Co-Immobilization of Glucose Dehydrogenase and Xylose Dehydrogenase as a New Approach for Simultaneous Production of Gluconic and Xylonic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Co-Immobilization of GDH and XDH
2.3. Activity and Kinetic Parameters of the Free and Immobilized Enzymes
2.4. Conversion of Glucose to Gluconic Acid and Xylose to Xylonic Acid Catalyzed by Free and Co-Immobilized Enzymes
2.4.1. Time Course of the Conversion of Glucose to Gluconic Acid and Xylose to Xylonic Acid
2.4.2. Effect of pH and Temperature on Enzymatic Conversion of Glucose to Gluconic Acid and Xylose to Xylonic Acid
2.4.3. Stability of the Free and Co-Immobilized GDH and XDH
2.4.4. Storage Stability and Reusability of the Free and Co-Immobilized GDH and XDH
2.5. Analytical Techniques
2.6. Statistical Analysis
3. Results
3.1. Enzyme Co-Immobilization and Characterization
3.2. Enzymes Co-Immobilization and Gluconic Acid and Xylonic Acid Production
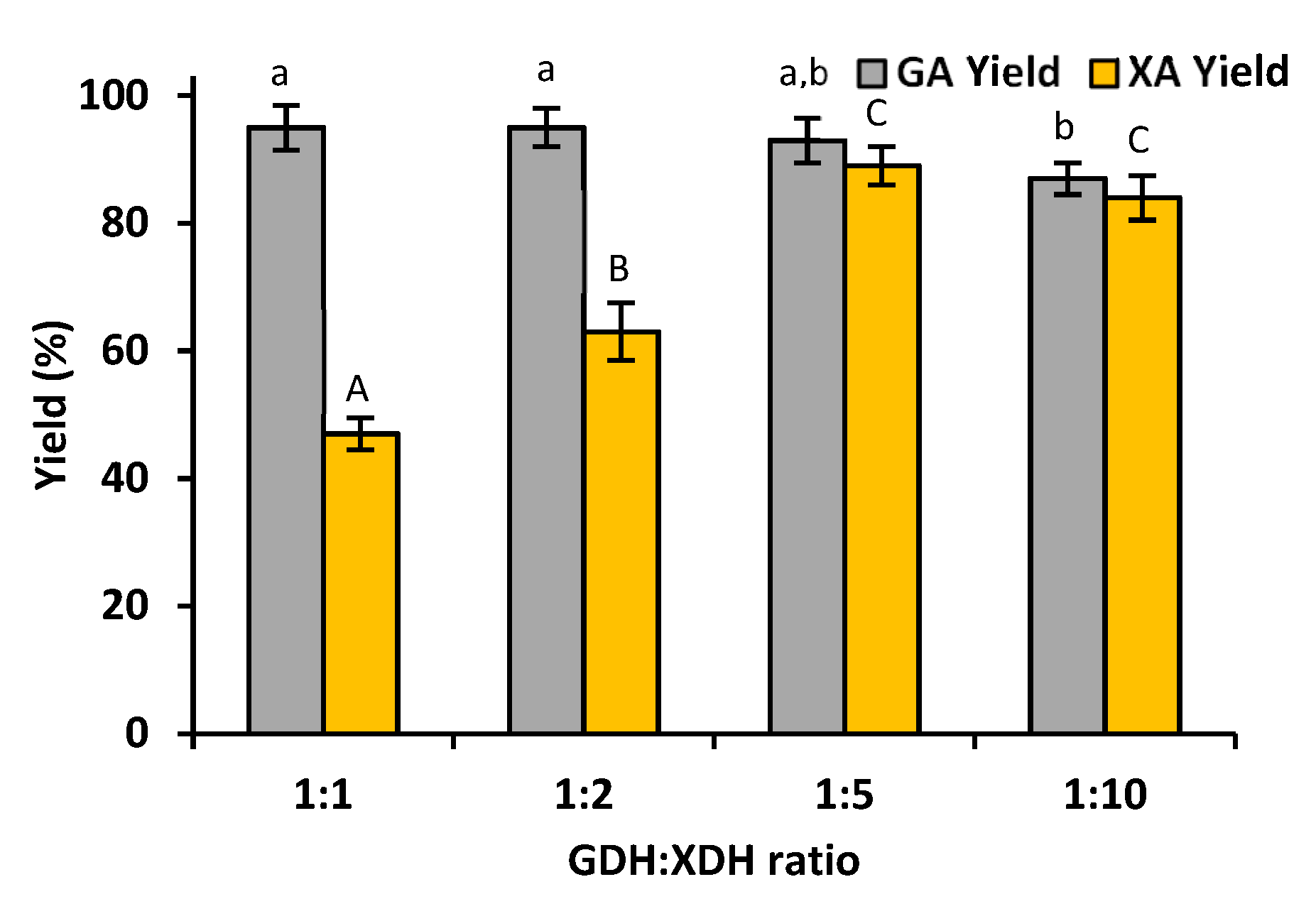
3.2.1. Effect of GDH:XDH Ratio on the Production of Gluconic Acid and Xylonic Acid
3.2.2. Time Course of The Production of Gluconic Acid and Xylonic Acid Catalysed by Co-Immobilized GDH and XDH.
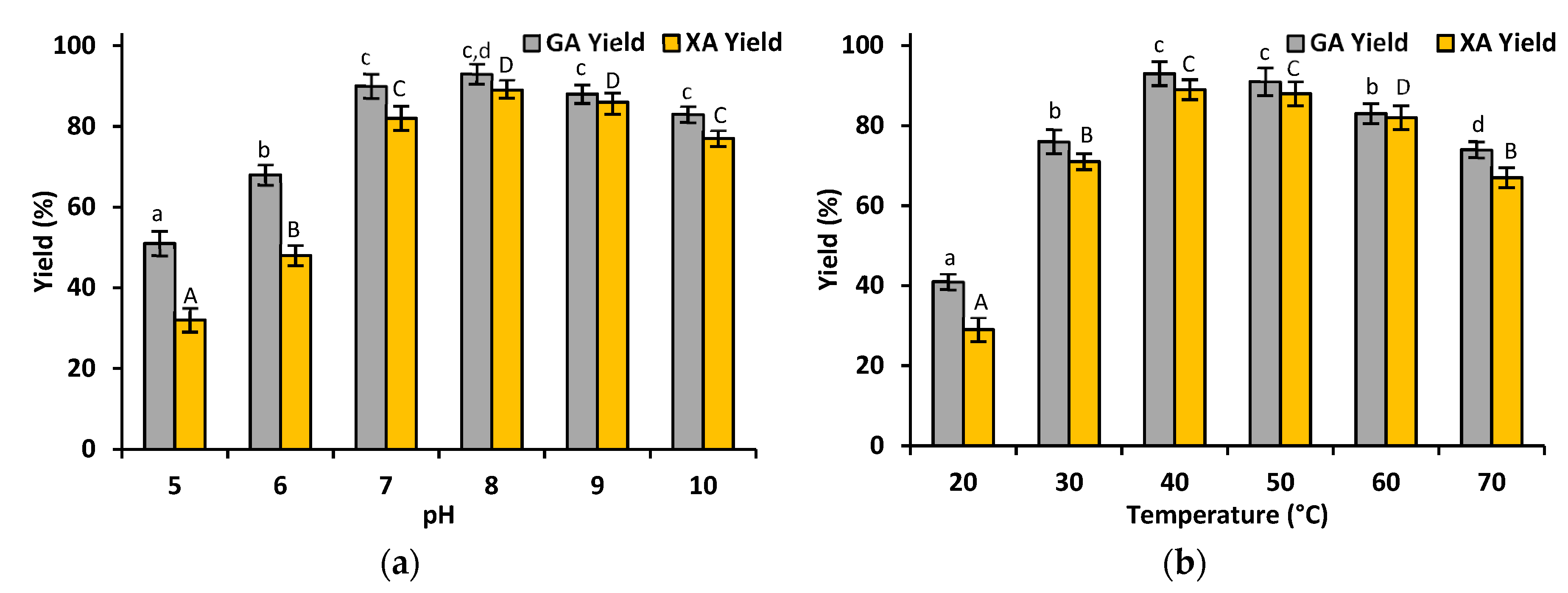
3.2.3. Effect of pH and Temperature on The Production of Gluconic and Xylonic Acid Catalyzed by Co-Immobilized GDH and XDH
