye. J. Electroanal. Chem. 2019, 832, 247–253. [Google Scholar] [CrossRef]
- Lu, Y.G.; Turner, S.; Verbeeck, J.; Janssens, S.D.; Wagner, P.; Haenen, K.; Van Tendeloo, G. Direct visualization of boron dopant distribution and coordination in individual chemical vapor deposition nanocrystalline b-doped diamond grains. Appl. Phys. Lett. 2012, 101, 41907–251910. [Google Scholar] [CrossRef][Green Version]
- Zhang, T.; Wang, L.; Sun, F.; Shen, B.; Zhang, Z. The effect of boron doping on the morphology and growth rate of micron diamond powders synthesized by hfcvd method. Diam. Relat. Mater. 2013, 40, 82–88. [Google Scholar] [CrossRef]
- Bernard, M.; Baron, C.; Deneuville, A. About the origin of the low wave number structures of the raman spectra of heavily boron doped diamond films. Diam. Relat. Mater. 2004, 13, 896–899. [Google Scholar] [CrossRef]
- Ushizawa, K.; Watanabe, K.; Ando, T.; Sakaguchi, I.; Nishitani-Gamo, M.; Sato, Y.; Kanda, H. Boron concentration dependence of raman spectra on {100} and {111} facets of b-doped cvd diamond. Diam. Relat. Mater. 1998, 7, 1719–1722. [Google Scholar] [CrossRef]
- Mortet, V.; Vlčková Živcová, Z.; Taylor, A.; Frank, O.; Hubík, P.; Trémouilles, D.; Jomard, F.; Barjon, J.; Kavan, L. Insight into boron-doped diamond raman spectra characteristic features. Carbon 2017, 115, 279–284. [Google Scholar] [CrossRef]
- Bernard, M.; Deneuville, A.; Muret, P. Non-destructive determination of the boron concentration of heavily doped metallic diamond thin films from raman spectroscopy. Diam. Relat. Mater. 2004, 13, 282–286. [Google Scholar] [CrossRef]
- Messner, M.; Walczyk, D.J.; Palazzo, B.G.; Norris, Z.A.; Taylor, G.; Carroll, J.; Pham, T.X.; Hettinger, J.D.; Yu, L. Electrochemical oxidation of metal carbides in aqueous solutions. J. Electrochem. Soc. 2018, 165, H3107–H3114. [Google Scholar] [CrossRef]
- Bauer, A.D.; Carlsson, J.O. Corrosion of chemically vapour deposited titanium cabide on an inert substrate. J. Phys. IV 1991, 2, C2-641–C2-648. [Google Scholar]
- Ali, M.; Ürgen, M. Surface morphology, growth rate and quality of diamond films synthesized in hot filament cvd system under various methane concentrations. Appl. Surf. Sci. 2011, 257, 8420–8426. [Google Scholar] [CrossRef]
- Swain, G.M. The susceptibility to surface corrosion in acidic fluoride media—A comparison of diamond, hopg, and glossy carbon electrodes. J. Electrochem. Soc. 1994, 141, 3382–3393. [Google Scholar] [CrossRef]
- Wild, C.; Herres, N.; Koidl, P. Texture formation in polycrystalline diamond films. J. Appl. Phys. 1990, 68, 973–978. [Google Scholar] [CrossRef]
- Wild, C.; Kohl, R.; Herres, N.; Müller-Sebert, W.; Koidl, P. Oriented cvd diamond films: Twin formation, structure and morphology. Diam. Relat. Mater. 1994, 3, 373–381. [Google Scholar] [CrossRef]
- Tamor, M.A.; Everson, M.P. On the role of penetration twins in the morphological development of vapor-grown diamond films. J. Mater. Res. 1994, 9, 1839–1849. [Google Scholar] [CrossRef]
- Paritosh; Srolovitz, D.J.; Battaile, C.C.; Li, X.; Butler, J.E. Simulation of faceted film growth in two-dimensions: Microstructure, morphology and texture. Acta Mater. 1999, 47, 2269–2281. [Google Scholar] [CrossRef]


| Gas | The Volume Flow Rate (sccm) | |
|---|---|---|
| The Nucleation Stage for 2 h | The Growth Stage for 8 h | |
| CH4 | 5, 6, 7, 8, 10, 14 | 3 |
| B Source | 0.4 | 0.4 |
| Gas | The Volume Flow Rate (sccm) | |
|---|---|---|
| The Nucleation Stage for 2 h | The Growth Stage for 8 h | |
| CH4 | 10 | 3 |
| B Source | 0.1, 0.2, 0.4, 0.6, 0.8 | |
| Gas | The Volume Flow Rate (sccm) | ||
|---|---|---|---|
| The Nucleation Stage for 2 h | The Growth Stage for 8 h | ||
| Process-1 | CH4 | 8 | 3 |
| B Source | 0.4 | 0.4 | |
| Process-2 | CH4 | 8 | 3 |
| B Source | 0.4 | 0.4 (for 6 h) and 0.1(for last 2 h) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.r.; Ding, M.h.; Zhang, L.; Yang, Z.l.; Lu, Y.; Tang, W.z. Optimizing the Microstructure and Corrosion Resistance of BDD Coating to Improve the Service Life of Ti/BDD Coated Electrode. Materials 2019, 12, 3188. https://doi.org/10.3390/ma12193188
Lu Xr, Ding Mh, Zhang L, Yang Zl, Lu Y, Tang Wz. Optimizing the Microstructure and Corrosion Resistance of BDD Coating to Improve the Service Life of Ti/BDD Coated Electrode. Materials. 2019; 12(19):3188. https://doi.org/10.3390/ma12193188
Chicago/Turabian StyleLu, Xin ru, Ming hui Ding, Lu Zhang, Zhi liang Yang, Yao Lu, and Wei zhong Tang. 2019. "Optimizing the Microstructure and Corrosion Resistance of BDD Coating to Improve the Service Life of Ti/BDD Coated Electrode" Materials 12, no. 19: 3188. https://doi.org/10.3390/ma12193188
APA StyleLu, X. r., Ding, M. h., Zhang, L., Yang, Z. l., Lu, Y., & Tang, W. z. (2019). Optimizing the Microstructure and Corrosion Resistance of BDD Coating to Improve the Service Life of Ti/BDD Coated Electrode. Materials, 12(19), 3188. https://doi.org/10.3390/ma12193188