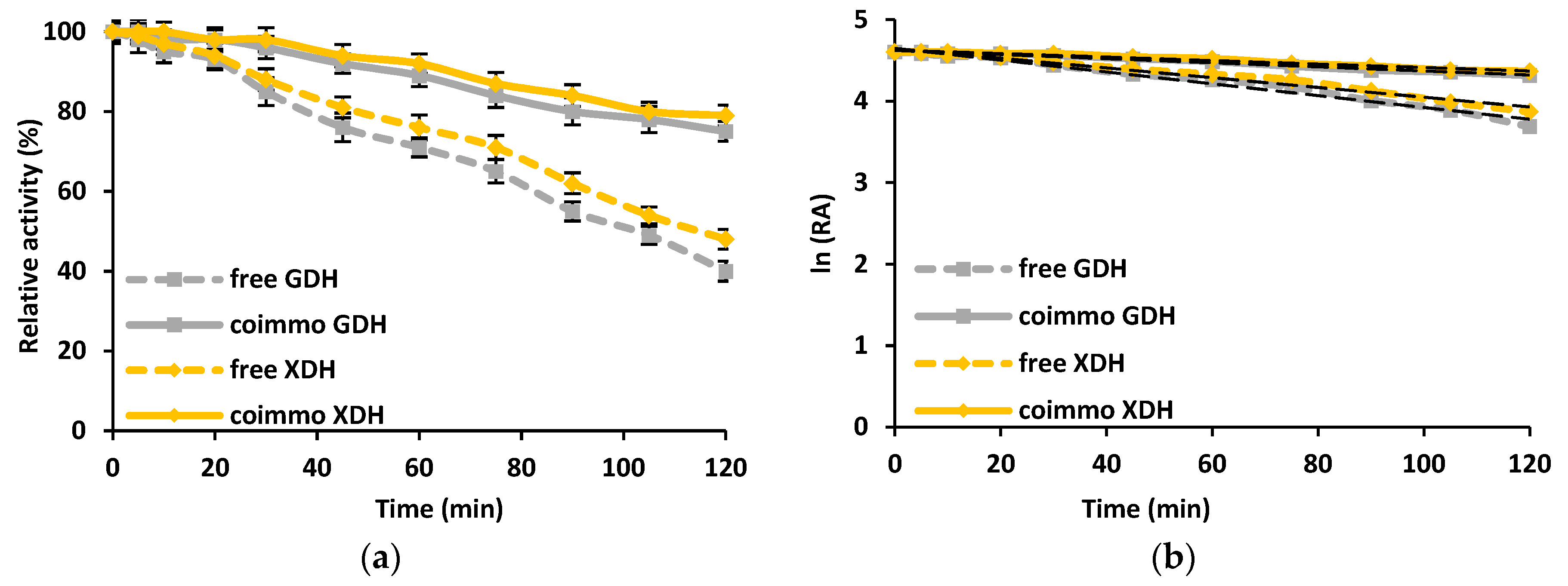
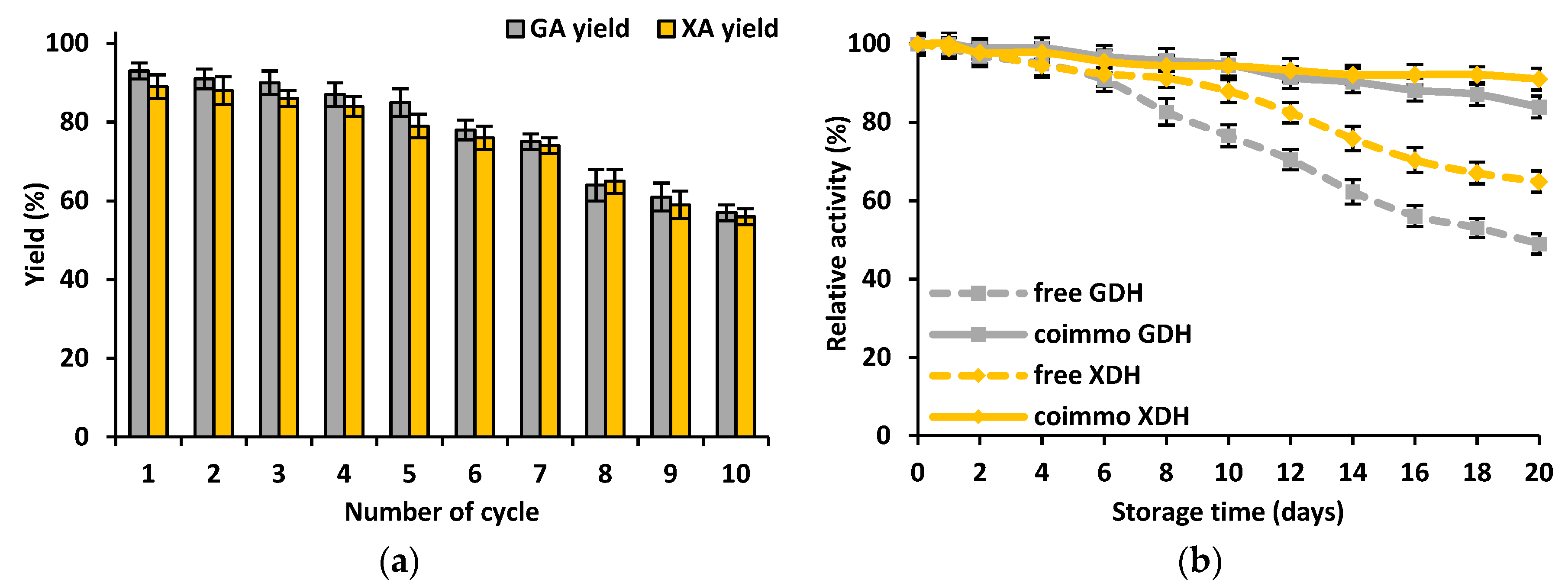
3.2.4. Stability of the Free and Co-Immobilized GDH and XDH
3.2.5. Storage Stability and Reusability of the Co-Immobilized GDH and XDH
4. Discussion
4.1. Enzyme Co-Immobilization and Characterization
4.2. Enzymes Co-Immobilization and Gluconic Acid and Xylonic Acid Production
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feliczak-Guzik, A.; Nowak, I. Mesoporous niobosilicates serving as catalysts for synthesis of fragrances. Cat. Today 2009, 142, 288–292. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Yu, C.; Tian, B.; Liu, X.; Fan, J.; Yang, H.; Zhao, D. Advances in mesoporous materials templated by nonionic block copolymers. In Nanoporous Materials: Science and Engineering; Lu, G.Q., Zhao, X.S., Eds.; Imperial College Press: London, Great Britain, 2004; pp. 115–136. [Google Scholar]
- Hartmann, M.; Kostrov, X. Immobilization of enzymes on porous silicas – benefits and challenges. Chem. Soc. Rev. 2013, 42, 6277–6289. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. Multifaceted strategy based on enzyme immobilization with reactant adsorption and membrane technology for biocatalytic removal of pollutants: A critical review. Biotechnol. Adv. in press.
- Górecka, E.; Jastrzębska, M. Immobilization techniques and biopolymer carriers. Biotechnol. Food Sci. 2011, 75, 65–86. [Google Scholar]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2012, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Fan, M. Immobilization of tetramethylguanidine on mesoporous SBA-15 silica: A heterogeneous basic catalyst for transesterification of soybean oil. Bioresour. Technol. 2013, 139, 388–392. [Google Scholar] [CrossRef]
- Roche, C.M.; Dibble, C.J.; Stickel, J.J. Laboratory-scale method for enzymatic saccharification of lignocellulosic biomass at high-solids loadings. Biotechnol. Biofuels 2009, 2, 28–37. [Google Scholar] [CrossRef]
- Jørgensen, H.; Pinelo, M. Enzyme recycling in lignocellulosic biorefineries. Biofuels Bioprod. Bioeng. 2017, 11, 150–167. [Google Scholar] [CrossRef]
- Sueb, M.S.M.; Zdarta, J.; Jesionowski, T.; Jonsson, G.; Meyer, A.S.; Jørgensen, H.; Pinelo, M. High-performance removal of acids and furans from wheat straw pretreatment liquid by diananofiltration. Sep. Sci. Technol. 2017, 52, 1901–1912. [Google Scholar] [CrossRef]
- Zdarta, J.; Pinelo, M.; Jesionowski, T.; Meyer, A. Upgrading of biomass monosaccharides by immobilized glucose dehydrogenase and xylose dehydrogenase. ChemCatChem 2018, 10, 5164–5173. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Singh, S. Immobilization and applications of glucose-6-phosphate dehydrogenase: A review. Prep. Biochem. Biotechnol. 2013, 43, 376–384. [Google Scholar] [CrossRef]
- Eslamipour, F.; Hejazi, P. Evaluating effective factors on activity and loading of immobilized a-amylase onto magnetic nanoparticles using response surface desirability approach. RSC Adv. 2016, 6, 20187–20197. [Google Scholar] [CrossRef]
- Gholamzadeh, P.; Ziarani, G.M.; Badiei, A. Immobilization of lipases onto the SBA-15 mesoporous silica. Biocatal. Biotransform. 2017, 35, 131–150. [Google Scholar] [CrossRef]
- Zdarta, J.; Feliczak-Guzik, A.; Siwińska-Ciesielczyk, K.; Nowak, I.; Jesionowski, T. Mesostructured cellular foam silica materials for laccase immobilization and tetracycline removal: A comprehensive study. Micropor. Mesopor. Mater. 2020, 291, 109688. [Google Scholar] [CrossRef]
- Sánchez-Moreno, I.; García-Junceda, E.; Hermida, C.; Fernández-Mayoralas, A. Development of a new method for d -xylose detection and quantification in urine, based on the use of recombinant xylose dehydrogenase from Caulobacter crescentus. J. Biotechnol. 2016, 234, 50–57. [Google Scholar] [CrossRef]
- Liu, J.; Pang, B.Q.W.; Adams, J.P.; Snajdrova, R.; Li, Z. Coupled immobilized amine dehydrogenase and glucose dehydrogenase for asymmetric synthesis of amines by reductive amination with cofactor recycling. ChemCatChem 2017, 9, 425–431. [Google Scholar] [CrossRef]
- Delgove, M.; Valencia, D.; Solé, J.; Bernaerts, K.; de Wildeman, S.; Guillén, M.; Álvaro, G. High performing immobilized Baeyer-Villiger monooxygenase and glucose dehydrogenase for the synthesis of ε-caprolactone derivative. Appl. Catal. A 2019, 572, 134–141. [Google Scholar] [CrossRef]
- Li, L.; Liang, B.; Li, F.; Shi, J.; Mascini, M.; Lang, Q.; Liu, A. Co-immobilization of glucose oxidase and xylose dehydrogenase displayed whole cell on multiwalled carbon nanotube nanocomposite films modified electrode for simultaneous voltammetric detection of D-glucose and D-xylose. Biosens. Bioelectron. 2013, 42, 156–162. [Google Scholar] [CrossRef]
- Baron, M.; Fontana, J.D.; Guimaraes, M.F.; Woodward, J. Stabilization and reutilization of Bacillus megaterium glucose dehydrogenase by immobilization. Appl. Biochem. Biotechnol. 1997, 63, 257–268. [Google Scholar] [CrossRef]
- Zhou, Q.Z.; Chen, X.D. Effects of temperature and pH on the catalytic activity of the immobilized β-galactosidase from Kluyveromyces lactis. Biochem. Eng. J. 2001, 9, 33–40. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. Developments in support materials for immobilization of oxidoreductases: A comprehensive review. Adv. Colloid. Interfac. Sci. 2018, 258, 1–20. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–17. [Google Scholar] [CrossRef]
- Zhang, D.H.; Zhang, Y.F.; Zhi, G.Y.; Xie, Y.L. Effect of hydrophobic/hydrophilic characteristics of magnetic microspheres on the immobilization of BSA. Colloid. Surface. B 2011, 82, 302–306. [Google Scholar] [CrossRef]
- Bachosz, K.; Synoradzki, K.; Staszak, M.; Pinelo, M.; Meyer, A.S.; Zdarta, J.; Jesionowski, T. Bioconversion of xylose to xylonic acid via co-immobilized dehydrogenases for conjunct cofactor regeneration. Bioorg. Chem. in press. [CrossRef]
- Gao, F.; Ding, H.; Xu, X.; Zhao, Y. A self-sufficient system for removal of synthetic dye by coupling of spore-displayed triphenylmethane reductase and glucose 1-dehydrogenase. Environ. Sci. Pollut. Res. Int. 2016, 23, 21319–21326. [Google Scholar] [CrossRef]
- Yamanaka, K.; Gino, M. Purification and properties of D-xylose dehydrogenase in bacteria. Hakko Kogaku Kaishi 1979, 57, 322–331. [Google Scholar]
- Secundo, F. Conformational changes of enzymes upon immobilisation. Chem. Soc. Rev. 2013, 42, 6250–6261. [Google Scholar] [CrossRef]
- Aissaoui, N.; Landoulsi, J.; Bergaoui, L.; Boujdaya, S.; Lambert, J. Catalytic activity and thermostability of enzymes immobilized on silanized surface: Influence of the crosslinking agent. Enzyme Microb. Technol. 2013, 52, 336–343. [Google Scholar] [CrossRef]
- Twala, B.V.; Sewell, B.T.; Jordaan, J. Immobilisation and characterisation of biocatalytic co-factor recycling enzymes, glucose dehydrogenase and NADH oxidase, on aldehyde functional ReSyn™ polymer microspheres. Enzyme Microb. Technol. 2012, 50, 331–336. [Google Scholar] [CrossRef]
- Zhuang, H.; Dong, S.; Zhang, T.; Tang, M.; Xu, N.; Sun, B.; Liu, J.; Zhang, M.; Yuan, Y. Study on alkaline protease immobilized on mesoporous materials. Asian J. Chem. 2014, 26, 1139–1144. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Zhou, G. Functionalization of mesoporous SBA-15 with APTES by co-condensation and its effect on immobilization of Candida rugose lipase. Key Eng. Mater. 2015, 645–646, 1261–1266. [Google Scholar] [CrossRef]
- Zhou, Q.Z.K.; Chen, X.D. Immobilization of β-galactosidase on graphite surface by glutaraldehyde. J. Food Eng. 2001, 48, 69–74. [Google Scholar] [CrossRef]
- Liu, X.H.; Du, X.; Feng, J.R.; Wu, M.B.; Lin, J.P.; Guan, J.; Wang, T.; Zhang, Z.H. Co-immobilization of Short-chain dehydrogenase/reductase and glucose dehydrogenase for the efficient production of (±)-ethyl mandelate. Catal. Lett. 2019, 149, 1710–1720. [Google Scholar] [CrossRef]
- Solé, J.; Caminal, G.; Schürmann, M.; Álvaroa, G.; Guilléna, M. Co-immobilization of P450 BM3 and glucose dehydrogenase on different supports for application as a self-sufficient oxidative biocatalyst. J. Chem. Technol. Biotechnol. 2019, 94, 244–255. [Google Scholar] [CrossRef]
- Karimi, B.; Emadi, S.; Safaria, A.A.; Kermaniana, M. Immobilization, stability and enzymatic activity of albumin and trypsin adsorbed onto nanostructured mesoporous SBA-15 with compatible pore sizes. RSC Adv. 2014, 4, 4387–4394. [Google Scholar] [CrossRef]
- Sahin, S.; Ozmen, I. Determination of optimum conditions for glucose-6-phosphate dehydrogenase immobilization on chitosan-coated magnetic nanoparticles and its characterization. J. Mol. Catal. B 2016, 133, S25–S33. [Google Scholar] [CrossRef]
- Mureşeanu, M.; Trandafir, I.; Băbeanu, C.; Pârvulescu, V.; Păun, G. Laccase immobilized on mesoporous silica supports as an efficient system for wastewater bioremediation. Environ. Protect. Eng. 2016, 42, 81–95. [Google Scholar]
- Wongvitvitchot, W.; Siamnikorn, K.; Pithakratanayothin, S.; Chaisuwan, T.; Wongkasemjit, S. Effective and reusable T. reesei immobilized on SBA-15 for monomeric sugar production from cellulose hydrolysis. Bioresour. Technol. Rep. 2019, 5, 199–205. [Google Scholar] [CrossRef]
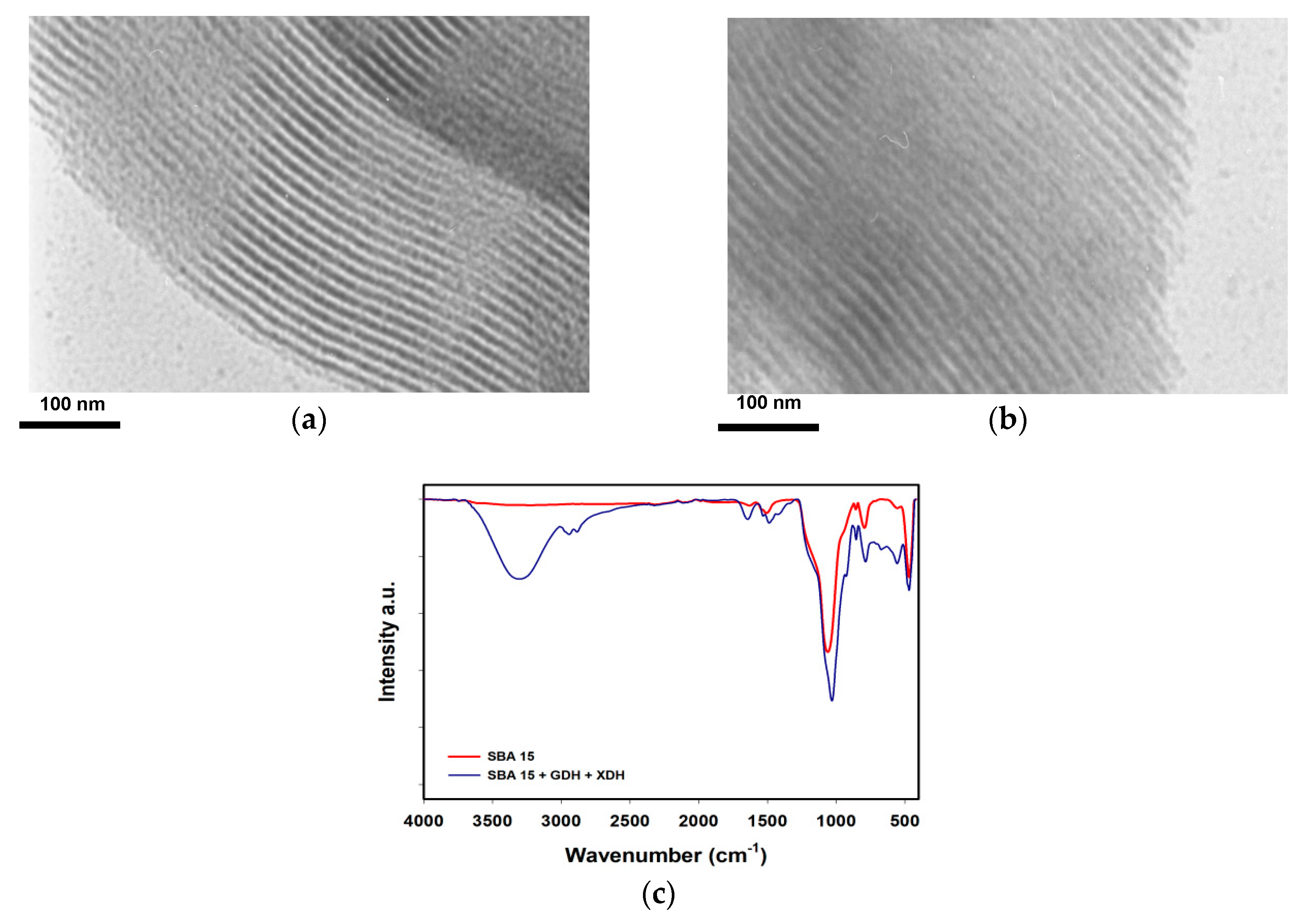

| Sample Name | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| SBA 15 | 564.8 | 0.804 | 20.362 |
| SBA 15 + GDH + XDH | 529.7 | 0.473 | 13.573 |
| Sample Name | KM (mM) | Vmax (U/mg) | Specific Activity (U/mg) |
|---|---|---|---|
| free GDH | 23.2 ± 1.5 | 6.4 ± 0.6 | 39.8 ± 2.3 |
| co-immobilized GDH | 30.1 ± 1.6 | 4.6 ± 0.4 | 25.9 ± 2.1 |
| free XDH | 0.116 ± 0.008 | 0.63 ± 0.09 * | 43.3 ± 1.8 * |
| co-immobilized XDH | 0.149 ± 0.011 | 0.48 ± 0.07 | 21.6 ± 2.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdarta, J.; Bachosz, K.; Degórska, O.; Zdarta, A.; Kaczorek, E.; Pinelo, M.; Meyer, A.S.; Jesionowski, T. Co-Immobilization of Glucose Dehydrogenase and Xylose Dehydrogenase as a New Approach for Simultaneous Production of Gluconic and Xylonic Acid. Materials 2019, 12, 3167. https://doi.org/10.3390/ma12193167
Zdarta J, Bachosz K, Degórska O, Zdarta A, Kaczorek E, Pinelo M, Meyer AS, Jesionowski T. Co-Immobilization of Glucose Dehydrogenase and Xylose Dehydrogenase as a New Approach for Simultaneous Production of Gluconic and Xylonic Acid. Materials. 2019; 12(19):3167. https://doi.org/10.3390/ma12193167
Chicago/Turabian StyleZdarta, Jakub, Karolina Bachosz, Oliwia Degórska, Agata Zdarta, Ewa Kaczorek, Manuel Pinelo, Anne S. Meyer, and Teofil Jesionowski. 2019. "Co-Immobilization of Glucose Dehydrogenase and Xylose Dehydrogenase as a New Approach for Simultaneous Production of Gluconic and Xylonic Acid" Materials 12, no. 19: 3167. https://doi.org/10.3390/ma12193167
APA StyleZdarta, J., Bachosz, K., Degórska, O., Zdarta, A., Kaczorek, E., Pinelo, M., Meyer, A. S., & Jesionowski, T. (2019). Co-Immobilization of Glucose Dehydrogenase and Xylose Dehydrogenase as a New Approach for Simultaneous Production of Gluconic and Xylonic Acid. Materials, 12(19), 3167. https://doi.org/10.3390/ma12193167










